Abstract
Early-life stress, such as maltreatment, institutionalization, and exposure to violence, is associated with accelerated telomere shortening. Telomere shortening may thus represent a biomarker of early adversity. Previous studies have suggested that responsive parenting may protect children from the negative biological and behavioral consequences of early adversity. This study examined the role of parental responsiveness in buffering children from telomere shortening following experiences of early-life stress. We found that high-risk children had significantly shorter telomeres than low-risk children, controlling for household income, birth weight, gender, and minority status. Further, parental responsiveness moderated the association between risk and telomere length, with more responsive parenting associated with longer telomeres only among high-risk children. These findings suggest that responsive parenting may have protective benefits on telomere shortening for young children exposed to early-life stress. Accordingly, this study has important implications for early parenting interventions.
Keywords: early-life stress, telomeres, child maltreatment, maternal sensitivity, parental behavior
Early-life stress is associated with negative physical and mental health outcomes, such as cardiovascular disease and depression (Cicchetti & Toth, 2005; Edwards, Holden, Felitti, & Anda, 2003; Melchior, Moffitt, Milne, Poulton, & Caspi, 2007). Researchers have turned their attention to identifying biological mechanisms that explain how early adversity (e.g., maltreatment, poverty, exposure to violence) increases risk for pathology later in life (Heim, Shugart, Craighead, & Nemeroff, 2010; Juster, McEwen, & Lupien, 2010; McCrory, De Brito, & Viding, 2010; Neigh, Gillespie, & Nemeroff, 2009). Several key biological systems, including the hypothalamic-pituitary adrenal (HPA) axis and the immune system, appear to play major roles in pathways leading from early adversity to disease (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; McEwen, 1998, 2008). More recent studies show that early-life stress can also cause changes at the cellular level, in the form of accelerated telomere shortening (Price, Kao, Burgers, Carpenter, & Tyrka, 2012; Shalev, 2012; Tyrka et al., 2010). In addition to identifying biomarkers of early adversity, there is a need to examine how environmental factors, such as responsive parenting, can protect children from the negative effects of early-life stress by preventing biological changes. In the current study, we examined telomere shortening in children identified as at high-risk for maltreatment following involvement in the Child Welfare System. Additionally, we examined the potential role of parental responsiveness in buffering the association between early-life stress and telomere shortening.
Telomeres are TTAGGG nucleotide repeats located at the ends of the chromosome (Figure 1; (Blackburn, 2001)). Telomeres function primarily to protect genomic DNA from damage during replication; however, they progressively shorten with each replication cycle, and thus, have been proposed as a marker for biological aging (McEachern, Krauskopf, & Blackburn, 2000). In normal cell lines, telomere shortening can progress to a critical point (e.g., the Hayflick Limit), which triggers cellular senescence (Harley, 1990; Hayflick & Moorhead, 1961). Many factors can influence telomere attrition, including oxidative stress. Of particular interest, oxidative stress can induce telomere shortening in somatic cells, or cells that do not exhibit cellular replication (von Zglinicki, 2002). Whereas telomerase, a ribonucleoprotein enzyme that adds TTAGGG repeats, aids in actively replenishing telomeric repeats during replication in germline and stem cells, this protein complex is not active in somatic cells. Thus, within somatic cell lines, telomere length and accelerated attrition induced by oxidative stress may represent a cumulative marker of chronic stress and provide a link between stress and age-related psychological disorders (O'Donovan et al., 2012; You et al., 2009).
Figure 1.
Simplified illustration of telomere sequences located at the end of chromosomes.
Stress or adversity experienced in childhood has been linked to reduced telomere length across several studies (For a review, see (Price, et al., 2012)). Most of these reports have examined associations between telomere length measured in adulthood and retrospective reports of childhood stressors (e.g., maltreatment, trauma, parental death, familial mental illness, parental unemployment) (Kananen et al., 2010; Kiecolt-Glaser et al., 2011; O'Donovan et al., 2011; Surtees et al., 2011; Tyrka, et al., 2010). Notably, only two studies to date have investigated the association between early adversity and telomere length measured during childhood. In the Bucharest Early Intervention Project, a randomized control trial of foster care versus continued institutional care, Romanian children's telomere length in middle childhood was inversely correlated with the amount of time spent living in an institution in early childhood (Drury et al., 2012). In another study, exposure to violence during childhood (i.e., witnessing domestic violence, experiencing frequent bullying, experiencing physical maltreatment) was associated with the erosion of telomere length between 5 and 10 years of age (Shalev, 2012; Shalev et al., 2012). Specifically, children who experienced two or more types of exposure to violence showed a significantly greater rate of telomere attrition compared to children with no exposure or one type of exposure. Taken together, these findings suggest a dose-dependent association between early-life stress and telomere shortening, with an increased number of adverse experiences, or greater duration of adversity, associated with accelerated telomere shortening (Price, et al., 2012).
