Abstract
When a mixture of superhelical DNA (RFI) of phage phiX174 am3 and fragments of single-stranded DNA from wild-type phiX174 was added to spheroplasts of E. coli carrying an amber suppressor, several percent of the progeny phage were recombinant. The yield of wild-type progeny was 10(3) to 10(4) times lower when the fragments came from phiX174 am3 or phage G4 am+, or when fragments were absent. Fewer recombinants were produced in proportion to the decrease in the fraction of RFI in samples treated with S1 nuclease, whereas the total yield of phage did not decrease. Transfection by fragments and superhelical DNA produced 20 to 100 times more recombinants than transfection by fragments and either nicked circular DNA or relaxed closed circular DNA. Transfection of a recA- strain by RFI DNA and fragments yielded 5-10% as many recombinants as transfection of a rec+ strain. This partial requirement for recA was bypassed by transfection with complexes of RFI AM3 DNA and am+ fragments made in vitro.
Full text
PDF

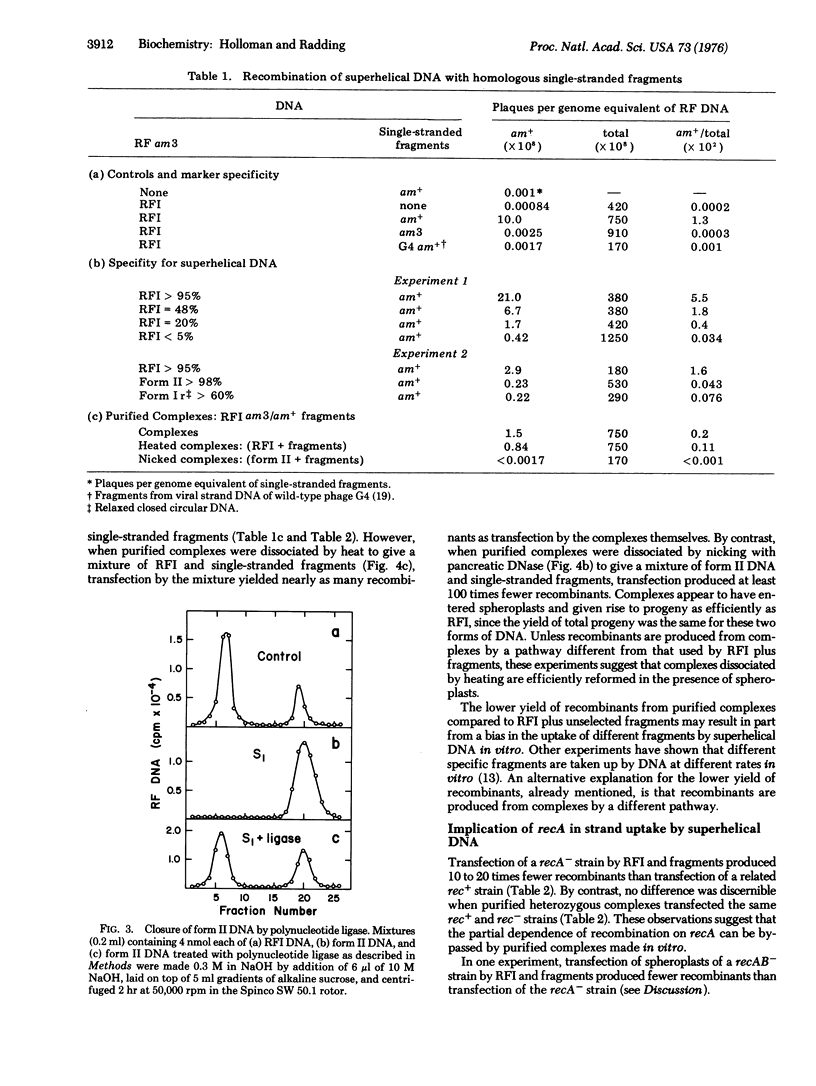
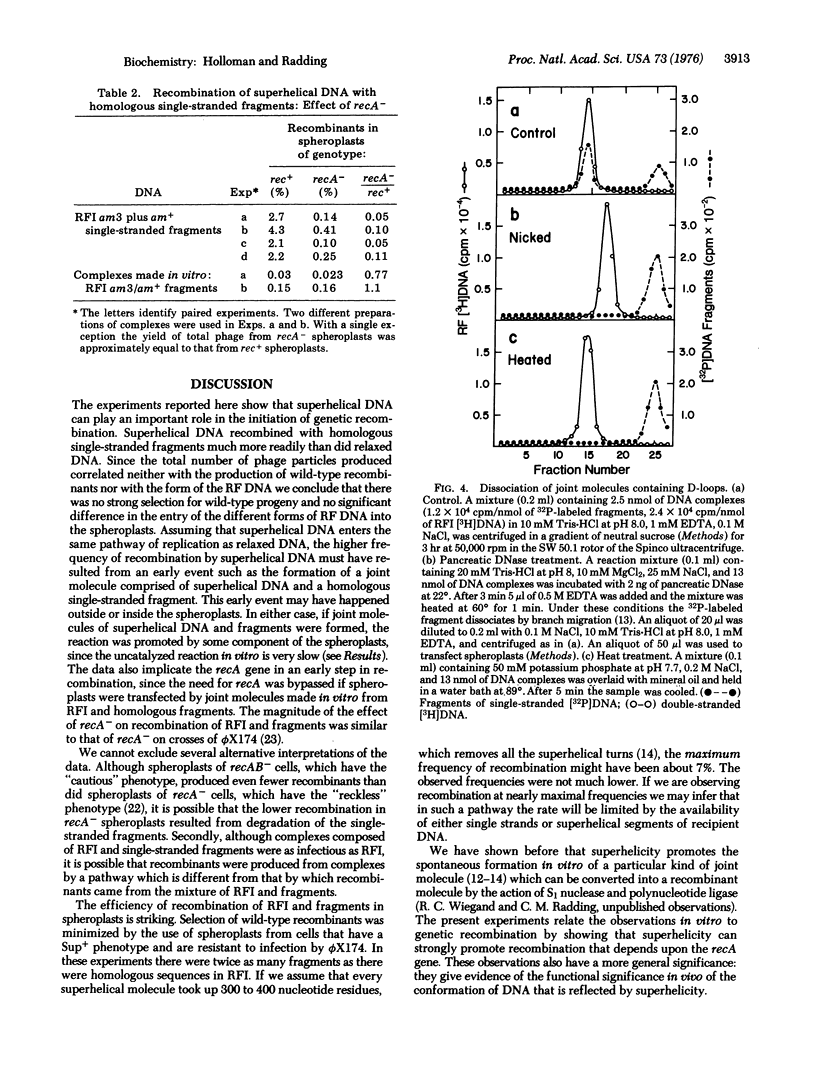

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Zuccarelli A. J., Davis G. C., Sinsheimer R. L. Genetic recombination in bacteriophage phi chi 174. J Virol. 1974 Apr;13(4):898–907. doi: 10.1128/jvi.13.4.898-907.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. Recombinant DNA molecules of bacteriophage phi chi174. Proc Natl Acad Sci U S A. 1975 Jan;72(1):235–239. doi: 10.1073/pnas.72.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R., Doermann A. H. Molecular and genetic recombination of bacteriophage T4. Annu Rev Genet. 1975;9:213–244. doi: 10.1146/annurev.ge.09.120175.001241. [DOI] [PubMed] [Google Scholar]
