Abstract
Objective
To evaluate a swallow preservation protocol (SPP) in which patients received swallow therapy before, during, and after radiation treatment and its efficacy in maintaining swallowing function in head and neck cancer patients.
Design
Case series with chart review.
Setting
Tertiary care academic medical center.
Subjects and Methods
Eighty-five patients who received radiation (RT) or chemoradiation (CRT) participated in the SPP from 2007 to 2012. Subjects were divided into 2 groups: compliant and noncompliant with SPP. At each SPP visit, the diet of each patient was recorded as regular (chewable), puree, liquid, or gastrostomy tube (G-tube) dependent, along with their compliance with the swallow exercises. Patients were stratified by age, gender, tumor stage, type of treatment, radiation dose, diet change, dysguesia, odynophagia, pain, and stenosis. Statistical analysis was performed comparing the 2 compliance groups in regards to swallowing-related outcomes at 1 month after completion of therapy.
Results
Fifty-seven patients were compliant and 28 were non-compliant with SPP during treatment. The compliant group had a higher percentage of patients tolerating a regular diet (54.4% vs 21.4%, P = .008), a lower G-tube dependence (22.8% vs 53.6%, P = .008), and a higher rate of maintaining or improving their diet (54.4% vs 25.0%, P = .025) compared to noncompliant patients.
Conclusion
A swallow preservation protocol appears to help maintain or improve swallow function in head and neck cancer patients undergoing RT or CRT. Patients who are able to comply with swallow exercises are less likely to worsen their diet, receive a G-tube, or develop stenosis.
Keywords: swallow therapy, swallow preservation, radiation and swallow
Introduction
Dysphagia is one of the most feared and common complications of radiation (RT) or chemoradiation (CRT) and one of the main predictors of poor posttreatment quality of life (QOL).1–3 The incidence of head and neck cancer treatment–related dysphagia is estimated to be 10 to 20,000 new cases per year.1 Posttreatment dysphagia occurs in at least 50% to 60% of patients and results from multiple factors, such as xerostomia, taste loss, stricture, fibrosis, and trismus, which worsen during treatment and for 3 to 6 months afterwards and usually tend to stabilize by 1 year.1,3 Newman et al4 reported a decline of normal swallowing ability in 17% of patients treated with concurrent CRT. Carrara-de Angelis et al5 reported significant oral and pharyngeal swallow dysfunction in 100% of patients evaluated by modified barium swallow study (MBSS) after undergoing laryngeal organ preservation protocol. Carnaby-Mann et al6 found significant deterioration of muscle composition of all patients on MRI after CRT treatment, irrespective of swallowing intervention. The overall sum of these deleterious effects leads to decreased oral caloric intake, reliance on feeding tubes, increased morbidity, decreased quality of life, and increased use of health care services.7
In the era of organ preservation, an increasing number of studies are focusing on ways of preventing posttreatment dysphagia. Carroll et al8 reported that pretreatment swallowing exercises are effective. Murphy et al9 found that patients referred for early swallowing therapy had improved outcomes. Other studies have since reported similar findings.10–13 However, the fact remains that current clinical interventions to address dysphagia in irradiated patients are limited, and those that do exist typically follow a therapeutic or rehabilitative model as opposed to a preventive one.8 Kotz et al14 reported a randomized controlled trial of 26 patients who participated in prophylactic swallowing exercises before and during CRT and found improved swallowing in the treatment group at 3 and 6 months post CRT, but not immediately posttherapy or at 9 and 12 months after CRT. However, this study was limited by a small sample size and significant lack of compliance in the treatment group; thus, the question remains whether improved compliance with prophylactic swallow exercises maintains or improves swallowing function at an earlier time point. It is postulated that even brief periods of oropharyngeal rest or lack of exercise during radiation therapy are associated with dysphagia. Furthermore, there may be a “window of opportunity” during which dysphagia rehabilitation may be most effective.9 Gillespie et al15 reported that patients who had been without oral intake for more than 2 weeks had worse swallowing outcomes.
