Abstract
Background. The objective of our study was to ascertain racial/ethnic disparities in Asian/Pacific Islanders (API) for non-small-cell lung cancer (NSCLC) clinicopathologic features and survival outcomes based on various tumor characteristics and treatment modalities. Method. SEER database identified invasive NSCLC cases from 2004 to 2010. Variables included American Joint Committee on Cancer (AJCC) stage 7, tumor grade, tumor size, histology, age, marital status, radiation, surgery, and reason for no surgery. The Kruskall-Wallis test and the Z test were used to examine differences between races/ethnicities and the referent, non-Hispanic white (NHW). Multivariate Cox proportional analyses were used to establish the weight of the prognostic significance contributing to disease-specific survival (DSS) in each AJCC stage. Result. Improved DSS was seen in API across stage I (HR: 0.78), stage II (HR: 0.79), and stage IV (HR: 0.86), respectively, compared to the referent NHW (P < 0.01). Prognosis was improved by being married, being female gender, AIS histology, and birth outside the US (P < 0.01). Conclusion. We have demonstrated improved survival among API in early stage and stage IV NSCLC. Further research is necessary to clarify the role of lifestyle and tumor biology for these differences.
1. Background
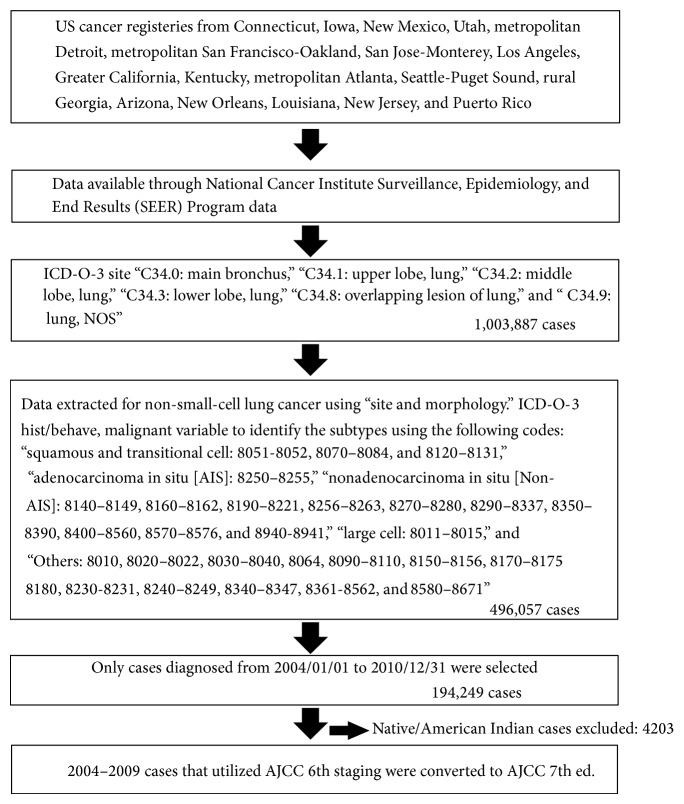
Lung cancer is the second most common cancer in both men and women with an estimated 224,210 cases expected to be diagnosed in 2014 in the United States (US) [1]. It is also the leading cause of cancer related deaths in the US accounting for 27% of all cancer related deaths [1]. The majority of lung cancer cases fall under the category of non-small-cell lung cancer (NSCLC) [1]. Figure 1 shows the selection of the non-small-cell lung cancer cases included in the study.
Figure 1.
Selection of the non-small-cell lung cancer cases included in the study.
Racial/ethnic disparities have been shown to influence survival outcomes in NSCLC [2–4]. Disparities in survival outcomes among racial/ethnic groups may be attributed to a complex interaction between genetic and lifestyle factors [4, 5]. Compared to non-Hispanic whites (NHW), Blacks have a higher incidence of lung cancer and more advanced disease at diagnosis with worse survival outcomes [6–8]. Despite a lower incidence, Hispanics are more likely to be diagnosed with advanced disease with poor outcomes compared to NHW [2, 9]. Asian/Pacific Islanders (API) have a lower incidence of NSCLC compared to NHW [10]. Interestingly, previous literature has shown that cancer related mortality is favorable in API compared to other racial/ethnic groups for early stage (stages IA and IB) NSCLC [11, 12]. However, survival outcomes in API and other racial/ethnic groups based on the recently published American Joint Committee on Cancer (AJCC) 7th edition have not been evaluated in detail [13].
In this study, our primary objective was to utilize an established large nationwide cancer registry to ascertain racial/ethnic disparities in NSCLC clinicopathologic features and survival outcomes.