Even in the face of early adversity, some children show remarkable resilience across socioemotional, behavioral, and physical health domains (Cicchetti, 2010; Masten, Best, & Garmezy, 1990). Responsive parenting has been implicated as a key protective factor for children who experience early-life stress (Afifi & Macmillan, 2011; Laucht, Esser, & Schmidt, 2001), and may serve as a buffer from physiological changes to the body's stress systems (Gunnar, Fisher, & The Early Experience, Stress, and Prevention Network, 2006). In one study, for example, children's exposure to a greater number of psychosocial (e.g., poverty) and physical (e.g., crowding) risk factors was associated with higher levels of allostatic load (Evans, Kim, Ting, Tesher, & Shannis, 2007), a measure of multiple physiological indicators of stress (McEwen, 2000). The association between risk exposure and allostatic load was found only among children with mothers low in responsiveness, as measured from youth reports of mothers’ instrumental and emotional support, as well as observed sensitivity (i.e., appropriate responses to children's cues) during a cooperative game. Similarly, another study found that maternal responsiveness moderated the associations between early-life risk (i.e., psychosocial disadvantage, low birth weight) and childhood behavioral problems (Laucht et al., 2001). Overall, infants born to psychosocially disadvantaged families showed more internalizing and externalizing problems throughout childhood than children without psychosocial risk. However, high quality early caregiving, rated through micro-level coding of mother and infant interactive behaviors, attenuated the effect of family adversity on children's behavior problems. For children from psychosocially disadvantaged families, higher maternal responsiveness was associated with fewer behavior problems. For children without family adversity, however, maternal responsiveness did not have an effect on children's behavior. In the same study, maternal responsiveness similarly moderated the effect of biological risk (i.e., low birth weight) on behavior problems. Very low birth weight children with high responsive mothers showed a decline in internalizing problems over time, whereas very low birth weight children with low responsive mothers showed an increase in internalizing problems over time. In the absence of this biological risk (i.e., very low birth weight), however, variability in maternal responsiveness showed no analogous effect on children's behavior problems. Together these studies offer a model for examining the protective role of parental responsiveness in preventing negative biological and behavioral sequalae for children who experience early adversity.
In the current study, we examined the role of parental responsiveness in protecting children from the damaging effects of early-life stress on telomere length measured in early childhood. Telomere length was compared between high-risk children, who were identified by the Child Welfare System as being at elevated risk for maltreatment, and low-risk comparison children. Parental responsiveness, coded from observed parent-child interactions, was examined as a moderator of the association between risk and telomere length. We focused specifically on parental responsiveness characterized by synchronous, or “serve and return,” interactions. Synchronous interactions in which a parent contingently responds to his or her child's cues, behaviors, and expressions, are thought to play an especially important role during early childhood in shaping brain circuitry, enhancing behavioral self-control, and maintaining physiological regulation (Feldman, 2007; Shonkoff & Bales, 2011).
Method
Participants
Participants included 100 children and 96 parents (4 parents had two children enrolled in the study). Of these participants, 89 were included in analyses (4 high-risk children and 7 low-risk children were excluded due to outlying telomere values, as described below). At the time of the present study, children ranged in age from 3.9 to 6.5 years old (M = 4.9, SD = .59). “High-risk” children (n = 51) were recruited from an ongoing longitudinal study examining the efficacy of an attachment-based intervention for infants involved in the Child Welfare System. High-risk children and their parents were initially referred to the program as infants (under 20 months old) following involvement in the Child Welfare System. The participating birth parent was the primary caregiver identified by the referring agency. Although children were identified by agencies as at-risk for being maltreated, children remained in their homes with their birth parents as part of the city's diversion from foster care program. Importantly, children in the “high risk” group did not necessarily experience maltreatment at the time of referral or subsequently. Limited access to families’ records prevented detailed characterization of children's history of early adversity; however, the conditions noted most often included child neglect, domestic violence, parental substance use, and inadequate housing. “Low-risk” comparison children (n = 38) were recruited from local childcare centers, through flyers distributed throughout the community, and through announcements posted on a University website. Parents in the “low-risk” group denied involvement with the Child Welfare System, as assessed through an initial screening question. All parents were mothers, with the exception of 2 fathers in the low-risk comparison group. See Table 1 for demographic characteristics of each group.
Table 1.
