Abstract
siRNA LOR-1284 targets the R2 subunit of ribonucleotide reductase (RRM2) and has shown promise in cancer therapy. In this study, transferrin (Tf) conjugated lipid nanoparticles (Tf-NP-LOR-1284) were synthesized by microfluidic hydrodynamic focusing (MHF) and evaluated for targeted delivery of LOR-1284 siRNA to acute myeloid leukemia (AML) cells. In vitro study showed that Tf-NP-LOR-1284 can protect LOR-1284 from serum nuclease degradation. Selective uptake of Tf-NP-LOR-1284 was observed in MV4–11 cells. In addition, qRT-PCR and Western blot results revealed that Tf-NP-LOR-1284 was more effective than free LOR-1284 in reducing the R2 mRNA and protein levels. Tf-NP-LOR-1284 showed prolonged circulation time and increased AUC after i.v. administration relative to free LOR-1284. Furthermore, Tf-NP-LOR-1284 facilitated increased accumulation at the tumor site along with decreased R2 mRNA and protein expression in a murine xenograft model. These results suggest that Tf-conjugated NPs prepared by MHF provide a suitable platform for efficient and specific thereapeutic delivery of LOR-1284 to AML.
Keywords: Microfluidics, Lipid nanoparticles, Transferrin, siRNA, Acute myeloid leukemia
1. Background
Acute myeloid leukemia (AML), one of the most common types of leukemia, is characterized by clonal accumulation and expansion of immature myeloid cells in the bone marrow. It is a lethal disease for which current therapy is of limited effectiveness. Small interfering RNAs (siRNA) are able to specifically silence gene expression and hold great promise as therapeutic agents for a variety of diseases including AML 1. LOR-1284, an siRNA targeting the R2 subunit of ribonucleotide reductase (RRM2), is considered a promising agent for overcoming drug resistance associated with AML 2, 3. LOR-1284 has demonstrated strong antitumor activity in several human tumor models, including renal cell carcinoma, melanoma, and colon adenocarcinoma 4. In vitro studies have shown that LOR-1284 mediated downregulation of R2, which coincides with a decrease in cellular proliferation and cell cycle inhibition in several human tumor cell lines including A498 (renal carcinoma), A2058 (melanoma), and HT-29 (colon adenocarcinoma), in a sequence-specific manner 4. Furthermore, in vivo studies have shown that LOR-1284 significantly inhibits the growth of murine xenograft tumors in a dose-dependent manner 4. These data suggests that LOR-1284 has great potential as a treatment for human cancers and leukemia. However, unlike antisense oligodeoxyribonucleotides (ODNs), which have yielded promising results in clinical trials 5, 6, siRNA treatment has yet to be evaluated in AML patients .
One of the major challenges in siRNA based therapy is systemic delivery to the tumor site 7. Due to a relatively high molecular weight (14–20 kDa) and high charge density, siRNA is unable to get across the cell membrane effectively. Furthermore, siRNA is susceptible to degradation by serum nucleases. Lipid nanoparticles (NPs), meanwhile, have been recognized as promising delivery vehicles for siRNA due to their biocompatibility and ease of production 8–10. Several technologies have been used in the production of siRNA loaded NPs; including detergent dialysis, ethanol dilution, freeze-thawing and thin-film hydration 11–13. NPs are synthesized by self-assembly upon mixing of cationic and anionic components. In conventional methods, i.e., bulk-mixing (BM), lipids and siRNAs spontaneously assemble into heterogeneous nanostructures based on electrostatic interactions, a process that often yields particles with a high degree of polydispersity. Microfluidics (MF) is a recent development in nanotechnology that is able to provide well-defined mixing parameters 14, 15. It is a powerful technology capable of manipulating liquid flows at the microliter scale, resulting in particles with much lower polydispersity. Recently, lipid-based ODN and siRNA nanoparticles have been produced in a microfluidic hydrodynamic focusing (MHF) device 14, 16
Tf receptor (TfR), also named CD71, is a transmembrane glycoprotein (180 kDa) commonly overexpressed on proliferating cells including AML 17, 18. Holo-Tf is an 80 kDa iron-transporting glycoprotein with high affinity for the TfR. TfR binding induces internalization of Tf-conjugated nanoparticles via receptor-mediated endocytosis. TfR expression can be upregulated by iron sequestration by a chelator, such as deferoxamine 19. Tf and antibody to TfR have been evaluated as targeting agents in many published studies for drug, gene, ODN, and siRNA delivery 6, 16, 20. Furthermore, Tf receptor targeted nanoparticles carrying plasmid DNA or ODN have been shown to have enhanced transfection efficiency in Tf-overexpressing tumor cells 21.
In this study, a Tf-NP based siRNA delivery system was introduced for targeted delivery of LOR-1284 to AML cells. MHF was used to prepare Tf-NP-LOR-1284. The serum stability and cytotoxicity of the NPs were evaluated by agarose gel electrophoresis and MTS, respectively. Cellular uptake and intracellular localization of Tf-NP-LOR-1284 were observed by flow cytometry and by confocal microscopy, respectively. The gene regulation effect of Tf-NP-LOR-1284 in vitro was evaluated by qRT-PCR and Western blot analyses. In vivo pharmacokinetics and biodistribution of Tf-NP-LOR-1284 in xenograft tumor-bearing nude mice was investigated. Finally, the downregulation of R2 mRNA and protein expression in vivo were studied following i.v. administration of Tf-NP-LOR-1284 in the xenograft model.
2. Methods
2.1. Materials
3β-[N-(N’, N’-dimethylaminoethane)-carbamoyl] cholesterol hydrochloride (DC-Chol), 1,2- Dioleyloxy-3-trimethylammonium propane (DOTMA) and methoxy-polyethylene glycol (MW 2000) distearoyl phosphatidylethanolamine (mPEG-DSPE) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). 1,2-Dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA) was purchased from Genzyme Pharmaceuticals (Cambridge, MA, USA). Egg phosphatidylcholine (egg PC) was obtained from Lipoid (Newark, NJ, USA). Human holo-Tf and 2-iminothiolane (Traut’s reagent), and other chemicals and reagents used were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All tissue culture media and supplies were obtained from Invitrogen (Carlsbad, CA, USA). All reagents used were of analytical grade or better. The MF chip was purchased from Translume, Inc (Ann Arbor, MI, USA). LOR-1284 siRNA (sense sequence: 5'GAGAGUAGGCGAGUAUCAGdTdT 3'), Cy3-, Cy5- and FAM- labeled LOR-1284, and negative control siRNA were custom synthesized by Qiagen (Germantown, Maryland, USA).
2.2. Preparation of Tf-NP-LOR-1284 by MHF
Cationic NPs were prepared by ethanol injection method 22. Briefly, cationic and helper lipids (DOTMA, DODMA or DC-Chol)/EggPC/mPEG-DSPE at molar ratio of 45/54/1 were dissolved in ethanol and quickly injected into HEPES buffer (20mM HEPES, pH 7.4). The ethanol was then removed by dialysis against HEPES buffer using a MWCO 10 kDa Float-A-Lyzer.
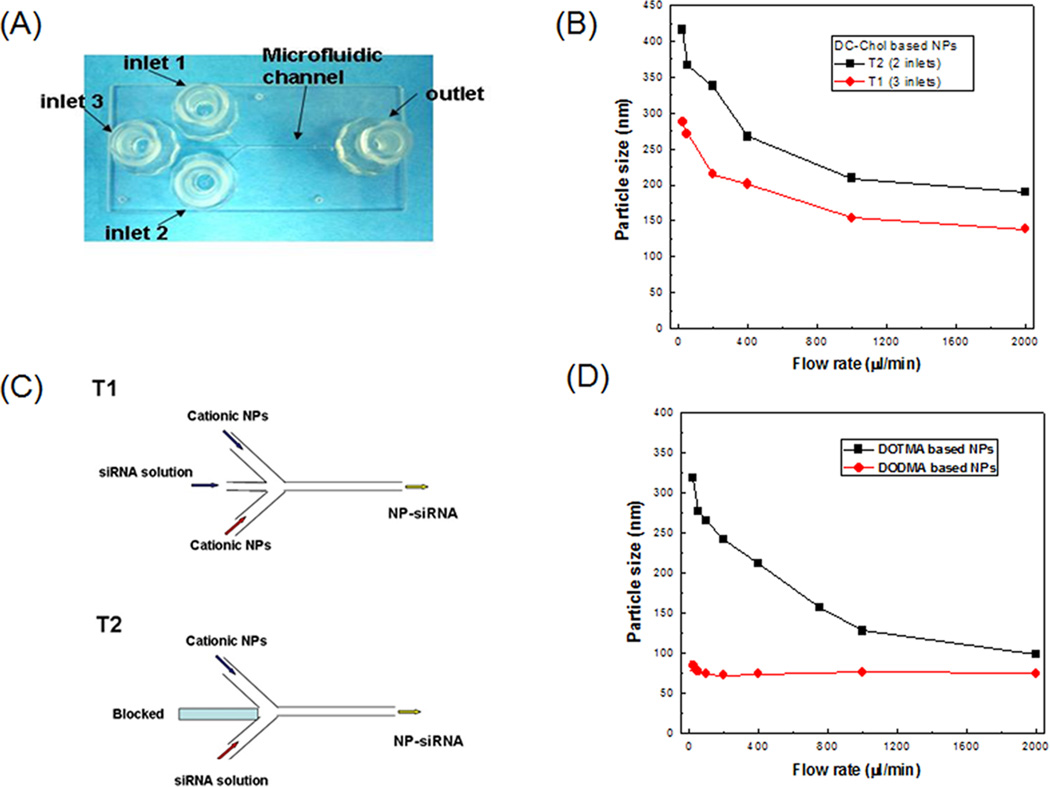
A microfluidic system with two syringe pumps and a 3-inlet microfluidic chip was constructed for the synthesis of NPs containing siRNAs. As shown in Figure 1a, a glass MF chip with a “Y”-channel design was used. The MHF preparation of siRNA-loaded NPs was performed by constant-rate infusion of cationic NPs and siRNA in HEPES buffer through the inlets. Two flow patterns were evaluated (Figure 1b). In the flow pattern of T1, the siRNA solution (25 µg/ml) was injected at inlet 3. Meanwhile, cationic NPs (125 µg/ml) were injected through inlets 1 and 2. The flow rates of three streams were identical. The resulting NP-siRNA was collected at the outlet port. In the flow pattern of T2, the siRNA solution (25 µg/ml) and the cationic NPs (250 µg/ml) were injected at the same flow rate in inlets 1 and 2, respectively. Inlet 3 was blocked. The resulting NP-siRNA was collected at the outlet port in which the weight ratio of total lipids: siRNA was 10:1. The effect of shear stress on MHF preparation of NP-siRNA was investigated by varying the flow rate from 25 to 2000 µl/min. The synthesis was repeated at least three times under each set of conditions.
