Abstract
Antibacterial and remineralizing dental composites and adhesives were recently developed to inhibit biofilm acids and combat secondary caries. It is not clear what effect these materials will have on dental pulps in vivo. The objectives of this study were to investigate the antibacterial and remineralizing restorations in a rat tooth cavity model, and determine pulpal inflammatory response and tertiary dentin formation. Nanoparticles of amorphous calcium phosphate (NACP) and antibacterial dimethylaminododecyl methacrylate (DMADDM) were synthesized and incorporated into a composite and an adhesive. Occlusal cavities were prepared in the first molars of rats and restored with four types of restoration: Control composite and adhesive; control plus DMADDM; control plus NACP; and control plus both DMADDM and NACP. At 8 or 30 days (d), rat molars were harvested for histological analysis. For inflammatory cell response, regardless of time periods, NACP group and DMADDM+NACP group showed lower scores (better biocompatibility) than control group (p = 0.014 for 8 d, p = 0.018 for 30 d). For tissue disorganization, NACP and DMADDM+NACP had better scores than control (p = 0.027) at 30 d. At 8 d, restorations containing NACP had tertiary dentin thickness (TDT) that was 5-6 fold that of control. At 30 d, restorations containing NACP had TDT that was 4-6 fold that of control. In conclusion, novel antibacterial and remineralizing restorations were tested in rat teeth in vivo for the first time. Composite and adhesive containing NACP and DMADDM exhibited milder pulpal inflammation and much greater tertiary dentin formation, than control adhesive and composite. Therefore, the novel composite and adhesive containing NACP and DMADDM are promising as a new therapeutic restorative system to not only combat oral pathogens and biofilm acids as shown previously, but also facilitate the healing of the dentin-pulp complex.
Keywords: Dental nanocomposite, bonding agent, calcium phosphate nanoparticles, quaternary ammonium methacrylate, rat tooth cavity, in vivo properties
1. Introduction
Dental resin composites are continuously being improved in physical and load-bearing properties and are increasingly being used clinically [1-3]. After being bonded to dental tissues with adhesives [4], the restorations are intended to function in the oral cavities durably. However, secondary caries at the tooth-restoration margins compromises the longevity [5-7]. Nearly half of all restorations fail within ten years, and replacing them accounts for 50-70% of all restorations performed [8,9]. Dental caries, a dietary carbohydrate-modified bacterial infectious disease, is a common bacterial infection in humans [10-12]. The basic mechanism of caries is demineralization of enamel and dentin via acid generated by bacterial biofilms [13-15]. Composites can not hinder bacteria colonization or combat demineralization. To address this issue, efforts are underway to develop antibacterial resins to reduce caries [16-21]. Novel polymers containing quaternary ammonium methacrylates (QAMs) were developed [17-23]. 12-methacryloyloxydodecylpyridinium bromide (MDPB) and methacryloxylethylcetyl dimethyl ammonium chloride (DMAE-CB) could copolymerize with other dental monomers to form antibacterial polymer matrices to reduce bacteria growth [17,18,22]. Recently, dimethylaminododecyl methacrylate (DMADDM) was synthesized and exhibited a stronger antibacterial efficacy [24]. A bonding agent containing DMADDM showed no decrease in antibacterial activity after 6 months of water-aging, compared with that at 1 day [25]. However, the in vivo properties of DMADDM resins need to be tested.
New restorative materials need to be compatible with the dentin-pulp complex. It is important to investigate the influence of antibacterial monomers on pulpal healing after cavity restoration. MDPB exhibited a low level of toxicity to human pulpal cells similar to triethyleneglycol dimethacrylate (TEGDMA) [26]. The incorporation of MDPB into a primer did not increase the toxicity on pulpal cells [27]. The inhibitory effects of MDPB on the proliferation and mineralization of odontoblast-like MDPC-23 mouse cells were lower than those of bisphenol A glycidyl methacrylate (BisGMA), indicating that MDPB had better biocompatibility than BisGMA [28]. For the new DMADDM, its median lethal concentration was between 20 to 40 μg/mL, 20-fold higher than that of BisGMA control, indicating a much lower cytotoxicity than BisGMA [29].
Most previous studies on biocompatibility of antibacterial monomers were in vitro. Animal models could provide an alternative to human models for the assessment of restoratives. Previous animal models used monkeys [30], dogs [31], ferrets [32], and rats [33]. Ethical and cost considerations have favored rat models by many researchers. Several studies showed that the healing of rat molar pulps after pulp-capping was histologically similar to humans and other animal species [34]. Rat molars could be considered anatomically, biologically and physiologically similar to human molars [34]. Previous studies used maxillary molars in rats, and class I [35-37] and class V preparations [38] were tested. However, there has been no report on investigating antibacterial QAM-containing adhesives and composites in a rat tooth model. It remains to be investigated whether the QAM-incorporating materials in deep cavity restorations would exert adverse influence on the dentin-pulp complex in vivo.
Another approach to combat caries is to impart remineralizing capability to restorations [39-45]. Previous studies incorporated calcium phosphate (CaP) particles of about 1-55 μm in sizes into resins [39,40]. These traditional CaP composites released calcium (Ca) and phosphate (P) ions and remineralized tooth lesions in vitro [39,40]. CaP nanoparticles of about 100 nm in size were also filled into resins [41,42]. Composites containing nanoparticles of amorphous calcium phosphate (NACP) with a high surface area released high levels of Ca and inorganic phosphate (Pi) ions while possessing flexural strength nearly two-fold those of traditional CaP composites [41,42]. However, to date, there has been no report on in vivo pulpal response to composite and adhesive containing antibacterial monomer and CaP nanoparticles.
The objectives of this study were to investigate novel antibacterial and remineralizing restoratives in a rat tooth model, and examine pulpal inflammation and tertiary dentin formation using nanocomposite and adhesive containing NACP and DMADDM. It was hypothesized that: (1) The antibacterial and remineralizing nanocomposite and adhesive will be more biocompatible with less pulpal inflammation that traditional composite and adhesive control; (2) Adding DMADDM into composite and adhesive with antibacterial activity will not adversely affect pulpal response, compared with control without DMADDM; (3) Adding NACP into composite and adhesive will reduce pulpal inflammation and greatly increase tertiary dentin formation.
