Abstract
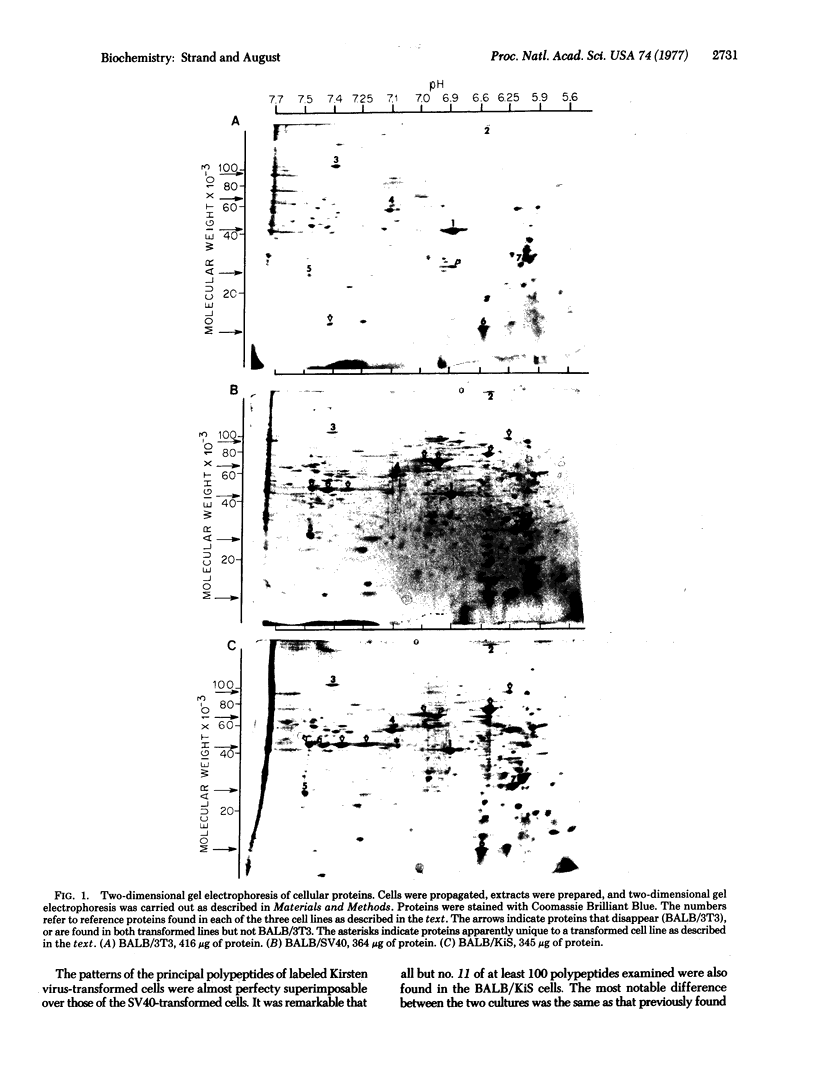
Proteins solubilized from normal BALB/3T3 cells and BALB/3T3 transformed by simian virus 40 or Kirsten sarcoma virus have been analyzed by two-dimensional gel electrophoresis and tryptic peptide mapping. A large fraction of the polypeptides of the virus-transformed cells, about 30%, were different from normal cells. In contrast to the marked differences between normal and transformed cells, the polypeptides of the DNA and RNA virus-transformed cells were almost identical. These findings were observed with polypeptides stained by Coomassie Blue, or labeled with [14C]glucosamine or [35S]methionine. Pulse-chase analysis showed that most of the polypeptides were stable during 20 hr of incubation. The identity of several polypeptides was confirmed by tryptic peptide mapping.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Lockwood T., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. II. Proteolytic and glycosidase activity and sublethal autolysis. Exp Cell Res. 1974 Jan;83(1):25–30. doi: 10.1016/0014-4827(74)90683-1. [DOI] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A. Electrophoretic analysis of substrate-attached proteins from normal and virus-transformed cells. Biochemistry. 1976 Sep 7;15(18):4094–4104. doi: 10.1021/bi00663a028. [DOI] [PubMed] [Google Scholar]
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Kijimoto S., Hakomori S. Enhanced glycolipid: -galactosyltransferase activity in contact-inhibited hamster cells, and loss of this response in polyoma transformants. Biochem Biophys Res Commun. 1971 Aug 6;44(3):557–563. doi: 10.1016/s0006-291x(71)80119-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Yamaguchi N., Kuchino T., Aoi Y. Alterations in surface glycoproteins and level of sialyltransferase of cells transformed by a temperature-sensitive mutant of simian virus 40. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4090–4094. doi: 10.1073/pnas.73.11.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Singer S. J. The specific cleavage of immunoglobulin polypeptide chains at cysteinyl residues. J Biol Chem. 1968 Apr 25;243(8):1777–1786. [PubMed] [Google Scholar]
- Smith H. S., Turner S., Leong J. A., Rigby P. W. Effect of passage in culture on a clone of BALB/c 3T3 cells transformed by simian virus 40. J Virol. 1976 Jul;19(1):146–153. doi: 10.1128/jvi.19.1.146-153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Antigenic properties of murine sarcoma virus-transformed BALB-3T3 nonproducer cells. J Exp Med. 1972 Mar 1;135(3):503–515. doi: 10.1084/jem.135.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry A. H., Culp L. A. Substrate-attached glycoproteins from normal and virus-transformed cells. Biochemistry. 1974 Jan 29;13(3):414–425. doi: 10.1021/bi00700a004. [DOI] [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]
- Wickus G., Gruenstein E., Robbins P. W., Rich A. Decrease in membrane-associated actin of fibroblasts after transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):746–749. doi: 10.1073/pnas.72.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]