Child Demographic Characteristics.
| Variable | Low-risk (n = 38) | High-risk (n = 51) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Male | 16 | 42% | 33 | 65% |
| Female | 22 | 58% | 18 | 35% |
| Ethnicity | ||||
| White | 8 | 21% | 5 | 10% |
| African American | 26 | 69% | 40 | 78% |
| Hispanic | 2 | 5% | 3 | 6% |
| Bi-racial | 2 | 5% | 3 | 6% |
| Mean (SD) | Min – Max | Mean (SD) | Min – Max | |
|---|---|---|---|---|
| Age (years) | 5.0 (.60) | 4.1 – 6.5 | 4.9 (.59) | 3.9 – 6.2 |
| Birth weight (grams) | 115.2 (18.5) | 67 – 147 | 113.6 (17.9) | 71 – 170 |
| Income | $55,835 (51,244) | $2,400-$200,000 | $14,526 (12,253) | $2,000-$60,000 |
| Log-transformed income | 4.6 (.45) | 3.8 – 5.3 | 4.0 (.34) | 3.3 – 4.8 |
Procedure
At the time of the present study, high-risk participants were already enrolled in a longitudinal study and had previously expressed interest in being contacted about additional research opportunities. Low-risk participants expressed interest via a phone call or email after receiving information from the recruitment sources described above. Research staff contacted parents by phone to describe the research protocol, and scheduled a home visit if parents were interested in participating. At the home visit, parent consent and child assent were obtained. Parents completed a demographic questionnaire to obtain information about parents’ and children's race/ethnicity and age, household income, and children's birth weight. Parents and children were videotaped during a 20-minute standardized parent-child interaction task to assess parental responsiveness. The research staff collected children's DNA by brushing each side of the child's cheeks for 20 seconds using SK-1 buccal swabs (Boca Scientific, Boca Raton, FL). Buccal swabs were temporarily stored in a small cooler with ice packs during transport to the laboratory. Parents received $50 and children received a small toy for their participation.
Measures
Telomere length
Buccal swabs were stored at −20°C until DNA was extracted. DNA was extracted via manufacturer's instructions with Puregene Buccal Cell Kit (Qiagen, Valencia, CA) with the only modification being incubation in Protinease K solution for 1.5 hours. The extracted DNA was quantified and assessed for purity via nanodrop spectrophotometry. Subsequently, aliquots of extracted DNA samples were diluted to 10 ng/μL and placed in −20°C for short-term storage. Relative telomere length was measured using a standard curve based quantitative PCR (qPCR) reaction as previously reported (Cawthon, 2002; O'Callaghan & Fenech, 2011) on a Bio-Rad CFX96 real-time PCR system. 20ng of sample DNA was assayed via primers specific to telomeres (T; ForwardTEL: 5’-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3’, ReverseTEL: 5’-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3’) and in a separate PCR plate against a single copy gene (S), acidic ribosomal phosphoprotein 36B4 (Forward36B4: 5’-CAGCAAGTGGGAAGGTGTAATCC-3’, Reverse36B4: 5’-CCCATTCTATCATCAACGGGTACAA-3’). For each participant, the (T) and (S) qPCR assays were carried out in triplicate on different 96-well plates. A participant's DNA was pipetted into the same well positions for the separate plates (T and S) to reduce well-to-well variability (36). Triplicate measurements outside of a one Ct deviation were excluded from the calculation of the sample average similar to O'Callaghan (37). Six dilutions of a reference DNA spanning 2x concentrations from 2.5 ng/μL to 80 ng/μL were used to construct a standard curve similar to Cawthon (Cawthon, 2002).
A T/S ratio was constructed for each sample by: (1) subtracting the average of the triplicate telomere samples Ct from the whole plate Ct average (ΔCtel); (2) subtracting the average triplicate 36B4 samples Ct from the whole plate Ct average (ΔC36B4); and, (3) taking the ratio of T to S ((2^(ΔCtel)) / (2^(ΔC36B4))). Efficiency for qPCR reactions was well within accepted ranges between plates for both telomere (SD = 5.8%) and single copy gene (SD = 1.3%). The inter-assay coefficient of variation for the standards in telomere PCR was 0.73% and for the 36B4 PCR was 0.44%, demonstrating very low measurement error. The intra-assay coefficient of variation for the individual triplicate samples analyzed for telomeres was 0.71% and for 36B4 was 0.46%. Each plate contained a representative sample of high-risk and low-risk children of which individual sample identity was blind to the researcher. After the T/S ratio was calculated, T/S ratios for each plate beyond 2 standard deviations were excluded from further analysis before collapsing across all T/S ratios. Any sample that was excluded from analysis (e.g., the T/S ratio was beyond 2 standard deviations, or amplified beyond the range of the standards) was re-assayed to confirm the Ct measurements obtained were replicable (n = 11). The Pearson correlation coefficient between the first and second triplicate measurements for the telomere PCR was r = .94 and for 36B4 PCR r = .92, p < .001 for both, indicating a high correlation between re-measurement of excluded samples and a low probability of experimenter error in the biochemical analysis.
