Abstract
Objectives
Many recent adhesives on the market exhibit reasonable clinical performance. Future innovations in adhesive materials should therefore seek out novel properties rather than simply modifying existing technologies. It is proposed that adhesive materials that are “bio-active” could contribute to better prognosis of restorative treatments.
Methods
This review examines the recent approaches used to achieve therapeutic polymers for dental adhesives by incorporating bio-active components. A strategy to maintain adhesive restorations is the focus of this paper.
Results
Major trials on therapeutic dental adhesives have looked at adding antibacterial activities or remineralization effects. Applications of antibacterial resin monomers based on quaternary ammonium compounds have received much research attention, and the loading of nano-sized bioactive particles or multiple ion-releasing glass fillers have been perceived as advantageous since they are not expected to influence the mechanical properties of the carrier polymer.
Significance
The therapeutic polymer approaches described here have the potential to provide clinical benefits. However, not many technological applications in this category have been successfully commercialized. Clinical evidence as well as further advancement of these technologies can be a driving force to make these new types of materials clinically available.
Keywords: Adhesives, Dental polymers, Bio-active, Antibacterial, Remineralization, QAC, Nano-particle
1. Incorporation of QAC-based resin monomers
Quaternary ammonium compounds (QACs) are a group of cationic antimicrobials widely used for numerous industrial and pharmaceutical purposes [1]. In 1994, to develop non-agent-releasing antibacterial dental resins, Imazato et al. combined alkylpyridinium, a type of QAC, with a methacrylate group, and successfully synthesized a novel dental resin monomer, 12-methacryloyloxydodecylpyridinium bromide (MDPB) [2] (Fig. 1). While the QAC group is responsible for the antibacterial activity of MDPB, the methacrylate group allows for copolymerization with other conventional monomers. Since antibacterial monomers are immobilized in the resin matrix and do not leach out after curing, incorporating these monomers imposes no negative influences on the mechanical properties of the carrier material [2]. Without releasing these active agents, QAC-based resinous materials can exhibit stable and long-term antibacterial effects [2].
Fig. 1.
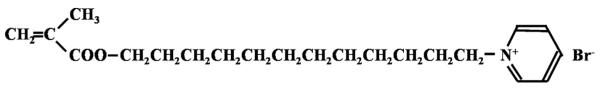
QAC-based antibacterial monomer MDPB.
1.1. Antibacterial effects
Experimental antibacterial adhesive systems were first prepared by incorporating MDPB into the primer of commercial self-etching adhesive Liner Bond 2 [3]. Since then, the antibacterial activity of this prototype has been investigated and confirmed by a number of in vitro studies. Based on the findings of this experimental material, Clearfil Protect Bond, employing a 5% MDPB-containing primer, was developed and commercialized (sold as Clearfil SE Protect in USA and Clearfil Mega Bond FA in Japan).
Before curing, the MDPB-containing primer can kill bacteria rapidly because of the bactericidal activity of unpolymerized MDPB. It can thereby act as a cavity disinfectant. When the primer containing MDPB was kept in direct contact with planktonic bacteria, all bacteria were killed within 30 s [3–5]. It is noteworthy that the Clearfil Protect Bond primer was able to penetrate a 500-μm-thick dentin block [6] and eradicate caries-related species inside the dentin [7]. In vivo studies using beagle dogs found that the MDPB-containing primer could also inactivate Streptococcus mutans in the cavity [8]. Since residual bacteria are one of the primary causes of secondary caries, the cavity-disinfecting effects of the MDPB-containing primer may improve the outcomes of restorative treatments of caries lesions.
After curing, MDPB-containing resins can inhibit the growth of bacteria that comes into contact with the material, thereby acting as a so-called “contact inhibitor” (Fig. 2). When S. mutans was incubated in contact with the cured primer/adhesive surface containing MDPB, the number of viable bacteria was significantly reduced [9,10]. However, materials containing MDPB only exhibited bacteriostatic, rather than bactericidal effects, against the contacting bacteria. Two possible reasons for the reduction in antibacterial activity after curing have been proposed; (i) the movement of the immobilized molecules is limited, and (ii) the density of the QAC group of MDPB exposed on the outer surface is not high enough to kill bacterial cells.
Fig. 2.
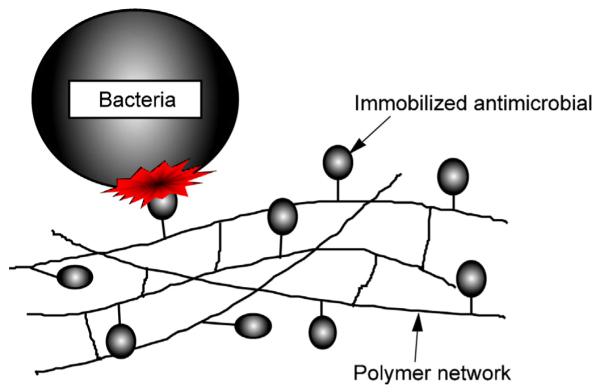
Antimicrobial immobilized in a polymer network by copolymerization of the antibacterial monomer with conventional methacrylate monomers; contact inhibition of bacteria.
