Abstract
Some health-related proteins such as lipoprotein lipase may be regulated by qualitatively different processes over the physical activity continuum, sometimes with very high sensitivity to inactivity. The most powerful process known to regulate lipoprotein lipase protein and activity in muscle capillaries may be initiated by inhibitory signals during physical inactivity, independent of changes in lipoprotein lipase messenger RNA.
Keywords: dose response, coronary heart disease (CHD), transcription, posttranslational, signaling, sedentary, aging
INTRODUCTION
Most of the public health concern is concentrated in the least active 20% to 30% of the population, and the number of very inactive people is growing rapidly. There is a need to identify at the cellular and molecular level the causal links explaining why physical inactivity raises the risk of coronary heart disease (CHD) by negatively impacting disease susceptibility proteins. The trend in recent years has been a shift away from promoting only the stereotypical endurance exercise training paradigm toward physical activity for enhancing health and avoiding the diseases caused by physical inactivity (8). The epidemiologic evidence showing that structured sessions of continuous high-intensity exercise are not essential for preventing disease caused by inactivity actually dates back to the classic vocational studies. What was interesting about some of those studies is that they contrasted disease outcomes between sedentary people with co-workers who were unintentionally exposed to enough repetitive physical activity throughout the course of each day to prevent coronary artery disease and mortality significantly. Those studies are consistent with the consensus statements that recently have deemphasized the necessity for intense exercise while emphasizing the benefit of maintaining moderate activity every day. There also has been much interest in the idea of accumulating as many minutes of moderate muscular activity throughout the day as possible, sometimes referred to as active living.
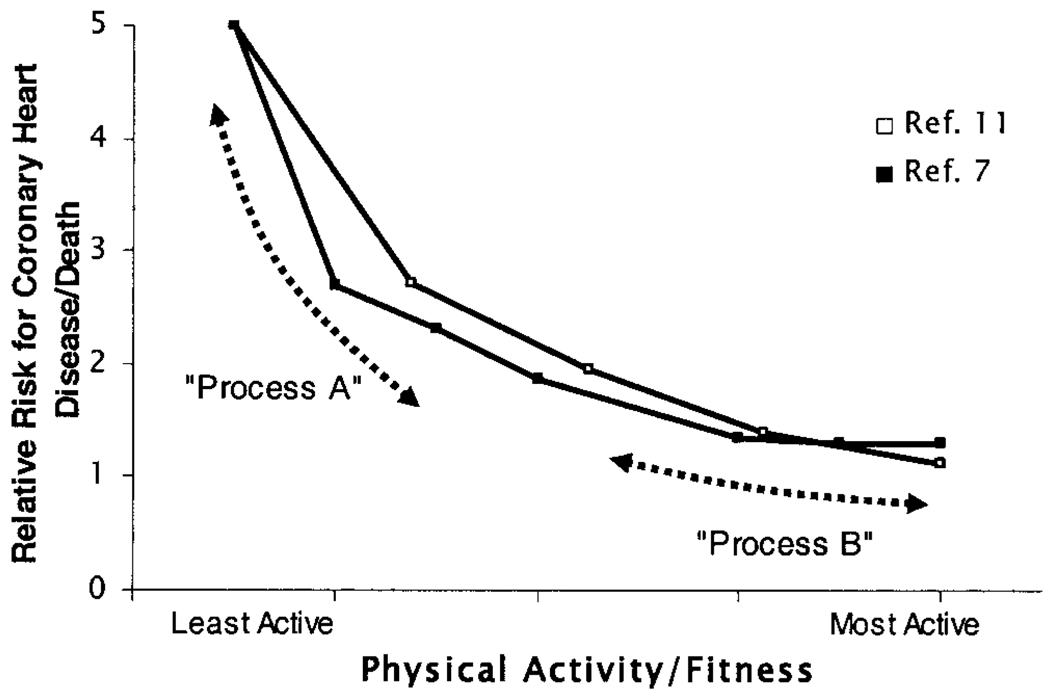
Epidemiologic studies of physical inactivity indicate that the greatest disease and mortality rates are in the least active people (Fig. 1), and thus understanding the physiological and molecular responses to inactivity is of high significance. Furthermore, it has been suggested that the steep improvements in disease likely would come from accumulating significant amounts of activity on most days, even if the intensity is moderate enough to reduce chances for injury and recidivism (7). However, there is less research that has sought to provide plausible cellular and molecular mechanisms regulating proteins that modify the risk of metabolic and cardiovascular diseases during inactivity. In this review, a good example of very potent effects of physical inactivity is illustrated.