Parental responsiveness
Parents and children were videotaped during a 20-minute semi-structured interaction task (NICHD Early Child Care Research Network, 1999, 2003). Parent-child dyads were asked to play with toys from three boxes in a set order. The first box contained a set of markers, construction paper, stickers, and stencils; the second box contained a cash register toy and dress-up clothes; and the third box contained a set of Duplo Legos. Parents were instructed to play with their children as they normally would, to present the boxes in the specified order, and to spend some time on each box. Undergraduate coders were blind to participants’ group and other information about the families. Parental responsiveness to non-distress was coded on a 5-point Likert scale, based on procedures used in previous studies (1999). At high levels of responsiveness, parents “follow the child's lead” by responding contingently to the child's signals, expressions, and behaviors in ways that are well timed and child-centered. At low levels, parents engage in non-synchronous interactions, in which they might appear detached and unresponsive, or over-stimulating and intrusive. All coders met acceptable levels of inter-rater reliability on practice videos. Twenty-five percent of the videotapes were double-coded and the Spearman correlation between coder scores was .80. Mean scores for double-coded videotaped were used for primary analyses. Parent responsiveness ranged from 1 to 5 (M = 3.03, SD = 1.01).
Results
Preliminary Analyses
Preliminary analyses examined between-group differences in demographic characteristics (i.e., child age, child gender [dummy coded as 0 for male, 1 for female], minority status [dummy coded as 0 for non-minority, 1 for minority], household income), as well as associations between demographic characteristics and variables of interest (i.e., telomere length, parental responsiveness). As mentioned above, high-risk children were recruited from an ongoing longitudinal study examining the effectiveness of an attachment-based parenting intervention through a randomized clinical trial. Given that there were no intervention effects on children's telomere length (p > .05), high-risk children from the experimental and control intervention groups were grouped together for primary analyses. A Chi-square test revealed no significant group difference in the distribution of children by minority status, and a t-test showed no group difference in children's age (p's > .05). A Chi-square test revealed that the high-risk group had a higher male-to-female ratio than the low-risk group, χ2 (1, 89) = 4.50, p < .05. On average, low-risk participants reported higher household income (log-transformed due to positive skew) than high-risk participants, t(1,87) = -6.39, p < .01. Telomere length was correlated with children's birth weight, r = .22, p < .05, but was not associated with child age, gender, minority status, or household income. Parental responsiveness was correlated with log-transformed household income, r = .23, p < .05. On average, parents of minority children demonstrated lower parental responsiveness than parents of non-minority children, t(1,87) = 1.98, p < .05. Parental responsiveness did not differ between low-risk and high-risk groups, and was not associated with child gender or age. Given the significant group differences and/or associations with primary variables of interest, log-transformed household income, birth weight, gender, and minority status were included as covariates in primary analyses.
Primary Analyses
Group differences in relative telomere length
An analysis of covariance was conducted to examine group differences in telomere length, controlling for log-transformed household income, children's birth weight, gender, and minority status. Telomere length was shorter in high-risk children compared to low-risk children, F(1, 83) = 4.37, p < .05 (See Figure 2). Due to concerns about the non-independence of data collected from siblings, analyses were also conducted excluding one child from each sibling pair. There were four sets of siblings in the full data set, but two sets of siblings had one child with outlying telomere data. Thus, only two children were excluded in this secondary analysis. The results remained significant when excluding one child from each sibling pair, F(1, 81) = 4.79, p < .05.
Figure 2.
Relative telomere length in Low-risk versus High-risk children. Error bars are ± S.E.M, *p <.05.
Parental responsiveness and telomere length
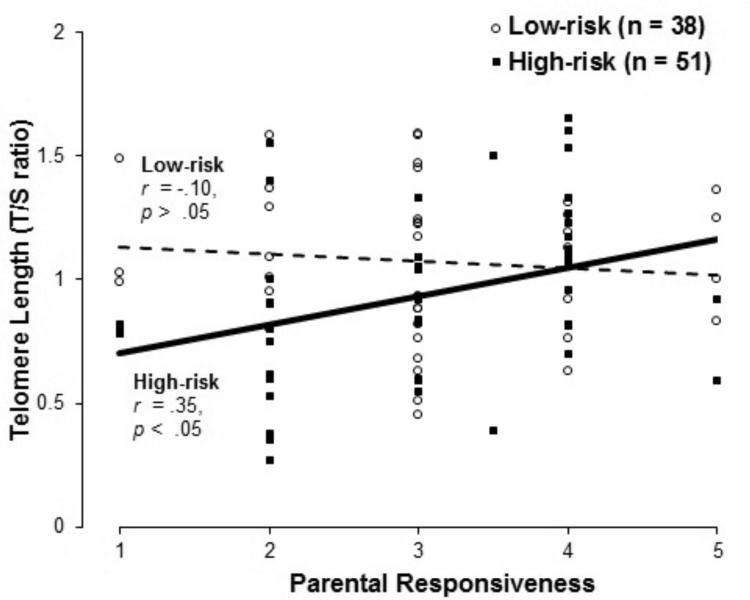