Figure 1. Synthesis of siRNA-loaded NPs by MHF.
(A) Photographs of the MF chip. The device consisted of three inlet ports and one outlet port. The inlet ports were connected to sterile syringes containing either cationic NPs or siRNA buffer solution. (B) Schematics for flow patterns T1 and T2. The effects of flow patterns and flow rates on the particle size of Tf-NP-siRNA for DC-Chol based NPs (C) and DOTMA, DODMA based NPs (D), respectively. Results are presented as mean ± SD of three independent experiments.
Tf was conjugated to the surface of NPs by post-insertion and the molar ratio of Tf/lipids was 1/1000 19, 23, 24. First, human holo-Tf was thiolated at its N-terminus with 2-iminothiolane (Traut’s reagent) in PBS (pH=8.0) and purified by gel filtration on a PD-10 column. The thiolated Tf (Tf-SH) was then reacted with micelles of Mal–PEG–DSPE at a protein-to-lipid molar ratio of 1:10 for 2 h at room temperature in PBS (pH=6.5) and dialyzed using a SpectraPor Float-A-Lyzer MWCO 5000Da (Spectrum Labs, Rancho Dominguez, CA) against 1× PBS (pH=7.4) to form Tf–PEG–DSPE. The Tf ligand was incorporated onto NPs through post-insertion of Tf-PEG-DSPE into the NP-siRNA at 37°C for 30 minutes. The encapsulation efficiency of siRNA in the NPs was determined by measuring free siRNA by the RiboGreen assay (Invitrogen, Carlsbad,CA)
2.3. Particle size and zeta potential measurements
The particle size of Tf-NPs was determined by dynamic light scattering (DLS) on a BI-200SM (Brookhaven Instruments Corp., Holtsville, NY) instrument under the intensity-weighted setting. The zeta potential of Tf-NPs was measured on a ZetaPALS zeta potential analyzer (Brookhaven Instruments Corp., Holtsville, NY) after dilution in deionized water.
2.4. Serum stability study of Tf-NP-LOR-1284
To evaluate the serum stability, Tf-NP-LOR-1284 were mixed with fetal bovine serum (FBS) at a 1:1 (v/v) ratio and incubated at 37 °C. The particle size of the incubation mixtures was then determined by DLS. The stability of Tf-NP-LOR-1284 was similarly measured by monitoring the changes in the mean particle diameter at 4 °C.
2.5. Cell culture and transfection study
MV4–11 cells, obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA), were cultured in RPMI 1640 media supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and L-glutamine at 37 °C in a humidified atmosphere containing 5% CO2. Cells were seeded in 6-well tissue culture plates at a density of 105 cells /well 24 h prior to treatment. Free Tf-NP-LOR-1284 were added to each well at 100 nM final concentration. Cells were incubated at 37 °C for 4 h, washed twice with PBS, cultured in fresh medium for another 24, 48, 72, or 96 h, and then analyzed for R2 mRNA levels using quantitative RT–PCR and/or R2 protein levels using Western blot. Free LOR-1284 and NP formulations containing a scrambled siRNA sequence were used as controls.
2.6. Cellular uptake of Tf-f-NPs by flow cytometry
To determine the cellular uptake of NPs by AML cells, fluorescence-labeled Tf-targeted NPs (Tf-f-NPs) were used. FAM labeled siRNA was encapsulated into NPs by MHF to obtain the Tf-f-NPs. The cellular uptake was determined by flow cytometry. Briefly, 60,000 cells were seeded in a 24-well plate and incubated at 37 °C for 24 h prior to treatment. After incubation with various Tf-f-NPs for 4 h, cells were rinsed three times with cold PBS and fixed in 4% para-formaldehyde solution. The cell fluorescence intensity was analyzed by a Beckman Coulter EPICS XL flow cytometer (Beckman Coulter, Pasadena, CA, USA). A minimum of 10,000 events were collected for each cell sample under the LIST mode. Free FAM-siRNA and NP-FAM-siRNA treated groups were used for comparison.
2.7. Cytotoxicity study by MTS
The cytotoxicity of Tf-NP-LOR-1284 was evaluated by MTS cell proliferation assay according to the manufacturer’s instructions (Promega, Madison, WI, USA). Briefly, the MV4–11 cells were seeded at a density of 5,000 cells/well (100 µl /well) in a 96 well plate. After 24 h, cells were rinsed with PBS and incubated with 100 µl cell culture medium containing different concentrations of Tf-NP-NC-siRNA, free LOR-1284, or Tf-NP-LOR-1284 (10, 25, 50, 100, 250, 500 and 1000 nM) at 37 °C for 48 h. Then, 20 µl of MTS reagent was added to each well and the cells were incubated at 37 °C for another 2 h. The absorbance at 490 nm was measured by an automated plate reader (Molecular Devices, Sunnyvale, CA, USA). The results of cell survival are presented as the percentage viability relative to untreated control.
2.8. Quantification of R2 mRNA by real-time quantitative RT–PCR
The effect of LOR-1284, delivered via Tf-NP-LOR-1284 on R2 mRNA level in AML cells, was evaluated by qRT–PCR. The total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), then cDNA was synthesized utilizing Superscript III (Invitrogen, Carlsbad, CA) and the real-time quantification PCR was performed with Taqman gene expression assay (Applied Biosystems, Foster City, CA) following manufacturers protocols. Expression of R2 gene was normalized to GAPDH. The comparative cycle threshold (CT) method, also known as ΔΔCT method, was used for relative quantification of gene expression.
2.9. Quantification of R2 protein by Western Blot
In order to determine R2 protein level, cells treated with free LOR-1284 or Tf-NP-LOR-1284 were lysed with lysis buffer containing a protease inhibitor cocktail (CalBiochem, San Diego, CA). Anti-R2 and anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Images were taken with Kodak X-OMAT film (Kodak, Rochester, NY, USA). In addition to the film, chemiluminescent signals of the protein bands were also directly quantified using the Chemi-Doc phosphor imager system (Bio-Rad, Hercules, CA, USA) with R2 protein levels normalized to GAPDH.
2.10. Pharmacokinetic (PK) study in murine model
The protocol for animal studies described in this article was approved by the Ohio State University's Institutional Animal Care and Use Committee. In order to determine the potential in vivo application of Tf-NPs, a PK study was carried out in a murine model. Plasma clearance kinetics of Tf-NP-LOR-1284 was determined by measuring the fluorescence intensity of Cy3-labeled LOR-1284 (Cy3-LOR-1284) in serum. Briefly, mice (6-weeks-old) were given tail vein i.v. bolus injections of 2.5 mg/kg Cy3-LOR-1284 in saline or in Tf-NP formulation. At indicated time points (5min, 15min, 30min, 1h, 2h, 4h, 10h, and 24 h), mice were sacrificed and blood and tissue samples were collected from three mice each. Plasma was separated from red blood cells via immediate centrifugation at 1000×g for 5 min. Samples were then treated with 1% SDS and heated to 95 °C for 5 min, followed by centrifugation at 12, 000×g for 5 min. The fluorescence of supernatant was determined on a microplate reader. The plasma half-life (T1/2), area under the curve (AUC), mean residence time (MRT), and total body clearance (CL) were calculated using Winnonlin software 25 (Pharsight Co., CA, USA).
2.11. In vivo biodistribution study by IVIS imaging and confocal microscopy
To study the in vivo biodistribution of Tf-NP-LOR-1284, NOD-SCID mice were used. MV4–11 cells (5 × 106) were subcutaneously inoculated into the flank of NOD-SCID mice. Palpable tumors ~1 cm3 in size developed within 4 weeks after inoculation. Cy5-LOR-1284 was encapsulated in Tf-NPs as a probe to investigate in vivo tissue biodistribution. Tf-NPs or NPs loaded with Cy5-LOR-1284 were injected i.v. through the tail vein at a dose of 2.5 mg/kg. Major organs including the liver, lung, kidney, spleen, and heart as well as tumor were harvested 4 h following injection. Tissue samples were fixed in 4% para-formaldehyde/PBS for 6 h and then placed into a 30% sucrose/PBS solution overnight at 4°C. The fluorescence signals of Cy5 emitted by the whole tissues were measured using a Xenogen IVIS-200 optical in vivo Imaging system (Caliper Life Sciences, Hopkinton, MA).