2. Materials and methods
2.1. Synthesis of NACP and DMADDM
NACP were synthesized via a spray-drying technique as previously described [41,42,44]. Briefly, a solution was prepared by dissolving calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and dicalcium phosphate anhydrous (CaHPO4) (J.T. Baker, Phillipsburg, NJ) into an acetic acid solution. This solution was sprayed through a nozzle into a heated chamber. The water and volatile acid were evaporated and expelled into an exhaust-hood [41,42,44]. The dried particles were collected by an electrostatic precipitator [44]. A previous study determined that the NACP surface area was 17.76 m2/g and mean particle size was 116 nm [44].
The synthesis of DMADDM was recently described [24,25]. Briefly, a modified Menschutkin reaction was used where a tertiary amine group was reacted with an organo-halide [23]. A benefit of this reaction is that the reaction products are generated in quantitative amounts and require minimal purification. Ten mmol of 1-(dimethylamino)docecane (DMAD, Tokyo Chemical Industry, Japan) and 10 mmol of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajec, Trevose, PA) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 °C for 24 h. The solvent was then evaporated in the air, yielding DMADDM as a clear, colorless, and viscous liquid [24,25]. The reaction and product of DMADDM were verified via Fourier transform infrared spectroscopy (FTIR) as described in a recent study [24].
2.2. Fabrication of composite and bonding agent
BisGMA and TEGDMA (Esstech, Essington, PA) at a mass ratio of 1:1 were rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate (all mass fractions) [44]. DMADDM was mixed with the photo-activated BisGMA-TEGDMA resin at 5% by mass: DMADDM/(BisGMA-TEGDMA + DMADDM) = 5%. This resin is referred to as BisGMA-TEGDMA-DMADDM. Barium boroaluminosilicate glass with a median particle size of 1.4 μm (Caulk/Dentsply, Milford, DE) was silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine [44]. NACP and glass particles were mixed into resin to fabricate the following composites:
Control composite: 30% BisGMA-TEGDMA resin + 70% glass.
DMADDM composite: 30% BisGMA-TEGDMA-DMADDM resin + 70% glass.
NACP composite: 30% BisGMA-TEGDMA + 35% glass + 30% NACP.
DMADDM + NACP composite: 30% BisGMA-TEGDMA-DMADDM + 35% glass + 30% NACP.
The parent adhesive system was Scotchbond Multi-Purpose bonding system (3M, St. Paul, MN), referred as “SBMP”. According to the manufacturer, SBMP adhesive contained 60-70% of BisGMA, 30-40% of 2-hydroxyethyl methacrylate (HEMA), tertiary amines and photo-initiator. SBMP primer contained 35-45% of HEMA, 10-20% of a copolymer of acrylic and itaconic acids, and 40-50% water. Four bonding agents were tested:
Control bonding agent: Unmodified SBMP primer and adhesive.
DMADDM bonding agent: SBMP primer + 5% DMADDM, SBMP adhesive + 5% DMADDM.
NACP bonding agent: Unmodified SBMP primer, SBMP adhesive + 30% NACP.
DMADDM + NACP bonding agent: SBMP primer + 5% DMADDM, SBMP adhesive + 5% DMADDM + 30% NACP.
The 5% quaternary ammonium was used following previous studies which showed strong antibacterial activity without negatively affecting dentin bond strength [25]. The 30% NACP in resin was shown to result in high levels of Ca and Pi ion release [44,46].
2.3. Rat tooth cavity model
Wistar rats (male, 3 months old, Harlan, Indianapolis, IN) were used for tooth cavity restorations following previous studies [34,37]. This model was approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee (IACUC, # 12-12-02). For anesthesia, Ketamine HCl (100 mg/mL, Butler Schein Animal Health, Dublin, OH) and Xylazine (5 g, Sigma-Aldrich, St. Louis, MO) were injected according to the dose recommended by the University of Maryland Veterinary Resources Program. The dose of Ketamine HCl was 80 mg/kg and the dose of Xylazine was 10 mg/kg. The rat was positioned in the dorsal position and a mouth retraction board was used to gain easier access to the maxillary first molars. Suction was used to remove debris and water during the procedure. Before cavity preparation, the teeth were cleaned mechanically with a small brush, chemically with sodium hypochlorite (5%), and disinfected with chlorhexidine digluconate (0.12%) [37]. Effort was made to keep the molars in a dry state and isolated from contamination. Aided with a stereomicroscope (ES High Power), occlusal cavities were prepared in two teeth per rat: the upper, right and left first molars [33,34]. A handpiece and a sterile 1/2 round carbide bur were used at a maximum speed of 3,000 rotations/min under continued water-cooling and suction [37]. A cavity of approximately 1 mm in width and 1 mm in depth (checked via a periodontal probe with a scale) is prepared. The cavity was etched with 37% phosphoric acid gel for 15 s. A primer was applied, and then an adhesive was applied and light-cured for 10 s (Optilux-VCL401, Demetron Kerr, Danbury, CT). Then, a composite was applied to fill the cavity and light-cured for 60 s. Rats were sacrificed at 8 or 30 d for histological analysis. The following four materials were tested.
Control group: Unmodified SBMP bonding agent control, and composite control.
DMADDM group: DMADDM bonding agent, and DMADDM composite.
NACP group: NACP bonding agent, and NACP composite.
DMADDM + NACP group: DMADDM + NACP in bonding agent and in composite. This constituted a 4 × 2 full factorial design, with four different materials, and two time periods (8 and 30 d). Six teeth (n = 6) per material per time period required 4 × 2 × 6 = 48 tooth cavities. Two tooth cavities per rat required 24 rats. The use of two teeth per rat followed previous studies [33,34]. The sample size was the number of tooth cavities, not the number of animals, following previous studies [51,52]. The sample size of n = 6 was also consistent with previous studies [47,52]. Examples of the rat tooth cavity preparation and restoration are shown in Fig. 1A-C.