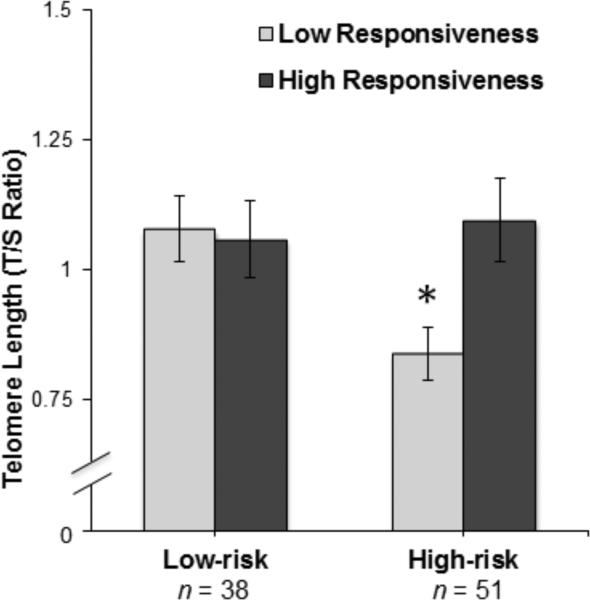
Multiple linear regression analyses were conducted to examine the association between parental responsiveness and telomere length. Covariates (i.e., log-transformed household income, children's birth weight, children's gender, children's minority status) were entered into Step 1 of the model. Risk group (low-risk vs. high-risk), parental responsiveness, and a Risk × Responsiveness interaction term were added in at Step 2, with telomere length as the dependent variable. The results are presented in Table 2. Step 1 accounted for 3.8% of the variance in telomere length and was not statistically significant. Step 2 accounted for 19.8% of the variance, representing a significant change in R-square, F(3, 82) = 5.44, p < .01. Notably, the Risk × Responsiveness interaction was significant (Standardized β = −.94, p < .01), indicating that parental responsiveness moderated the association between risk group and telomere length (See Figures 3a and 3b). To further examine this interaction, analyses were conducted separately for the high- and low-risk groups. Parental responsiveness predicted telomere length among high-risk children, with more responsive parenting associated with longer telomeres (r = .35, p < .05), but not among low-risk children (r = −.10, p > .05). The results remained significant when excluding the second child of the two sibling pairs.
Table 2.
Linear Regression Model for Telomere Length.
| Variable | B | SE | t | p |
|---|---|---|---|---|
| Step 1 | ||||
| Log-transformed income | −.04 | .08 | −.05 | .66 |
| Birth weight | .004 | .002 | 2.03 | .05 |
| Gender | .14 | .07 | 2.02 | .05 |
| Minority status | −.04 | .11 | −.34 | .74 |
| (Constant) | .68 | .46 | 1.48 | .14 |
| Step 2 | ||||
| Risk group | .74 | .21 | 3.44 | .001 |
| Responsiveness | .12 | .04 | 2.91 | .005 |
| Risk × Responsiveness | −.18 | .07 | −2.77 | .007 |
| (Constant) | .62 | .50 | 1.23 | .22 |
Note. R2 = .08 for Block 1 (p > .05); ΔR2 = .14 for Block 2 (p < .01).
Figure 3a.
Moderating role of parental responsiveness (graphed continuously) on the association between early-life risk and telomere length. Trend lines show that parental responsiveness predicted telomere length in high-risk children, but not in low-risk children. Data points represent individual participant's relative telomere length (along the y-axis) by parental responsiveness score (along the x-axis). For the 25% of participants with double-coded parental responsiveness data, plotted data points reflect the average of both coders’ scores, which is why 2 scores fall between major scale points.
Figure 3b.
Moderating role of parental responsiveness on the association between early-life risk and telomere length (graphed as median split for illustrative purposes). Error bars are ± S.E.M, *p < .05.
Discussion
Telomere length was significantly shorter in high-risk children with previous involvement in the Child Welfare System, relative to low-risk comparison children. Further, parental responsiveness moderated the association between early adversity and telomere length, with higher parental responsiveness predicting longer telomeres only among high-risk children. These findings remained significant after controlling for household income, birth weight, gender, and minority status. Taken together, these findings support the critical role of high quality parenting behavior in modifying the biological impact of early-life stress.
Early-life stress appears to affect telomere biology very early in childhood, by 4 to 6 years of age, suggesting that changes in telomere length may manifest at a much earlier age than has been typically assessed. In peripheral blood mononuclear cells (PBNCs), telomeres are thought to shorten at an accelerated rate in early-life (e.g. approximately 170 base-pairs per year) when compared to adult life (e.g. approximately 30-50 base-pairs per year) (Zeichner et al., 1999). However, the actual rate of loss and the factors governing inter-individual variability in the rate of loss in similarly aged persons are still unclear (Benetos et al., 2001; Frenck, Blackburn, & Shannon, 1998; Hewakapuge, van Oorschot, Lewandowski, & Baindur-Hudson, 2008). In the current study, we found no association between age and telomere length, perhaps due to the intentionally restricted age range or developmental period studied. Prospective longitudinal studies, that include multiple repeated measurements of telomere length from infancy through childhood, are needed to more systematically characterize normative and deviant patterns of telomere shortening.