For confocal imaging analysis, fixed tissue samples were then placed into a block holder containing OCT freezing medium (Fisher Scientific, Pittsburgh, PA, USA) and flash-frozen in liquid nitrogen. Tissue sections were counterstained with Alexa-488 phalloidin (13 nM, Life Technologies) and Hoechst 33342 (1 µM, Life Technologies) dyes in PBS for 20 min. The slides were mounted with the anti-fade reagent (Life Technologies) and analyzed by Olympus FV1000 Filter confocal microscope 26 (Olympus Optical Co., Tokyo, Japan). Tissues from non-tumor bearing mice (normal mice) were used as a control.
2.12. In vivo down-regulation study in murine leukemia models
The therapeutic efficacy of Tf-NP-LOR-1284 was investigated in SCID mice with xenograft MV4–11 tumors. Briefly, the tumor model was established in nude mice by subcutaneous implantation with 5×106 MV4–11 cells. Mice were randomized into different treatment groups (5 mice per group) to avoid cage effects. The mice developed tumors of ~50 mm3 within 14 days. For the in vivo down-regulation study, mice were injected with Tf-NPs carrying LOR-1284 siRNA or negative control siRNA (Tf-NP-NC-siRNA) at a dose of 2.5 mg/kg every 3 days starting from day 14 after inoculation by via tail vein.
2.13. Statistical analysis
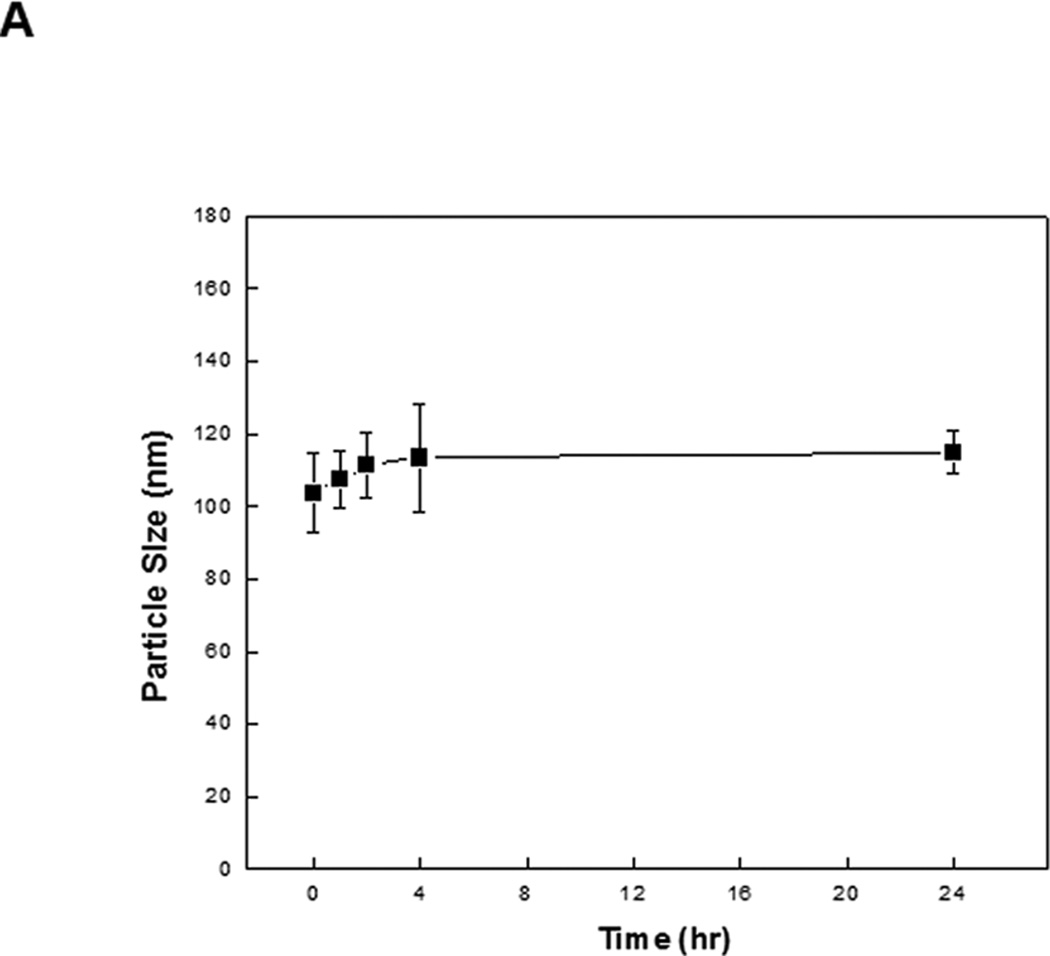
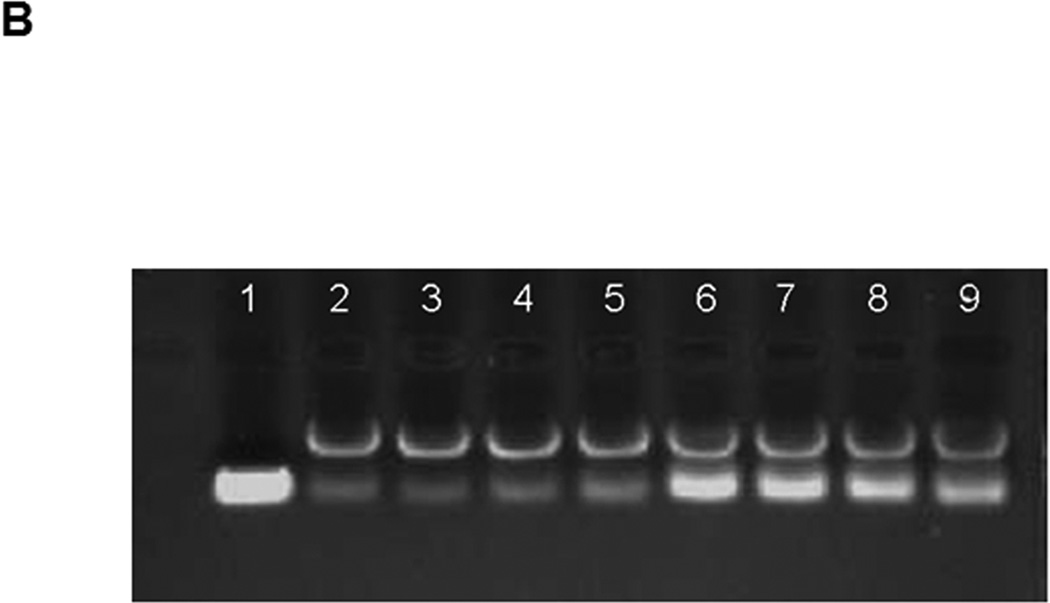
Data points are presented as the mean ± standard deviation (S.D.) of triplicates or quadruplicates unless otherwise indicated. in Figure 2A, there was no significant change in the average size of Tf-NP-LOR-1284 over 24 h when incubated with 50% serum, suggesting that the complexes remained intact. To further determine whether Tf-NP-LOR-1284 had increased resistance to nuclease digestion compared to the free siRNA, a serum protection assay was conducted by agarose gel electrophoresis. As shown in Figure 2B, after 8 h of exposure to serum, 100% of LOR-1284 remained intact with Tf-NP-LOR-1284, whereas less than 40% of free LOR-1284 remained intact. The dimmer siRNA bands for Tf-NP-LOR-1284 might be due to incomplete particle disruption by the 1% SDS rather than the degradation of the Experimental groups were compared using Student's t-test and one-way ANOVA with post hoc tests. A p-value of 0.05 was used as a cutoff for statistical significance.
Figure 2. In vitro evaluation of Tf-NP-siRNA.
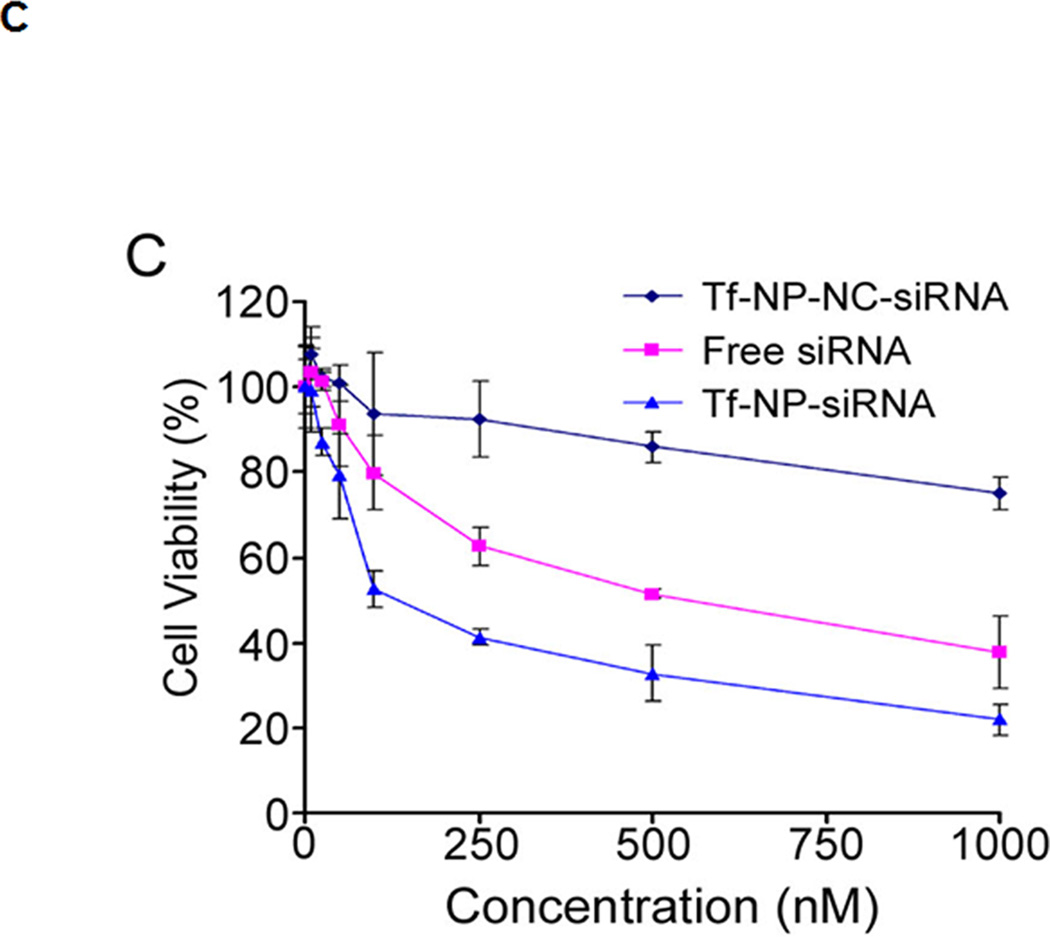
(A) The stability of Tf-NP-siRNA in serum. (B) Stability of Tf-NPs on siRNA in serum by agarose gel electrophoresis. The lanes are: 1. free siRNA without serum incubation. 2–5. Tf-NP-siRNA incubated for 0.5, 1, 2, or 4 h in serum. 6–9. free siRNA incubated for 0.5, 1, 2, or 4 h in serum. The bands above the siRNA bands in lanes 2–9 were due to SDS micelles. (C) Cytotoxicity study of Tf-NP-siRNA on MV4–11 by MTS. MV4–11 cells were transfected by Tf-NP-NC-siRNA, free siRNA, or Tf-NP-siRNA for 48 h. Results are presented as mean ± SD of three independent experiments.