2. Methods
We used the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Result (SEER) cancer registry that collects large observational data across 18 cancer registry sites. The database was accessed using the SEER*Stat 8.1.5, http://seer.cancer.gov/seerstat, assessed May 01, 2014. To be eligible, we identified patients diagnosed between 01/01/2004 to 31/12/2010 with NSCLC (ICD-O-3 Site C34.0–C34.9) based on the selected histology codes: squamous and transitional cell: 8051-8052, 8070–8084, and 8120–8131, adenocarcinoma in situ [AIS]: 8250–8255, nonadenocarcinoma in situ [Non-AIS]: 8050, 8140–8149, 8160–8162, 8190–8221, 8256–8263, 8270–8280, 8290–8337, 8350–8390, 8400–8560, 8570–8576, and 8940-8941, large cell: 8011–8015, and “Others”: 8010, 8020–8022, 8030–8040, 8046, 8090–8110, 8150–8156, 8170–8175, 8180, 8230-8231, 8240–8249, 8340–8347, 8561-8562, and 8580–8671. We utilized the time period stated, due to the ability to restage the tumors to the latest AJCC 7th edition using data from the collaborative stage variables. To investigate any existing treatment or tumor racial/ethnic disparities and disease-specific survival (DSS), racial/ethnic groups were categorized as NHW, Hispanics, Blacks, and API. Clinicopathologic characteristics included age at diagnosis, gender, birth country, marital status, tumor grade, tumor size, AJCC stage, and histology. Treatment variables included radiation, surgery, and radiation/surgery sequence. Decade long time intervals “<30 years,” “30–39,” “40–49,” “50–59,” “60–69,” “70–79,” and “>80 years” were used to categorize age. Marital status was described using “Single,” “Married,” or “Others,” a term that is inclusive of divorced, widowed, or separated individuals. Birth within the US or outside was used to monitor “the immigration effect.” The variable “Tumor size” reflected the size of the tumor mass and was classified categorically to “<30 mm”, “30–50 mm”, “50–70 mm”, “>70 mm” or in cases with no recorded tumor mass to “No mass was found”. All forms of radiation were collectively grouped as “Radiation received,” while all forms of cancer directed surgeries were coded collectively as “Surgery performed.” Cases after 2010 were excluded to allow a minimum of 12 months of follow-up period.
2.1. Statistical Analysis
The Kruskall-Wallis nonparametric test was employed to examine the differences that may exist among various racial/ethnic groups and tumor characteristics. The difference between the racial/ethnic groups and the reasons for no surgery was measured using Fisher's exact test. The end point was DSS which was measured in months from the date of diagnosis to death due to lung cancer or censoring, which included either being alive, lost to follow-up, or died due to other causes.
Multivariate Cox proportional hazard models were used to establish the weight of different characteristics (grade, age at diagnoses, tumor size, histology, marital status, race/ethnicity, gender, radiation, surgery, and radiation/surgery sequence) on prognostic significance contributing to the survival in each respective AJCC stage. The Z test was employed to examine the proportional differences that may exist between the referent NHW and other race/ethnic groups. The models were constructed using IBM SPSS Statistical software, version 21.0 (IBM Corp. Released 2012, IBM SPSS Statistics for Windows, Version 21.0, Armonk, NY: IBM Corp.).
3. Results
Our database yielded 190,046 patients with NSCLC: 145646 (76.6%) NHW, 10350 (5.4%) Hispanics, 22525 (11.9%) Blacks, and 11525 (6.1%) API (Table 1).
Table 1.
Baseline demographic and clinicopathologic of the study cohort.
| Characteristics | NHW (145464) | Hispanic (10350) | Black (22525) | API (11525) | ||||
|---|---|---|---|---|---|---|---|---|
| f | % | f | % | f | % | f | % | |
| Grade (P < 0.01) | ||||||||
| Grade I | 8027 | 5 | 632 | 5.4* | 817 | 3.3* | 724 | 5.7* |
| Grade II | 26678 | 16.5 | 1849 | 15.7 | 3665 | 14.8* | 2106 | 16.6 |
| Grade III | 44361 | 27.5 | 3158 | 26.8 | 6968 | 28.2 | 3250 | 25.7* |
| Grade IV | 3590 | 2.2 | 258 | 2.2 | 554 | 2.2 | 233 | 1.8* |
| Unknown | 78658 | 48.8 | 5871 | 49.9* | 12710 | 51.4* | 6355 | 50.2* |
| AJCC stage 7 (P < 0.01) | ||||||||
| Stage I | 28649 | 17.8 | 1588 | 13.5* | 3062 | 12.4* | 1815 | 14.3 |
| Stage II | 21121 | 13.1 | 1345 | 11.4* | 3150 | 12.7* | 1481 | 11.7* |
| Stage IIIa | 22119 | 13.7 | 1468 | 12.5* | 3711 | 15.0* | 1514 | 12.0* |
| Stage IIIb | 4830 | 3 | 330 | 2.8 | 899 | 3.6* | 469 | 3.7* |
| Stage IV | 68745 | 42.6 | 5619 | 47.7* | 11703 | 47.4* | 6246 | 49.3* |
| Histology (P < 0.01) | ||||||||
| Squamous cell/transitional cell carcinoma | 38005 | 23.6 | 2265 | 19.2* | 5955 | 24.1 | 1999 | 15.8* |
| Adenocarcinoma in situ [AIS] | 6678 | 4.1 | 587 | 5.0* | 686 | 2.8* | 794 | 6.3* |
| Nonadenocarcinoma in situ [non-AIS] | 61860 | 38.3 | 4931 | 41.9* | 9512 | 38.5 | 6282 | 49.6* |