Figure 1.
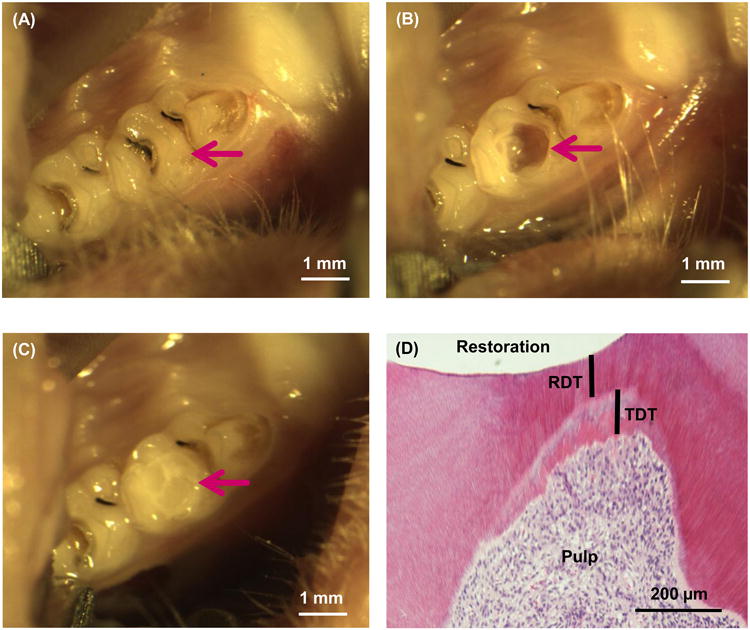
Rat tooth cavity model. (A) Rat right and left molar teeth were used. (B) Occlusal cavity was prepared. (C) Cavity was restored with bonding agent and composite and light-cured. (D) Example of histological image showing the remaining dentin thickness (RDT) and tertiary dentin thickness (TDT) after 30 d.
2.4. Histological analyses
At 8 or 30 d post-operation, the animals were sacrificed via CO2 inhalation [37]. These time periods were selected because a previous study showed that inflammatory processes of rat pulps subsided in 8 d, reparative dentinogenesis was initiated in 2 weeks, and a thick dentin bridge formed in the subsequent 2 weeks. The maxillary molar teeth and the surrounding bone were dissected, fixed in formalin (2.5%) and demineralized for 8 weeks with EDTA [47] Then the samples were exposed to ascending concentrations of alcohol (70, 90 and 100%), cleared in methyl salicilate and submerged in paraffin for 12 h [48] Sections with a thickness of 5 μm were cut in a buccolingual direction along the long axis of the tooth at every 50 μm. The sections were used for Haematoxylin and Eosin (H&E) staining and histological analysis [48] The sections were evaluated using a light microscope (Eclips e600, Nikon, Tokyo, Japan) to examine two types of histological features according to predefined criteria as stated in Tables 1 and 2, following previous reports [49,50]. Scoring was done in a blind manner; the first author scored the samples, and then a colleague in the oral histology field reexamined all samples.
Table 1. Definition of inflammatory cell response scores.
| Inflammatory cell response | characterization |
|---|---|
| Score I | None or a few scattered inflammatory cells present in the pulp area corresponding to the axial wall, characteristic of normal tissue. |
| Score II | Slight inflammatory cell infiltrate with polymorphonuclear (PMNs) or mononuclear leukocytes (MNLs). |
| Score III | Moderate inflammatory cell infiltrate involving the coronal pulp. |
| Score IV | Severe inflammatory cell infiltrate or abscess involving coronal pulp. |
Table 2. Definition of tissue disorganization scores.
| Tissue disorganization | characterization |
|---|---|
| Score I | Normal tissue |
| Score II | Odontoblastic layer disorganized but central pulp normal |
| Score III | Total disorganization of the pulp tissue morphology |
| Score IV | Pulp necrosis |
The remaining dentin thickness (RDT) and the tertiary dentin thickness (TDT) were measured for all sections using Adobe Photoshop software CS (Adobe Systems, San Jose, CA). RDT refers to the minimum thickness of the regular dentin along the direction of dentin tubules between the cavity floor and the pulp chamber (not including the newly-formed tertiary dentin). TDT was measured as the maximum tertiary dentin thickness between the regular dentin and the pulp. An example of RDT and TDT in a rat tooth restoration is shown in Fig. 1D. For RDT and TDT, six teeth for each group at each time period were measured (mean ± sd; n = 6).
2.5 Statistical analysis
The RDT and TDT values were analyzed statistically by two-way ANOVA (material type and time period). The mean ranks of the two histological features in Table 3 were calculated for each group and subjected to nonparametric Kruskall-Wallis analysis and the Mann-Whitney U-test. The critical value was set at α = 0.05.
Table 3. Rat tooth pulp scores of the present study.
(Number of tooth samples in each group at each time period that were rated at each score)
| Histopathological event | Group | 8-day scores | 30-day scores | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| I | II | III | IV | I | II | III | IV | ||
| Inflammatory cell response | Control | 0 | 2 | 4 | 0 | 0 | 5 | 1 | 0 |
| DMADDM | 1 | 2 | 3 | 0 | 1 | 4 | 1 | 0 | |
| NACP | 2 | 4 | 0 | 0 | 4 | 2 | 0 | 0 | |
| DMADDM+NACP | 2 | 4 | 0 | 0 | 4 | 2 | 0 | 0 | |
| Tissue disorganization | Control | 0 | 6 | 0 | 0 | 1 | 5 | 0 | 0 |
| DMADDM | 0 | 6 | 0 | 0 | 0 | 5 | 1 | 0 | |
| NACP | 2 | 4 | 0 | 0 | 5 | 1 | 0 | 0 | |
| DMADDM+NACP | 2 | 4 | 0 | 0 | 5 | 1 | 0 | 0 | |
3. Results
The scores of rat tooth pulp response for the groups and time periods are listed in Table 3. For inflammatory cell response, regardless of the time periods, NACP group and DMADDM+NACP group showed lower scores (better biocompatibility) than control group (p = 0.014 for 8 d, p = 0.018 for 30 d). DMADDM group had similar scores to control group (p = 0.465 for 8 d, p = 0.589 for 30 d).
