Abstract
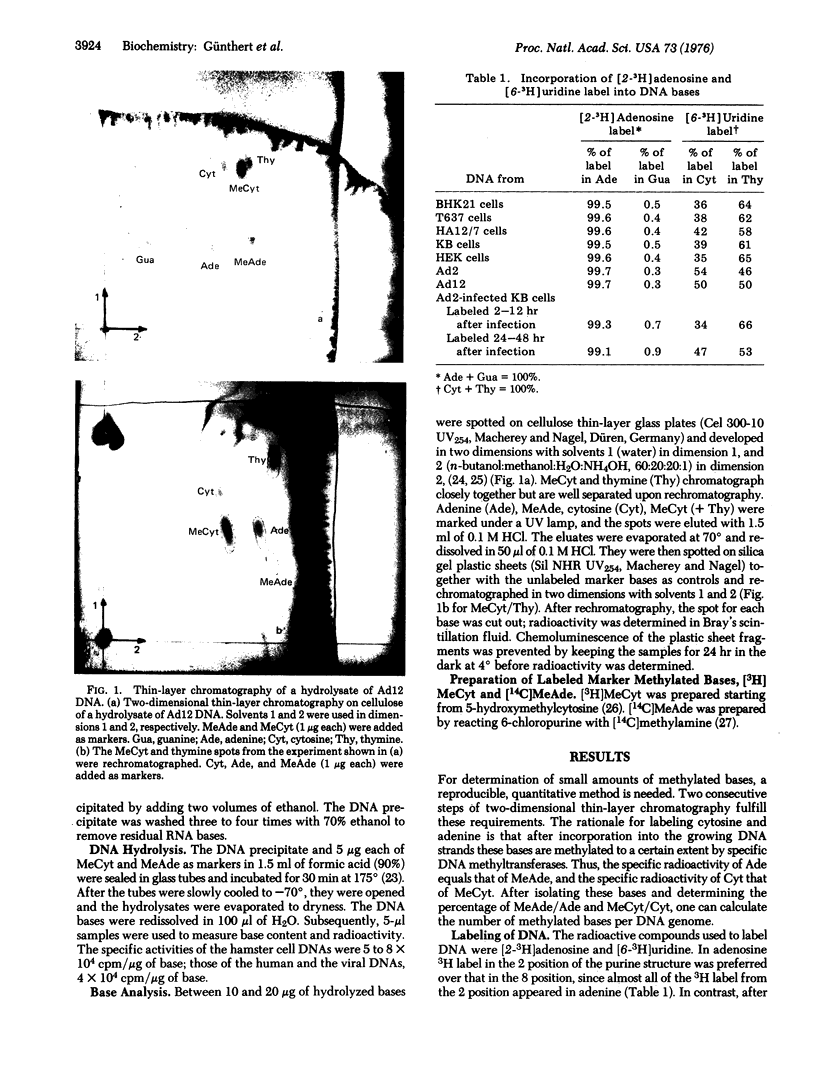
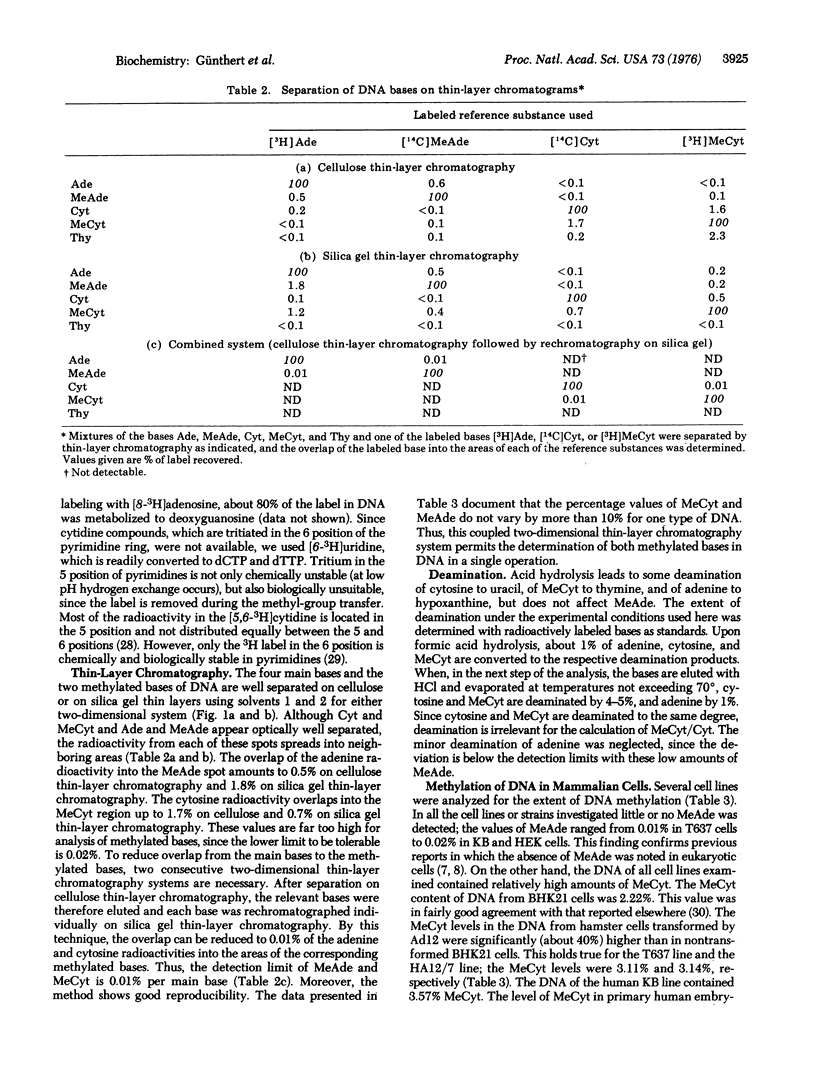
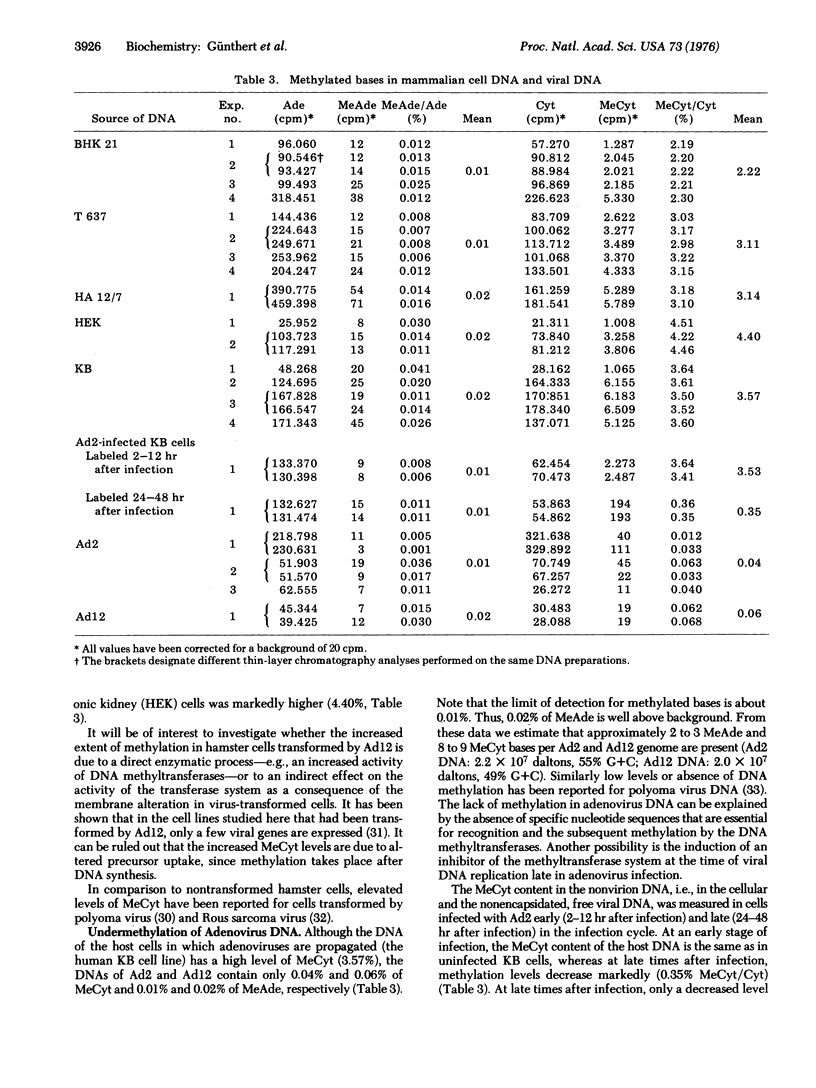
DNAs of adenovirus type 2 and type 12 contain low amounts of methylated bases (0.01 and 0.02% N6-methyl-adenine per adenine, if any, and 0.04 and 0.06% 5-methylcytosine per cytosine for type 2 and type 12, respectively), whereas the DNA of the mammalian host cells contains much more 5-methylcytosine (3.57% for human KB cells). The DNA of hamster cells transformed by adenovirus type 12 contains 3.11 and 3.14% 5-methycytosine (HA12/7 and T627 cells, respectively), whereas the DNA from untransformed hamster cells (BHK21 cells) contains 2.22% 5-methylcytosine. In the DNA of human and hamster cells, little, if any, N6-methyladenine was detected. Methylation of DNA was determined by a sensitive method based on two consecutive steps of two-dimensional thin-layer chromatography of the radioactively labeled DNA bases. By this procedure the detection limits of 5-methylcytosine and N6-methyladenine could be lowered to 0.01% per main base.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. Delayed methylation of DNA in developing sea urchin embryos. Nat New Biol. 1973 Jul 4;244(131):27–29. doi: 10.1038/newbio244027a0. [DOI] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- BROOM A. D., TOWNSEND L. B., JONES J. W., ROBINS R. K. PURINE NUCLEOSIDES. VI. FURTHER METHYLATION STUDIES OF NATURALLY OCCURRING PURINE NUCLEOSIDES. Biochemistry. 1964 Apr;3:494–500. doi: 10.1021/bi00892a005. [DOI] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- EIDINOFF M. L., REILLY H. C., KNOLL J. E., MARRIAN D. H. Hydrolysis products of nucleic acids labeled with tritium; preparation by biosynthesis. J Biol Chem. 1952 Dec;199(2):511–516. [PubMed] [Google Scholar]