| Large cell carcinoma | 5483 | 3.4 | 356 | 3.0* | 1013 | 4.1* | 315 | 2.5* |
| Others | 49288 | 30.6 | 3629 | 30.8* | 7548 | 30.5 | 3278 | 25.9* |
| Age | ||||||||
| Mean ± standard deviation | 68.86 ± 11.239 | 67.40 ± 12.395* | 64.65 ± 11.467* | 68.05 ± 12.315* | ||||
| Median (range) | 70 (15–99) | 69 (15–99) | 65 (15–99) | 69 (20–99) | ||||
| Age grouping (P < 0.01) | ||||||||
| <30 | 203 | 0.1 | 73 | 0.6* | 41 | 0.2 | 34 | 0.3* |
| 30–39 | 815 | 0.5 | 170 | 1.4* | 192 | 0.8* | 192 | 1.5* |
| 40–49 | 7353 | 4.6 | 761 | 6.5* | 2022 | 8.2* | 707 | 5.6* |
| 50–59 | 24812 | 15.4 | 1924 | 16.3* | 6279 | 25.4* | 2210 | 17.4* |
| 60–69 | 47243 | 29.3 | 3253 | 27.6* | 7734 | 31.3* | 3278 | 25.9* |
| 70–79 | 51282 | 31.8 | 3640 | 30.9 | 5806 | 23.5* | 3910 | 30.9* |
| 80+ | 29606 | 18.4 | 1947 | 16.5* | 2640 | 10.7* | 2337 | 18.4 |
| Gender (P < 0.01) | ||||||||
| Male | 85908 | 53.3 | 6407 | 54.4* | 14086 | 57.0* | 7241 | 57.2* |
| Female | 75406 | 46.7 | 5361 | 45.6* | 10628 | 43.0* | 5427 | 42.8* |
| Birth country (P < 0.01) | ||||||||
| United States | 108817 | 67.5 | 3816 | 32.4* | 18836 | 76.2* | 2190 | 17.3* |
| Outside the United States | 6565 | 4.1 | 4106 | 34.9* | 376 | 1.5* | 7369 | 58.2* |
| Unknown | 45932 | 28.5 | 3846 | 32.7* | 5502 | 22.3* | 3109 | 24.5* |
| Marital status (P < 0.01) | ||||||||
| Single | 16835 | 10.4 | 1815 | 15.4* | 7212 | 29.2* | 1200 | 9.5* |
| Married | 85907 | 53.3 | 6074 | 51.6 | 8299 | 33.6* | 8216 | 64.9* |
| Others | 53186 | 33 | 3452 | 29.3* | 8136 | 32.9 | 2851 | 22.5* |
| Unknown | 5386 | 3.3 | 427 | 3.6* | 1067 | 4.3* | 400 | 3.2 |
| Tumor size (P < 0.01) | ||||||||
| No tumor found | 713 | 0.4 | 55 | 0.5 | 74 | 0.3* | 45 | 0.4 |
| ≤30 mm | 49950 | 31 | 3147 | 26.7* | 6205 | 25.1* | 3528 | 27.8* |
| >30 mm and ≤50 mm | 36401 | 22.6 | 2470 | 21.0* | 5449 | 22.0* | 3008 | 23.7* |
| >50 mm and ≤70 mm | 19615 | 12.2 | 1461 | 12.4 | 3399 | 13.8* | 1577 | 12.4 |
| >70 | 14848 | 9.2 | 1160 | 9.9* | 3060 | 12.4* | 1122 | 8.9 |
| Unknown | 393787 | 24.7 | 3475 | 29.5* | 6527 | 26.4* | 3388 | 26.7* |
* P < 0.05 using Z test when c/w NHW.
Ca.: carcinoma; f: frequency; P: P value; %, percentage; mm: millimeter; API: Asian Pacific Islanders; NHW: non-Hispanic whites.
3.1. Stage
Compared to NHW stage I diagnosis (17.8%), Blacks had the least proportion (12.4%) preceded by Hispanics (13.5%) and API (14.3%) (P < 0.05). NHW had the most stage II diagnosis (13.1%), followed by Blacks (12.7%), API (11.7%), and Hispanics (11.4%) (P < 0.05). Blacks had the highest stage III diagnoses (20.5%), followed by the referent NHW (18.5%), Hispanics (17.7%), and API (17.2%) (P < 0.05). API had the highest stage IV diagnoses (49.3%), followed by Hispanics (47.7%), Blacks (47.4%), and the referent NHW (42.6%), respectively.
3.2. Grade
Compared to the referent NHW's Grades 1 (5.0%) and 2 (16.5%) statuses, Blacks had the least amount of Grade 1 (3.3%) and Grade 2 (14.8%) tumors, while Hispanics (5.4%) and API (5.7%) both had relatively greater Grade 1 representations, respectively (P < 0.05). Regarding high grade tumors, API had significantly the lowest proportions of both Grade 3 (25.7%) and Grade 4 (1.8%) cases, compared to NHW. Hispanics also had lower Grade 3 (26.8%) diagnoses, compared to NHW, while Blacks had greater proportions (28.2%).
3.3. Histology
Squamous and transitional cell diagnoses were significantly less common in API (15.8%) and Hispanics (19.2%) compared to NHW (23.6%) (P < 0.05). Although Blacks had greater shares (24.1%), this was nonsignificant. Compared to NHWs' AIS (4.1%) and Non-AIS (38.3%) histological diagnoses, Hispanics had greater proportions (5.0% and 41.9%), with API having the greatest representation (6.3% and 49.6%). Alternatively, Blacks yielded the fewest AIS (2.8%) cases in our study (P < 0.05). For large cell carcinoma in the referent group (3.4%), both API (2.5%) and Hispanics (3.0%) ranked lower, while a greater share was found among Blacks (4.1%) (P < 0.05).
3.4. Age
Compared to NHW's later mean age at diagnosis of 68.86 years ± 11.239, an earlier onset was observed among API (68.05 ± 12.315 years), Hispanics (67.40 ± 12.395 years), and Blacks (64.65 ± 11.467 years) (P < 0.05). Greater than 50% of cases among Blacks were seen in the 5th (25.4%) and 6th (31.3%) decades, respectively, compared to the majority of the cases that presented later in the 6th and 7th decades among other ethnicities (P < 0.05).
3.5. Marital Status
In our study, Blacks (29.2%) had the highest “single” status, followed by Hispanics (15.4%), and NHW (10.5%), with lowest observations noted among API (9.5%). Blacks (32.9%) and NHW (33.0%) had higher “Others” status, with relative lower proportions observed amongst Hispanics (29.3%) and API (22.5%) (P < 0.05). Finally, married individuals were significantly more common among AIP (64.9%) and less common among Blacks (33.6%) compared to NHW (33.0%).