To date, the majority of studies on dysphagia prevention during RT or CRT for head and neck cancer have not reported swallowing outcomes in patients who performed swallowing exercises before and during treatment. In this study, we retrospectively evaluated the effectiveness of swallow exercises (Swallow Preservation Therapy) before and during RT or CRT in preservation of swallowing function after completion of treatment. Specifically, we evaluated if there was a significant difference in diet and swallowing-related parameters in a group compliant with swallow therapy versus a noncompliant group at 1 month posttherapy.
Methods
Patient Selection
This study was approved by the Institutional Review Board. A retrospective review of our clinical database of head and neck cancer patients treated with either RT or CRT and who participated in the swallow preservation protocol (SPP) between 2007 and 2012 was performed. We excluded patients who had previous surgery, inadequate follow-up, or significantly missing data. The cancer therapy and SPP were performed at the David Geffen School of Medicine at the University of California, Los Angeles (an academic tertiary care medical referral center). As part of the SPP, every patient referred for radiation therapy at our institution was evaluated by a speech-language pathologist (SLP) prior to treatment. The demographic data and other relevant information including primary tumor site, stage of tumor, type of treatment (RT or CRT), radiation dose administered to the primary site, and so on were also recorded.
Swallow Preservation Protocol
All patients underwent pretreatment swallow assessment 2 weeks prior to cancer treatment that included education about their cancer and expected treatment side effects, assessment for pretreatment dysphagia, and introduction of an exercise program. The purpose of the swallow exercises was to maintain range of motion of oral, pharyngeal, and laryngeal structures involved in swallowing and counter the radiation fibrosis that leads to restricted range of motion resulting in dysphagia.10 It is worth noting that one major goal of the program was to encourage patients to continue oral intake as much as possible, despite dysguesia and odynophagia, and thus at each weekly session the importance of swallow exercises was reinforced by the SLP with the focus aimed at increasing adherence and assessing performance of swallow exercises.10
A swallowing exercise timeline is outlined in Table 1. A complete swallow preservation exercise set consisted of the following previously described maneuvers8, 14: gargling liquid for 10 seconds 10 times, effortful swallow 10 times, Mendelsohn maneuver 10 times, chug-a-lug 3 ounces at once, tongue protrusion 10 times, tongue press 10 times, and Shaker head lift 3 times. These swallow preservation exercise sets were to be performed 3 times daily (3 sets) except for Shaker exercise, which was to be performed once per day (1 set). The last SPP clinical visit was at 2 months posttreatment.
Table 1.
Swallow therapy schedule as part of the swallowing preservation protocol.a
| Visit Number |
First Visit |
Second Visit until Completion of SPP |
Posttreatment Visits |
Posttreatment Visits (by 2 months up to 4 total visits) |
|---|---|---|---|---|
| Visit time | 2 weeks before RT/CRT treatment | 1 week after start of treatment (weekly) | 1 month after RT/CRT treatment | 2 months after RT/CRT treatment |
| Goals of visit | Swallow assessment, including diet recorded | Diet monitoring (encouragement of continued oral intake) | 1 month posttreatment diet recorded | 2 month posttreatment diet recorded by way of MBSS and swallow evaluations in clinic |
| Treatment education (eg, expected side effects)/swallow program education | Compliance with exercises (based on competency level during first visit)/exercise education | Exercise education | Exercise education | |
| Dietician referral (patients who are noted to have weight loss) | Signs of malnutrition are reported to oncologist | Weight recorded | Weight recorded |
Abbreviations: SPP, swallowing preservation protocol; MBSS, modified barium swallow study; RT/CRT, radiation/chemoradiation.
Patient’s weight, pain, compliance with swallow exercises, and diet were assessed at each follow-up period by the speech-language pathologist and recorded in the therapy progress notes.
Definition of Compliance
Patients were grouped as either compliant or noncompliant with swallowing preservation exercises. Compliance was based on patients’ self-report: each patient received a form to track their exercises and the form was brought to each weekly SPP visit. We defined compliance as performance of at least 1 full set of exercises per day and noncompliance as less than 1 full set per day. We did not attempt to separate the compliant group into fully compliant (ie, performed all 3 sets per day) versus partially compliant (performed less than 3 full sets but more than 1 full set). To our knowledge, there are no previous data in the literature that correlate a certain level of compliance with outcomes.