MDPB-containing adhesives have been suggested to be effective in root caries arrestment and dental pulp preservation. This is attributed to their lesion-disinfecting effects and bacteriostatic functionality on contact with bacteria after curing. In a S. mutans-induced artificial root caries model, a MDPB-containing adhesive completely prevents lesion progress [11]. As for pulp preservation, it has been confirmed using beagle dog models that the antibacterial primer containing MDPB can kill bacteria in the cavity, thus maintaining pulp vitality and primary odontoblastic function in infected, non-exposed and exposed cavities [8,12].
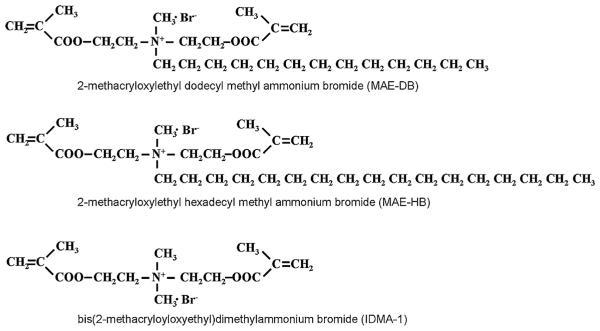
Besides MDPB, several other QAC monomers have been developed that can be utilized in resinous dental materials. Methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB, Fig. 3), synthesized by Chen’s group, provided a commercial etch and rinse adhesive with stable antibacterial activities that does not damage the bonding capacity [13,14]. In recent years, significant efforts have been devoted to developing QAC monomers with improved properties. For instance, QAC monomers with two methacrylate groups have been synthesized to enhance the polymerization capacity [15–17] (Fig. 4). Antibacterial monomers with radio-opacity have also been developed using iodine as a counter ion [18,19] (Fig. 5).
Fig. 3.
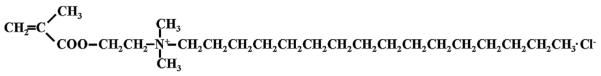
QAC-based monomer DMAE-CB.
Fig. 4.
QAC-based monomers with two polymerizable groups.
Fig. 5.
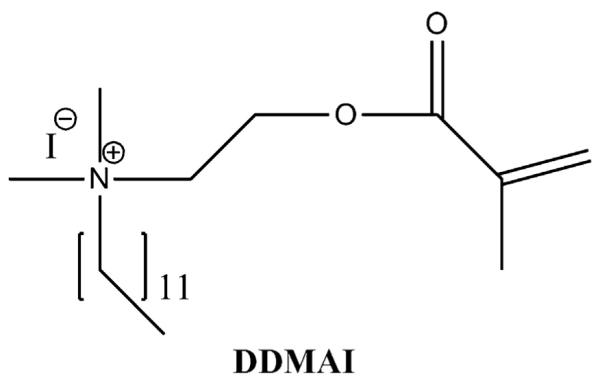
QAC monomer with iodine as a counter ion.
1.2. Inhibitory effects against matrix metalloproteinases
Although modern adhesives can achieve satisfying immediate bonding to dentin, they demonstrate a loss of bond strength over time. Enzymatic degradation of the collagen matrix by host-derived matrix metalloproteinases (MMPs) plays a significant role in the destruction of the bonded interface [20]. One strategy to improve the durability of resin–dentin bonds is to use inhibitors that inactivate MMPs at the bonded interface [21]. Chlorhexidine has been found to be a non-specific MMPs inhibitor [22], and applying this agent to adhesives has been reported to be beneficial for the preservation of the resin–dentin bonds in vitro [23–25]. Similar to chlorhexidine, a QAC disinfectant of benzalkonium chloride effectively inhibited both soluble recombinant MMPs and matrix-bound dentin MMPs [26]. Tezvergil-Mutluay et al. speculated that QAC monomers may inhibit MMP activity. Using both soluble rhMMP-9 and matrix-bound endogenous MMPs, they found that QAC monomers, including MDPB, exhibited MMP inhibition behavior that was comparable to that of chlorhexidine [27]. Noticeably, MDPB at 5%, which is the concentration utilized for the primer of commercial adhesive Clearfil Protect Bond, achieved 89% inhibition of soluble rhMMP-9 and approximately 90% inhibition of matrix-bound MMPs. Compared with chlorhexidine or benzalkonium chloride, which may leach out from bonded interfaces over time, polymerizable MMP-inhibitors are advantageous as they can be retained in the hybrid layer for years by copolymerization. Several investigations into bond durability, including in vivo studies, revealed that the MDPB-containing adhesive produced a more durable interface than conventional adhesives [28,29]. Such improved durability achieved by MDPB-containing adhesives may be partially explained by the inhibitory effects of MDPB on MMPs.