- Eskin B., Lautenberger J. A., Linn S. Host-controlled modification and restriction of bacteriophage T7 by escherichia coli B. J Virol. 1973 Jun;11(6):1020–1023. doi: 10.1128/jvi.11.6.1020-1023.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK R. M., FINK K. Relative retention of H3 and C14 labels of nucleosides incorporated into nucleic acids of Neurospora. J Biol Chem. 1962 Sep;237:2889–2891. [PubMed] [Google Scholar]
- Günthert U., Stutz J., Klotz G. Restriction and modification in B. subtilis. The biochemical basis of modification against endo R. Bsu R restriction. Mol Gen Genet. 1975 Dec 30;142(3):185–191. doi: 10.1007/BF00425644. [DOI] [PubMed] [Google Scholar]
- KLEIN A., SAUERBIER W. ROLE OF METHYLATION IN HOST CONTROLLED MODIFICATION OF PHAGE T1. Biochem Biophys Res Commun. 1965 Feb 3;18:440–445. doi: 10.1016/0006-291x(65)90728-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W. The 5-methylcytosine content of DNA: tissue specificity. J Cell Physiol. 1971 Aug;78(1):33–36. doi: 10.1002/jcp.1040780106. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Crathorn A. R., Shah S. A., Smith B. A. Biomethylation of deoxyribonucleic acid in cultured human tumour cells (HeLa). Methylated bases other than 5-methylcytosine not detected. Biochem J. 1972 Jun;128(1):133–138. doi: 10.1042/bj1280133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Differential methylation of mitochondrial and nuclear DNA in cultured mouse, hamster and virus-transformed hamster cells. In vivo and in vitro methylation. J Mol Biol. 1973 Oct 15;80(1):155–175. doi: 10.1016/0022-2836(73)90239-8. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- RANDERATH K. TWO-DIMENSIONAL SEPARATION OF NUCLEIC ACID BASES ON CELLULOSE LAYERS. Nature. 1965 Feb 27;205:908–908. doi: 10.1038/205908a0. [DOI] [PubMed] [Google Scholar]
- Rosenwirth B., Tjia S., Westphal M., Doerfler W. Incomplete particles of adenovirus. II. Kinetics of formation and polypeptide composition of adenovirus type 2. Virology. 1974 Aug;60(2):431–437. doi: 10.1016/0042-6822(74)90337-7. [DOI] [PubMed] [Google Scholar]
- Rubery E. D., Newton A. A. DNA methylation in normal and tumour virus-transformed cells in tissue culture. I. The level of DNA methylation in BHK21 cells and in BHK21 cells transformed by polyoma virus (PyY cells). Biochim Biophys Acta. 1973 Sep 28;324(1):24–36. doi: 10.1016/0005-2787(73)90247-5. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Strohl W. A., Rouse H., Teets K., Schlesinger R. W. The response of BHK21 cells to infection with type 12 adenovirus. 3. Transformation and restricted replication of superinfecting type 2 adenovirus. Arch Gesamte Virusforsch. 1970;31(1):93–112. doi: 10.1007/BF01241669. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Mazin A. L., Vasilyev V. K., Belozersky A. N. The content of 5-methylcytosine in animal DNA: the species and tissue specificity. Biochim Biophys Acta. 1973 Mar 28;299(3):397–403. doi: 10.1016/0005-2787(73)90264-5. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Interactions of adenovirus type 12 with host cell chromosomes. Prog Exp Tumor Res. 1973;18:240–259. doi: 10.1159/000393169. [DOI] [PubMed] [Google Scholar]