Figure 1.
Relationship between physical activity and the risk for death, and two hypothetical processes that influence this risk. Summary of two studies (□ref. (11);■ref. (7)) that demonstrate that those with the lowest fitness and activity are at a much greater risk for death and CHD. The most salient molecular and cellular underlying processes responsible for the causal links between increased CHD and physical inactivity likely will be found by studying candidate processes over a range of inactivity and activity that includes the most physically inactive (process A). Theoretically, process A may be operative at higher levels of activity as well. Process B hypothetically would buttress health further (i.e., moderately high intensity exercise), but it is not necessarily the same mechanism as process A. If this is indeed true that sometimes there are distinct mechanisms regulating disease-related proteins across the physical activity continuum, then exercise studies only examining the effect of moderately high exercise intensity may not detect some of the most potent processes (e.g., process A) responsible for the strong association of physical inactivity with CHD. The key concept is that the middle quintile (“average person”) generally does not formally exercise train, but health is strongly impacted by becoming less active because of the physiologic responses to inactivity (“inactivity physiology”).
LIPOPROTEIN LIPASE
In this review, we focused on describing the enzyme lipoprotein lipase (LPL) because it is one of the few proteins studied over a wide range of the physical activity continuum and also is linked to a risk for CHD. Although not without controversy regarding the mechanisms, many studies in humans and animals have documented a strong and inverse relationship between LPL activity and CHD. LPL is an enzyme that binds to circulating lipoproteins when present on the vascular endothelium and is essential for hydrolysis of the triglyceride contained in lipoproteins. Compelling evidence by others has shown that the loss of LPL activity at the vascular endothelium impairs optimal tissue-specific uptake of lipoprotein-derived fatty acids and may contribute to the risks frequently observed during metabolic diseases such as obesity, type II diabetes, and most importantly, CHD. Even partial reductions in LPL activity because of specific polymorphisms have been reported to increase significantly the odds ratio for death and greater CHD in human studies as much as fivefold over healthy controls (14). Studies experimentally raising LPL activity provide evidence for less diet-induced atherosclerosis in transgenic rabbits (4), less diabetic hyperlipidemia (11), and less dietary-induced adiposity in transgenic mice overexpressing muscle-specific LPL activity (9). Numerous studies examining a loss of LPL activity have noted other diverse effects caused by genetic mutations in mice, cats, minks, or humans, and after pharmacologic inhibition of LPL activity.
Given that many laboratories have been identifying the functions of LPL and have documented an inverse relationship between LPL activity and CHD, there is now a great need to identify the physiologic processes that determine LPL activity. Herein we summarize a model for recent research regarding significant reductions in skeletal muscle LPL activity during acute and chronic inactivity (2), with aging (1,6), and how it is increased significantly by walking (2) and run training (5). In keeping with the guidelines of this journal, we discuss a novel integrative concept and hypothesis regarding physical activity and inactivity that is supported by two to three recent peer-reviewed papers, and thus do not intend to slight valuable historical contributions by others.
REGULATION OF LPL BY ACUTE AND CHRONIC PHYSICAL INACTIVITY
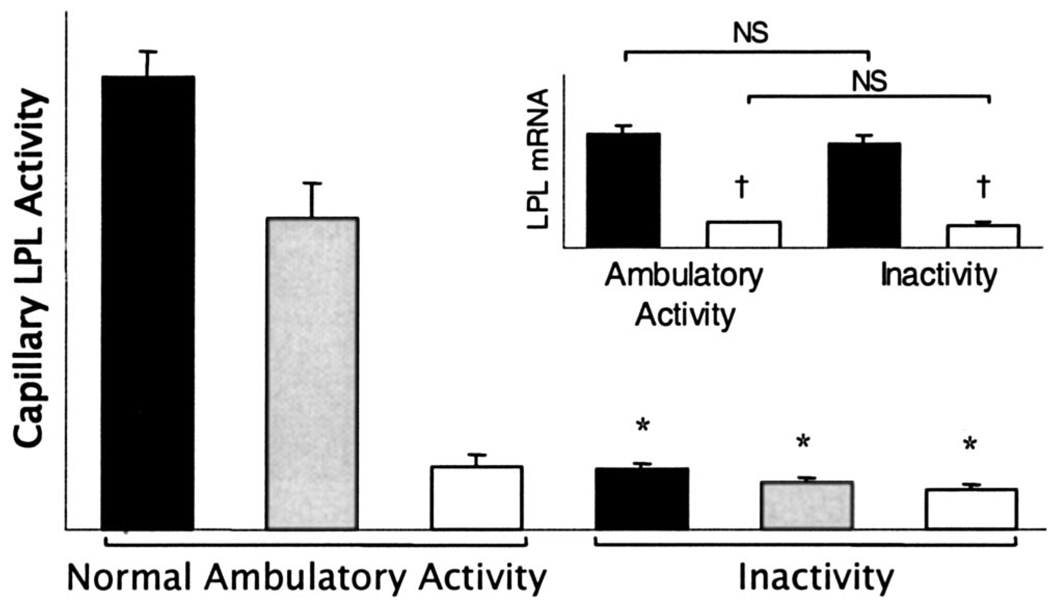