As increasing evidence continues to support the association between various forms of childhood stress and telomere shortening at the group level, more work is needed to understand individual-level factors that offer protective benefits or contribute to risk. Here, we find that responsive parenting plays a major role in buffering high-risk children from the negative effects of early adversity on telomere length. Our findings complement previous studies, which have showed that the adverse effects of psychosocial, biological, and physical risk experienced in childhood are attenuated in the context of an available and supportive caregiver (Evans et al., 2007; Laucht et al., 2001). A number of studies support the role of synchronous parent-child interactions as a key factor in children's development of biological and behavioral self-regulation (Feldman, 2007; Raver, 1996; Rocissano, Lynch, & Slade, 1987; Winberg, 2005). However, further research is needed to examine whether other aspects of responsive parenting (e.g., sensitivity to distress) or just positive caregiving more generally, offer the same buffering effects.
Importantly, these findings highlight that intervention programs for high-risk children should be implemented early and should aim to enhance positive parenting behaviors, specifically parent-child synchrony. Early parenting interventions have been shown to prevent biological dysregulation, as assessed through diurnal cortisol production, among children who experience early adversity. For example, Cicchetti, Rogosch, Toth, and Sturge-Apple (2011) found that maltreated children who received early preventative interventions, including Child-Parent Psychotherapy or a psychoeducational parenting intervention, maintained morning levels of cortisol that were similar to non-maltreated comparison children. Maltreated children who received care as usual, however, showed increasingly lower morning cortisol levels over time. These results parallel those found by Fisher, Stoolmiller, Gunnar, and Burraston (2007), in which a family-based intervention (i.e., Multidimensional Treatment Foster Care for Preschoolers) prevented the blunting of the diurnal cortisol rhythm typically seen among foster children. Future studies can examine whether similar interventions designed to improve parental responsiveness have an effect on stabilizing or even increasing telomere length. By incorporating multiple assessments of telomere length (i.e., before the intervention, after the intervention, and at follow-up intervals), these studies can characterize intervention effects on telomere length over time.
Currently, the mechanisms regulating reduced telomere length following early-life stress are not well understood (Shalev, 2012). Dysregulation of the hypothalamic-pituitary adrenal (HPA) axis may represent one mechanism through which stress leads to telomere attrition. The HPA axis mounts a response to acute stressors, resulting in increased circulating levels of glucocorticoids (i.e. cortisol in humans) (Gunnar & Quevedo, 2007). Increased circulating levels of glucocorticoids result in increased production of free reactive oxygen species, which can yield single-strand breaks in telomeres that are unable to be repaired (Houben, Moonen, van Schooten, & Hageman, 2008; Petersen, Saretzki, & von Zglinicki, 1998; You et al., 2009). Repeated exposure to oxidative stress increases the frequency of these breaks in telomeres (Petersen et al., 1998). Recent evidence supports this association between acute psychosocial stress, cortisol, and telomere length. For example, greater cortisol reactivity, following exposure to an acute stressor, is found to predict shorter telomere length in postmenopausal women (Tomiyama et al., 2012). Interestingly, in early childhood, most children appear to go through a stress hyporesponsive period, during which cortisol does not increase following stressors (For a review, see Gunnar, Talge, & Herrera, 2009). This period of cortisol hyporesponsivity may be similar to that observed in rodents from postnatal day 4 to 14 (Moriceau, Roth, & Sullivan, 2010; Sapolsky & Meaney, 1986), and presumably protects the developing brain from the deleterious effects of elevated cortisol (Gunnar et al., 2006). However, for children without a responsive caregiver, particularly those with disorganized attachments, cortisol continues to increase to stressors in early childhood (Bernard & Dozier, 2010; Hertsgaard, Gunnar, Erickson, & Nachmias, 1995; Spangler & Grossmann, 1993). This repeated exposure to cortisol in childhood via increased reactivity of the HPA axis may contribute to telomere shortening in children from high-risk environments, particularly in the absence of responsive caregivers.
In addition to mounting a stress response, the HPA axis regulates circadian patterns of activity by maintaining a diurnal rhythm of cortisol production (Gunnar & Cheatham, 2003). Typically, cortisol peaks approximately 30 minutes after wake-up, and decreases across the day to a bedtime nadir (Gunnar & Donzella, 2002; Larson, White, Cochran, Donzella, & Gunnar, 1998). Children who experience early-life stress have perturbed patterns of diurnal cortisol production, often marked by lower wake-up levels and blunted wake-up to bedtime slopes. Although this blunted diurnal pattern has been observed in foster children and previously institutionalized children (Bruce, Fisher, Pears, & Levine, 2009; Bruce, Kroupina, Parker, & Gunnar, 2000; Carlson & Earls, 1997; Dozier et al., 2006; Fisher, Gunnar, Dozier, Bruce, & Pears, 2006), it is especially evident in maltreated children that continue to live with their high-risk birth parents (Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010). In a study of postmenopausal women, flatter diurnal cortisol slopes were associated with shorter telomere length (Tomiyama et al., 2012). Thus, telomere shortening may be associated with dysregulation of both the stress reactivity and diurnal functions of the HPA system.