3.6. Birth Country
Significant majority of the API (58.2%) were born outside the United States (US). A greater proportion of Hispanics (34.9%), compared to NHW (4.1%), and Blacks (1.5%) are born outside (P < 0.05).
3.7. Tumor Size
“No tumor was found” in 0.3% of the Black population, compared to (0.4%) NHW (P < 0.05). Both NHW (31.0%) and API (27.8%) had the greater proportion of tumor ≤30 mm, compared to Blacks (25.1%) and Hispanics (26.7), respectively. A similar trend of proportionality was observed in tumors greater than 30 mm but not more than 50 mm, with API (23.7%) and NHW (22.6%) being higher, compared to Blacks (22.0%) and Hispanics (21.0%) (P < 0.05). Alternatively, Blacks had relatively greater proportion of tumors greater than 50 mm, followed by Hispanics, NHW, and API, respectively.
3.8. Radiation
With regard to stage I NSCLC cases, radiation was less frequently utilized in both API (11.0%) and Hispanics (12.2%) compared to NHW (16.3%) whereas greater proportions of Blacks (18.9%) were treated with radiation (P < 0.01). This trend was also observed in stage II cases, with relatively more NHW (33.9%) and Blacks (36.9%) than API (26.5%) and Hispanics (28.6%) receiving radiation as part of their treatment (P < 0.01). Higher rates of Blacks (57.3%) had radiation as part of the treatment in stage III followed by NHW (55.3%), API (50.5%), and Hispanics (48.2%) (P < 0.01). Similarly, Blacks (44.9%) had the highest proportions of radiation utilization compared to NHW (44.1%), API (41.0%), and Hispanics (39.1%) in the stage IV cohort (P < 0.01).
3.9. Surgery
For AJCC stage I cases, a greater proportion of API (80.4%) were treated with cancer directed surgery compared to Hispanics (75.4%), NHW (75.6%), and Blacks (65.7%) (P < 0.01). Blacks (35.1%) had the lowest rate of surgical treatment in stage II while NHW (47.3%) had the highest rate followed by API (44.7%) and Hispanics (44.7%) (P < 0.01). Compared to NHW (17.0%), lower rates of surgical treatment in Blacks (12.0%) were observed while API (20.4%) and Hispanics (19.4%) had greater proportions that underwent surgery (P < 0.01). NHW (4.5%) had the highest proportion of cancer directed surgery compared to Hispanics (3.8%), API (3.6%), and Blacks (3.5%) in stage IV NSCLC (P < 0.01).
3.10. Reason for No Surgery
The most common reason for no surgery for all ethnicities was because it was “not recommended.” This reason was proportionally more common among API and least among Blacks (P < 0.05) for all AJCC stages except stage I (P < 0.05). In contrast, API had the highest proportion of refusal for surgical treatment in early stage NSCLC patients (P < 0.05).
3.11. Survival Analyses
Multivariate Cox proportional models were utilized to analyze the variables contributing to the DSS among different AJCC stage.
3.12. Patient Demographics
Demographic variables that had improved survival at each AJCC stage were; female gender, and being married, (P < 0.05). Immigrants born outside the US had significant improved survival outcome in comparison to US born patients. Patients with stage II diagnosed at age 70–79 (hazard ratio [HR]: 4.077, P < 0.05) and >80 (HR: 5.14, P < 0.05) had poor outcomes; Patients >80 years had worsened survival among stage IV (HR: 1.626, P < 0.05). API had a significantly improved survival in stage I (HR: 0.775, P < 0.05), stage II (HR: 0.791, P < 0.05), and stage IV (HR: 0.858, P < 0.05). This improvement was not observed in stage III (HR: 0.966, P > 0.1). Unlike API, both Hispanics and Blacks did not have impact on the survival favorably compared to NHW (Table 3).
Table 3.
Multivariate Cox proportional analysis used to ascertain the contributions of the demographic, clinicopathologic, and treatment features to the DSS among the different AJCC stages.
