Abstract
We report the isolation of a bacterium from Galleria mellonella larva and its identification using genome sequencing and phylogenomic analysis. This bacterium was named Alcaligenes faecalis strain MOR02. Microscopic analyses revealed that the bacteria are located in the esophagus and intestine of the nematodes Steinernema feltiae, S. carpocapsae, and H. bacteriophora. Using G. mellonella larvae as a model, when the larvae were injected with 24,000 CFU in their hemocoel, more than 96% mortality was achieved after 24 h. Additionally, toxicity assays determined that 1 μg of supernatant extract from A. faecalis MOR02 killed more than 70% G. mellonella larvae 96 h after injection. A correlation of experimental data with sequence genome analyses was also performed. We discovered genes that encode proteins and enzymes that are related to pathogenicity, toxicity, and host/environment interactions that may be responsible for the observed phenotypic characteristics. Our data demonstrates that the bacteria are able to use different strategies to colonize nematodes and kill insects to their own benefit. However, there remains an extensive group of unidentified microorganisms that could be participating in the infection process. Additionally, a nematode-bacterium association could be established probably as a strategy of dispersion and colonization.
1. Introduction
Nematodes have a global impact on ecosystems and economies; however, parasitic nematodes also can have many beneficial impacts on human interests and health [1]. For example, entomopathogenic nematodes (EPNs) that belong to the families Steinernematidae and Heterorhabditidae are commercially used as biological control agents for crop pests [2]. The EPNs Heterorhabditis and Steinernema have unique mechanisms to associate with and transmit bacteria to insect hosts; specifically, these nematodes have a symbiotic association with pathogenic bacteria from the Photorhabdus and Xenorhabdus genera, respectively [3]. In this relationship, the nematode provides the bacteria with nutrients, protection, and environmental dispersion. Meanwhile, the bacteria provide nutrition to the nematode through available insect-derived nutrients as well as antimicrobial compounds that prevent the development of bacteria, fungi, and yeast in the insect. For example, Photorhabdus produces bacteriocins and lumicins [4, 5].
Both nematodes and bacteria can infect and kill insects. Once the infective juvenile reaches the insect hemocoel, they release the symbiotic bacteria into a rich medium that grows the bacterial cells. The bacterial cells then release antimicrobial compounds, toxins, and exoenzymes causing the insect death usually within 24–48 h [6, 7].
Although it has been postulated that the nematode-bacteria interaction is unique some nonsymbiotic bacteria that are able to coinhabit or colonize the insect cadaver and the nematode have been reported. Lysenko and Weiser [8] isolated bacteria associated with S. carpocapsae, such as Alcaligenes, Pseudomonas, and Acinetobacter spp., which also are pathogenic to Galleria mellonella larvae. More recently, the bacteria Flavobacterium sp., Providencia vermicola, and Alcaligenes faecalis were isolated from the nematode Rhabditis blumi [9].
Several bacterial species have also been identified from hemolymph of insect cadavers infected with EPNs as well as from infective juvenile EPNs [4].
In this study, we report the isolation and identification of the bacteria A. faecalis strain MOR02 from G. mellonella dead larva recovered from soil samples in Tenango, Morelos, Mexico. The pathogenicity and toxicity of this bacterium were also analyzed and we determined that A. faecalis MOR02 causes mortality in G. mellonella larvae 24 h after injection. Additionally, the protein extract from a supernatant culture of the bacteria is toxic in G. mellonella larvae 96 h after injection. We also microscopically observed the association of A. faecalis MOR02 and nematodes from the genera Steinernema and Heterorhabditis. We performed genome sequencing on A. faecalis MOR02, and the bioinformatic data analysis supports the phenotypic characteristics of the bacteria.
2. Materials and Methods
2.1. Bacterial Isolation from the Nematode
The bacteria were isolated from the hemolymph of a G. mellonella larvae cadaver found in the soil of Tenango (Santa Ana), Morelos, Mexico, by Guadalupe Peña. G. mellonella larvae were disinfected with ethanol 70% and the contents were streaked on LB media plates for bacterial growth. The isolated bacteria were grown at 30°C at 250 rpm overnight and then were grown on LB media plates.
Bacterial genomic DNA extraction was performed using the UltraClean Microbial DNA Isolation kit (MOBIO).
2.2. Bioinformatic Methods
2.2.1. Assembly
For the sequencing of the A. faecalis MOR02 genome, sequencing on the Genome Analyzer IIx (GAIIx) Illumina platform was performed by the UUSMD (Unidad Universitaria de Secuenciación Masiva de DNA, Instituto de Biotecnología, UNAM). The 19,250,362 paired-end reads were assembled de novo using the SPAdes program (version 3.1.1) and 23 contigs were generated with a 315-fold median coverage depth.
2.2.2. Annotation
The RAST (Rapid Annotation using Subsystem Technology) version 2.0 (http://rast.nmpdr.org/) [10], RNAmmer version 1.2 (http://www.cbs.dtu.dk/services/RNAmmer/) [11], tRNAscan-SE version 1.21 (http://lowelab.ucsc.edu/tRNAscan-SE/) [12], and ARAGORN (http://mbioserv2.mbioekol.lu.se/ARAGORN/) [13] servers were used for genome annotation and the prediction of rRNA and tRNA genes, respectively. Clusters of Orthologous Groups of proteins (COG) [14] and Gene Ontology (GO) [15, 16] annotations were performed using a BLAST search against the downloaded databases. KEGG Orthology (KO) [17, 18] annotation was performed at the KEGG Automatic Annotation Server (KAAS) with the Bidirectional Best Hits (BBH) method (http://www.genome.jp/kegg/kaas/). PFAM annotation was performed at the EMBL-EBI batch search server (http://pfam.xfam.org/search).
2.2.3. Phylogeny and Identification
The isolated 16S rRNA sequence was identified using the RNAmmer version 1.2 server [11] (http://www.cbs.dtu.dk/services/RNAmmer/) on contig1 (722,086 bp). This sequence was used for a BLASTN search (http://blast.ncbi.nlm.nih.gov/) using the MEGABLAST algorithm to search for nonredundant (nr) nucleotide [19] databases. The higher significant alignments reported were downloaded for phylogenetic analysis. A full tree with 165 sequences and a representative tree with 25 sequences of the genera Alcaligenes and closely related bacteria were constructed using all 16S rRNA sequences. These sequences were first aligned using MUSCLE server version 3.7 (http://phylogeny.lirmm.fr/phylo_cgi/one_task.cgi?task_type=muscle) [20] and the resulting alignment was analyzed using the phylogenetic analysis program MEGA version 6.0 [21]. A maximum likelihood method was used to calculate distances and the neighbor-joining method was used to infer the evolutionary tree and bootstrap values were also calculated using 1000 replicates.