Statistical Analysis
At each session, patients’ diet was recorded, along with their ability to perform the swallow exercises. Diets were recorded as regular (chewable), puree, liquid, or gastrostomy tube (G-tube) dependent. The patients were then categorized by compliance and were analyzed for significant trends in swallowing related parameters. Descriptive statistics were used to characterize the sample. For comparing continuous variables, 2-tailed, unequal variance, t tests were used. For comparing categorical and ordinal variables, Fisher’s exact tests were used; for tables larger than 2 by 2, extended versions of the Fisher’s exact test were used. A P value of less than .05 was considered statistically significant.
Results
Patient Characteristics
Eighty-five patients participated in the UCLA SPP from 2007 to 2012. Of these, 18 patients received RT alone and 67 received CRT. Fifty-seven patients were compliant with the swallow program, while 28 were noncompliant. Patient age ranged from 22 to 91 years (mean 60, median 59, SD 13). There were more male patients (N = 66) than female patients (N = 19). There was no significant difference in gender between the compliant and noncompliant groups (P = .274). There was also no significant difference between the 2 groups in other baseline characteristics, including gender, age, weight, tumor stage, treatment type (RT or CRT), radiation dose to primary tumor site, and pretreatment diet. Other baseline characteristics are shown in Table 2.
Table 2.
Comparison of baseline characteristics between compliant and noncompliant patient groups with associated P values.
| All (n = 89) | Compliant (n = 58) | Noncompliant (n = 31) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. or Mean |
Range, SD | No. or Mean |
Range, SD | No. or Mean |
Range, SD | P Value | ||
| Male | 66 | 42 | 24 | .273 | ||||
| Female | 19 | 15 | 4 | |||||
| Age | 60 | 22–91, 13 | 60 | 22–87, 13 | 61 | 34–91, 14 | .581 | |
| Weight (kg) | 80.1 | 44.5–152.4, 19.2 | 80.9 | 44.5–152.4, 19.8 | 81.2 | 47.2–113.3, 18.4 | .938 | |
| RT | 18 | 13 | 5 | .779 | ||||
| CRT | 67 | 44 | 23 | |||||
| Radiation dose (cGy) | 6433 | 1260–7200, 1049 | 6467 | 1260–7200, 892.1 | 6364 | 1890–6950, 1330 | .712 | |
| Subsite | NP | 15 | 12 | 3 | .308 | |||
| OC | 5 | 2 | 3 | |||||
| OP | 53 | 33 | 20 | |||||
| Laryn | 8 | 7 | 1 | |||||
| UP | 4 | 3 | 1 | |||||
| Stage | 0 | 1 | 1 | 0 | .898 | |||
| 1 | 2 | 1 | 1 | |||||
| 2 | 3 | 2 | 1 | |||||
| 3 | 14 | 9 | 5 | |||||
| 4 | 53 | 37 | 16 | |||||
| Unk | 12 | 7 | 5 | n/a | ||||
| Pretreatment diet | Chew | 69 | 49 | 20 | .290 | |||
| Puree | 9 | 5 | 4 | |||||
| Liquid | 2 | 1 | 1 | |||||
| G-tube | 5 | 2 | 3 | |||||
Abbreviations: SD, standard deviation; n/a, not applicable; unk, unknown stage; NP, nasopharynx; OC, oral cavity; OP, oropharynx; laryn, larynx; UP, unknown primary.