3. Results
3.1 Synthesis and optimization of the NP formulation by MHF
The effects of flow pattern (T1 and T2) and flow rate on the formation of Tf-NP-LOR-1284 were investigated in order to optimize the synthetic method. The three typical cationic lipids (DC-Chol, DODMA and DOTMA) were examined to condense siRNA by MHF. Taking DC-Chol as the cationic lipid, Figure 1c shows that the particle size decreased with increasing flow rate for both flow patterns. The particle sizes of Tf-NP-LOR-1284 made by T1 pattern (three inlets) was much smaller than those made by T2 pattern (two inlets). Therefore, the T1 pattern was selected for further study. Briefly, the LOR-1284 solution (25 µg/ml) was injected at inlet 3 and the cationic NPs (125 µg/ml) were injected through inlets 1 and 2 at the same time. As shown in Figure 1d, the particle sizes of resulting Tf-NP-siRNA ranged from 100 nm to 400nm when the DOTMA cationic lipid was used. However, the particle size of Tf-NP-siRNA made from DODMA cationic lipid remained almost unchanged with the flow rates ranging from 25 µl/min to 2000 µl/min. These results indicate that the particle size of Tf-NP-siNRA in MHF was dependent on both the used cationic lipid and the flow rates. In the following experiments, the DODMA cationic lipid was used because of the smaller particle size of the NPs based on DODMA. The loading efficiencies for T1 and T2 were 91.5±4.5 % and 87.7±3.4%, respectively.
3.2. In vitro evaluation of Tf-NP-LOR-1284
Tf-NP-LOR-1284 was evaluated for serum stability. As shown siRNA because the intensity stayed the same despite escalating incubation time in the serum. These results suggest that the Tf-NP-LOR-1284 formulation provides protection for LOR-1284 from serum degradation and thus is potentially suitable for in vivo applications.
The cytotoxicity of Tf-NP-NC-siRNA, free LOR-1284, and Tf-NP-LOR-1284 were evaluated in vitro. MV4–11 cells were treated with Tf-NP-NC-siRNA, free LOR-1284, or Tf-NP-LOR-1284 at various concentrations (0–1000 nM). At 24h post-transfection, the cell viability was measured by MTS assay. As shown in Figure 2C, no significant cytotoxicity of Tf-NP-NC-siRNA to MV4–11 cells was detected at the range of 5~250nM, while a slightly diminished cell viability was observed at very high concentrations (500 and 1000nM). Since the concentrations of siRNA used were lower than 250nM, the vehicle-related cytotoxicity could be neglected in our tests. In contrast, free LOR-1284 exhibited moderate cytotoxicity and Tf-NP-LOR-1284 caused the significant inhibitory effects on MV4–11 cells when compared with Tf-NP-NC-siRNA. The IC50 for Tf-NP-LOR-1284 was 105.27 nM while the IC50 for free LOR-1284 was 647.2 nM. Taken together, these results suggest that the TfR-mediated targeting increased the cytotoxicity of Tf-NP-LOR-1284.
3.3. Cellular uptake and intracellular location of Tf-NP-LOR-1284
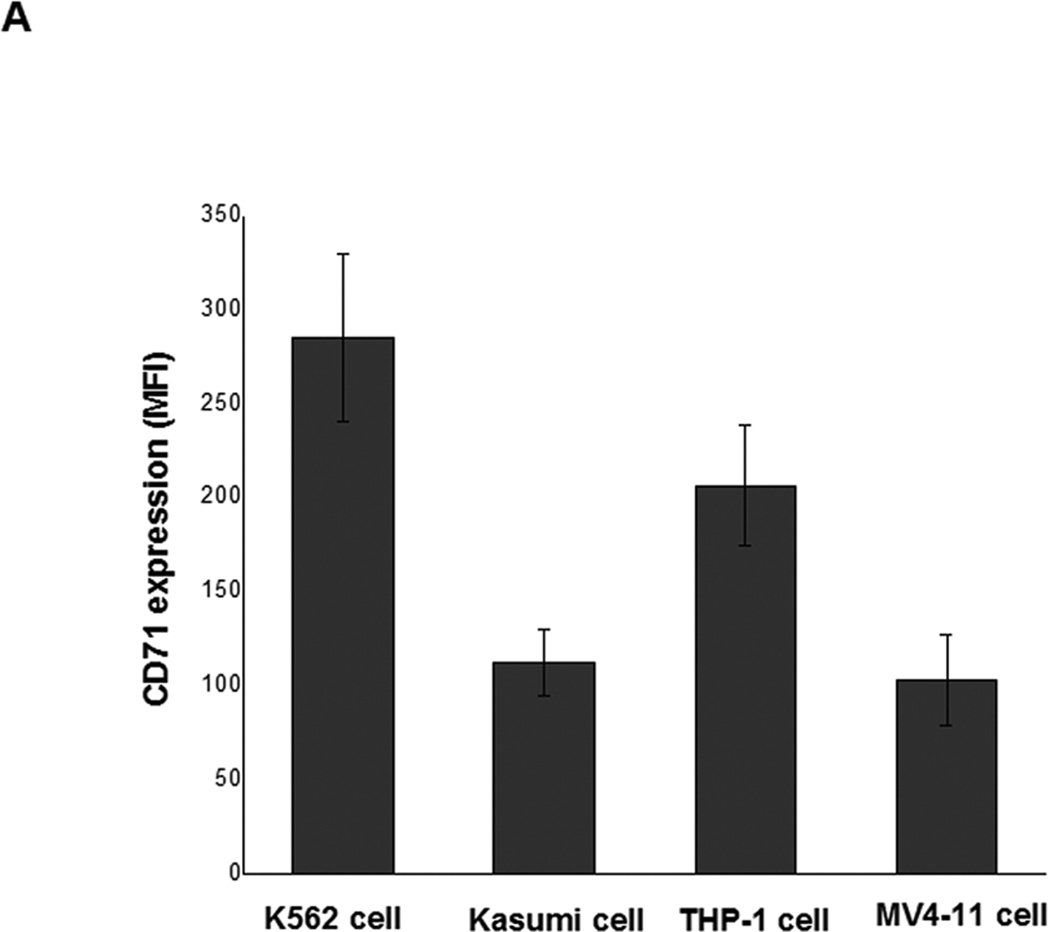
One major challenge in achieving efficient LOR-1284 transfection is the targeted-delivery of siRNA to specific cells. Therefore, Tf was used as targeting ligand for specific delivery of LOR-1284 to AML cells. Since the delivery efficiency of Tf-NPs is strongly dependent on the Tf receptor (TfR) on the cell surface, TfR expression levels on various AML cell lines (MV411, K562, THP-1 and Kasumi cells) were first evaluated before further testing. Cells were surface-stained with PE-labeled anti-TfR (anti-CD71) monoclonal antibodies (BD Biosciences, San Jose, CA) for 30 min on ice, followed by flow cytometry analysis. Results showed that TfR was overexpressed on the four tested AML cell lines (Figure 3A).
Figure 3. Cellular uptake and intracellular location of Tf-NP-siRNA in MV4–11 cells.