2.2.4. Phenotypic Characterization
Three tests were performed as part of phenotypic characterization: the swarming motility test, chitinase activity assay, and esterase/lipase hydrolysis test.
To assess swarming motility, we slightly modified a previously reported method [22]. Briefly, a plate of trypticase soy broth (TSB) (Bioxon) and 0.5% agar (Bioxon) was inoculated in the center with a drop of a bacteria enriched liquid culture that was incubated at 25°C for 7 days. E. coli DH5α that have reportedly poor motility were used as a control [23].
For the chitinase activity assay, a sterile solution containing colloidal chitin was prepared as follows: 2 g colloidal chitin was dissolved in 100 mL of a solution prepared containing 0.5 g casein peptone (Bioxon), 0.5 g yeast extract (Bioxon), 0.1 g KH2PO4 (High Purity) 0.01 g MgSO4 ·7H2O (J.T. Baker), 1.5 g agar (Bioxon), and pH adjusted to 6.5. A drop of an enriched culture of A. faecalis MOR02 was inoculated on the plates and incubated at 37°C until a white halo of degradation was observed in the center of the plate. E. coli DH5α cells were used as a negative control. The streptococcus thermophiles activity of the bacteria were assessed using the Tween 80 hydrolysis test that was performed as previously reported [24].
2.2.5. Cell Transformation
Electrocompetent A. faecalis MOR02 cells were prepared and transformed using electroporation with the plasmid pT4-23S-Cherry. This plasmid has the coding sequence of mCherry under the control of a 23S promoter and a kanamycin resistance cassette (30 μg/mL). We have previously constructed this plasmid based on the pT4-mCherry plasmid, which has coding sequence of mCherry under the control of the trc promoter and a kanamycin resistance cassette (30 μg/mL). After 3 days of growth at 37°C, positive red clones were observed and fluorescence microscopic observation was performed using a filter Nikon B-2A (Nikon Eclipse E4000). We subsequently referred to this transform as A. faecalis MOR02-Cherry.
2.2.6. Bacterial Pathogenic Assays
For all pathogenic assays, 100 mL of LB medium (kanamycin 100 mg/mL) was inoculated with a fresh preinoculum of A. faecalis MOR02-Cherry; the cells were then grown at 37°C overnight. When an OD600 of 1.4–1.6 was reached, the cells were collected using centrifugation at 5,000 g at 4°C for 20 min. Serial dilutions were performed in LB broth to obtain dilutions of 108, 109, 1010, and 1011, which corresponded to bacterial suspensions at 2.4 × 104–2.4 × 107 CFU/mL. The same conditions were used for negative control E. coli DH5α transformed with pT4-mCherry, a plasmid with coding sequence of mCherry under the control of the trc promoter and a kanamycin resistance cassette (30 μg/mL).
The pathogenicity assessment was performed using the injection method on sixth-instar G. mellonella larvae. For injection assays, 10 μL of bacterial suspensions was injected into the dorsal region of the third from last abdominal segment of the larvae using a 0.3 mL/cc insulin syringe. Before each pathogenic assay, the presence of A. faecalis MOR02-Cherry in cultures used to inject the G. mellonella larvae was verified using fluorescence microscopy (Nikon Eclipse E4000).
LB broth and E. coli DH5α cells carrying the pT4-mCherry plasmid were used as a negative control. Additionally, damage caused by the puncture was also assessed in the larvae. Each bacterial suspension was tested using 10 insect larvae in three independent experiments.
The injected larvae were incubated at 26°C in presence of small pieces of diet [for 500 mg of diet, 97.5 mL bee honey, 20 mL sterile glycerol (J.T. Baker), 37.5 g sterile wheat bran, 73 g sterile rice cereal, and 73 g yeast extract (Pronat Ultra)]. Mortality was assessed every 24 h after injection. We considered dead or dying larvae when they did not show any movement after being pricked with a toothpick as well as a black appearance due to necrosis.
At 24 and 48 h after injection the inner contents of the G. mellonella larvae were streaked on LB media Petri dishes supplemented with kanamycin 100 mg/mL and grown at 37°C for 48 h. A. faecalis MOR02-Cherry cells were identified by the presence of red fluorescence. In each case, three independent experiments were performed.
2.2.7. Protein Precipitation and Toxicity Assay
The proteins present in 300 mL of supernatant from A. faecalis MOR02-Cherry cultures were precipitated using cold acetone and were incubated at −20°C overnight. The acetone cultures were centrifuged at 5,000 g at 4°C and the protein pellet was obtained and washed twice with cold acetone. After the acetone evaporated, the pellet was resuspended in Tris 10 mM pH 7.2. Proteins were quantified using the Bradford reagent (BioRad) and a standard curve of bovine serum albumin (BSA).
To assess protein toxicity, sixth-instar G. mellonella larvae were injected in the hemocoel with 0.5 , 1, 2, and 4 μg extracted proteins (final volume injected in each larvae, 20 μL). The negative controls were larvae injected with BSA at the same protein concentration and Tris 10 mM pH 7.2. We also used larvae that were only punctured with the syringe to evaluate damage due to the injection. For each protein concentration, 10 larvae were injected and three independent experiments were performed.
2.2.8. Association of A. faecalis Strain MOR02 with Nematodes
To establish whether an association between the nematode and bacteria existed, second stage infective juvenile S. feltiae, S. carpocapsae, and H. bacteriophora nematodes (Entonem, Koppert) were hydrated using sterilized distilled water and then were put in contact with A. faecalis MOR02-Cherry. The bacteria cells were previously grown on LB media dishes supplemented with kanamycin (100 mg/mL) for 48 h at 37°C.
In 60 × 15 mm Petri dishes, the nematodes (approximately fifty) and a loopful of bacteria were mixed and incubated at 20°C for 18 h. After this, the mCherry protein in the nematodes was excited at 555 nm and emission fluorescence was collected at 605 nm using a bandpass of 40X (Nikon, TE300).