For tissue disorganization, the scores at 8 d showed no significant difference among the four material groups (p = 0.204). At 30 d, NACP group and DMADDM+NACP group had lower scores (better biocompatibility) than control group (p = 0.027). The scores at 30 d were lower than the scores at 8 d, although the difference was not significant (p = 0.317 for control and DMADDM group, p = 0.093 for NACP and DMADDM+NACP group).
Representative histological images of H&E stained sections at 8 d are shown in Fig. 2: (A-D) Control group, DMADDM group, NACP group, and DMADDM + NACP group, respectively; and (E-H) the same four groups, at a higher magnification. Control and DMADDM group exhibited disruption of the odontoblast layer associated with a medium inflammatory response in the pulp. Blood vessels were observed, and a thin layer of tertiary dentin could be found. NACP group and DMADDM+NACP group had generally normal pulp tissues, with much thicker tertiary dentin than Control.
Figure 2.
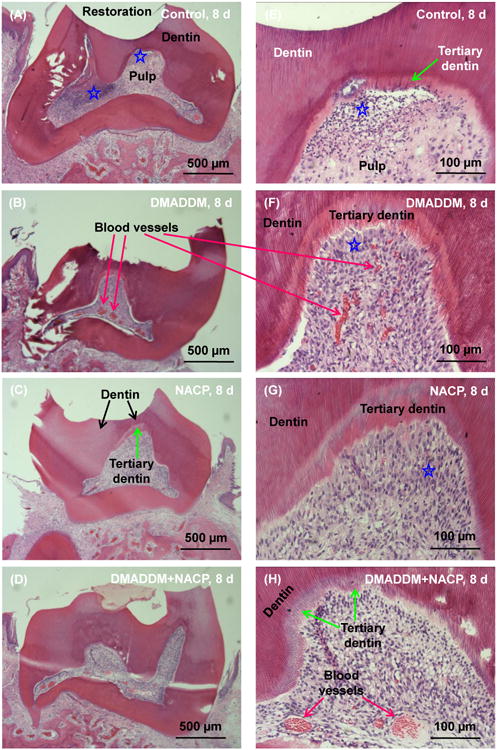
Histological analyses of rat tooth cavities at 8 d. (A-D) Control group, DMADDM group, NACP group, and DMADDM + NACP group. (E-H) The same four groups at a higher magnification. Control and DMADDM group exhibited disruption of the odontoblast layer associated with a medium inflammatory response in the pulp. Blood vessels were observed, and a thin layer of tertiary dentin could be found. NACP group and DMADDM+NACP group had generally normal pulp tissues, with much thicker tertiary dentin than Control. Stars indicate areas with inflammatory cell infiltration.
Accidental mechanical pulp exposure during cavity preparation was found in only one sample of the DMADDM+NACP group. All other samples of all groups showed a remaining dentin layer between restoration and pulp, without pulp perforation. The one sample with pulp perforation is shown in Fig. 3. Some of the restorative material and several dentin pieces were visible in the pulp chamber and were mixed with the pulp tissues. Even with direct contact of the DMADDM+NACP restorative material with pulp tissues, there was still only mild pulpal response with a disrupted odontoblast layer and scattered inflammatory cell infiltration, similar to other samples without pulp perforation. This corresponds to a pulpal inflammation response of score II, and tissue disorganization of score II, as defined in Tables 1 and 2. In addition, it appears that proteins secreted by the pulp cells were in an early stage of being calcified to form tertiary dentin, as indicated in Fig. 3C. This result suggests that the new antibacterial and remineralizing nanocomposite and bonding agent did not cause severe pulpal inflammation and did not compromise the pulp cells' repair and regeneration capability.
Figure 3.
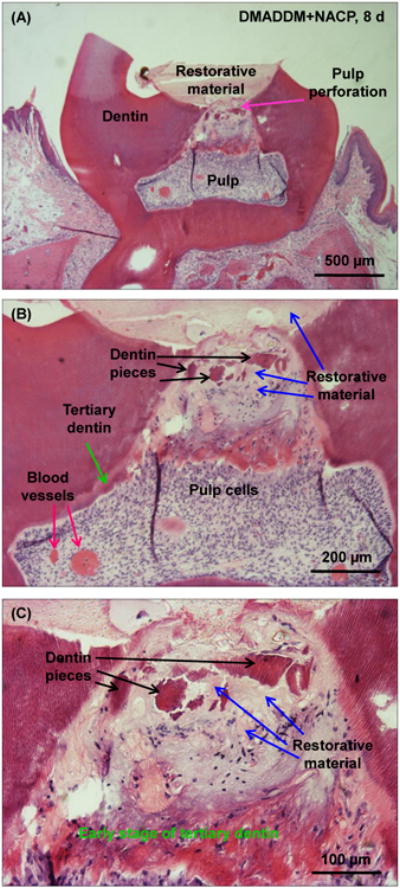
All samples showed remaining dentin layer between restoration and pulp, except one sample of DMADDM+NACP at 8 d which had pulp perforation: (A) lower, (B) medium and (C) higher magnification. With pulp exposure, some restorative materials and dentin pieces were pushed into pulp chamber. Nonetheless, there was mild pulpal response similar to other samples without pulp perforation, indicating that the new antibacterial and remineralizing nanocomposite and bonding agent were biocompatible.
Representative H&E histological images at 30 d are shown in Fig. 4. Control and DMADDM group exhibited slight chronic inflammatory pulp responses with dilated blood vessels. Tertiary dentin thickness of these two groups increased, albeit modestly, when compared with their counterparts at 8 d.
Figure 4.