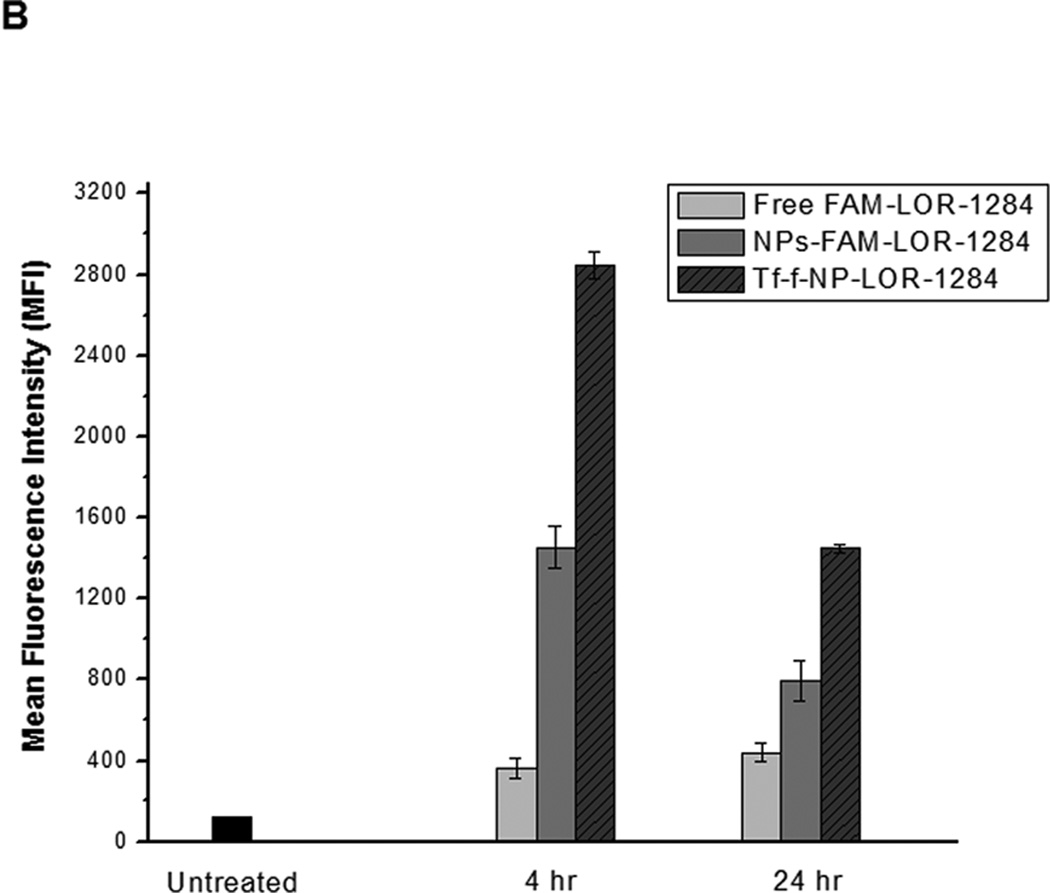
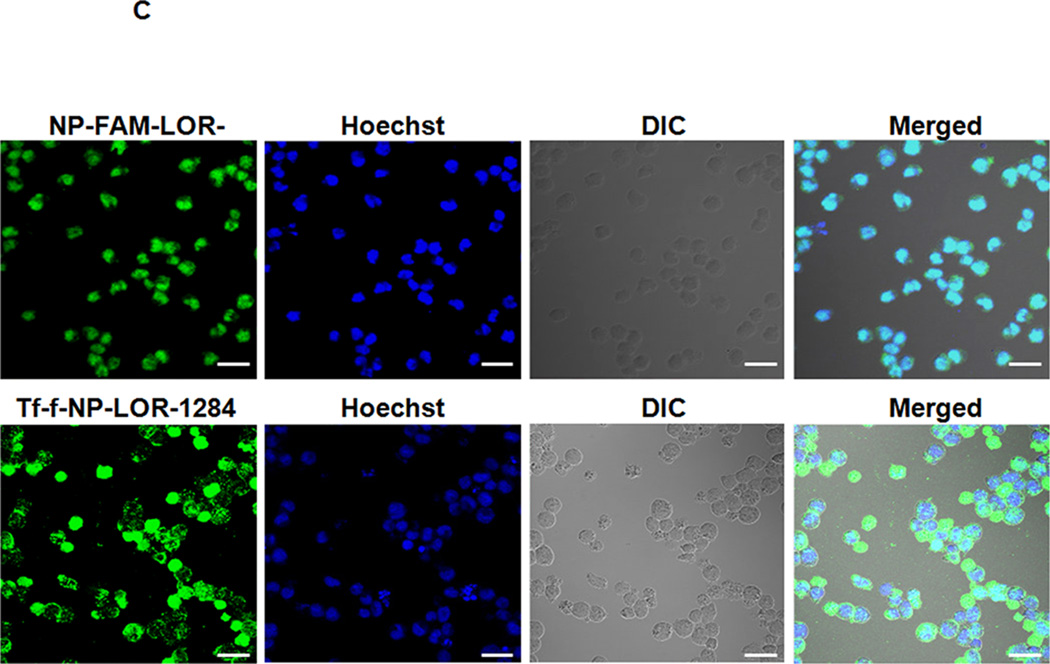
(A) Flow cytometry analysis on expression levels of TfR (also known as CD71) on the surface of AML cells. Cells were surface stained with PE-labeled anti-TfR (anti-CD71) monoclonal antibodies (BD Biosciences, San Jose, CA) for 30min on ice, followed by flow cytometry analysis. (B) Cellular uptake of FAM-labeled Tf-NP-siRNA by flow cytometry. (C) Intracellular localization of Tf-NP-siRNA 4 h after transfection by confocal microscopy.
The FAM labeled Tf-NP-LOR-1284 (Tf-f-NP-LOR-1284) was prepared as described in Methods. The mean fluorescence intensity (MFI) of cells treated with Tf-f-NP-LOR-1284 (100 nM) at 4 h and 24h was analyzed by flow cytometry. As shown in Figure 3B, the MFI of MV4–11 cells treated by Tf-f-NP-LOR-1284 was much higher than those of the control groups, which meant that cellular uptake of LOR-1284 was significantly improved by this delivery vehicle. For Tf-NPs, the cellular uptake was more rapid than free siRNA due to TfR-mediated uptake. At 24h, The Tf-NPs might have lost the iron bound to the Tf, which in turn resulted in the recycling of the Tf-NP back to the cellular surface and its release from the cell. In contrast, there was continuous uptake of the free siRNA. However, it should be noted that at all time points Tf-NP facilitated greater uptake of siRNA than the non-targeted control and the free siRNA. Furthermore, the intracellular trafficking of Tf-f-NP-LOR-1284 in cells was determined by confocal microscopy (Figure 3C). The green (FAM-LOR-1284) fluorescence was clearly visualized in cells treated with FAM-LOR-1284 loaded Tf-f-NPs, indicating efficient uptake of Tf-f-NP-LOR-1284.
3.4. Tf-NP-LOR-1284-mediated gene silencing in AML cells
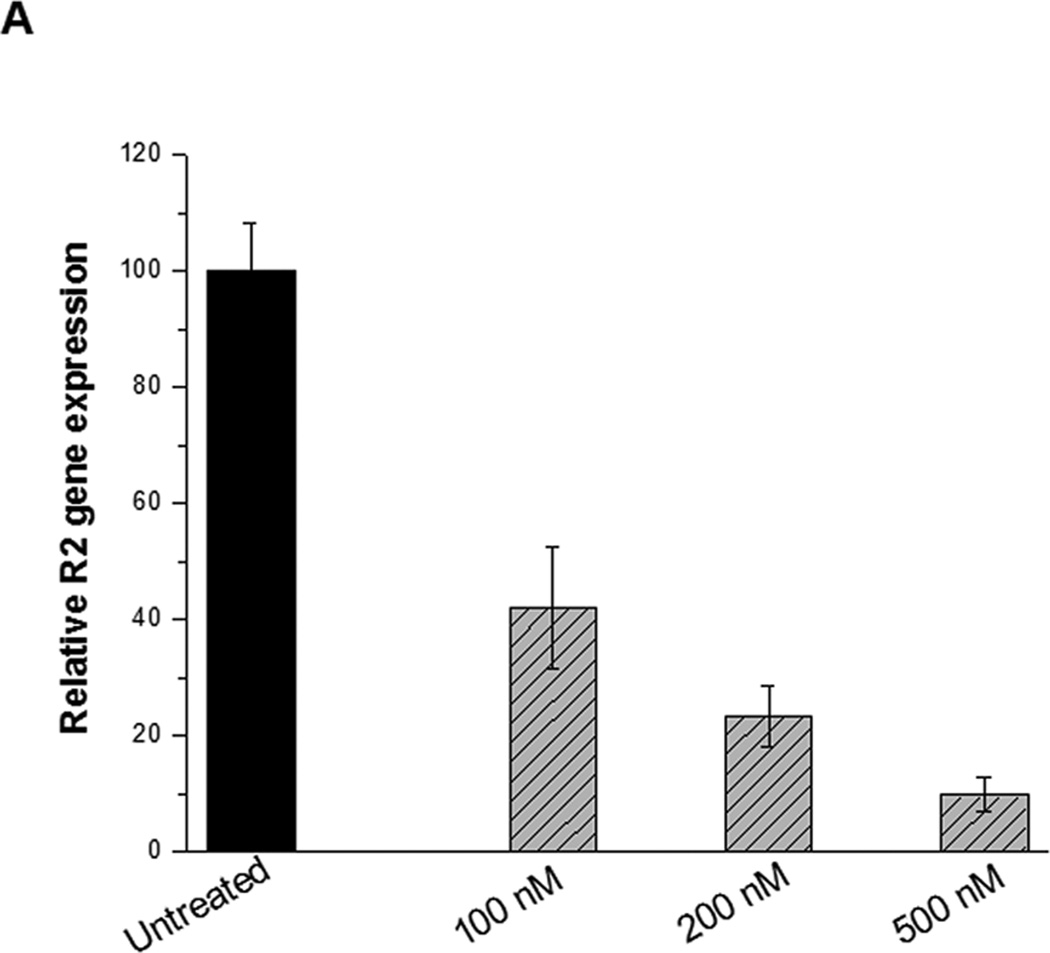
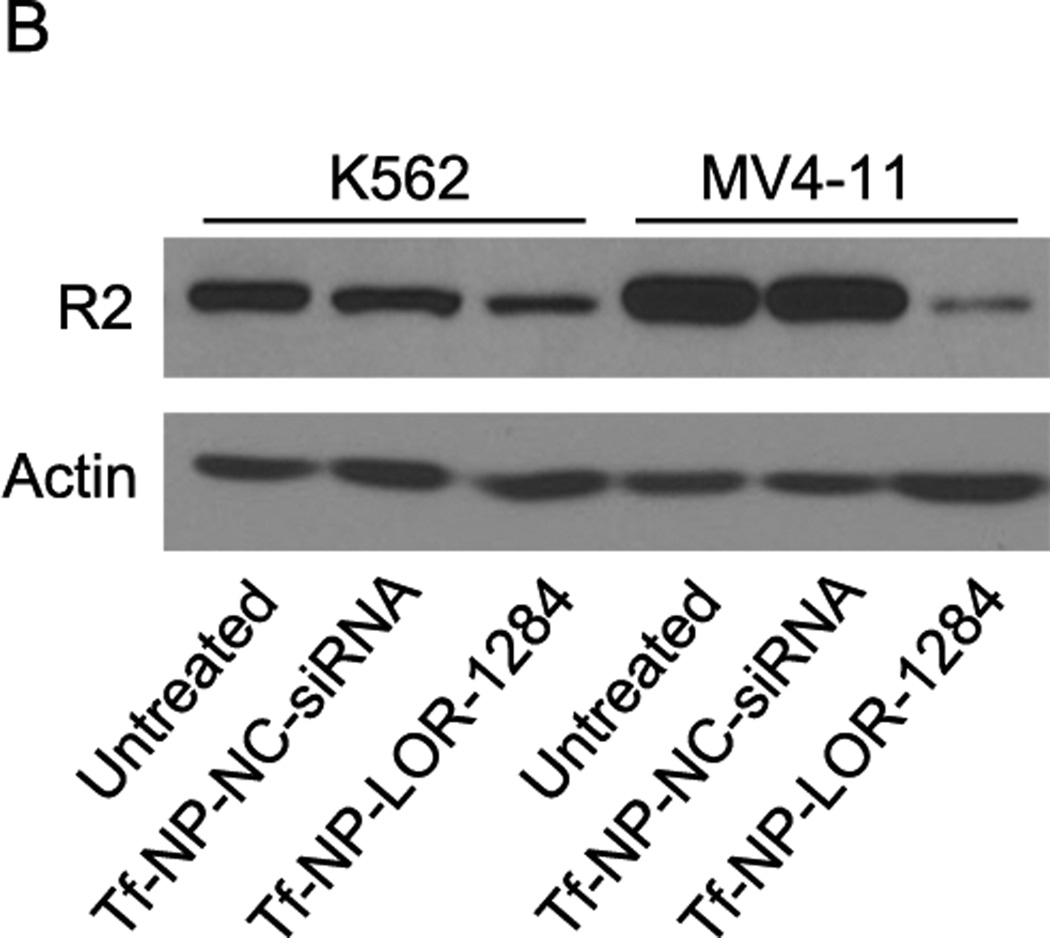
Next, we examined whether Tf-NP-LOR-1284 uptake resulted in target gene silencing in AML cells. MV4–11 cells were incubated with various concentrations of Tf-NP-LOR-1284 for 24h, and the R2 mRNA levels were measured by qRT-PCR. Figure 4A shows that the R2 mRNA level, relative to the level of untreated cells, was reduced in a concentration-dependent manner when free LOR-1284 was applied. The down-regulation efficiency of R2 by LOR-1284 siRNA was significantly enhanced when it was delivered with the NPs. At 250nM, the down-regulation of R2 by Tf-NP-LOR-1284 was nearly 80%, while the efficiency of free LOR-1284 at the same concentration was only 50%. From 250 to 1000 nM, the efficiency did not increase significantly. Overall, the results suggest that NP delivery systems have potent transfection efficiency for