2.3. Statistical Analysis
All statistical analyses were performed using Minitab 15 Statistical Software. A one-way analysis of variance [25] was used to analyze differences in mortality when different amounts of supernatant extract were used in the bioassays (1, 2, and 4 μg). The same analysis was performed to assess differences in mortality among different injected CFU numbers in the larvae. To determine significant differences among the means, a Tukey test was performed. The significance threshold was set at P < 0.05.
3. Results
3.1. Bacterial Isolation
After the bacteria were isolated from the larva, they were grown in liquid LB medium and then were streaked on Petri dishes with solid LB medium. From these isolates, one colony was selected and used for further genomic DNA extraction and identification.
3.2. Genome Sequencing
The genome of the isolated microorganism was sequenced using the Genome Analyzer IIx (GAIIx) Illumina platform, with a random subset of 19,250,362 paired-end reads (315X coverage). The genome was assembled using the SPAdes program (version 3.1.1) into 23 contigs that were deposited in the GenBank database. We obtained a draft genome with a total length of 4,402,705 bp in the 23 contigs that had a GC content of 56.4% [26].
3.3. Identification
The genome draft was analyzed on the RAST server (version 2.0) [10] and contains 4,019 coding sequences (CDS) including 52 tRNAs. Using the servers RAST, ARAGORN [27] (http://mbio-serv2.mbioekol.lu.se/ARAGORN/), and RNAmmer 1.2 [11] (http://www.cbs.dtu.dk/services/RNAmmer/) to analyze the contig1 sequence (722,086 bp) we identified the presence of the genes coding for tRNA-Val(gac) [121,713-121,789], tRNA-Leu(tag) [324,903-324,987] and tRNA-Met(cat) [456,223-456,301], in addition to 5S rRNA [609,890-610,001], 23S rRNA [610,185-613,067] tRNA-Ala(tgc) [613,447-613,522], tRNA-Ile(gat) [613,534-613,610], and 16S rRNA [613,709-615,233] in that order (Figure S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/570243). This arrangement is closely similar to the one reported for an A. faecalis subsp. faecalis NCIB 8687 contig (GenBank accession number AKMR01000044; 6,924 bp), but without the presence of the tRNA-Val-GAC gene described in that strain. The sequence of the 16S rRNA gene (1,524 bp) reported here is 99% identical to several A. faecalis strains that are reported in the GenBank database. These strains are described as being involved with (1) converting iminodiacetonitrile to iminodiacetic acid, (2) phenol degradation, (3) host of S. thermophilus, or (4) synthesis of (R)-(-)-mandelic acid and its derivatives from racemates by enantioselective degradation, among other activities. However, these isolated strains have not been completely characterized or published.
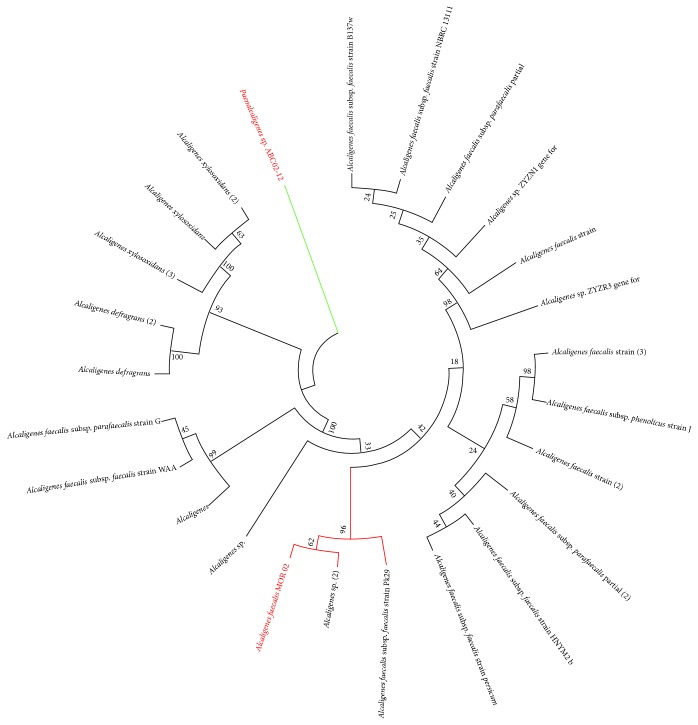
Based on the results of the 16S ribosomal DNA (rDNA) sequence (Figures 1 and S2), this strain was the closest to (i) A. faecalis BC2000 (GenBank accession number AY662683.1; homology 99%, based on 16S rDNA), which is one of a group of bacteria isolated from rhizosphere soil. BC2000 has been proven to have functions on plant growth-promotion and antagonism against plant parasitic nematodes; (ii) Alcaligenes sp. F78 (GenBank accession number EU443097.1; homology 99%, based on 16S rDNA), isolated from a mycorrhizosphere bacteria conglomerate; (iii) Alcaligenes sp. ECU0401 (GenBank accession number EF535732.1; homology 99%, based on 16S rDNA), a nitrilase producer; and (iv) Alcaligenes sp. PGBS001 (GenBank accession number EU622578.1; homology 99%, based on 16S rDNA) isolated from a microbial community decomposing wheat straw under aerobic conditions (Figure S2).
Figure 1.
16S rRNA gene phylogeny of 24 Alcaligenes spp., including A. defragrans, A. xylosoxidans, and several substrains of A. faecalis. In addition, a related bacterium isolated from larvae guts, Paenalcaligenes sp. ABC02-12, was used as an outgroup (green line). Maximum likelihood method was used to compute the evolutionary distances and the neighbor-joining method was used to infer the evolutionary history. Bootstrap percentages are given in the nodes (number of bootstrap replicates: 1000). A. faecalis strain MOR_02 is shown in red.
We conclude that our strain is an A. faecalis and designated it as A. faecalis MOR02 (GenBank accession number JQCV00000000, http://www.ncbi.nlm.nih.gov/nuccore/JQCV00000000.1/).