A recent study (2) tested the hypothesis that one reason why physical inactivity may impair lipid metabolism is because of reduced LPL activity in weight-bearing skeletal muscles that otherwise could be prevented by maintaining the nonfatiguing contractions that can occur during normal ambulatory activities in rats. The study found that the amount of LPL activity in skeletal muscles is remarkably sensitive to physical inactivity and activity (Fig. 2). This is especially evident in the capillary beds of oxidative muscle sections that are known from EMG and blood flow studies to be recruited at the lower intensities frequently occurring in everyday life. In these studies, there was no muscle atrophy. The reduction in muscle LPL activity during physical inactivity, experimentally robust because it occurs during both acute and chronic inactivity, is evident in both rats and mice and was induced using two different models of decreased contractile activity (hindlimb unloading and acute unilateral tenotomy). A general conclusion to draw from this is that there is a potent regulatory process at the lower end of the physical activity continuum controlling LPL activity (2).
Figure 2.
LPL activity and mRNA (inset) in different muscle fiber types after reduced physical activity compared with normal control ambulatory activity. LPL activity was determined in a rat slow-twitch red soleus muscle known to be most extensively recruited during low to moderate ambulatory activity (■; N = 35), fast-twitch red quadriceps ( ; N = 7), and fast-twitch white quadriceps muscle sections least recruited (□; N = 6) during ambulatory activity (2). *P < 0.05 indicates significant difference between reduced activity and ambulatory activity. †P < 0.05 indicates significantly lower LPL mRNA in fast-twitch white muscle than in red oxidative soleus muscle. NS, no significant difference in LPL mRNA between inactivity and ambulatory activity measured by Northern blotting (2) and by real-time polymerase chain reaction (3).
; N = 7), and fast-twitch white quadriceps muscle sections least recruited (□; N = 6) during ambulatory activity (2). *P < 0.05 indicates significant difference between reduced activity and ambulatory activity. †P < 0.05 indicates significantly lower LPL mRNA in fast-twitch white muscle than in red oxidative soleus muscle. NS, no significant difference in LPL mRNA between inactivity and ambulatory activity measured by Northern blotting (2) and by real-time polymerase chain reaction (3).
The marked reduction of skeletal muscle LPL activity during inactivity does not involve a decreased expression of LPL messenger RNA (mRNA) in those same muscles (Fig. 2). Furthermore, the low muscle LPL activity after inactivity can be reversed after a single session of treadmill walking at a moderate intensity (8 meters·min−1, ≤50% of V̇O2 max, performed intermittently with 30 min of rest each hour over 4 h). The increase in LPL activity during walking does not involve an increase in LPL mRNA concentration (2). LPL activity within the capillary beds increases several fold after walking exercise even when transcription is pharmacologically suppressed (2).
The known steps involved in regulating LPL activity during inactivity in young rats are very similar to the decreases in skeletal muscle LPL activity in older rats (1). Aged rats have significantly lower LPL specifically in the postural muscles (both capillary and total LPL activity and protein). As also seen in sedentary young rats, aged animals do not have a decrease in LPL mRNA concentration. Aged humans and rats develop relatively greater postprandial hypertriglyceridemia (6). Less physical activity (spontaneous activity) is a common stereotype of the aged in all species of which we are aware. Potential reversal of some of the negative effects of aging on LPL and plasma lipid metabolism with exercise in humans has been reviewed (6).