| Characteristics | Stage I | Stage II | Stage IIIa | Stage IIIb | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | HR | P | HR | P | |
| Grade | ||||||||||
| Grade I | Referent | Referent | Referent | Referent | Referent | |||||
| Grade II | 1.589 | P < 0.01 | 1.15 | P < 0.01 | 1.049 | P > 0.05 | 1.482 | P < 0.01 | 1.24 | P < 0.01 |
| Grade III | 1.836 | P < 0.01 | 1.348 | P < 0.01 | 1.208 | P < 0.01 | 1.507 | P < 0.01 | 1.467 | P < 0.01 |
| Grade IV | 1.884 | P < 0.01 | 1.426 | P < 0.01 | 1.346 | P < 0.01 | 1.27 | P > 0.05 | 1.525 | P < 0.01 |
| Histology | ||||||||||
| Squamous cell/transitional cell Ca. | Referent | Referent | Referent | Referent | Referent | |||||
| Adenocarcinoma in situ [AIS] | 0.642 | P < 0.01 | 0.739 | P < 0.01 | 0.623 | P < 0.01 | 0.539 | P < 0.05 | 0.739 | P < 0.01 |
| Nonadenocarcinoma in situ | 0.928 | P < 0.05 | 0.966 | P > 0.05 | 0.887 | P < 0.01 | 0.956 | P > 0.05 | 0.935 | P < 0.01 |
| Large cell carcinoma | 1.088 | P > 0.05 | 1.079 | P > 0.05 | 0.927 | P > 0.05 | 1.049 | P > 0.05 | 1.05 | P > 0.05 |
| Others | 0.896 | P < 0.05 | 0.983 | P > 0.05 | 0.906 | P < 0.01 | 0.952 | P > 0.05 | 1.05 | P < 0.05 |
| Age grouping | ||||||||||
| <30 | Referent | Referent | Referent | Referent | Referent | |||||
| 30–39 | 1.252 | P > 0.05 | 2.795 | P > 0.05 | 1.332 | P > 0.05 | 0.879 | P > 0.05 | 1.04 | P > 0.05 |
| 40–49 | 1.992 | P > 0.05 | 3.091 | P > 0.05 | 1.477 | P > 0.05 | 1.298 | P > 0.05 | 1.111 | P > 0.05 |
| 50–59 | 2.091 | P > 0.05 | 3.257 | P > 0.05 | 1.426 | P > 0.05 | 1.354 | P > 0.05 | 1.215 | P > 0.05 |
| 60–69 | 2.28 | P > 0.05 | 3.455 | P > 0.05 | 1.535 | P > 0.05 | 1.338 | P > 0.05 | 1.28 | P > 0.05 |
| 70–79 | 2.639 | P > 0.05 | 4.077 | P < 0.05 | 1.726 | P > 0.05 | 1.486 | P > 0.05 | 1.409 | P > 0.05 |
| 80+ | 3.025 | P > 0.05 | 5.14 | P < 0.05 | 2.083 | P > 0.05 | 1.795 | P > 0.05 | 1.626 | P < 0.05 |
| Gender | ||||||||||
| Male | Referent | Referent | Referent | Referent | Referent | |||||
| Female | 0.833 | P < 0.01 | 0.831 | P < 0.01 | 0.845 | P < 0.01 | 0.8 | P < 0.01 | 0.854 | P < 0.01 |
| Birth country | ||||||||||
| United States | Referent | Referent | Referent | Referent | Referent | |||||
| Outside the United States | 0.86 | P < 0.01 | 0.915 | P > 0.05 | 0.8 | P < 0.01 | 0.825 | P < 0.05 | 0.875 | P < 0.01 |
| Marital status | ||||||||||
| Single | Referent | Referent | Referent | Referent | Referent | |||||
| Married | 0.874 | P < 0.01 | 0.915 | P < 0.05 | 0.887 | P < 0.01 | 0.771 | P < 0.01 | 0.842 | P < 0.01 |
| Others | 1.012 | P > 0.05 | 1.001 | P > 0.05 | 0.969 | P > 0.05 | 0.922 | P > 0.05 | 0.968 | P > 0.05 |
| Races | ||||||||||
| Non-Hispanic whites | Referent | Referent | Referent | Referent | Referent | |||||
| Hispanics | 0.916 | P > 0.05 | 0.966 | P > 0.05 | 1.059 | P > 0.05 | 1.019 | P > 0.05 | 1.023 | P > 0.05 |
| Blacks | 0.954 | P > 0.05 | 1.043 | P > 0.05 | 0.972 | P > 0.05 | 1.026 | P > 0.05 | 0.969 | P > 0.05 |
| Asians and Pacific Islander | 0.775 | P < 0.01 | 0.791 | P < 0.01 | 0.939 | P > 0.05 | 1.03 | P > 0.05 | 0.858 | P < 0.01 |
| Radiation | ||||||||||
| Radiation not received | Referent | Referent | Referent | Referent | Referent | |||||
| Radiation received | 0.693 | P < 0.01 | 0.623 | P < 0.01 | 0.586 | P < 0.01 | 0.651 | P < 0.01 | 0.917 | P < 0.01 |
| Cancer directed surgery | ||||||||||
| Not performed | Referent | Referent | Referent | Referent | Referent | |||||
| Performed | 0.231 | P < 0.01 | 0.282 | P < 0.01 | ||||||
HR: hazard ratio; P: P value; Ca.: carcinoma.
3.13. Clinicopathologic Features
Higher grade was uniformly associated with poor prognosis across all the stages (P < 0.05). However both AIS and Non-AIS diagnoses (with the exception of stage II, HR: 0.966, P > 0.05) were both associated with improved survival compared to the referent squamous and transitional diagnosis, with AIS being the more favorable diagnosis (Table 2).
Table 2.
Baseline treatment characteristics of the racial/ethnic racial cohorts among the AJCC stages.