- Fox M. S. On the mechanism of integration of transforming deoxyribonucleate. J Gen Physiol. 1966 Jul;49(6):183–196. doi: 10.1085/jgp.49.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Evolution of phi-chi 174. Isolation of four new phi-chi-like phages and comparison with phi-chi 174. Virology. 1974 Mar;58(1):272–289. doi: 10.1016/0042-6822(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Holloman W. K., Wiegand R., Hoessli C., Radding C. M. Uptake of homologous single-stranded fragments by superhelical DNA: a possible mechanism for initiation of genetic recombination. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2394–2398. doi: 10.1073/pnas.72.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. Toward a general theory of genetic recombination in DNA. Adv Genet. 1971;16:325–348. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr Replication and molecular recombination of T-phage. Annu Rev Microbiol. 1975;29:355–376. doi: 10.1146/annurev.mi.29.100175.002035. [DOI] [PubMed] [Google Scholar]
- Paszewski A. Gene conversion: observations on the DNA hybrid models. Genet Res. 1970 Feb;15(1):55–64. doi: 10.1017/s0016672300001373. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- Wackernagel W. An improved spheroplast assay for lambda-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972 Apr;48(1):94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Godson G. N., Radding C. M. Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem. 1975 Nov 25;250(22):8848–8855. [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]