Distribution of Functional LPL Protein in Muscle
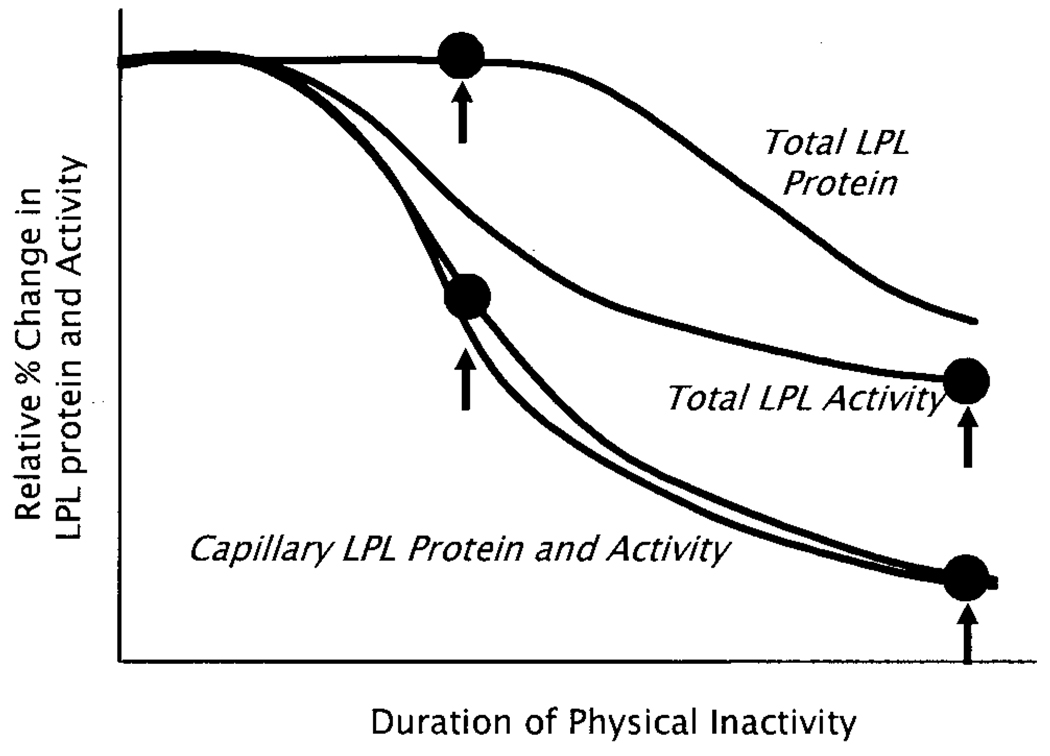
LPL protein is distributed spatially between the site of synthesis (myocyte) and the critical site for physiologic interactions with circulating lipoproteins (capillary endothelium). Total LPL is measured in tissue homogenates. The LPL protein and activity richly concentrated in the capillary beds can be extracted rapidly when small pieces of muscle are bathed in a heparin-containing medium (2) or when the vasculature of the intact rat hindlimb is perfused with heparin (Hutchins, K.C., and M.T. Hamilton, unpublished observations, 2004). Importantly, during physical inactivity, there is a more rapid onset and greater percentage decrease in LPL associated with the capillary pool compared with the LPL in whole muscle (Fig. 3), suggesting a possible posttranslational regulation of the distribution of LPL. The LPL protein in capillaries decreases approximately 50% before the total LPL begins to decrease, and the LPL mRNA does not decrease at any point up to even 11 d (2).
Figure 3.
Skeletal muscle LPL mass and activity in response to physical inactivity. Measurement of the temporal changes in LPL mRNA, protein mass, and activity in both the capillary pool and total tissue at the onset and throughout a day of physical inactivity gives insight into the underlying mechanisms triggering the changes in LPL activity. After an initial lag period of approximately 4 h, there is a precipitous loss of capillary LPL protein that is closely paralleled by a simultaneous decrease in capillary LPL activity (measured in both soleus and red quadriceps muscles at many time points) (2). Coincident with the early events, there is likely a decrease in intracellular-specific LPL activity because total tissue LPL protein does not change initially, yet total tissue LPL activity decreases. Eventually, total LPL protein decreases. Importantly, the loss of LPL activity during physical inactivity does not require a decrease in LPL mRNA concentration (Fig. 4).
The loss of LPL activity is localized to skeletal muscles with reduced contractile activity (2). The most oxidative muscle regions with normally the greatest amount of LPL activity experience approximately a 95% loss of the capillary LPL activity after physical inactivity (Table 1, Fig. 2). Thus, very important processes that determine the functional capillary LPL require a sufficient volume of moderate intensity contractile activity.
TABLE 1.