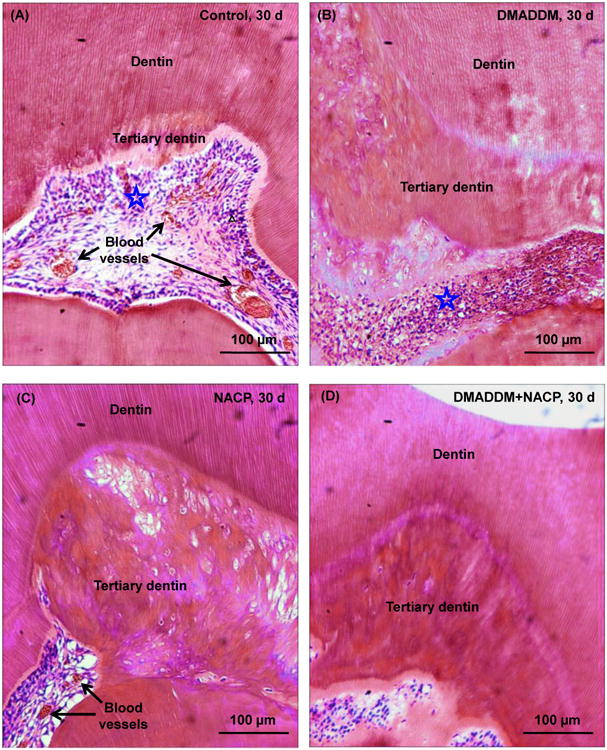
Representative H&E images at 30 d: (A) Control group, (B) DMADDM group, (C) NACP group, and (D) DMADDM + NACP group. Stars indicate areas with inflammatory cells. Blood vessels are indicated by arrows. Control and DMADDM exhibited slight inflammatory responses. NACP and DMADDM+NACP showed normal pulp tissue without inflammatory response, and greater tertiary dentin thickness.
In contrast, NACP group and DMADDM+NACP group at 30 d showed normal pulp tissue with much greater tertiary dentin thickness. Examination of all samples (n = 6) for each group indicated that all the samples that contained NACP had better pulpal response, noticeably less pulpal inflammation, and much more tertiary dentin formation, than samples without NACP. These results demonstrated that NACP in bonding agent and composite decreased pulpal inflammation and increased tertiary dentin thickness, while DMADDM had little effect, compared with the control using a commercial bonding agent and a glass-filled composite.
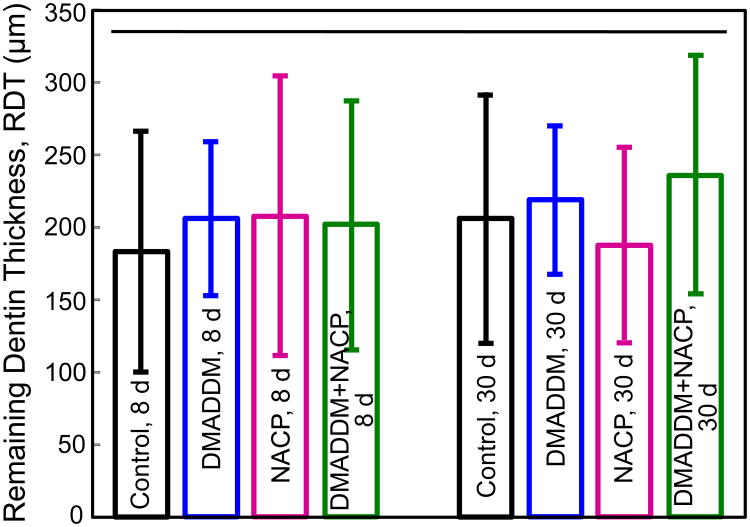
The RDT values measured from the H&E histological images are plotted in Fig. 5 (mean ± sd; n = 6). Two-way ANOVA results for the RDT data are shown in Table 4: Neither the two variables (material type and time period) nor their interaction were statistically significant. In general, the mean RDT values were approximately 200 μ m, showing that deep cavities can be reproducibly prepared in rat teeth with a similar and consistent thickness of remaining dentin, suitable for testing pulpal response.
Figure 5.
Remaining dentin thickness (RDT) measured from H&E histological images (mean ± sd; n = 6). Horizontal line indicates values that are not significantly different from each other (p > 0.1). These data showed that deep cavities with RDT of about 200 μm were reproducibly prepared in rat teeth in this animal model.
Table 4. Two-way ANOVA analysis for remaining dentin thickness (RDT)*.
| Source | Type III Sum of Squares | DF | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 8001.421 | 7 | 1143.060 | .196 | .985 |
| 1977742.803 | 1 | 1977742.803 | 338.692 | .000 | |
| Material type | 4380.393 | 3 | 1460.131 | .250 | .861 |
| Time period | 506.963 | 1 | 506.963 | .087 | .770 |
| Material * Time | 4444.581 | 3 | 1481.527 | .254 | .858 |
| Error | 233574.190 | 40 | 5839.355 | ||
| Total | 2328845.988 | 48 | |||
| Corrected Total | 241575.611 | 47 |
R Squared =.033.
DF: degree of freedom. Sig.: significance.
This Two-way ANOVA table for RDT data showed that neither individual factors (material type and time period) nor their interaction were statistically significant. Hence all tooth cavities had similar RDT.
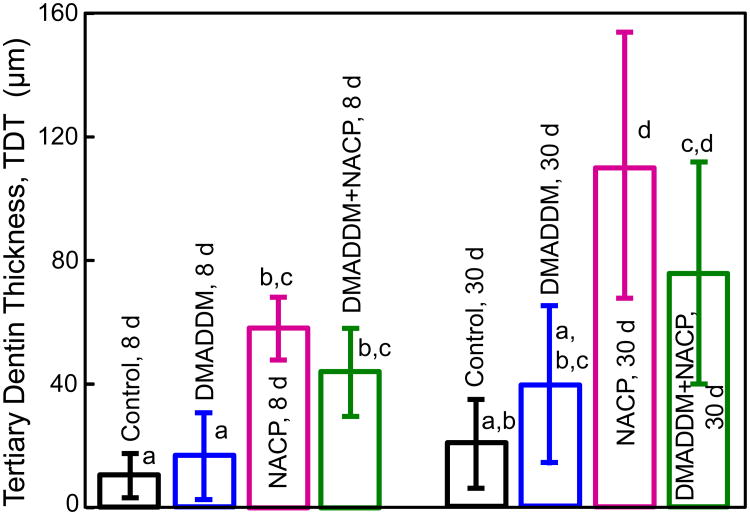
TDT results from the H&E histological images are plotted in Fig. 6 (mean ± sd; n = 6). Two-way ANOVA results for TDT are shown in Table 5: The two variables (material type and time period) had significant effects on TDT, while there was no significant interaction between material type and time period. At 8 d, NACP group and DMADDM+NACP group had TDT that were approximately 5-6 fold that of control. At 30 d, all groups showed an increasing trend in TDT compared with 8 d. NACP group and DMADDM+NACP group had TDT that were approximately 4-6 fold that of Control.