Diet before and after treatment
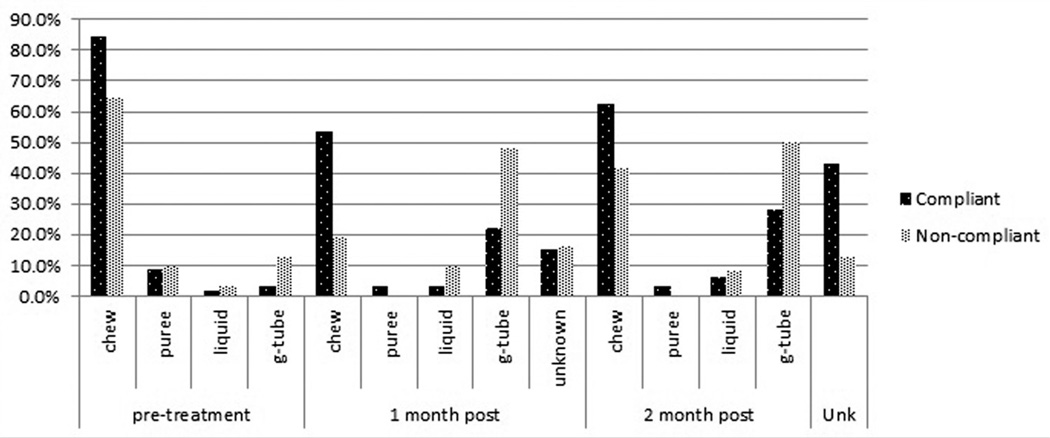
There was no difference in pretreatment diet between the 2 groups (P = .290). Swallowing-related outcomes was evaluated 1 month after completion of therapy. Thirty-one of 57 (54.4%) compliant patients were tolerating a regular chewable diet, compared to only 6 of 28 (21.4%) noncompliant patients (P = .008). Furthermore, only 13 of 57 (22.8%) compliant patients were noted to be G-tube dependent, compared to 15 of 28 (53.6%) noncompliant patients (P = .008).
We attempted to evaluate dietary outcomes at 2 months posttreatment. However, at this timeline there was a notably higher dropout rate in the compliant group, 25 of 57, compared to the noncompliant group, 4 of 28 (P = .057). Of those who continued to follow up at 2 months, 23 of 32 (71.9%) compliant patients remained on some form of oral diet (chewable, puree, or liquid), compared to 12 of 24 (50%) noncompliant patients (P = .510). Assuming that all patients who dropped out at the 2-month follow-up were tolerating a form of oral diet, significantly more compliant patients (48 of 57 or 84%) than noncompliant patients (16 of 28 or 57%) were on an oral diet (P = .014). A comparison of pre- and posttreatment diets for the compliance groups is provided in Figure 1.
Figure 1.
Comparison of pre- and posttreatment diets for compliant and noncompliant patients. Unknown diet at 2 months is due to patient dropout from swallow preservation protocol visits.
Diet change
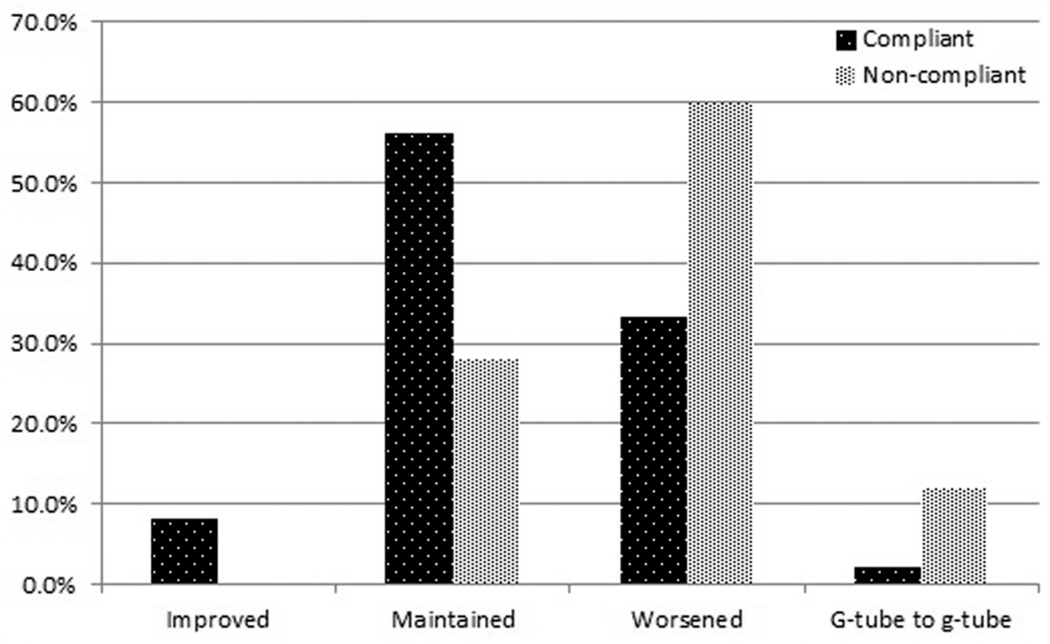
We defined change in diet as a step up or step down in diet when diets were ranked in the following order with chewable (regular) being the best: G-tube, liquid, puree, chewable. Maintenance of diet was defined as no change in diet from pre- to posttreatment. The exception to this was if a patient remained on a G-tube diet from pretreatment to post-treatment, which was considered in its own category. There was a significant difference in diet change, with more of the compliant patients maintaining or improving their diet from pretreatment to 1 month posttreatment when compared to the noncompliant patients (P = .025). The trend in diet change between the 2 groups is shown in Figure 2.