Lipoprotein lipase regulatory processes at the two ends of the physical activity continuum
| Referent Control | ||
|---|---|---|
|
|
||
| Effects of Physical Inactivity | Effects of Intense Exercise | |
| LPL mRNA | No change in LPL mRNA in any red oxidative or white glycolytic muscles |
↑ LPL mRNA in all white glycolytic leg muscles; no change in oxidative muscles of rats with already high levels of ambulatory activity and LPL mRNA |
| LPL protein | ↓ LPL protein concentration without ↓ in LPL mRNA concentration |
↑ LPL protein may be partly because ↑ LPL mRNA in glycolytic muscles |
| LPL activity | ↓ 10–20 times in LPL activity in red oxidative muscle sections and 50% ↓ white glycolytic sections |
2–3 times ↑ in LPL activity in white glycolytic fibers; no change in red oxidative muscles when compared with referent ambulatory control |
| Time course for LPL activity | In most oxidative red muscles, ↓ LPL activity begins 4–6 hours after initiating inactivity, reaches steady state after ~18 hrs; chronic (11 days) and acute (12–18 hours) have similar LPL effects |
↑ LPL activity after exercise that does not even begin to return to ambulatory control levels 27 hours after last exercise bout |
Changes in lipoprotein lipase elicited by both physical inactivity and quantified intense exercise were made relative to the normal ambulatory active state of cage animals engaged largely in walking, standing, and rearing. These comparisons of responses to physical inactivity and exercise were made in the same rat strain (i.e., Sprague-Dawley), gender (i.e., female), and age (i.e., 2–4 months). Physical inactivity was induced by hindlimb unloading for 1 hr to 11 days of intermittent unloading at 10 hr/day (2) and intense exercise consisted of running at 56 m/min, 3.5 hr/day, and 7 days/week for 2–3 weeks (5). Table 1 illustrates the principles essential for understanding how skeletal muscle LPL is regulated across two distinct ranges of the physical activity dose response continuum. Using the same type of animals as the referent control for physical inactivity and exercise stimuli, it is apparent that the physical inactivity responses are not simply the opposite of the exercise responses. For example, intense running increases LPL, mRNA, protein, and activity, primarily in white glycolytic muscle sections and not in red oxidative muscles, whereas the responses to physical inactivity decrease LPL activity in all muscle sections with the greatest effect in red oxidative fibers but does not decrease LPL mRNA concentration. Models involving inactivity have ranged from 6 to 18 hours of acute inactivity to 11 days of partial reductions in normal ambulatory activity (10 hr/day) (2). LPL, lipoprotein lipase; ↑, increase; ↓, decrease.
LPL is the essential enzyme for lipolysis of triglycerides in circulating lipoproteins and can have a variety of effects on metabolism (4,9,12,13). Thus, it is not surprising that there is a large decrease in radiolabeled triglyceride lipoprotein uptake locally into skeletal muscle with low LPL activity (2). Sometimes, the removal of normal ambulatory activities decreases plasma HDL cholesterol concentration, with two studies reporting approximately 20% reductions (2,15). Importantly, human CHD is greatest in the individuals who are the least physically active (Fig. 1), and LPL may provide one reason for this.