LOR-1284 in the tested AML cell line. The Tf-NP-LOR-1284 mediated gene silencing effect in MV4–11 was further studied by Western blot. A high level of R2 protein expression was detected in untreated MV4–11 cells as shown in Figure 4B. As expected, Tf-NP-LOR-1284 can dramatically reduce the R2 protein level for both MV4–11 and K562 cell lines (Figure 4B). The Tf-NP-NC-siRNA had no effect on R2 protein level. In summary, these results suggest that Tf-NP-LOR-1284 can significantly increase the gene silencing effect of LOR-1284 siRNA in AML cells.
Figure 4. Tf-NP-siRNA mediated gene silencing in MV4–11 and K562 cells.
(A) Concentration-dependent effect of Tf-NP-siRNA on R2 mRNA knockdown by qRT-PCR. (B) The effect of Tf-NP-siRNA on downregulation of R2 protein relative to control groups by Western blot. Cells were transfected by Tf-NP-NC-siRNA (200 nM) or Tf-NP-siRNA (200 nM) for 48 h.
3.5. Pharmacokinetic study
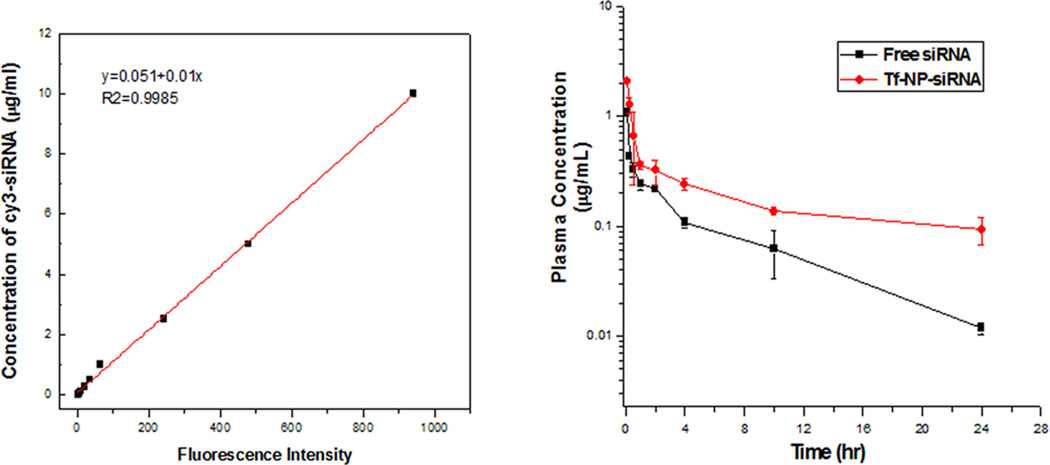
A standard curve of fluorescence intensity versus concentration of Cy3-LOR-1284 (Figure 5A) was established to determine the Cy3-LOR-1284 concentration of various samples in serum. The plasma half-life (T1/2), the area under the curve (AUC), the mean residence time (MRT), and total body clearance (CL) were calculated using a two-compartmental model by Winnonlin software (Pharsight Co., CA). The circulation time of Tf-NP-LOR-1284 was evaluated by measuring plasma clearance of Cy3-LOR-1284-Tf-NPs in normal ICR mice. As shown in Table 1, Tf-NP-LOR-1284 intravenously administered to mice at 2.5 mg/kg resulted in a peak plasma concentration (Cmax) of 2.72 µg/mL. At 24 h after i.v. administration, 20% of the injected Cy3-LOR-1284-Tf-NPs remained in the plasma, yielding a plasma half-life of about 10.2 h (Figure 5B). The AUC was 5.5 h·µg/mL. In contrast, the peak plasma concentration (2.27 µg/mL) was lower than Cy3-LOR-1284-Tf-NPs in the free LOR-1284 group. Moreover, only 1% of the free Cy3-LOR-1284 was detected in the plasma 24 h after the i.v. injection, yielding a plasma half-life of about 2.93 h. Thus, the circulation time of LOR-1284 was extended 3-fold when incorporated into Tf-NPs. The AUC in the free LOR-1284 group was only 1.58 h·µg/mL, indicating that the free Cy3-LOR-1284 was rapidly cleared from circulation. These results indicate that the NP encapsulation may extend the circulation time of siRNAs.
Figure 5. Pharmacokinetic study of Cy3 labeled Tf-NP-siRNA in ICR mice.
(A) The standard curve of MFI versus the concentration of Cy3-siRNA. (B) The plasma concentrations of Cy3-labeled Tf-NP-siRNA and free Cy3-siRNA after injection. Data represents the mean ± SD (n=3).
Table 1.