3.4. Genome Annotation
Our draft genome provided us with information concerning the potential proteins involved with several of the observed phenotypes. The 4,019 CDS reported by the RAST server (version 2.0) were compared with the Clusters of Orthologous Groups of proteins (COG), Gene Ontology (GO) [15], KEGG Orthology [17], and PFAM databases. After the predicted proteins were classified based on these different approaches, we use several keywords (e.g., symbiosis, pathology, intracellular survival, toxicity, and antibiotic production) to cluster the candidates with similar functions. Finally, the candidate proteins were grouped and are summarized in Figure 5.
Figure 5.
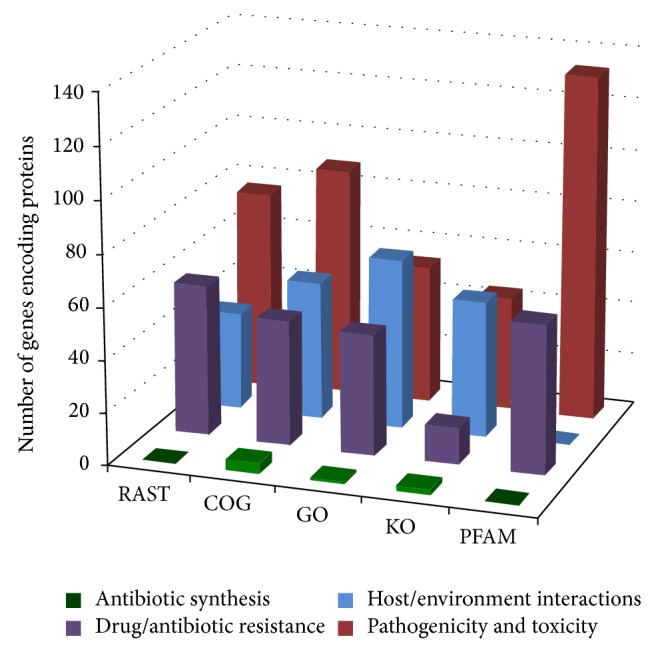
The number of genes encoding proteins and their classification according to information retrieved from the RAST server and COG, GO, KO, and PFAM databases.
The most abundant proteins found in our search were those involved with pathogenesis and toxicity, followed by drug/antibiotic resistance proteins. Table 2 shows the nonredundant (nr) proteins retrieved from RAST server (version 2.0) and analyzed databases. The host/environment interactions subgroup includes proteins related to flagellar movement and taxis, which could participate in the observed A. faecalis MOR02 swarming movement. The proteins related to both activities are chemotaxis proteins, chemotaxis response protein, flagellin, flagellar transcriptional activators, flagellar biosynthetic proteins, flagellar basal-body rod proteins, and flagellum specific ATP synthase, among others (Table S1).
Table 2.
Categorization of genes encoding proteins that were identified after an analysis on the RAST server and COG, GO, KO, and PFAM databases. All proteins are nonredundant.
| Subgroups | Number genes encoding proteins |
|---|---|
| Host/environment interactions | |
| Host factor | 2 |
| Taxis | 19 |
| Flagellar | 42 |
| Signal transduction | 34 |
| Starvation | 11 |
| Metabolism of xenobiotics | 9 |
| Pathogenicity and toxicity | |
| Peptidases | 54 |
| Proteases | 55 |
| Lipases/esterases | 88 |
| Hemolysins | 7 |
| Invasins | 2 |
| Virulence | 9 |
| Toxins | 9 |
| Chitin deacetylase | 1 |
| Secretion system | 29 |
| Pathogenesis | 7 |
| Antibiotic synthesis | 7 |
| Drug/antibiotic resistance | |
| Drug/antibiotic transporter | 68 |
| Resistance | 66 |
| Beta-lactamase | 20 |
| Related to penicillin | 12 |
| Dihydrofolate reductase | 3 |
The subgroup pathogenicity and toxicity primarily include peptidases, proteases, lipases/esterases, hemolysins, virulence factors, and toxins. The chitinase activity of A. faecalis MOR02 may be related to a chitin deacetylase protein found in the COG database, whereas the activity on Tween 80 may be due to the presence of lipases/esterases. As we reported, the supernatant extract of A. faecalis MOR02 was toxic to G. mellonella larvae; this toxicity must be due to proteins that are excreted, specifically toxins, proteases, and peptidases. In the bacteria genome we found some genes that encode proteins that could be participating in such toxic activities. In the GO database, we found that there is evidence that the TolR protein acts as a toxin transporter and a toxin secretion ATP-binding protein; therefore, it may participate in pathogenesis. We also found proteins in the type II secretion pathway, a system that Gram-negative bacteria use to release enzymes or toxins (Tables S2, S3, and S4). Finally, in the antibiotic synthesis and drug/antibiotic resistance subgroups, we found genes that encode proteins related to antibiotic synthesis and resistance, such as proteins involved in mitomycin antibiotic biosynthesis, ABC-type bacteriocin/antibiotic exporters, beta-lactamases, MATE (multidrug and toxic compound extrusion) efflux family proteins, and multidrug resistance transporters. We also found other proteins that were more specific, such as chloramphenicol acetyltransferases or translation elongation factor G that has a known function in tetracycline resistance, among others. We also found additional proteins related to drug resistance such as bacitracin, bleomycin, and fusaric acid in PFAM database (Tables S1, S3, and S5). All of these proteins were reported to participate in the mechanisms that bacteria possess to cope with antibiotics.
3.5. Phenotypic Characterization
Extracellular chitinase production was discovered when we observed a white halo of chitinase activity after incubating A. faecalis MOR02 with chitin. Esterase activity was observed when A. faecalis MOR02 used Tween 80 as a substrate and fatty acids were released. These results confirm the chitinolytic and esterase activity of A. faecalis that has previously been reported [28, 29]. Swarming motility was observed in TSA (Trypticase Soy Agar) plates inoculated with the bacteria, where zones of consolidation or terraces, commonly known as bull's eye, were observed. Concurrent with this observation we also observed a greenish slime surrounding the bacterial growth.
3.6. mCherry Protein Expression in A. faecalis MOR02
To analyze the possible association of A. faecalis MOR02 with nematodes we transformed bacteria with plasmid pT4-23S-mCherry. The transformed bacteria were referred to as A. faecalis MOR02-Cherry and mCherry expression was corroborated using fluorescence microscopy.