CONTRASTING THE EFFECTS OF INACTIVITY WITH THE EFFECTS OF INTENSE EXERCISE
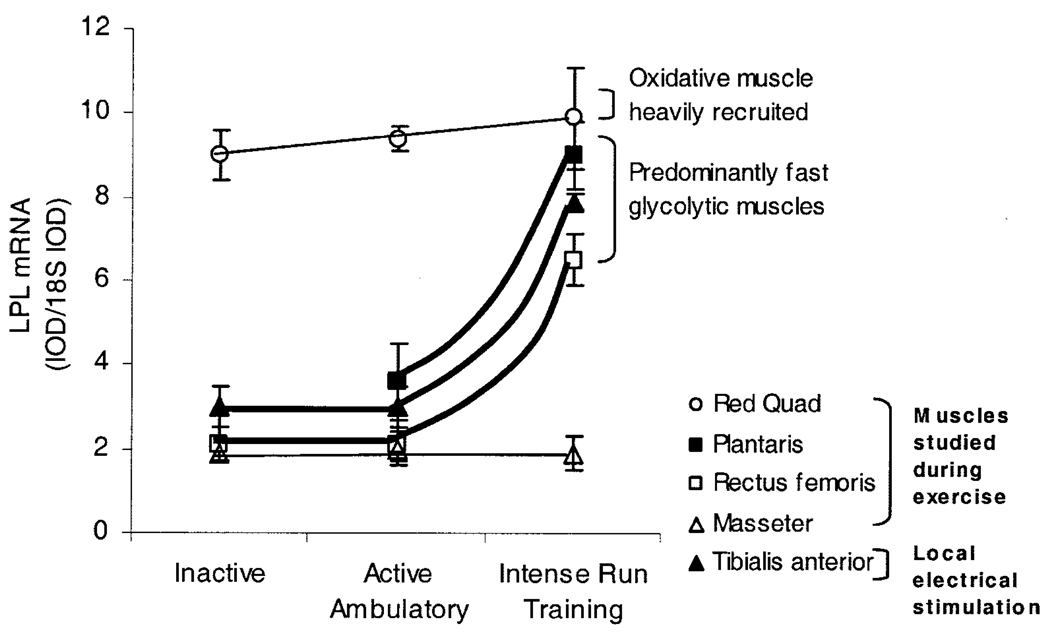
There are many contrasts for LPL regulation between the effects of running intensely (56 ± 1 m·min−1, 11 km·d−1, 2–3 wk, ≥80% V̇O2 max) and the reversible effects of physical inactivity or walking slowly (Table 1). In contrast to the effect of inactivity, high-intensity running (Fig. 4) certainly can increase LPL mRNA expression significantly (approximately twofold to threefold increases in all examined white glycolytic muscles in the leg) (5). As a contrast between the low and high end of the physical activity spectrum (Table 1), there is an apparent threshold for the processes increasing LPL mRNA, because LPL mRNA did not change in any muscle type when comparing inactivity with lower intensity ambulatory activity (Fig. 2 and Fig 4). However, it also is important to note that there is not an apparent threshold intensity for the process regulating capillary LPL activity, because even the normal ambulatory activity in the cage or walking at 8 meters·min−1 maintains LPL activity many fold more compared with the LPL activity in the inactive muscle (Fig. 2). All muscle types in the rat (especially oxidative muscles) had greater LPL activity during control ambulatory activity than when sedentary.
Figure 4.
Pretranslational process increasing LPL mRNA is activated in white glycolytic muscle by intense run training and electrical stimulation. This pretranslational process (increased LPL mRNA) is activated by intense run training or intense electrical stimulation of a motor neuron, but this effect requires intense contractions and occurs only in predominantly fast-twitch muscles (5). The run training averaged 56 ± 1 m·min−1 (>80%V̇O2max) for 3.4 hr·d−1 and was compared with the same muscles of nonrunning ambulatory control rats. In other rats, a muscle was electrically stimulated for 4 hr·d−1 to mimic run training. The run training stimuli did not increase LPL mRNA in a muscle that is not used in locomotion (masseter). Note that LPL mRNA was altered only at the high end of the physical activity continuum and did not decrease at the low end of the continuum.
In contrast, the running only increased LPL activity and protein in the fast white muscles and had no effect on any of the oxidative muscle sections (5). The differences listed in Table 1 are important to note because they illustrate differences at the two ends of the physical activity continuum for the type of myocytes affected, and they show differences in molecular processes such as differential expression of LPL mRNA.
There are now several sets of data pointing to local signals regulating skeletal muscle over the entire range of the dose-response continuum (2,5). This is in contrast to the long-held speculation that hormones are sufficient to change muscle LPL with exercise. Taken together, local contractile activity likely is sufficient and necessary to explain both the decreases in LPL activity during inactivity and the increases in LPL activity during training.
A CONCEPT OF INACTIVITY PHYSIOLOGY: ACUTE AND CHRONIC RESPONSES TO PHYSICAL INACTIVITY
Inactivity physiology includes the study of the biological responses to physical inactivity and is critical for elucidating the mechanisms operating at the key lower end of this continuum where most of the change in disease occurs. As suggested by Figure 1, persons with an average level of physical activity (the median quintile in the center of the graph) are expected to have a significant increase in cardiovascular disease and mortality when becoming more physically inactive (moving toward the steep left side of the graph). The least active people and people in the middle quintile for physical activity and fitness generally do not report exercising regularly. We introduce the concept that responses to physical inactivity (inactivity physiology) can cause significant negative effects on many specific cellular and molecular processes important for disease-related proteins, and sometimes these processes are qualitatively different from what is known about more intense exercise training. Although this is not currently a well-appreciated concept gauging from the number of research publications, we expect it will be a fruitful area of future research.