Figure 6.
Tertiary dentin thickness (TDT) measured from H&E histological images (mean ± sd; n = 6). Values with different letters are significantly different from each other (p < 0.05). At each time period, there was no significant difference between Control and DMADDM (p > 0.1). There was no difference between NACP and DMADDM+NACP (p > 0.1). NACP and DMADDM+NACP had TDT that were 5-6 fold that of Control. For each group, TDT at 30 d was about twice that at 8 d.
Table 5. Two-way ANOVA analysis for tertiary dentin thickness (TDT)*.
| Source | Type III Sum of Squares | DF | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 49944.110 | 7 | 7134.873 | 13.164 | .000 |
| 103808.668 | 1 | 103808.668 | 191.529 | .000 | |
| Material type | 35937.269 | 3 | 11979.090 | 22.102 | .000 |
| Time period | 10600.926 | 1 | 10600.926 | 19.559 | .000 |
| Material * Time | 3405.916 | 3 | 1135.305 | 2.095 | .116 |
| Error | 21679.938 | 40 | 541.998 | ||
| Total | 175432.716 | 48 | |||
| Corrected Total | 71624.048 | 47 |
R Squared =.697.
DF: degree of freedom. Sig.: significance.
The Two-way ANOVA table for TDT data showed that the two variables (material type and time period) had significant effects on TDT, while their interaction was not significant.
4. Discussion
The present study investigated novel antibacterial and remineralizing nanocomposite and bonding agent in vivo for the first time, and determined the pulp inflammation and tertiary dentin formation of restorations containing DMADDM and NACP in tooth cavities in rats. Considering that recurrent caries at the tooth-restoration margins is a primary reason for restoration failure, antibacterial and remineralizing restorative materials would be highly desirable. DMADDM was synthesized recently and showed strong bactericidal activity against oral and perioral bacteria [29]. A primer and an adhesive containing 5% of DMADDM inhibited microcosm biofilm growth, metabolic activity and lactic acid production [24]. A DMADDM-NACP nanocomposite inhibited biofilm metabolic activity and lactic acid without compromising mechanical strength [53]. The present study focused on the biocompatibility of the new restoratives in the dentin-pulp complex using a rat model. The antibacterial and remineralizing nanocomposite and adhesive were more biocompatible with less pulpal inflammation than control composite and adhesive. DMADDM-containing composite and adhesive generated pulp response similar to control group. NACP-incorporating materials, including NACP group and DMADDM+NACP group, generated milder pulp inflammation and much thicker tertiary dentin, than control group.
In a previous study, dental pulp in dogs capped with adhesive produced variable response, characterized by moderate to severe inflammatory reaction in most teeth for 7, 21 and 65 day observation periods [52]. Rat molar teeth are another useful model to provide valuable data on pulp reaction after restoration [34]. In the present study, a deep occlusal cavity was prepared in rat molars with remaining dentin thickness of approximately 200 μm suitable for examining pulp response to the restorative materials. The present study showed mild inflammatory reaction at 8 d for control group and DMADDM group, and slight inflammatory reaction at 30 d. These responses were milder than the moderate to severe inflammatory reaction in the dog model in a previous study [52]. The difference may be partially due to the exceptional resilience and self-reparative capacity of the rat pulp. In addition, the present study employed deep cavity to elicit pulp reaction with a remaining dentin layer between cavity floor and the pulp, while in the previous dog study [52], the pulp was perforated and directly capped with adhesive which might generate a more severe inflammatory response.
The control group used a commercial bonding agent and a composite filled with glass particles. Its components, including BisGMA, TEGDMA and HEMA, were shown to cause a moderate level of cytotoxicity in vitro to cultured cells [54]. They could cause inhibitory effects on DNA synthesis, total protein content and protein synthesis. In clinical practice, restorative materials are applied to dentin which is a biologic composite of apatite in a collagen matrix with a fluid-filled tubular structure connecting to the pulp [55]. The etching process removes the smear layer and opens dentinal tubules to facilitate the infiltration of monomers, thus irritating the dentin-pulp complex. This might partially explain the disruption of the odontoblast layer and a mild-to-medium inflammatory response at 8 d in the present study. Furthermore, evidence of dentinal fluid flow after the application of adhesive suggests that the polymerized adhesive could not completely seal the deep vital dentin [56,57]. Unpolymerized monomers may be released out of the polymer matrix [58] and diffuse through the tubules into the pulp [59], causing damage to pulpal tissues [60]. Therefore, with the release of unpolymerized monomer, the irritation to pulp could persist and cause chronic inflammatory response, as observed in the 30 d groups. However, the pulpal inflammation at 30 d was milder than that at 8 d, indicating that there was likely an initial relatively high-rate of release of unpolymerized monomers decreasing with time.