Pharmacokinetic parameters of Tf–NP–Cy3–siRNA and free Cy3–siRNA after i.v. bolus administration at 1 mg per kg bw
| Parameters | Unit | Free siRNA | Tf–NP–siRNA |
|---|---|---|---|
| Cmax | µg ml−1 | 2.27 | 2.72 |
| t1/2α | h | 0.06 | 0.18 |
| t1/2β | h | 2.93 | 10.2 |
| Vss | ml kg−1 | 2.38 | 2.37 |
| CL | ml kg−1 h | 0.63 | 0.18 |
| AUC | h µg ml−1 | 1.58 | 5.46 |
| MRT | h | 3.78 | 12.96 |
3.6. In vivo biodistribution of Tf-NP-LOR-1284 in tumor-bearing murine models
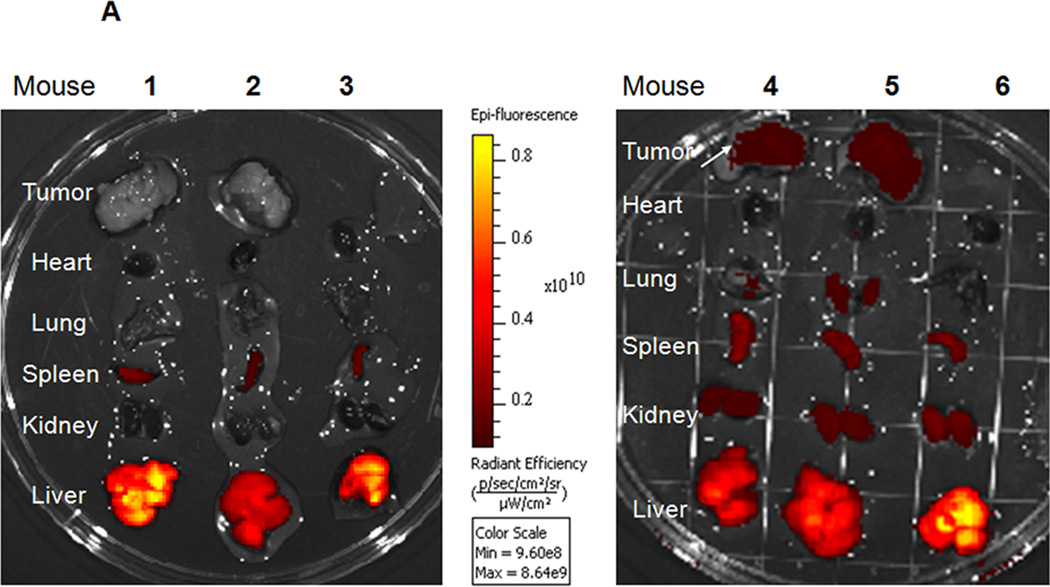
Tf targeting effect on biodistribution of LOR-1284-NPs was evaluated. As shown in Figure 6A, the NP-LOR-1284 without Tf targeting preferentially accumulated in the liver for both tumor bearing mice (1 and 2) and normal mouse (3). Inspection of other organs revealed minor Cy5-fluorescence in the spleen, kidney, heart and lung with levels several fold lower than that in the liver. The accumulation of NPs carrying Cy5-LOR-1284 in tumor (mouse 1 and 2) was relatively low compared to that in liver and other organs, though the tumor size remained close to 1 cm3. We further investigated the effect of Tf targeting on in vivo biodistribution of NP-Cy5 LOR-1284 In normal SCID mouse 6, the distribution of Tf-NP-Cy5 LOR-1284 was similar to that of NP-Cy5 LOR-1284 in mouse 3, which indicated localization in the liver. However, the fluorescence intensity of Tf-NP-Cy5 LOR-1284 was significantly increased in the tumor tissue (mouse 4 and 5) compared to NP-Cy5 LOR-1284 in tumor bearing mice (mouse 1 and 2 in Figure 6A), suggesting improved tumor accumulation through Tf targeting.
Figure 6. In vivo distribution study of Cy5 labeled Tf-NP-siRNA.
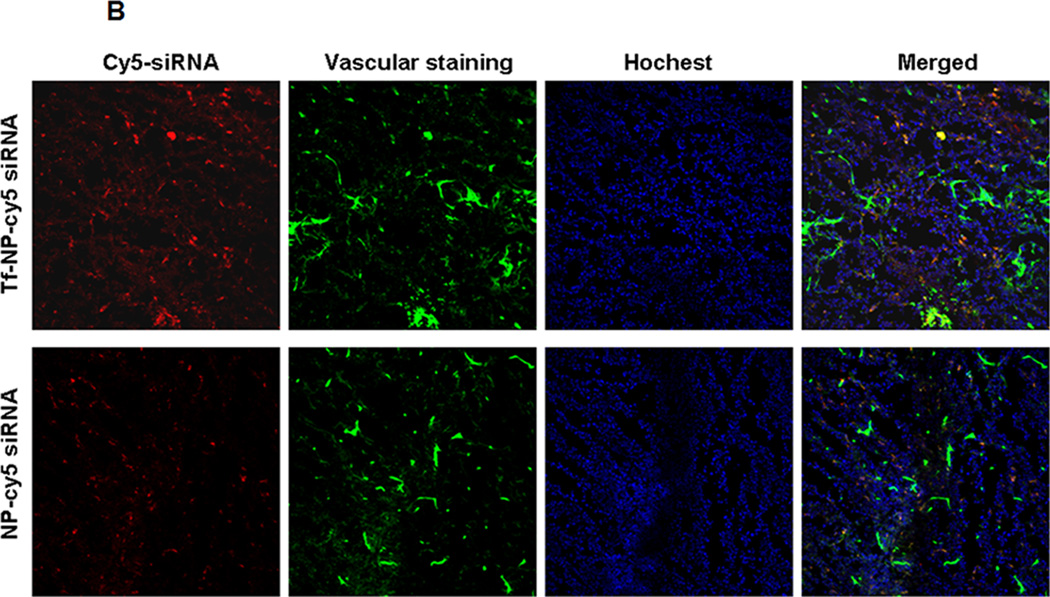
(A) Tf-mediated tumor targeting distribution of Cy5-siRNA by IVIS imaging. 1–2: Cy5-labeled NP-siRNA in NOD-SCID mice. 3: Cy5-labeled NP-siRNA in normal SCID mice. 4–5: Cy5-labeled Tf-NP-siRNA in NOD-SCID mice. 3: Cy5-labeled Tf-NP-siRNA in normal SCID mice. (B) Cy5-labeled Tf-NP-siRNA in NOD-SCID mice analyzed by confocal microscopy.
Besides the quantitative analysis of biodistribution by IVIS imaging, the distribution of Tf-NPs or NPs mediated LOR-1284 delivery was evaluated using confocal microscopy. As shown in Figure 6B, the Tf-NPs mediated greater accumulation of Cy5-LOR-1284 in the tumor compared to NP-Cy5 LOR-1284 in tumor, which validated the observation in IVIS pictures. Thus, although most Cy5-LOR-1284 loaded in NPs went into the liver, it was nonetheless concluded that the targeting effect of Tf was able to improve accumulation in the tumor tissue.
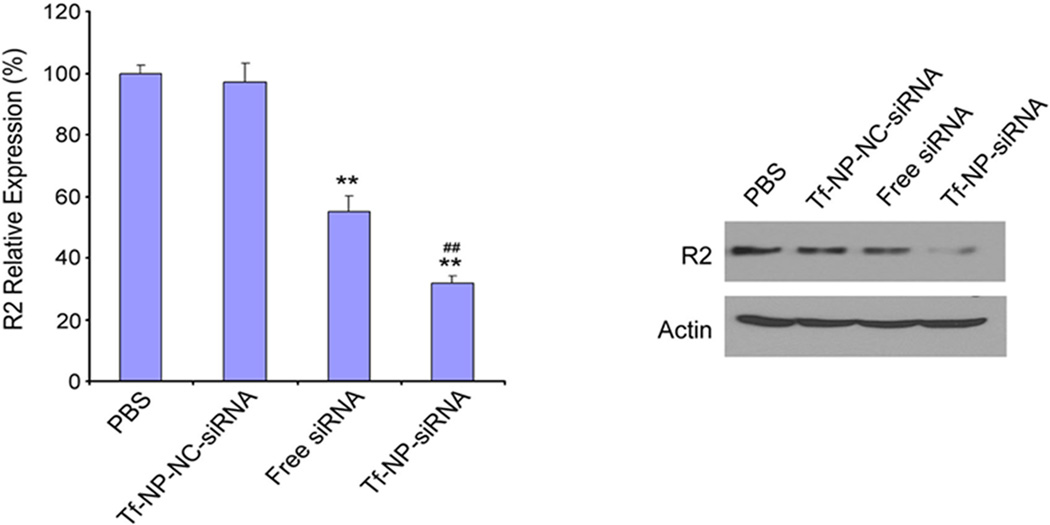
3.7. In vivo gene silencing Tf-NP-LOR 1284 siRNA
The in vivo gene silencing effect of Tf-NP-LOR-1284 was evaluated in nude mice inoculated with MV4–11 cells 14 days prior to treatment. Tf-NP-LOR-1284 was administered by i.v. injection via tail vein at a dose of 2.5 mg/kg LOR-1284 every three days. As expected, the R2 mRNA level in the Tf-NP-LOR-1284 treatment group was significantly lower than in the control group, while the free LOR-1284 group moderately inhibited R2 mRNA expression (Figure 7A). Moreover, the R2 protein level was decreased by 86% with Tf-NP-LOR-1284 treatment when compared with the control group, as shown in Figure 7B. These data further showed that Tf-NP-LOR-1284 was more effective than free LOR-1284.
Figure 7. In vivo down-regulation study of Tf-NP-LOR-1284 in tumor-bearing NOD-SCID mice.
(A) R2 mRNA expression of tumors in different groups. (B). R2 protein expression of tumors in different groups.
4. Discussion
RR is a valuable target for AML because it plays a critical role in nucleoside metabolism and thus DNA replication. Human RR is composed of two subunits: RR M1 subunit (R1) and M2 subunit (R2). Although both subunits are essential for its catalytic activity, only R2 protein level fluctuates with the cell cycle while R1 remains relatively stable. Numerous studies have shown that R2 protein overexpression is associated with malignant and metastatic status of tumor cells including AML, and R2 has been recognized as a target for AML therapy. LOR-1284 has been developed as an anticancer agent by targeting RRM2. However, its inefficient systemic delivery has greatly hampered its potential application in the clinic. In this study, we successfully developed a Tf-modified NPs synthesized by a novel microfluidic hydrodynamic focusing method for LOR-1284 siRNA delivery.