3.7. Pathogenicity of A. faecalis MOR02 to G. mellonella Larvae
To assess the ability of A. faecalis MOR02 to grow and survive within the hemocoel larvae as well as to test its pathogenicity, 10 μL of different bacterial suspensions of A. faecalis MOR02-Cherry was injected intolarvae. As shown in Table 1, larvae injected with 240 and 2,400 CFU had 3.33% mortality at 72 h after injection and 6.67% at 48 h after injection, respectively. With 2,400 CFU, only 33.33% mortality was observed at >96 h after injection. Conversely, larvae injected with 24,000 CFU had 96.67% mortality at 24 h after injection, while 100% mortality was observed using 240,000 CFU.
Table 1.
Mortality assessment in the pathogenicity assay of A. faecalis MOR02 and E. coli DH5α (carrying plasmid pT4-mCherry) on G. mellonella larvae.
| Larvae treatment | Mortality (%) | |||
|---|---|---|---|---|
| A. faecalis MOR02-Cherry | 24 h after injection | 48 h after injection | 72 h after injection | >96 h after injection |
| Puncture | 0 | 0 | 0 | 0 |
| LB broth | 0 | 0 | 0 | 0 |
| 240 CFU | 0 | 0 | 3.33 ± 4.71 | 0 |
| 2,400 CFU | 0 | 6.67 ± 4.71a | 6.67 ± 9.43a | 33.33 ± 9.42a |
| 24,000 CFU | 96.67 ± 4.71a | 3.33 ± 4.71a | ∗ | ∗ |
| 240,000 CFU | 100a | ∗ | ∗ | ∗ |
|
| ||||
| E. coli DH5α | 24 h after injection | 48 h after injection | 72 h after injection | >96 h after injection |
|
| ||||
| Puncture | 0 | 10 | 0 | 0 |
| LB broth | 0 | 0 | 0 | 0 |
| 240 CFU | 0 | 0 | 0 | 0 |
| 2,400 CFU | 0 | 0 | 0 | 0 |
| 24,000 CFU | 0 | 0 | 0 | 0 |
| 240,000 CFU | 0 | 0 | 0 | 0 |
aSignificant differences between values. Note: the percentage values shown are accumulative. ∗At this time all larvae were dead.
We did not observe any mortality as a result of injection puncture, injection of LB broth or E. coli DH5α cells.
3.8. Toxicity of the Supernatant Protein Extract of A. faecalis MOR02
A toxicity comparison of four different amounts of supernatant protein extracts was assayed using sixth-instar G. mellonella larvae as the host. All doses had toxic activity as indicated by the presence of dead larvae at 96 h after injection. Necrotic tissue was also observed in the insects, as well as a change in body size and consistency. At 96 h after injection, 73.3, 83.3, and 83.3% of the larvae were dead using 1, 2, and 4 μg protein extract, respectively. Mortality was not observed in larvae that were punctured or injected with BSA (1, 2, and 4 μg) and the Tris 10 mM pH 7.2 controls resulted in 10% mortality (Figure 2).
Figure 2.
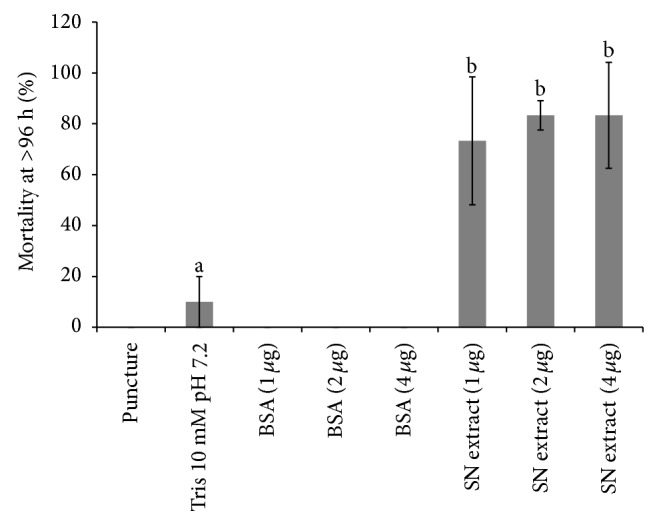
Toxicity of the supernatant (SN) extract of A. faecalis MOR02 on G. mellonella larvae. At 96 h after injection, 73.3, 83.3, and 83.3% of larvae were dead using 1, 2, and 4 μg toxin extract, respectively. There were no significant differences between the treatment groups. BSA: bovine serum albumin; puncture: larvae only punctured with a syringe. Different letters indicate significant differences. Bars represent standard deviation of three independent experiments.
3.9. The Association of A. faecalis Strain MOR02 with Nematodes
A microscopic analysis of the nematodes showed that A. faecalis MOR02-Cherry is located inside the nematodes S. feltiae, S. carpocapsae,and H. bacteriophora after 18 h at 20°C. We also observed fluorescence outside of the nematodes in the presence of Escherichia coli expressing mCherry protein (negative control) (Figure 3). The fluorescence in S. feltiae nematodes was observed along the esophagus and intestine (Figure 4).
Figure 3.
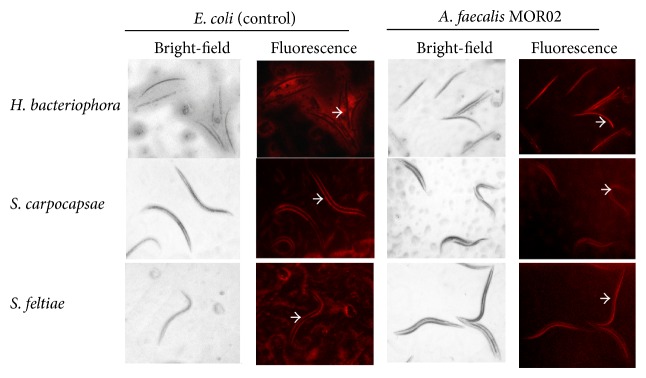
Association of A. faecalis MOR02 IJ2 nematodes. Bright-field and fluorescence microscopy analyses of the association of E. coli and A. faecalis MOR02-Cherry with H. bacteriophora, S. carpocapsae, and S. feltiae. In E. coli (negative control), fluorescence is observed outside nematodes (white arrows), whereas A. faecalis MOR02 fluorescence is located inside the nematodes (white arrows). Gut autofluorescence of H. bacteriophora is not clearly observed due to red fluorescence of A. faecalis MOR02.
Figure 4.