Figure 2.
Comparison of diet change for the compliant and non-compliant groups. There was a significant difference in diet change, with more of the compliant patients maintaining or improving their diet from pretreatment to 1 month posttreatment when compared to the noncompliant patients (P = .025).
Other posttreatment measures
No significant difference in weight change was found between compliant and noncompliant patients at 1 month (P = .563). There was no significant difference in weight change between the compliant and the noncompliant patients who remained in the SPP at 2 months (P = .289). However, within the compliant group, those who continued to follow up at 2 months had significantly more weight loss as compared to those who dropped out after the 1-month follow-up (P = .05). In addition, there was no significant difference in weight loss between G-tube (N = 28) and non–G-tube patients (N = 57), (P = .981) at 1 month. However, there was a significant difference in the incidence of stenosis between the 2 groups (compliant group N = 4 and noncompliant group N = 9, P = .012). The number of patients who expressed xerostomia, dysguesia, or odynophagia was also not significantly different between the compliance groups. Furthermore, the average pain level on swallowing (scale of 1–10, with 10 being the worst pain) was not significantly different between the compliance groups. These measures are summarized in Table 3.
Table 3.
Comparison of posttreatment measures between compliant and noncompliant groups with associated P values.
| Compliant (n = 57) | Noncompliant (n = 28) | ||||||
|---|---|---|---|---|---|---|---|
| 1 Month | 2 Month | ||||||
| N=85 | 1 Month | 2 Months | 1 Month | 2 Months | P Value | P Value | |
| Weight loss | 8.0% (SD 7.5) | 14.1% (SD 19.1) | 8.6% (SD 9.5) | 8.6% (SD 9.2) | .771 | .189 | |
| Stenosis (for all follow-up time) | Yes | 4 | 9 | n/a | .012* | ||
| No | 54 | 22 | |||||
| Aspiration | Yes | 2 | 4 | 3 | 6 | .999 | .14 |
| No | 7 | 18 | 6 | 8 | |||
| Xerostomia | Yes | 17 | 13 | 8 | 9 | .604 | .999 |
| No | 25 | 15 | 17 | 12 | |||
| Dysguesia | Yes | 17 | 13 | 5 | 8 | .188 | .398 |
| No | 24 | 14 | 19 | 15 | |||
| Odynophagia | Yes | 21 | 15 | 14 | 11 | .807 | .79 |
| No | 23 | 16 | 12 | 15 | |||
| Pain level (0–10 scale) | 3.5 (SD 2.2) | 4.4 (SD 2.6) | 3.4 (SD 2.5) | 2.7 (SD 1.3) | .927 | .156 | |
Asterisk indicates statistically significant difference.
Discussion
The majority of patients who undergo RT and CRT experience significant side effects and complications, both during treatment and for extended periods of time after treatment.1 Shortly after starting radiation therapy, patients develop mucositis, radiation dermatitis, and edema of the soft tissues, resulting in pain, copious mucus production, xerostomia, and tissue swelling, which contributes to acute dysphagia.9 As the acute effects resolve, late effects including fibrosis, lymphedema, and damage to neural structures begin to manifest. Both acute and late effects result in adverse sequelae including aspiration, feeding tube dependence, and nutritional deficiencies.9
The primary aim of this study was to evaluate whether a swallow preservation program during RT/CRT maintains posttreatment swallow function. Our results demonstrate that compliance with swallow therapy during RT/CRT is beneficial in maintaining swallowing function. We demonstrate that the real benefit of compliance with swallow exercises before and during RT and CRT is that the swallowing function is better preserved at the conclusion of therapy and thus patients benefit immediately from improvement or maintenance of swallowing as they do not have to wait 3 to 12 months after therapy for swallowing potential to return, as had been suggested by Kotz et al.14 There was a significant difference between the compliant group versus the noncompliant group in the number of patients who tolerated a higher level of posttreatment diet. In other words, attending the program, fully committing to the exercises, and monitoring by experienced staff appear to preserve swallow function. We noted no difference in the incidence of xerostomia, dysguesia, odynophagia, pain level, and weight loss between these 2 groups, so compliance appears to be an independent predictive factor. That is not to say, however, that compliance cannot be affected by pain, nausea, or other complications and side effects of CRT, as patients have different tolerances for these factors.