Another possible mechanism through which early-life stress may lead to telomere shortening is via changes to markers of immune functioning. In addition to being associated with glucocorticoids, production of reactive oxygen species is associated with increased production of proinflammatory cytokines (Floyd et al., 1999). Research has shown an inverse relationship between telomerase activity and the increased production of proinflammatory cytokines, which may also influence telomere biology in relation to psychosocial stress in early-life (Beyne-Rauzy et al., 2005; Danese, Pariante, Caspi, Taylor, & Poulton, 2007). Multi-method studies, in which biological samples across systems (i.e., cortisol, proinflammatory cytokines, and telomeres) are collected, can further inform our understanding of the mechanisms by which glucocorticoids and proinflammatory cytokines may influence telomere length (e.g., via changes in telomerase, hTERT, TRF1/2, or TNF-α activity).
The present research benefits from a number of methodological strengths. Notably, reports of risk exposure and parental responsiveness were not contingent on parent-report or self-report. In fact, agencies within the Child Welfare System identified and referred the high-risk children due to risk of neglect and maltreatment suspected during infancy. Additionally, parental responsiveness was measured observationally through psychometrically sound and well validated procedures (NICHD Early Child Care Research Network, 1999, 2003). This study also had limitations as described by other research examining telomere length (Drury et al., 2012; Shalev et al., 2012). First, the use of a cross-sectional versus longitudinal design presents a limitation in examining the stress-related reduction of telomere length over time. Second, the use of qPCR to measure relative telomere length can produce high variability, although variation in the measurement of our samples was low. However, relative telomere length measurement using qPCR has several advantages in that it requires a small amount of the participant's DNA, is specific to only telomeric regions (vs. subtelomeric regions as measured through the terminal restriction fragment method), and offers a high-throughput method for measuring many samples in a short period of time (Hewakapuge, van Oorschot, Lewandowski, & Baindur-Hudson, 2008). Third, limited information regarding the type, severity, and chronicity of adverse experiences of the high-risk children in our study prevented examination of the possible dose-dependent associations between early-life stress and telomere length, as well as the examination of maltreatment subtype on telomere reduction. Limited information about the timing of participants’ experiences of early adversity also prevents the identification of possible sensitive periods in development, during which telomeres may be particularly susceptible to stress.
Despite these limitations, our research highlights the buffering role of parenting behavior on telomere biology following early life-stress. This study has important implications for preventative interventions that are implemented early and specifically target parental responsiveness, and also suggests that changes to telomere biology may be an additional way to measure the efficacy of early interventions for high-risk children.
Acknowledgements
This research was supported by funding from Edna Bennett Pierce and from NIH grants R01 MH074374, R01 MH084135, and R01 MH052135 to M. Dozier.
Footnotes
Citation: Asok, A., Bernard, K., Roth, T. L., Rosen, B., & Dozier, M. (In press). Parental responsiveness moderates the association between early-life stress and reduced telomere length. Development and Psychopathology.