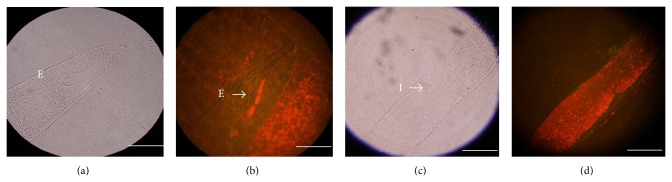
Microscopic analysis of the association of A. faecalis MOR02 with IJ2 S. feltiae. (a) Bright-field microscopy of the anterior section of the nematode; E, esophagus. (b) Fluorescence microscopy of the anterior section of the nematode showing the bacteria located in the nematode esophagus; the arrow points to A. faecalis MOR02-Cherry inside the nematode esophagus; (c) Bright-field microscopy of the nematode intestine; I, intestine. (d) Fluorescence microscopy of the intestine with bacterial colonization. Scale bar, 25 μm.
4. Discussion
We recently identified a bacterium that we named A. faecalis MOR02 that was isolated from a nematode found in a G. mellonella larva cadaver.
The chitinase and esterase activity that were observed as part of the phenotypic characterization of A. faecalis MOR02 are supported by the presence of genes related to these activities within its genome. One such gene is chitin deacetylase that has been reported in fungi and insects, but only in the bacteria Bacillus sp., Serratia sp., and members of the family Vibrionaceae [30–32]. This enzyme catalyzes the deacetylation of chitin to produce acetate and chitosan, which indicates that this enzyme has potential biotechnological applications for controlling fungal plant pathogens or insect pests in agriculture. Both chitinase and esterase activities may assist the bacteria when degrading insect body components, which we observed as a floppy phenotype (massive loss of body turgor) in G. mellonella larvae infected with A. faecalis MOR02.
The swarming motility by flagella in A. faecalis MOR02 has been reported in this species as well as in alphaproteobacteria, gammaproteobacteria, and firmicutes. Actually, this behavior is considered a pathogenic factor for eubacteria, such as Proteus mirabilis, Pseudomonas aeruginosa, Serratia liquefaciens, and S. marcescens [33–35]. Several proteins, such as flagellin and those related to flagellar body composition, were also identified in the databases we analyzed.
The diverse associations in nematodes are of interest because they represent examples of diversity, adaptation, and evolution in nature. When we examined the association between A. faecalis MOR02 and the nematodes S. feltiae, S. carpocapsae, and H. bacteriophora, we observed that fluorescence clearly shows that A. faecalis MOR02 is located in the digestive tract of nematodes, whereas the control E. coli was observed outside on the cuticles of nematodes. This result demonstrates the specificity of the bacteria-nematode interaction. The results of the pathogenicity assays indicated that A. faecalis MOR02 is lethal when directly injected into the hemocoel of larvae. However, the controls LB broth and E. coli DH5α did not affect insect mortality or changes in body consistency or development. The pathogenicity of A. faecalis in insects has been previously reported; Park et al. [9] observed less than 30% mortality in fourth-instar G. mellonella larvae at 48 h after haemocoelic injection with 103, 104, and 105 bacteria cells of A. faecalis. In this study, we observed 96% mortality 24 h after injection of 24,000 CFU in sixth-instar larvae. All the dead larvae had necrosis and a softened consistency, which suggests that A. faecalis MOR02 enter the hemocoel and disperse throughout the insect body, excreting toxic compounds that cause the observed damage. In the A. faecalis MOR02 genome we found many genes that encode proteins in the type II secretion system that is conserved in Gram-negative bacteria. The proteins secreted by this pathway include proteases, pectinases, phospholipases, lipases, and toxins. Many of these proteins are associated with tissue destruction, which contributes to cell damage and disease [36, 37].
It is necessary to identify the compounds that cause toxicity after the bacteria enter the insect larvae. Genes that encode proteases and peptidases have been identified in the A. faecalis MOR02 genome. However, when we examined the bibliography for other candidates not classified in the above-mentioned databases we identified the protein peg.814 that has high similarity (87%) to the HIP57 protein from Xenorhabdus budapestensis, a protein homologous to the GroEL chaperone. X. budapestensis HIP57 caused G. mellonella larval bodies to blacken and die [38], a phenotype very similar to the phenotype observed for A. faecalis strain MOR02 in our experiments. It has been proposed that, in addition to the molecule chaperon role of GroEL, HIP57 could possess another novel function as a toxin insecticide [38]. Because of the results of the phenoloxidase (PO) activity analysis of G. mellonella larvae injected with HIP57 X. budapestensis, the author suggested that HIP57 activates the PO cascade, which provides an extensive defense mechanism that is potentially responsible for G. mellonella larval death. The mode of action of this protein that has high similarity to GroEL and injectable toxicity to G. mellonella causes an increased phenoloxidase activity innate immune response that is stimulated by the injection [39]. Because different toxins have different activities against different pests, the proteases and toxins in A. faecalis MOR02 allow the exploration of novel options of pest control. It will be interesting in the future to mutate peg.814 and analyze its effects on G. mellonella.
In addition to the established associations of Heterorhabditis and Steinernema with Photorhabdus and Xenorhabdus, respectively, in this report, we describe the association between bacteria and nematodes. Although we found A. faecalis MOR02 associated with S. feltiae, S. carpocapsae, and H. bacteriophora, the function of these bacteria when they are associated with nematodes remains to be elucidated. It appears that the bacteria assist with the insect infection process, as well as with bacteria considered primary symbionts, as we demonstrated in the pathogenicity and toxicity assays. The reason that A. faecalis MOR02 is not able to establish a permanent symbiotic association with nematodes may be because it lacks the genes important for colonization. Easom et al. [40] analyzed mutants that had reduced ability to colonize the guts of infective juvenile (IJ) nematodes. They identified six different genetic loci (pbgPE operon, galE, galU, asmA, hdfR, and proQ) that were involved with the mutualistic colonization of IJ H. bacteriophora by P. luminescens. When we performed a search of these genes in the A. faecalis MOR02 genome we found two genes encoding proteins with 66% and 71% similarity to GalU (glucose-1-phosphate uridyltransferase or UDP-glucose pyrophosphorylase) and GalE (UDP-glucose 4-epimerase), respectively. The activities of these proteins are important for the production of polysaccharides, an important factor for colonization that is considered an important virulence factor in Gram-negative pathogens [40–43]. Therefore, it is likely that the presence of these two proteins enables the bacteria to colonize the gut of nematodes temporarily.