| NHW (28649) | Hispanics (1588) | Blacks (3062) | API (1815) | |||||
|---|---|---|---|---|---|---|---|---|
| f | % | f | % | f | % | f | % | |
| AJCC stage I | ||||||||
| Radiation (P < 0.01) | ||||||||
| Radiation not received | 23652 | 82.6 | 1385 | 87.2* | 2450 | 80.8* | 1601 | 88.2* |
| Radiation received | 4666 | 16.3 | 194 | 12.2* | 578 | 18.9* | 200 | 11 |
| Unknown | 331 | 1.2 | 9 | 0.6* | 34 | 1.1 | 14 | 0.8 |
| Cancer directed surgery (P < 0.01) | ||||||||
| Not performed | 6961 | 24.3 | 389 | 24.5 | 1033 | 33.7* | 354 | 19.5* |
| Performed | 21582 | 75.6 | 1197 | 75.4 | 2012 | 65.7* | 1459 | 80.4* |
| Reason for no surgery (P < 0.05)a | ||||||||
| Died | 21 | 0.3 | 1 | 0.3 | 3 | 0.3 | 0 | 0 |
| Not recommended | 6014 | 86.3 | 328 | 84.3 | 873 | 84.5 | 294 | 83.1 |
| Patient refusal | 450 | 6.4 | 28 | 7.1 | 81 | 7.8 | 41 | 11.6* |
| Unknown | 582 | 8.4 | 34 | 8.7 | 93 | 9 | 21 | 5.9 |
|
| ||||||||
| NHW (21121) | Hispanics (1345) | Blacks (3150) | API (1481) | |||||
| f | % | f | % | f | % | f | % | |
|
| ||||||||
| AJCC stage II | ||||||||
| Radiation (P < 0.01) | ||||||||
| Radiation not received | 13590 | 64.3 | 941 | 70.0* | 1954 | 62.0* | 1075 | 72.6* |
| Radiation received | 7159 | 33.9 | 384 | 28.6* | 1144 | 36.3* | 392 | 26.5* |
| Unknown | 372 | 1.8 | 20 | 1.5 | 52 | 1.7 | 14 | 0.9* |
| Cancer directed surgery (P < 0.01) | ||||||||
| Not performed | 11083 | 52.7 | 743 | 55.3 | 2038 | 64.9* | 818 | 55.3* |
| Performed | 9937 | 47.3 | 601 | 44.7 | 1101 | 35.1* | 661 | 44.7* |
| Reason for no surgery (P < 0.05)a | ||||||||
| Died | 37 | 0.3 | 5 | 0.7 | 8 | 0.4 | 2 | 0.2 |
| Not recommended | 10089 | 91 | 672 | 90.4 | 1826 | 89.6* | 760 | 92.9* |
| Patient refusal | 308 | 3.1 | 21 | 2.8 | 56 | 2.7 | 28 | 3.4 |
| Unknown | 750 | 6.7 | 46 | 6.1 | 159 | 7.8 | 30 | 3.7* |
|
| ||||||||
| NHW (26949) | Hispanics (1798) | Blacks (4610) | API (1983) | |||||
| f | % | f | % | f | % | f | % | |
|
| ||||||||
| AJCC stage III | ||||||||
| Radiation (P < 0.01) | ||||||||
| Radiation not received | 11542 | 42.8 | 908 | 50.5* | 1885 | 40.9* | 955 | 48.2* |
| Radiation received | 14909 | 55.3 | 867 | 48.2* | 2641 | 57.3* | 1002 | 50.5* |
| Unknown | 498 | 1.8 | 23 | 1.3 | 84 | 1.8 | 26 | 1.3* |
| Cancer directed surgery (P < 0.01) | ||||||||
| Not performed | 22228 | 83 | 1448 | 80.6* | 4037 | 88.0* | 1577 | 79.6* |
| Performed | 4562 | 17 | 349 | 19.4* | 548 | 12.0* | 403 | 20.4* |
| Reason for no surgery (P < 0.01)a | ||||||||
| Died | 52 | 0.2 | 1 | 0 | 9 | 0.2 | 2 | 0.1 |
| Not recommended | 20685 | 93.1 | 1353 | 93.4 | 3713 | 92.0* | 1504 | 95.4* |
| Patient refusal | 353 | 1.6 | 20 | 1.4 | 53 | 1.3 | 21 | 1.3 |
| Unknown | 1297 | 5.8 | 75 | 5.2 | 287 | 7.1* | 53 | 3.7* |
|
| ||||||||
| NHW (68745) | Hispanics (5619) | Blacks (11703) | API (6246) | |||||
| f | % | f | % | f | % | f | % | |
|
| ||||||||
| AJCC stage IV | ||||||||
| Radiation (P < 0.01) | ||||||||
| Radiation not received | 37450 | 54.5 | 3356 | 59.7* | 6285 | 53.7 | 3628 | 58.1* |
| Radiation received | 30306 | 44.1 | 2195 | 39.1* | 5259 | 44.9 | 2562 | 41.0* |
| Unknown | 989 | 1.4 | 68 | 1.2 | 159 | 1.4 | 56 | 0.9* |
| Cancer directed surgery (P < 0.01) | ||||||||
| Not performed | 65265 | 95.5 | 5399 | 96.2* | 11233 | 96.5* | 6014 | 96.4* |
| Performed | 3107 | 4.5 | 213 | 3.8* | 410 | 3.5* | 222 | 3.6* |
| Reason for no surgery (P < 0.01)a | ||||||||
| Died | 141 | 0.2 | 14 | 0.3 | 28 | 0.3 | 14 | 0.2 |
| Not recommended | 61220 | 93.8 | 5112 | 94.7* | 10331 | 92* | 5796 | 96.3* |
| Patient refusal | 766 | 1.2 | 41 | 0.8* | 138 | 1.2 | 58 | 0.9 |
| Unknown | 3511 | 5.4 | 239 | 4.4* | 796 | 7.1* | 156 | 2.6* |
* P < 0.05 using Z test when c/w NHW; aFisher's exact test was used to test difference among the races.
f: frequency; P: P value; %, percentage; API: Asian Pacific Islanders; NHW: non-Hispanic whites.
3.14. Treatment Modality
Treatment with radiation was associated with favorable 5-year prognosis (stage I HR: 0.693; stage II HR: 0.623; stage III HR: 0.60, and stage IV: 0.917, P < 0.01). Surgical treatment favorably impacted stage I (HR: 0.231), and stage II (HR: 0.282) survival respectively, (P < 0.01).