There is a critical time period during early radiation treatment when side effects (odynophagia, mucositis, xerostomia, etc) limit the patient’s desire and ability to swallow, and even brief periods of oropharyngeal rest or lack of exercise during CRT may be associated with prolonged dysphagia. While the optimal timing of swallowing therapy has not yet been established,9,14 our data suggest that maintenance of swallowing during RT and CRT maintains the swallowing function at the end of treatment. The high dropout rate in the compliant group and the minimal dropout in the non-compliant group support this notion, as it is our belief that those who are swallowing well tend not to follow up. This may also be supported by the weight loss data trend within the compliant group where those who continued to follow up had significantly more weight loss than those who dropped out. However, we recognize that swallow exercises are likely needed long after completion of radiotherapy as radiation fibrosis and other complications are a long-term sequelae and during therapy the problems are largely acute inflammatory reactions, edema, and mucositis.
The goal of a pretreatment and concurrent swallowing preservations program is to maximize the patient’s ability to overcome the temptation to decrease oral intake and, if possible, avoid placing a feeding tube. Placement of a feeding tube during therapy has been shown to be associated with a higher incidence of esophageal stenosis, and our study supports those findings.16,17 Significantly fewer of the compliant patients in our series, 13 of 57, were noted to be G-tube dependent posttherapy, whereas more than half, 15 of 28, of the noncompliant patients were G-tube dependent. Weight loss in G-tube patients was not significantly different from that of non–G-tube patients. The overall health benefits of a G-tube diet cannot be argued, although patients who do take a non-oral diet may be more predisposed to dysphagia and esophageal stenosis.
The development of upper esophageal stenosis is important in the progression of dysphagia.16,17 Compliance is likely to be decreased in patients who develop stenosis and cannot tolerate swallowing during treatment therapy.16,17 Another possibility is that those patients who were noncompliant and stopped swallowing during therapy were the ones who developed full stenosis. In our study, 13 of the 85 patients were noted to develop esophageal stenosis. Only 4 of those patients were in the compliant group, and all of those patients were able to tolerate an oral diet based on the MBSS result at 2 months posttherapy. Of the 9 patients in the noncompliant group, 6 were completely G-tube dependent. Prevention of esophageal stenosis by more active swallow assessments, office-based esophageal endoscopy, and further focused swallow therapy and support in the sub-group of patients who receive G-tubes during therapy is one of the updated goals of our SPP. We believe that the significant dropout rate in the compliant group was secondary to those subjects tolerating adequate diet and no longer needing follow-up. Our data support this notion in that those compliant patients who did not drop out after 1 month had significantly more weight loss than their counterparts in the compliant group who did drop out. The low dropout rate in the noncompliant patients is likely due to continued need for swallowing therapy and support. Future prospective trials would shed light in this regard.
The limitations of our study include a short longitudinal follow-up period, as we were limited by our protocol, which followed patients only for 2 months posttherapy. Ideally, a prospective study would be conducted to follow patients for a year or more posttreatment. However, our focus was primarily on swallow preservation immediately after therapy, and we suspect this benefit is maintained long term. We also did not assess some other objective findings from MBSS that may be preserved by swallow preservation exercises, such as epiglottic inversion, tongue base contraction, and hyolaryngeal elevation.8 A further important limitation is that we did not specifically assess QOL data. However, we believe that assessment of diet tolerance at 1 and 2 months after treatment gives objective and useful measure of a patient’s swallowing function, regardless of pain and other subjective symptoms obtained from QOL instruments. Since persistent dysphagia is recognized as a complication of RT and CRT, it is essential for patients to be seen on a regular basis. Early pretreatment referral for evaluation by a physician or an SLP specializing in dysphagia management is critical to ensure adequate assessment of swallow function and to begin education and therapy to maintain swallow function during treatment. Our SPP includes weekly visits to follow progress and identify swallowing issues before serious complications arise. Reasons for noncompliance in patients include social, medical, psychological, financial, and insurance issues, and overcoming these obstacles should be one of the goals of therapy and future studies as well.