5. Conclusions
In this work, we demonstrate pathogenicity and toxicity of bacteria isolated from the hemolymph of G. mellonella larvae identified as A. faecalis MOR02. These bacteria are pathogen to larvae insect and excrete toxic compounds and enzymes as chitinases that could be helping in the infection process. A possible bacteria-nematode association that is not inherited to progeny may be a strategy of dispersion that could be occurring in the environment.
Genomic analysis shows that these bacteria possess a wide repertoire of enzymes related to toxicity with a potential to be used as a biocontrol compound. We are now investigating the role of the protein peg.814 as a candidate in the toxicity of the bacteria.
Supplementary Material
Figure S1: Graphical representation of a 5712 bp fragment from the contig1 showing the disposition of the transfers RNA genes tRNA-Val(gac) [121, 713-121, 789], tRNA-Leu(tag)[324, 903-324, 987], tRNA-Met(cat) [456, 223-456, 301] and tRNA Ala(tgc) [613, 447-613, 522], tRNA-Ile(gat) [613, 534-613, 610] in addition to the ribosomal genes 5S rRNA [609, 890-610, 001], 23S rRNA[610, 185-613, 067] and16SrRNA[613, 709-615, 233]
Figure S2: A phylogenetic analysis of one hundred and sixty five 16S rRNA sequences (available upon request) aligned with MUSCLE server version 3, calculating distances with the maximum likelihood method and inferring the evolutionary tree with the neighbor-joining method using 1000 replicates to calculate bootstrap values
Table S1: Functional analysis of the Alcaligenes faecalis Strain MOR02 proteome, comparing the genome with the annotation of the server RAST
Table S2: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the Gene Ontology (GO) server
Table S3: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the PFAM server.
Table S4: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the KEGG Orthology (KO) server.
Table S5: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the Clusters of Orthologous Groups of proteins (COG) server.
Acknowledgments
The authors are grateful to Jorge Abimael Valle-Hernández for statistical analysis assistance and Luis Fernando Lozano-Aguirre Beltrán (Center of Genomics Sciences-National University of México) for bioinformatic assistance.re
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Murfin K. E., Dillman A. R., Foster J. M., et al. Nematode-bacterium symbioses-cooperation and conflict revealed in the ‘omics’ age. Biological Bulletin. 2012;223(1):85–102. doi: 10.1086/BBLv223n1p85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro-Ilan D. I., Han R., Dolinksi C. Entomopathogenic nematode production and application technology. Journal of Nematology. 2012;44(2):206–217. [PMC free article] [PubMed] [Google Scholar]
- 3.Poinar G. O. Nematodes for Biological Control of Insects. Boca Raton, Fla, USA: CRC Press; 1979. [Google Scholar]
- 4.Gouge D. H., Snyder J. L. Temporal association of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) and bacteria. Journal of Invertebrate Pathology. 2006;91(3):147–157. doi: 10.1016/j.jip.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Chaston J. M., Suen G., Tucker S. L., et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: convergent lifestyles from divergent genomes. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027909.e27909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnell A. M., Stock S. P. Heterorhabditis, Steinernema and their bacterial symbionts—lethal pathogens of insects. Nematology. 2000;2(1):31–42. doi: 10.1163/156854100508872. [DOI] [Google Scholar]
- 7.Vaaje-Kolstad G., Horn S. J., Sørlie M., Eijsink V. G. H. The chitinolytic machinery of Serratia marcescens—a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS Journal. 2013;280(13):3028–3049. doi: 10.1111/febs.12181. [DOI] [PubMed] [Google Scholar]
- 8.Lysenko O., Weiser J. Bacteria associated with the nematode Neoaplectana carpocapsae and the pathogenicity of this complex for Galleria mellonella larvae. Journal of Invertebrate Pathology. 1974;24(3):332–336. doi: 10.1016/0022-2011(74)90140-2. [DOI] [PubMed] [Google Scholar]
- 9.Park H. W., Kim Y. O., Ha J.-S., et al. Effects of associated bacteria on the pathogenicity and reproduction of the insect-parasitic nematode Rhabditis blumi (Nematoda: Rhabditida) Canadian Journal of Microbiology. 2011;57(9):750–758. doi: 10.1139/w11-067. [DOI] [PubMed] [Google Scholar]
- 10.Meyer F., Paarmann D., D'Souza M., et al. The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9, article 386 doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagesen K., Hallin P., Rødland E. A., Stærfeldt H.-H., Rognes T., Ussery D. W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schattner P., Brooks A. N., Lowe T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research. 2005;33(2):W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laslett D., Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Research. 2004;32(1):11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatusov R. L., Fedorova N. D., Jackson J. D., et al. The COG database: an updated vesion includes eukaryotes. BMC Bioinformatics. 2003;4, article 41 doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman M., Banu S. S., Gomathinayagam S., Raj G. D. Lesion scoring technique for assesing the virulence and pathogenicity of Indian field isolates of avian Eimeria species. Veterinarski Arhiv. 2011;81(2):259–271. [Google Scholar]
- 16.Ashburner M., Ball C. A., Blake J. A., et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalafalla R. E., Müller U., Shahiduzzaman M., et al. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitology Research. 2011;108(4):879–886. doi: 10.1007/s00436-010-2129-y. [DOI] [PubMed] [Google Scholar]
- 18.Ogata H., Goto S., Sato K., Fujibuchi W., Bono H., Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 1999;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schares G., Pantchev N., Barutzki D., Heydorn A. O., Bauer C., Conraths F. J. Oocysts of Neospora caninum, Hammondia heydorni, Toxoplasma gondii and Hammondia hammondi in faeces collected from dogs in Germany. International Journal for Parasitology. 2005;35(14):1525–1537. doi: 10.1016/j.ijpara.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Edgar R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5, article 113 doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giammanco G. M., Grimont P. A. D., Grimont F., Lefevre M., Pignato S. Phylogenetic analysis of the genera Proteus, Morganella and Providencia by comparison of rpoB gene sequences of type and clinical strains suggests the reclassification of Proteus myxofaciens in a new genus, Cosenzaea gen. nov., as Cosenzaea myxofaciens comb. nov. International Journal of Systematic and Evolutionary Microbiology. 2011;61(7):1638–1644. doi: 10.1099/ijs.0.021964-0. [DOI] [PubMed] [Google Scholar]