Recently, microfluidics has been investigated for the controllable synthesis of ODN-loaded NPs 14, 16, 27–29. Due to its small channel dimensions and typically low volumetric flow rates, microfluidic devices generally operate under laminar flow. This allows for mixing to be strictly confined to diffusion between laminar streams, thus producing reproducible mixing conditions.
To achieve higher specificity, NPs can be surface modified with ligands that specifically recognize receptors on tumor cells. Receptor-mediated endocytosis has the potential for effective delivery of LOR-1284 into specific organs or cellular subtypes. Studies have already shown that TfR is an attractive marker for tumor cell targeting in gene therapy. Here, Tf was conjugated to the NP for targeted delivery of LOR-1284 to AML cells overexpressing TfR. Although the targeted NPs have shown limited advantage over non-targeted NPs in solid tumor models due to the limitation of the extravasation rate of NPs from systemic circulation, Tf-NP-LOR-1284 are superior to free LOR-1284 in terms of deliver efficiency. The enhancement in the in vivo gene silencing may be attributed to its improvement in serum stability, prolonged circulation time, enhanced permeability and retention (EPR) effect, and TfR mediated cellular uptake. A possible mechanism of Tf-f-NP- LOR-1284 delivery is shown in Scheme 2.
We have recently shown target-cell selective delivery of an antisense ODN using folate receptor and Tf receptor-targeted NPs, respectively 23, 30. The NPs exhibited prolonged circulation time in vivo and receptor-dependent uptake and target downregulation in vitro. The potential challenges associated with NPs include rapid clearance of the particles by the reticuloendothelial system (RES), vehicle related cytotoxicity, and elicitation of cytokine response due to activation of toll-like receptors (TLRs). In summary, optimized siRNA delivery systems with high efficacy and selectivity and rational siRNA design for RNA interference (RNAi) may lead to future clinical success of RNAi therapy. In this study, we successfully developed a Tf-modified NP using a novel microfluidic hydrodynamic focusing method (MHF) for LOR-1284 siRNA targeted delivery and R2 gene silencing therapy in AML. The MHF technology offers a simple, affordable, and reproducible method for the manufacture of Tf-NP-LOR-1284 and demonstrated potentially superior production of NPs with more uniform structure, narrow size distribution, and high LOR-1284 loading efficiency. The MHF synthesized Tf-NP-LOR-1284 have excellent serum stability, high transfection efficiency, and superior tumor targeting, which efficiently and specifically downregulate R2 in vitro and in vivo. Moreover, Tf-NP-LOR-1284 was found to be more effective in tumor growth inhibition and prolonging mouse survival than Tf-NP-NC-siRNA. Therefore, MHF method provides a promising strategy for the reproducible production of siRNA-based nanoparticle therapeutics and may have the potential for clinical application in AML therapy.
Acknowledgments
This work was supported by NIH/NCI SBIR contract HHSN261201000051C to Nanomaterials Innovation Ltd. We thank Brian Kemmenoe and Sara Cole at CMIF of OSU for advice and assistance in generating the confocal data.
This work was supported by NIH/NCI SBIR contract HHSN261201000051C to Nanomaterial Innovation Ltd.
Reference
- 1.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 2.Gosselin MA, Guo W, Lee RJ. Bioconjug Chem. 2001;12:989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 3.Klisovic RB, Blum W, Wei X, Liu S, Liu Z, Xie Z, Vukosavljevic T, Kefauver C, Huynh L, Pang J, Zwiebel JA, Devine S, Byrd JC, Grever MR, Chan K, Marcucci G. Clin Cancer Res. 2008;14:3889–3895. doi: 10.1158/1078-0432.CCR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avolio TM, Lee Y, Feng N, Xiong K, Jin H, Wang M, Vassilakos A, Wright J, Young A. Anticancer Drugs. 2007;18:377–388. doi: 10.1097/CAD.0b013e328013c04f. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Sherwin T, Brown WL, Stockley PG. Nanomedicine. 2005;1:67–76. doi: 10.1016/j.nano.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Liu S, Yu B, Golan S, Koh CG, Yang J, Huynh L, Yang X, Pang J, Muthusamy N, Chan KK, Byrd JC, Talmon Y, Lee LJ, Lee RJ, Marcucci G. Mol Pharm. 2010;7:196–206. doi: 10.1021/mp900205r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landry B, Aliabadi HM, Samuel A, Gul-Uludag H, Jiang X, Kutsch O, Uludag H. PLoS One. 2012;7:e44197. doi: 10.1371/journal.pone.0044197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong WH, Park K, Lee MY, Lee H, Sung DK, Hahn SK. Biomaterials. 2013;34:542–551. doi: 10.1016/j.biomaterials.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 9.Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J. Biomaterials. 2013;34:196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Yu B, Hsu SH, Zhou C, Wang X, Terp MC, Wu Y, Teng L, Mao Y, Wang F, Xue W, Jacob ST, Ghoshal K, Lee RJ, Lee LJ. Biomaterials. 2012;33:5924–5934. doi: 10.1016/j.biomaterials.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JS, MacKay JA, Szoka FC., Jr Bioconjug Chem. 2003;14:420–429. doi: 10.1021/bc025625w. [DOI] [PubMed] [Google Scholar]
- 12.Aditya NP, Patankar S, Madhusudhan B, Murthy RS, Souto EB. Eur J Pharm Sci. 2010;40:448–455. doi: 10.1016/j.ejps.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Hinrichs WL, Mancenido FA, Sanders NN, Braeckmans K, De Smedt SC, Demeester J, Frijlink HW. Int J Pharm. 2006;311:237–244. doi: 10.1016/j.ijpharm.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Yu B, Zhu J, Xue W, Wu Y, Huang X, Lee LJ, Lee RJ. Anticancer Res. 2011;31:771–776. [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Lee RJ, Lee LJ. Methods Enzymol. 2009;465:129–141. doi: 10.1016/S0076-6879(09)65007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh CG, Zhang X, Liu S, Golan S, Yu B, Yang X, Guan J, Jin Y, Talmon Y, Muthusamy N, Chan KK, Byrd JC, Lee RJ, Marcucci G, Lee LJ. J Control Release. 2010;141:62–69. doi: 10.1016/j.jconrel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollia P, Samara M, Stamatopoulos K, Belessi C, Stavroyianni N, Tsompanakou A, Athanasiadou A, Vamvakopoulos N, Laoutaris N, Anagnostopoulos A, Fassas A. Leuk Res. 2003;27:1101–1103. doi: 10.1016/s0145-2126(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 18.Kollia P, Stavroyianni N, Stamatopoulos K, Zoi K, Viniou N, Mantzourani M, Noguchi CT, Paterakis G, Abazis D, Pangalos C, Loukopoulos D, Yataganas X. Br J Haematol. 2001;115:19–24. doi: 10.1046/j.1365-2141.2001.03065.x. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Liu S, Yu B, Golan S, Koh CG, Yang J, Huynh L, Yang X, Pang J, Muthusamy N, Chan KK, Byrd JC, Talmon Y, Lee LJ, Lee RJ, Marcucci G. Mol Pharm. 2011;7:196–206. doi: 10.1021/mp900205r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Yu B, Weecharangsan W, Piao L, Darby M, Mao Y, Koynova R, Yang X, Li H, Xu S, Lee LJ, Sugimoto Y, Brueggemeier RW, Lee RJ. Int J Pharm. 2010;390:234–241. doi: 10.1016/j.ijpharm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Liu Y, Tai HC, Zhu J, Ding H, Lee RJ. Int J Pharm. 2011;404:205–210. doi: 10.1016/j.ijpharm.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu B, Mao Y, Bai LY, Herman SE, Wang X, Ramanunni A, Jin Y, Mo X, Cheney C, Chan KK, Jarjoura D, Marcucci G, Lee RJ, Byrd JC, Lee LJ, Muthusamy N. Blood. 2013;121:136–147. doi: 10.1182/blood-2012-01-407742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Koh CG, Liu S, Pan X, Santhanam R, Yu B, Peng Y, Pang J, Golan S, Talmon Y, Jin Y, Muthusamy N, Byrd JC, Chan KK, Lee LJ, Marcucci G, Lee RJ. Mol Pharm. 2009;6:221–230. doi: 10.1021/mp800149s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Schwind S, Yu B, Santhanam R, Wang H, Hoellerbauer P, Mims A, Klisovic R, Walker AR, Chan KK, Blum W, Perrotti D, Byrd JC, Bloomfield CD, Caligiuri MA, Lee RJ, Garzon R, Muthusamy N, Lee LJ, Marcucci G. Clin Cancer Res. 2013;19:2355–2367. doi: 10.1158/1078-0432.CCR-12-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Xu S, Teng L, Yung B, Zhu J, Ding H, Lee RJ. Anticancer Res. 2011;31:1521–1525. [PubMed] [Google Scholar]
- 26.Yang Z, Sun W, Hu K. Biochim Biophys Acta. 2009;1793:1868–1875. doi: 10.1016/j.bbamcr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay G, Jeon A, Dong Y, Whitehead KA, Anderson DG. J Am Chem Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 28.Leung AK, Hafez IM, Baoukina S, Belliveau NM, Zhigaltsev IV, Afshinmanesh E, Tieleman DP, Hansen CL, Hope MJ, Cullis PR. J Phys Chem C Nanomater Interfaces. 2012;116:18440–18450. doi: 10.1021/jp303267y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belliveau NM, Huft J, Lin PJ, Chen S, Leung AK, Leaver TJ, Wild AW, Lee JB, Taylor RJ, Tam YK, Hansen CL, Cullis PR. Mol Ther Nucleic Acids. 2013;1:e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu SJ, Marcucci G, Lee RJ. Anticancer Res. 2006;26:1049–1056. [PubMed] [Google Scholar]