4. Discussion
This study utilized the SEER database to examine racial/ethnic disparities in NSCLC clinicopathologic features and stage-based survival outcomes. API were more likely to be diagnosed with AIS histology but yet presented with late stage disease. Our analysis showed that cancer directed surgery and radiation therapy were significantly less likely to be offered to API compared to NHW. Despite this, compared to NHW, API had increased disease-specific survival for early stage (I and II) and stage IV NSCLC. This analysis determined that survival disparities are also seen in API based on the recent AJCC 7th edition staging system. Previous retrospective analyses have shown API to have decreased mortality compared to NHW for stage I disease, with an overall survival advantage regardless of smoking status which is consistent with our results [7–9]. Our analysis also found increased survival in API with stage II disease compared to NHW. Stage IV disease was seen more frequently in API than in NHW with lower rates of cancer directed surgery and radiation therapy. Despite this, there was a survival advantage for API compared to NHW in stage IV NSCLC which is consistent with prior studies [14].
Improved outcomes in API may be attributed to favorable demographic and clinicopathologic features demonstrated in our analysis including being married, birth outside of the US, AIS histology, and earlier age at diagnosis. Regarding treatment modalities in stage IV, despite improved survival, the API cohort was less likely to receive cancer directed surgery compared to NHW. Pertinently, there was greater proportion of surgery which was not part of the treatment plan. According to Chang et al., API as a group had better overall survival after NSCLC diagnosis compared to NHW, and single marital status was associated with decreased survival in the API population, which is consistent with our results [8].
NSCLC is a heterogeneous disease that is influenced by genetic, lifestyle, and socioeconomic elements. These elements are likely major factors in the disparate presentations and outcomes among different racial/ethnic groups. Smoking status is an important prognostic indicator, with an improvement in overall and disease-specific survival in never smokers compared to patients with a smoking history [9, 11]. Response to therapy including surgery, chemotherapy, and radiation is also improved in never smokers even in advanced disease [9]. Unlike NHW and black patients diagnosed with NSCLC, a relatively high percentage of never smokers are seen in the API US population [12]. However, besides smoking status, additional factors may account for improved outcomes because Asian ethnicity independently is a favorable prognostic indicator for overall survival in both smokers and never smokers [9]. Lower socioeconomic status (SES) is associated with increased lung cancer incidence [15]. In addition to a higher prevalence of smoking in lower SES groups, they are unlikely to receive adequate health care. In prior observational studies, Blacks were less likely to receive surgery, chemotherapy, or radiation for stage III disease and were less likely to receive chemotherapy for stage IV disease in comparison to NHW [16–18]. In our study, cancer directed surgery was less likely to be offered to Blacks compared to NHW. However, our study is unique in that it demonstrates that radiation is more significantly likely to be administered to Blacks diagnosed with NSCLC. Poor access to quality health care is a major factor in racial/ethnic disparities, which have shown that when equivalent health care access is provided, survival outcomes become comparable [3, 13, 19, 20]. Further research is necessary to determine whether lung cancer treatment is suboptimal in API residing in the US.
Overexpression of the epidermal growth factor receptor (EGFR) leading to aberrant tyrosine kinase mediated signaling is implicated in approximately 70% of NSCLC cases and is associated with a poor prognosis [21]; EGFR tyrosine kinase inhibitors (TKI) were developed as a potential therapeutic option to improve outcomes. A greater understanding of the activity of TKI has led to the discovery that the efficacy of these inhibitors is dependent on the presence of EGFR activating mutations instead of the degree of EGFR overexpression. EGFR activating mutations are seen more commonly in females, AIS histology, never or light smokers, and East Asians [22, 23]. The prevalence of EGFR activating mutations in other racial/ethnic groups such as Blacks and NHW appears to be highly variable [24–26]. Improved survival in API potentially could be due to the presence of these mutations; however, randomized controlled trials have not shown an overall survival benefit with TKI therapy in the adjuvant, stage III maintenance, first-line metastatic, and second-line treatment settings [27–31].
This study has several limitations. It is a retrospective analysis where data was collected by medical record review. This could have led to incorrect classification of race/ethnicity and tumor classification. In our analysis, we came across cases with insufficient data labeled “NOS.” However, we found the number of missing cases to be proportional among different racial/ethnic groups. In addition, we were not able to account for both genetic and lifestyle factors linked to NSCLC including testing for EGFR, KRAS, and ALK mutations, familial history, smoking history, and occupational exposure to carcinogens. We were also not able to determine specific chemotherapy regimens given to patients.
This is the first SEER analysis to utilize the recent AJCC 7th edition to determine survival outcomes in API compared to NHW. Improved survival outcomes were seen in API for both early and advanced stage disease. Interestingly, in all stages, except for stage III, there was a significant survival benefit. This may be due to an insufficient sample size but also may be due to disparities in tumor biology and lifestyle factors specific for this stage. Further research is necessary to gain a better understanding of the NSCLC outcomes in the API population residing in the US.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Raz D. J., Gomez S. L., Chang E. T., et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. Journal of Thoracic Oncology. 2008;3(12):1391–1397. doi: 10.1097/jto.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganti A. K., Subbiah S. P., Kessinger A., Gonsalves W. I., Silberstein P. T., Loberiza F. R., Jr. Association between race and survival of patients with non-small-cell lung cancer in the united states veterans affairs population. Clinical Lung Cancer. 2014;15(2):152–158. doi: 10.1016/j.cllc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan C. R., Meram A. D., Proctor C. D., Wu H., Zhu K., Marrogi A. J. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiology Biomarkers and Prevention. 2006;15(1):25–31. doi: 10.1158/1055-9965.epi-05-0537. [DOI] [PubMed] [Google Scholar]
- 4.Wisnivesky J. P., McGinn T., Henschke C., Hebert P., Iannuzzi M. C., Halm E. A. Ethnic disparities in the treatment of stage I non-small cell lung cancer. American Journal of Respiratory and Critical Care Medicine. 2005;171(10):1158–1163. doi: 10.1164/rccm.200411-1475oc. [DOI] [PubMed] [Google Scholar]
- 5.SEER Stat Fact Sheets: Lung and Bronchus Cancer. Surveillance, Epidemiology, and End Result, 2014, http://seer.cancer.gov/statfacts/html/lungb.html.