- 23.Wood T. K., González Barrios A. F., Herzberg M., Lee J. Motility influences biofilm architecture in Escherichia coli . Applied Microbiology and Biotechnology. 2006;72(2):361–367. doi: 10.1007/s00253-005-0263-8. [DOI] [PubMed] [Google Scholar]
- 24.Kilburn J. O., O'Donnell K. F., Silcox V. A., David H. L. Preparation of a stable mycobacterial tween hydrolysis test substrate. Journal of Applied Microbiology. 1973;26(5):p. 826. doi: 10.1128/am.26.5.826-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aylward F. O., Tremmel D. M., Starrett G. J., et al. Complete genome of Serratia sp. strain FGI 94, a strain associated with leaf-cutter ant fungus gardens. Genome Announcements. 2013;1(2) doi: 10.1128/genomeA.00239-12.e0023912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Mendoza A., Lozano-Aguirre Beltrán LF., Martínez-Ocampo F., Quiroz-Castañeda RE., Dantán-González E. A newly sequenced Alcaligenes faecalis strain: implications for novel temporal symbiotic relationships. Genome Announcements. 2014;2(6) doi: 10.1128/genomeA.01246-14.e01246-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laslett D., Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Research. 2004;32(1):11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annamalai N., Rajeswari M. V., Vijayalakshmi S., Balasubramanian T. Purification and characterization of chitinase from Alcaligenes faecalis AU02 by utilizing marine wastes and its antioxidant activity. Annals of Microbiology. 2011;61(4):801–807. doi: 10.1007/s13213-011-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrity G., Brenner D. J., Staley J. T., et al. Bergey's Manual of Systematic Bacteriology: Volume Two: The Proteobacteria (Part C) New York, NY, USA: Springer; 2006. [Google Scholar]
- 30.Hunt D. E., Gevers D., Vahora N. M., Polz M. F. Conservation of the chitin utilization pathway in the Vibrionaceae. Applied and Environmental Microbiology. 2008;74(1):44–51. doi: 10.1128/aem.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y., Park R. D., Muzzarelli R. A. A. Chitin deacetylases: properties and applications. Marine Drugs. 2010;8(1):24–46. doi: 10.3390/md8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur K., Dattajirao V., Shrivastava V., Bhardwaj U. Isolation and characterization of chitosan-producing bacteria from beaches of Chennai, India. Enzyme Research. 2012;2012:6. doi: 10.1155/2012/421683.421683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahlen S. D. Serratia infections: from military experiments to current practice. Clinical Microbiology Reviews. 2011;24(4):755–791. doi: 10.1128/cmr.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanks R. M. Q., Lahr R. M., Stella N. A., et al. A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0057634.e57634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phung L. T., Trimble W. L., Meyer F., Gilbert J. A., Silver S. Draft genome sequence of Alcaligenes faecalis subsp. faecalis NCIB 8687 (CCUG 2071) Journal of Bacteriology. 2012;194(18):p. 5153. doi: 10.1128/jb.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandkvist M. Type II secretion and pathogenesis. Infection and Immunity. 2001;69(6):3523–3535. doi: 10.1128/IAI.69.6.3523-3535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nivaskumar M., Francetic O. Type II secretion system: a magic beanstalk or a protein escalator. Biochimica et Biophysica Acta—Molecular Cell Research. 2014;1843(8):1568–1577. doi: 10.1016/j.bbamcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Yang J., Zeng H. M., Lin H. F., et al. An insecticidal protein from Xenorhabdus budapestensis that results in prophenoloxidase activation in the wax moth, Galleria mellonella . Journal of Invertebrate Pathology. 2012;110(1):60–67. doi: 10.1016/j.jip.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Castagnola A., Stock S. P. Common virulence factors and tissue targets of entomopathogenic bacteria for biological control of lepidopteran pests. Insects. 2014;5(1):139–166. doi: 10.3390/insects5010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Easom C. A., Joyce S. A., Clarke D. J. Identification of genes involved in the mutualistic colonization of the nematode Heterorhabditis bacteriophora by the bacterium Photorhabdus luminescens . BMC Microbiology. 2010;10, article 45 doi: 10.1186/1471-2180-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou Y., Feng S., Xu C., et al. The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Veterinary Microbiology. 2013;162(1):278–284. doi: 10.1016/j.vetmic.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Jiang S.-S., Lin T.-Y., Wang W.-B., Liu M.-C., Hsueh P.-R., Liaw S.-J. Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrobial Agents and Chemotherapy. 2010;54(5):2000–2009. doi: 10.1128/aac.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramjeet M., Cox A. D., Hancock M. A., et al. Mutation in the LPS outer core biosynthesis gene, galU, affects LPS interaction with the RTX toxins ApxI and ApxII and cytolytic activity of Actinobacillus pleuropneumoniae serotype 1. Molecular Microbiology. 2008;70(1):221–235. doi: 10.1111/j.1365-2958.2008.06409.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Graphical representation of a 5712 bp fragment from the contig1 showing the disposition of the transfers RNA genes tRNA-Val(gac) [121, 713-121, 789], tRNA-Leu(tag)[324, 903-324, 987], tRNA-Met(cat) [456, 223-456, 301] and tRNA Ala(tgc) [613, 447-613, 522], tRNA-Ile(gat) [613, 534-613, 610] in addition to the ribosomal genes 5S rRNA [609, 890-610, 001], 23S rRNA[610, 185-613, 067] and16SrRNA[613, 709-615, 233]
Figure S2: A phylogenetic analysis of one hundred and sixty five 16S rRNA sequences (available upon request) aligned with MUSCLE server version 3, calculating distances with the maximum likelihood method and inferring the evolutionary tree with the neighbor-joining method using 1000 replicates to calculate bootstrap values
Table S1: Functional analysis of the Alcaligenes faecalis Strain MOR02 proteome, comparing the genome with the annotation of the server RAST
Table S2: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the Gene Ontology (GO) server
Table S3: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the PFAM server.
Table S4: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the KEGG Orthology (KO) server.
Table S5: Analysis of the Alcaligenes faecalis strain MOR02 proteome in the Clusters of Orthologous Groups of proteins (COG) server.