References
- Afifi TO, Macmillan HL. Resilience following child maltreatment: a review of protective factors. Canadian Journal of Psychiatry. 2011;56(5):266–272. doi: 10.1177/070674371105600505. [DOI] [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2 Part 2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Archives of Pediatrics and Adolescent Medicine. 2010;164(5):438–443. doi: 10.1001/archpediatrics.2010.54. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M. Examining infants' cortisol responses to laboratory tasks among children varying in attachment disorganization: Stress reactivity or return to baseline? Developmental Psychology. 2010;46(6):1771–1778. doi: 10.1037/a0020660. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyne-Rauzy O, Prade-Houdellier N, Demur C, Recher C, Ayel J, Laurent G, Mansat-De Mas V. Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood. 2005;106(9):3200–3205. doi: 10.1182/blood-2005-04-1386. doi: 10.1182/blood-2005-04-1386. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–673. doi: 10.1016/s0092-8674(01)00492-5. doi: S0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51(1):14–23. doi: 10.1002/dev.20333. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Kroupina M, Parker S, Gunnar MR. The relationships between cortisol patterns, growth retardation, and developmental delays in postinstitutionalized children.. Paper presented at the International Conference on Infant Studies; Brighton, UK. 2000. [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:145–154. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Development and Psychopathology. 2011;23:789–800. doi: 10.1017/S0954579411000307. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of Nattional Academy of Sciences. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. doi: 0610362104 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Levine S. Foster children's diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11(2):189–197. doi: 10.1177/1077559505285779. doi: 11/2/189 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Nelson CA. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Molecular Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. doi: 10.1038/mp.2011.53 mp201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry. 2003;160(8):1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48(3-4):329–354. doi: 10.1111/j.1469-7610.2006.01701.x. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Annals of New York Academy of Science. 2006;1094:215–225. doi: 10.1196/annals.1376.023. doi: 1094/1/215 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinolgy. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Jaffery F, Maidt L, Robinson K, Pye Q, Stewart C. Increased oxidative stress brought on by pro-inflammatory cytokines in neurodegenerative processes and the protective role of nitrone-based free radical traps. Life Sciences. 1999;65(18-19):1893–1899. doi: 10.1016/s0024-3205(99)00443-9. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. Brain and behavior interface: Stress and the developing brain. Infant Mental Health Journal. 2003;24(3):195–211. doi: 10.1002/Imhj.10052. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. doi: S0306453001000452. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA, The Early Experience, Stress, and Prevention Network Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Developmental Psychopathology. 2006;18(3):651–677. [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB. Aging of cultured human skin fibroblasts. Methods for Molecular Biology. 1990;5:25–32. doi: 10.1385/0-89603-150-0:25. doi: 10.1385/0-89603-150-0:25. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Research. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology. 2010;52(7):671–690. doi: 10.1002/dev.20494. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66(4):1100–1106. [PubMed] [Google Scholar]
- Hewakapuge S, van Oorschot RA, Lewandowski P, Baindur-Hudson S. Investigation of telomere lengths measurement by quantitative real-time PCR to predict age. Legal Medicine (Tokyo) 2008;10(5):236–242. doi: 10.1016/j.legalmed.2008.01.007. doi: 10.1016/j.legalmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radical Biology & Medicine. 2008;44(3):235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. doi: PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology. 1998;33(4):327–337. doi: 10.1002/(SICI)1098-2302(199812)33:4<327::AID-DEV4>3.0.CO;2-S. [PubMed] [Google Scholar]
- Laucht M, Esser G, Schmidt MH. Differential development of infants at risk for psychopathology: The moderating role of early maternal responsivity. Developmental Medicine and Child Neurology. 2001;43(5):292–300. doi: 10.1017/s0012162201000561. [DOI] [PubMed] [Google Scholar]
- Masten A, Best K, Garmezy N. Resilience and development: Contributions from the study of children who overcome adversity. Development and Psychopathology. 1990;2:425–444. [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. doi: 10.1111/j.1469-7610.2010.02271.x JCPP2271. [DOI] [PubMed] [Google Scholar]
- McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annual Review of Genetics. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–15. doi: 10.1016/j.metabol.2008.07.006. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Journal of Epidemiology. 2007;166(8):966–974. doi: 10.1093/aje/kwm155. doi: kwm155 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma, Violence, and Abuse. 2009;10(4):389–410. doi: 10.1177/1524838009339758. doi: 1524838009339758 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network Early child care and mother-child interaction from 36 months through first grade. Infant Behavior and Development. 2003;26:345–370. [Google Scholar]
- NICHD Early Child Care Research Network Child care and mother-child interaction in the first 3 years of life. Developmental Psychology. 1999;35(6):1399–1413. [PubMed] [Google Scholar]
- O'Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biological Procedures Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26(4):573–579. doi: 10.1016/j.bbi.2012.01.007. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Experimental Cell Research. 1998;239(1):152–160. doi: 10.1006/excr.1997.3893. doi: S0014482797938933. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.025. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC. Relations between social contingency in mother-child interaction and 2-year-olds' social competence. Developmental Psychology. 1996;32(5):850–859. doi: Doi 10.1037/0012-1649.32.5.850. [Google Scholar]
- Rocissano L, Lynch V, Slade A. Dyadic Synchrony and Toddler Compliance. Developmental Psychology. 1987;23(5):698–704. doi: Doi 10.1037/0012-1649.23.5.698. [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.32. doi: 10.1038/mp.2012.32 mp201232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Bales SN. Science does not speak for itself: Translating child development research for the public and its policymakers. Child Development. 2011;82(1):17–32. doi: 10.1111/j.1467-8624.2010.01538.x. doi: 10.1111/j.1467-8624.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann KE, Early Experience, Stress, and Prevention Network Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64(5):1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66(11):1152–1162. doi: 10.1093/gerona/glr112. doi: glr112 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Epel E. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & Behavior. 2012;106(1):40–45. doi: 10.1016/j.physbeh.2011.11.016. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. doi: S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Winberg J. Mother and newborn baby: Mutual regulation of physiology and behavior - A selective review. Developmental Psychobiology. 2005;47(3):217–229. doi: 10.1002/dev.20094. doi: 10.1002/Dev.20094. [DOI] [PubMed] [Google Scholar]