Behaviors involving nonfatiguing muscle contractions that are not part of intentional exercise raise skeletal muscle energy demand locally in the working muscle fibers more than an order of a magnitude. These nonexercise behaviors are sometimes called active living in epidemiologic studies, and the influence on whole body metabolic rate has been called nonexercise activity thermogenesis (10). Nonexercise activity thermogenesis primarily is the result of low- to moderate-intensity ambulatory activity (≤50% of V̇O2 max), can account for a large percentage of the 24-hr metabolic rate, is highly variable between individuals, and can be sufficient to prevent diet-induced obesity (10). We propose that even independent of body fat, accumulation of contractile activity in an active lifestyle may stimulate some potent biochemical and molecular processes important for preventing the effects of inactivity on chronic metabolic diseases (e.g., process A in Fig. 1). Regulation of the fraction of capillary LPL in muscle may be one such mechanism, because removing the normal intermittent ambulatory activity caused a precipitous decrease in LPL activity (Fig. 2), which was reversible by reestablishing moderate ambulatory activity.
Cellular Signals Stimulated by Physical Inactivity
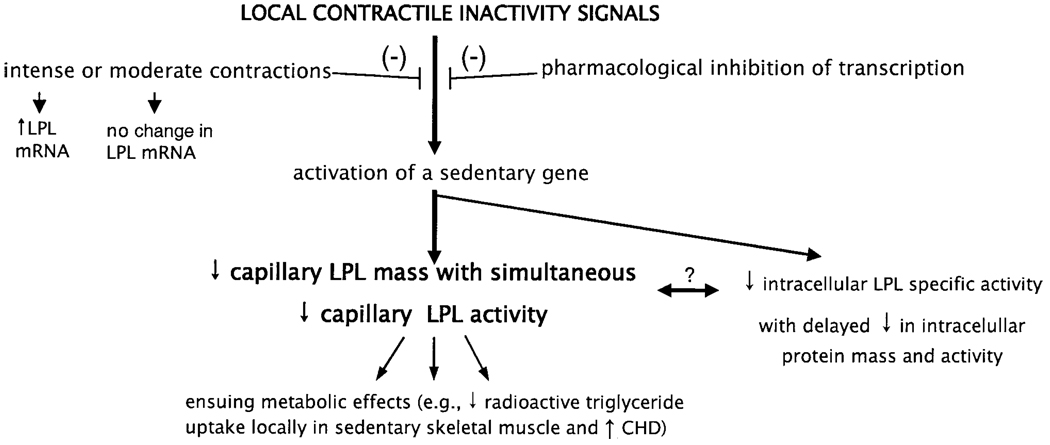
A common misconception we have noted from discussions with students and colleagues is that the reduction in the concentration of specific proteins during contractile inactivity likely is part of a generalized effect in concert with atrophy. However, LPL protein decreases in acute and chronic models of reduced contractile activity that do not cause any atrophy (2). Many mRNAs, proteins, and signal pathways are stimulated by contractile inactivity. One recent study (3) examined the expression of thousands of genes soon after becoming sedentary and found that there were at least as many genes that were upregulated in muscle as there were downregulated genes. Therefore, correcting this misconception is sometimes important to elucidate the signals causing the effects of inactivity. As an example, recent data have led to the novel hypothesis that there is a regulatory factor upregulated during inactivity that suppresses the amount of capillary LPL in muscle (2) (Fig. 5).
Figure 5.
Potential steps involved in decreasing LPL during contractile inactivity. The decrease in muscle LPL does not occur because of less LPL mRNA concentration (Figure 2). The pharmacologic inhibition of transcription prevented the decrease in LPL activity in sedentary muscle, but did not affect LPL activity in muscle of ambulatory and exercising rats (2). There is also potentially a reduction in intracellular LPL specific activity that may be involved. Conversely, the decrease in intracellular LPL mass may be a consequence of a process that causes a rapid loss of LPL mass at the capillary endothelium. Light or intense muscle contractions can prevent the upregulation or activation of a gene(s) that suppresses capillary LPL mass and activity (as described in text) (2). Loss of capillary LPL activity can reduce plasma triglyceride uptake and plasma HDL cholesterol concentration in some conditions and likely has other ensuing effects on lipid and carbohydrate metabolism.