Compared with the commercial adhesive and glass-filled composite control, the addition of antibacterial monomer DMADDM into adhesive and composite did not aggravate the pulp reaction, which exhibited a mild-to-medium inflammatory pulp response at 8 d and mild chronic inflammatory pulp response at 30 d. In a previous study, the cytotoxicity of DMADDM was demonstrated to be 20-fold lower than that of BisGMA control [29]. After polymerization, the DMADDM monomer was expected to be covalently-bonded in the polymer matrix, and the eluent of the cured DMADDM-incorporating resin was of a low concentration and showed a low cytotoxicity, similar to a commercial control without DMADDM [61]. These previous studies, together with the in vivo results of the present study, suggest that the incorporation of DMADDM did not adversely influence the biocompatibility of the composite and adhesive. Moreover, the antibacterial material showed better biocompatibility according to the TDT values, which might be due to the elimination of the irritation derived from bacterial metabolic activity.
Regarding the RDT and TDT values, deep cavities were prepared in monkey teeth in a previous study with RDT ranging from 211 to 353 μm [30]. These values were similar to the RDT of 182-238 μm of the present study, suitable for testing pulpal response to the restorative materials. In a previous study, the newly-formed TDT was 81-153 μm at 5 weeks, and increased to 149-238 μm at 8 weeks [30] In the present study, the rat molars had TDT of 22-111 μm at 30 d, comparable to the 5-week TDT of the previous study. It should be noted that different animals, and different types of teeth of the same animal, might generate different results. For example, in a study using rat incisors to test direct pulp-capping via mineral trioxide aggregate (MTA), desirable regenerative ability was obtained with TDT of 200 μm [51]. These TDT values were greater than the TDT of the present study using molar teeth, which might be due to the superior regenerative capacity of the continuously-growing rat incisors.
Compared to the control, all materials that contained NACP, including NACP group and DMADDM+NACP group, generated greater TDT and displayed better biocompatibility with normal pulp tissues or milder inflammations. CaP-based biomaterials are important for hard tissue repairs due to their excellent biocompatibility and bioactivity [39-45,62]. For example, calcium phosphate cement exhibited the potential to stimulate osteogenesis [63] and promote odontoblastic differentiation of human dental pulp cells (HDPCs) [64]. For bonding agent containing NACP that flowed into dentinal tubules, the addition of NACP might help absorb monomers and limit their diffusion into the pulp, thereby buffering the cytotoxicity of the monomers. This may have contributed to the reduced pulpal inflammation for NACP group and DMADDM+NACP group, compared with control group. The improved biocompatibility supports a desirable usage of NACP in dentin bonding agents as well as in composites. In addition, in pulp-capping, a previous study showed that a CaP-based material promoted pulp healing and dentin repair in vivo [62]. The Ca and Pi ions eluted from the calcium phosphate in the vicinity of the exposed pulp, along with the results that NACP could cause an elevated alkaline pH locally, could enhance calcification and promote tertiary dentin formation [43]. These factors likely contributed to the great increase in tertiary dentin thickness in the NACP group and the DMADDM+NACP group of the present study. The milder pulp inflammation and thicker tertiary dentin, together with remineralization and inhibition of biofilm acids shown in previous studies [24,25,45], indicate that the NACP group and the DMADDM+NACP group are promising for use in dental restorations. Further study needs to investigate if similar benefits of the reduced pulp inflammation and enhanced tertiary dentin formation can be achieved in humans via the NACP and the DMADDM+NACP.
5. Conclusions
Novel antibacterial and remineralizing restoratives were investigated in a rat tooth cavity model for the first time. Nanocomposite and adhesive containing DMADDM and NACP exhibited milder pulpal inflammation and much greater tertiary dentin formation, than traditional adhesive and composite. Adding NACP in adhesive and composite decreased pulpal inflammation; adding DMADDM had little effect on pulpal inflammation, compared with that using a commercial adhesive and a glass-filled composite. All restorations containing NACP, including NACP group and DMADDM+NACP group, had tertiary dentin thicknesses that were 4-6 fold that of control. Therefore, the incorporation of NACP and DMADDM had four benefits: (1) Remineralization and (2) antibiofilm and inhibition of acid production shown previously [24,25,45], and (3) decreased pulpal inflammation and (4) increased tertiary dentin shown in the present study. Novel composites and bonding agents containing NACP and DMADDM are promising as a new therapeutic restorative system to combat oral pathogens, inhibit biofilm acids and caries, and facilitate the healing of the dentin-pulp complex.
Acknowledgments
We are indebted to Prof. Man-Kyo Chung and Chen Chen for help with the animal experiments. We thank Drs. Laurence C. Chow, Joseph M. Antonucci, Sheng-Lin Gibson and Nancy Lin for discussions. We are grateful to Esstech (Essington, PA) for kindly donating the BisGMA and TEGDMA monomers. This study was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China 81100772 (FL), and a bridge funding from the University of Maryland School of Dentistry (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 2.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dent Mater. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Mjor IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–6. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS). June 17-19, 2004, Oregon Health and Science University, Portland, Oregon. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 9.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9:31–6. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 10.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 12.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–99. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 13.Featherstone JD. The continuum of dental caries--evidence for a dynamic disease process. J Dent Res. 2004;83 Spec No C:C39–42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 14.Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 15.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–73. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 16.Sarasam AR, Brown P, Khajotia SS, Dmytryk JJ, Madihally SV. Antibacterial activity of chitosan-based matrices on oral pathogens. J Mater Sci Mater Med. 2008;19:1083–90. doi: 10.1007/s10856-007-3072-z. [DOI] [PubMed] [Google Scholar]