- 6.Saeed A. M., Toonkel R., Glassberg M. K., et al. The influence of hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: an analysis of the survival, epidemiology, and end results database. Cancer. 2012;118(18):4495–4501. doi: 10.1002/cncr.26686. [DOI] [PubMed] [Google Scholar]
- 7.Ou S.-H. I., Zell J. A., Ziogas A., Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110(7):1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 8.Chang E. T., Shema S. J., Wakelee H. A., Clarke C. A., Gomez S. L. Uncovering disparities in survival after non-small-cell lung cancer among Asian/Pacific Islander ethnic populations in California. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(8):2248–2255. doi: 10.1158/1055-9965.epi-09-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiology study of never-smokers with non-small cell lung cancer (NSCLC): High percentages of Asian and Hispanic female never-smokers and the significance of Asian ethnicity. OncoLink: the Web's first cancer resource, University of Pennsylvania, Abramson Cancer Center, Philadelphia, Pa, USA, 1994–2014, http://www.oncolink.org/conferences/article.cfm?c=3&s=48&ss=268&id=1761.
- 10.Schwartz A. G., Cote M. L., Wenzlaff A. S., Land S., Amos C. I. Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. Journal of Thoracic Oncology. 2009;4(10):1195–1201. doi: 10.1097/jto.0b013e3181b244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferketich A. K., Niland J. C., Mamet R., et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;119(4):847–853. doi: 10.1002/cncr.27824. [DOI] [PubMed] [Google Scholar]
- 12.Ou S.-H. I., Ziogas A., Zell J. A. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. Journal of Thoracic Oncology. 2009;4(9):1083–1093. doi: 10.1097/jto.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 13.Zheng L., Enewold L., Zahm S. H., et al. Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(10):1841–1847. doi: 10.1158/1055-9965.epi-12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cetin K., Ettinger D. S., Hei Y.-J., O'Malley C. D. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the surveillance, epidemiology and end results program. Clinical Epidemiology. 2011;3(1):139–148. doi: 10.2147/clep.s17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong M. L., Clarke C. A., Yang J., Hwang J., Hiatt R. A., Wang S. Incidence of non-small-cell lung cancer among California hispanics according to neighborhood socioeconomic status. The Journal of Thoracic Oncology. 2013;8(3):287–294. doi: 10.1097/jto.0b013e31827bd7f5. [DOI] [PubMed] [Google Scholar]
- 16.Shugarman L. R., MacK K., Sorbero M. E. S., et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Medical Care. 2009;47(7):774–781. doi: 10.1097/mlr.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 17.Goulart B., Reyes C., Fedorenko C., et al. Referral and treatment patterns among patients with stages III and IV non-small-cell lung cancer. Journal of Oncology Practice. 2013;9(1):42–50. doi: 10.1200/JOP.2012.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy D., Liu C.-C., Xia R., et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–2211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 19.Zullig L. L., Carpenter W. R., Provenzale D. T., et al. The association of race with timeliness of care and survival among Veterans Affairs health care system patients with late-stage non-small cell lung cancer. Cancer Management and Research. 2013;5(1):157–163. doi: 10.2147/CMAR.S46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant A. S., Cerfolio R. J. Impact of race on outcomes of patients with non-small cell lung cancer. Journal of Thoracic Oncology. 2008;3(7):711–715. doi: 10.1097/jto.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 21.Meert A.-P., Martin B., Delmotte P., et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. European Respiratory Journal. 2002;20(4):975–981. doi: 10.1183/09031936.02.00296502. [DOI] [PubMed] [Google Scholar]
- 22.Gazdar A. F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jänne P. A., Johnson B. E., Lynch T., et al. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clinical Cancer Research. 2006;12(14):4416s–4420s. doi: 10.1158/1078-0432.ccr-06-0555. [DOI] [PubMed] [Google Scholar]
- 24.Nomura M., Shigmatsu H., Li L., et al. Polymorphisms, mutations, and amplification of the EGFR gene in non-small cell lung cancers. PLoS Medicine. 2007;4(4, article e125) doi: 10.1371/journal.pmed.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauml J., Mick R., Zhang Y., et al. Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist? Lung Cancer. 2013;81(3):347–353. doi: 10.1016/j.lungcan.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cote M. L., Haddad R., Edwards D. J., et al. Frequency and type of epidermal growth factor receptor mutations in African Americans with non-small cell lung cancer. Journal of Thoracic Oncology. 2011;6(3):627–630. doi: 10.1097/jto.0b013e31820a0ec0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng R., Hasan B., Mittmann N., et al. Economic analysis of NCIC CTG JBR.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer—a report of the working group on economic analysis, and the lung disease site group, National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology. 2007;25(16):2256–2261. doi: 10.1200/jco.2006.09.4342. [DOI] [PubMed] [Google Scholar]
- 28.Kelly K., Chansky K., Gaspar L. E., et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. Journal of Clinical Oncology. 2008;26(15):2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 29.Giaccone G., Herbst R. S., Manegold C., et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. Journal of Clinical Oncology. 2004;22(5):777–784. doi: 10.1200/jco.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Herbst R. S., Giaccone G., Schiller J. H., et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. Journal of Clinical Oncology. 2004;22(5):785–794. doi: 10.1200/jco.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 31.Thatcher N., Chang A., Parikh P., et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) The Lancet. 2005;366(9496):1527–1537. doi: 10.1016/s0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]