Hypothesis That a Short-Lived Inhibitor Protein Decreases LPL in Sedentary Muscle
Because LPL activity apparently decreases in the capillaries of muscle because of a posttranslational process (Table 1), we sought to begin to explore the hypothesis that there is an inhibitor expressed in sedentary muscle that can decrease LPL function rapidly (Fig. 5). Treatment of rats for 6 h with actinomycin D (a transcriptional inhibitor) did not decrease LPL mRNA in either white or red muscle (2). Actinomycin D treatment also did not change LPL activity in ambulatory control rats, nor did it attenuate an eightfold increase in LPL activity during treadmill walking for 4 h after physical inactivity. However, in sedentary rats, the transcriptional inhibition prevented the decrease in capillary LPL activity (2). This implies that a short-lived inhibitor protein for posttranslational regulation of LPL is induced only by physical inactivity stimuli.
SUMMARY
There is much to be learned from new insights regarding the molecular and physiological mechanisms responsible for the relationship between different diseases and physical inactivity and activity. Because epidemiologic studies demonstrate that inactivity contributes significantly to chronic metabolic diseases, we envision that there will be an emergence of “inactivity physiology” studies identifying additional key cellular mechanisms responsible for regulating disease-susceptibility proteins. Using LPL as an example, it is possible that some of the most potent processes regulating proteins modulating risk for disease will be evident at the low end of the physical activity continuum, and qualitatively different processes also may be operating during structured programs of more intense exercise training.
Acknowledgments
Supported by the National Space Biomedical Research Institute.
References
- 1.Bey L, Areiqat E, Sano A, Hamilton MT. Reduced lipoprotein lipase activity in postural skeletal muscle during aging. J. Appl. Physiol. 2001;91:687–692. doi: 10.1152/jappl.2001.91.2.687. [DOI] [PubMed] [Google Scholar]
- 2.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: A molecular reason to maintain daily low-intensity activity. J. Physiol. 2003;551:673–682. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bey L, Akunuri N, Zhao P, Hoffman EP, Hamilton DG, Hamilton MT. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol. Genomics. 2003;13:157–167. doi: 10.1152/physiolgenomics.00001.2002. [DOI] [PubMed] [Google Scholar]
- 4.Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 2001;276:40071–40079. doi: 10.1074/jbc.M105456200. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am. J. Physiol. 1998;275:E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int. J. Sport. Nutr. Exerc. Metab. 2001;11(suppl):S97–S104. doi: 10.1123/ijsnem.11.s1.s97. [DOI] [PubMed] [Google Scholar]
- 7.Haskell WL, Wolffe JB. Memorial Lecture. Health consequences of physical activity: Understanding and challenges regarding dose-response. Med. Sci. Sports Exerc. 1994;26:649–660. doi: 10.1249/00005768-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Haskell WL. Physical activity and disease prevention: Past, present, and future: a personal perspective. Exerc. Sports Sci. Rev. 2003;31:109–110. doi: 10.1097/00003677-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Jensen DR, Schlaepfer IR, Morin CL, Pennington DS, Marcell T, Ammon SM, Gutierrez-Hartmann A, Eckel RH. Prevention of diet-induced obesity in transgenic mice overexpressing skeletal muscle lipoprotein lipase. Am. J. Physiol. 1997;273:R683–R689. doi: 10.1152/ajpregu.1997.273.2.R683. [DOI] [PubMed] [Google Scholar]
- 10.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 12.Shimada M, Ishibashi S, Gotoda T, Kawamura M, Yamamoto K, Inaba T, Harada K, Ohsuga J, Perrey S, Yazaki Y, Yamada N. Overexpression of human lipoprotein lipase protects diabetic transgenic mice from diabetic hypertriglyceridemia and hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 1995;15:1688–1694. doi: 10.1161/01.atv.15.10.1688. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi K, Inoue Y, Hagi A, Murase T. The novel compound NO-1886 elevates plasma high-density lipoprotein cholesterol levels in hamsters and rabbits by increasing lipoprotein lipase without any effect on cholesteryl ester transfer protein activity. Metabolism. 1997;46:257–260. doi: 10.1016/s0026-0495(97)90250-x. [DOI] [PubMed] [Google Scholar]
- 14.Wittrup HH, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999;99:2901–2907. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- 15.Yanagibori R, Kondo K, Suzuki Y, Kawakubo K, Iwamoto T, Itakura H, Gunji A. Effect of 20 days’ bed rest on the reverse cholesterol transport system in healthy young subjects. J. Intern. Med. 1998;243:307–312. doi: 10.1046/j.1365-2796.1998.00303.x. [DOI] [PubMed] [Google Scholar]