- 17.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res. 2009;88:372–6. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 19.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B Appl Biomater. 2012;100:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. J Mater Sci Mater Med. 2012;23:1553–61. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 22.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 23.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2013 doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. J Dent. 2013 doi: 10.1016/j.jdent.2013.03.011. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. [DOI] [PubMed] [Google Scholar]
- 27.Imazato S, Tarumi H, Ebi N, Ebisu S. Cytotoxic effects of composite restorations employing self-etching primers or experimental antibacterial primers. J Dent. 2000;28:61–7. doi: 10.1016/s0300-5712(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 28.Nishida M, Imazato S, Takahashi Y, Ebisu S, Ishimoto T, Nakano T, et al. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials. 2010;31:1518–32. doi: 10.1016/j.biomaterials.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Weir MD, Fouad AF, Xu HH. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 2013;41:881–91. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CF, White KC, Ramus DL, Farmer JB, Snuggs HM. Reparative dentin: factors affecting its deposition. Quintessence Int. 1992;23:257–70. [PubMed] [Google Scholar]
- 31.Tziafas D, Alvanou A, Papadimitriou S, Gasic J, Komnenou A. Effects of recombinant basic fibroblast growth factor, insulin-like growth factor-II and transforming growth factor-beta 1 on dog dental pulp cells in vivo. Arch Oral Biol. 1998;43:431–44. doi: 10.1016/s0003-9969(98)00026-0. [DOI] [PubMed] [Google Scholar]
- 32.Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale. 1990;18:123–9. [PubMed] [Google Scholar]
- 33.Six N, Lasfargues JJ, Goldberg M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 2000;28:413–22. doi: 10.1016/s0300-5712(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 34.Dammaschke T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim. 2010;44:1–6. doi: 10.1258/la.2009.008120. [DOI] [PubMed] [Google Scholar]
- 35.Fachin EV, Scarparo RK, Pezzi AP, Luisi SB, Sant'ana Filho M. Effect of betamethasone on the pulp after topical application to the dentin of rat teeth: vascular aspects of the inflammation. J Appl Oral Sci. 2009;17:335–9. doi: 10.1590/S1678-77572009000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dammaschke T, Stratmann U, Fischer RJ, Sagheri D, Schafer E. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. 2010;41:e62–71. [PubMed] [Google Scholar]
- 37.Dammaschke T, Stratmann U, Fischer RJ, Sagheri D, Schafer E. Proliferation of rat molar pulp cells after direct pulp capping with dentine adhesive and calcium hydroxide. Clin Oral Investig. 2011;15:577–87. doi: 10.1007/s00784-010-0409-7. [DOI] [PubMed] [Google Scholar]
- 38.Kawagishi E, Nakakura-Ohshima K, Nomura S, Ohshima H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006;326:111–22. doi: 10.1007/s00441-006-0230-4. [DOI] [PubMed] [Google Scholar]
- 39.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–66. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 40.Langhorst SE, O'Donnell JN, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu HH, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC, et al. Nano DCPA-whisker composites with high strength and Ca and PO(4) release. J Dent Res. 2006;85:722–7. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. Strong nanocomposites with Ca, PO(4), and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katoh Y, Suzuki M, Kato C, Shinkai K, Ogawa M, Yamauchi J. Observation of calcium phosphate powder mixed with an adhesive monomer experimentally developed for direct pulp capping and as a bonding agent. Dent Mater J. 2010;29:15–24. doi: 10.4012/dmj.2009-023. [DOI] [PubMed] [Google Scholar]
- 44.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dammaschke T, Stratmann U, Wolff P, Sagheri D, Schafer E. Direct pulp capping with mineral trioxide aggregate: an immunohistologic comparison with calcium hydroxide in rodents. J Endod. 2010;36:814–9. doi: 10.1016/j.joen.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003;36:225–31. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 49.de Souza Costa CA, Teixeira HM, Lopes do Nascimento AB, Hebling J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. J Biomed Mater Res B Appl Biomater. 2007;81:175–84. doi: 10.1002/jbm.b.30651. [DOI] [PubMed] [Google Scholar]
- 50.Costa CA, Ribeiro AP, Giro EM, Randall RC, Hebling J. Pulp response after application of two resin modified glass ionomer cements (RMGICs) in deep cavities of prepared human teeth. Dent Mater. 2011;27:e158–70. doi: 10.1016/j.dental.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Orhan EO, Maden M, Senguuven B. Odontoblast-like cell numbers and reparative dentine thickness after direct pulp capping with platelet-rich plasma and enamel matrix derivative: a histomorphometric evaluation. Int Endod J. 2012;45:317–25. doi: 10.1111/j.1365-2591.2011.01977.x. [DOI] [PubMed] [Google Scholar]
- 52.Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005;33:639–47. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent Mater. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanks CT, Wataha JC, Parsell RR, Strawn SE. Delineation of cytotoxic concentrations of two dentin bonding agents in vitro. J Endod. 1992;18:589–96. doi: 10.1016/S0099-2399(06)81328-2. [DOI] [PubMed] [Google Scholar]
- 55.Pashley DH, Carvalho RM. Dentine permeability and dentine adhesion. J Dent. 1997;25:355–72. doi: 10.1016/s0300-5712(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 56.Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, et al. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater. 2007;23:705–13. doi: 10.1016/j.dental.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho RM, et al. Single-bottle adhesives behave as permeable membranes after polymerization. I. In vivo evidence. J Dent. 2004;32:611–21. doi: 10.1016/j.jdent.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Floyd CJ, Dickens SH. Network structure of Bis-GMA- and UDMA-based resin systems. Dent Mater. 2006;22:1143–9. doi: 10.1016/j.dental.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Cetinguc A, Olmez S, Vural N. HEMA diffusion from dentin bonding agents in young and old primary molars in vitro. Dent Mater. 2007;23:302–7. doi: 10.1016/j.dental.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 60.de Souza Costa CA, do Nascimento AB, Teixeira HM. Response of human pulps following acid conditioning and application of a bonding agent in deep cavities. Dent Mater. 2002;18:543–51. doi: 10.1016/s0109-5641(01)00089-6. [DOI] [PubMed] [Google Scholar]
- 61.Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of dental research. 2013 doi: 10.1177/0022034513502053. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickens SH, Flaim GM, Schumacher GE, Eichmiller FC, Schafer DR, Rutherford RB. Preclinical effectiveness of a novel pulp capping material. J Endod. 2010;36:1222–5. doi: 10.1016/j.joen.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruhe PQ, Hedberg-Dirk EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 64.Lee SK, Lee SI, Park JH, Jang JH, Kim HW, Kim EC. Effect of calcium phosphate cements on growth and odontoblastic differentiation in human dental pulp cells. J Endod. 2010;36:1537–42. doi: 10.1016/j.joen.2010.04.027. [DOI] [PubMed] [Google Scholar]