Conclusion
Swallow preservation exercises before and during RT and CRT for head and neck cancer appear to maintain swallow function. Specifically, compliance with a swallow preservation protocol leads to a faster return to normal diet and prevention of future esophageal stenosis. Larger and longer-term, prospective, randomized studies in the future are needed for assessment of the relationship between swallow preservation therapy and changes in QOL. However, swallowing exercises and encouragement to continue oral intake during RT and CRT should still be emphasized to every patient for optimal maintenance and recovery of swallow function.
Acknowledgments
Sponsorships: None.
Funding source: This study was supported in part by Grant No. RO1 DC011300 from the National Institutes of Health.
Footnotes
This article was presented at the American Head and Neck Society’s 8th International Conference on Head and Neck Cancer; June 22–24, 2012; Toronto, Ontario, Canada.
Author Contributions
Victor M. Duarte, analysis and interpretation of data, drafting article, revising critically, acquisition of data; Dinesh K. Chhetri, analysis and interpretation of data, revising critically, final approval; Yuan F. Liu, acquisition of data, revising critically methods and results; Andrew A. Erman, analysis and interpretation of data, revising critically methods and discussion; Marilene B. Wang, conception and design, revising critically, final approval.
Disclosures
Competing interests: None.
References
- 1.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 2.Maurer J, Hipp M, Schäfer C, Kolbl O. Dysphagia. Impact on quality of life after radio (chemo) therapy of head and neck cancer. Strahlenther Onkol. 2011;187:744–749. doi: 10.1007/s00066-011-2275-x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas L, Moore EJ, Olsen KD, Kasperbauer JL. Long-term quality of life in young adults treated for oral cavity squamous cell cancer. Ann Otol Rhinol Laryngol. 2012;121:395–401. doi: 10.1177/000348941212100606. [DOI] [PubMed] [Google Scholar]
- 4.Newman L, Vieira F, Schwiezer V, et al. Eating and weight changes following chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 1998;124:589–592. doi: 10.1001/archotol.124.5.589. [DOI] [PubMed] [Google Scholar]
- 5.Carrara-de Angelis E, Peher O, Barros AP, Nishimoto IN, Kowalski LP. Voice and swallowing in patients enrolled in a larynx preservation trial. Arch Otolaryngol Head Neck Surg. 2003;129:733–738. doi: 10.1001/archotol.129.7.733. [DOI] [PubMed] [Google Scholar]
- 6.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:210–219. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 7.McColloch NL, Carroll WR, Magnuson JS. Pretreatment dysphagia protocol for the patient with head and neck cancer undergoing chemoradiation. Dysphagia. 2010;19:53–56. [Google Scholar]
- 8.Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 9.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus CL, Logemann JA, Pauloski BR, et al. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106:1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JB, Emerton S, Kolbinson DA, et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck. 1999;21:1–11. doi: 10.1002/(sici)1097-0347(199901)21:1<1::aid-hed1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30:148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauloski BR, Rademaker AW, Logemann JA, et al. Swallow function and perception of dysphagia in patients with head and neck cancer. Head Neck. 2002;24:555–565. doi: 10.1002/hed.10092. [DOI] [PubMed] [Google Scholar]
- 14.Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg. 2012;138:376–382. doi: 10.1001/archoto.2012.187. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie MB, Brodsky MB, Day TA, Lee Fu-Shing, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362–1367. doi: 10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hunter JG, Lauretano L, Shellito P. Percutaneous endoscopic gastrostomy in head and neck cancer patients. Ann Surg. 1987;210:42–46. doi: 10.1097/00000658-198907000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–812. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]