2. Incorporation of nanoparticles
2.1. Antibacterial effects of silver nanoparticles
The antibacterial, antifungal, and antiviral actions of silver ions have been extensively investigated. Silver ions have been considered for applications as an antibacterial component in resinous dental materials. However, polymers filled with silver ions typically release a burst of ions and lose their antibacterial activity within a short period [30]. Compared with free silver ions, reduced silver nanoparticles incorporated into a polymeric matrix provide a large reservoir of silver ions that can be released in a more controlled manner at a steady rate, allowing for long-term antibacterial effects [31]. The direct incorporation of silver nanoparticles into a polymer matrix is a common strategy for preparing antibacterial resinous materials [32]. However, silver nanoparticles are difficult to disperse, as nano-sized particles tend to aggregate. In 2011, a new technique for preparing dental polymers with evenly dispersed silver nanoparticles was described using coupling photo-initiated free radical polymerization of dimethacrylates with in situ silver ion reduction [33]. The experimental composites containing 0.08% of silver nanoparticles exhibited a 40% reduction in bacterial coverage [33].
As opposed to QAC monomer-containing resins whose bacteriostatic effects depend on the direct contact of bacteria with the material surface, resinous materials loaded with silver nanoparticles can inhibit bacteria on its surface as well as bacteria suspended in the culture medium away from the surface [34]. Therefore, QAC monomers and silver nanoparticles could show complimentary behavior for inhibiting bacteria. Experimental adhesives containing both QAC monomers and silver nanoparticles exhibited significantly enhanced antibacterial potency before and after curing compared with adhesives that used either agent alone [35–40].
2.2. Remineralization by calcium phosphate nanoparticles
To develop resinous materials with remineralization capabilities, calcium and phosphate ion-releasing fillers can be incorporated. The release and precipitation of calcium and phosphate can enhance the formation of hydroxyapatite (Ca10(PO4)6(OH)2), which is the structural prototype for the major mineral component of teeth. In vitro studies revealed that methacrylate-based composites containing calcium phosphate fillers could release calcium and phosphate ions to supersaturated levels for apatite precipitation, and thus could effectively remineralize tooth lesions [41–44]. However, calcium phosphates containing resinous materials have the drawback of low mechanical strength. To develop experimental composites with high Ca and PO4 release rates and with acceptable mechanical properties, Xu et al. combined nano-sized dicalcium phosphate anhydrous (DCPA) with silica-fused whiskers as co-fillers [45–48]. As the nanoparticles have a high surface area, high levels of Ca and PO4 can be released with a relatively small amount of DCPA filler. This leaves room in the resin for a significant amount of silica-fused whiskers that can reinforce the mechanical properties. However, the silica-fused whisker-reinforced nanocomposite is relatively opaque with a whitish color owing to a refractive index mismatch between the whiskers and resin, and cannot be light-cured [49].
Amorphous calcium phosphate nanoparticles (NACP) were synthesized and combined with barium boroaluminosilicate glass particles, a typical dental glass filler similar to those in hybrid composites, to yield light-curable, weight-bearing, Ca and PO4-releasing composites [50,51]. One advantage of the composites containing calcium phosphate fillers is that they are “smart” and could release relatively high amounts of Ca and PO4 when the pH is reduced from neutral to cariogenic of pH 4.0 [50]. Furthermore, these materials can rapidly neutralize the acidic medium, increasing the pH from 4.0 to 5.69 within 10 min [52]. The “smart” release of Ca and PO4 as well as the neutralizing effects are promising material attributes to combat acid attack-induced mineral loss. A recent in vitro study using a 30-day demineralization/remineralization cyclic regimen found that NACP nano-composites could effectively remineralize the demineralized human enamel. These remineralization effects were 4-fold that of a commercial fluoride-releasing composite [53].
Based on these successful composites, Xu et al. conducted an approach to combine amorphous calcium phosphate with antibacterial components (QAC monomer or silver nanoparticles) (Fig. 6), and achieved a novel dental bonding agent with remineralization capacity and long-lasting antibacterial activity [54–57]. Such new materials having both remineralization and antibacterial properties may be of great benefit to preserve durable bonding interfaces and fight against secondary caries.
Fig. 6.
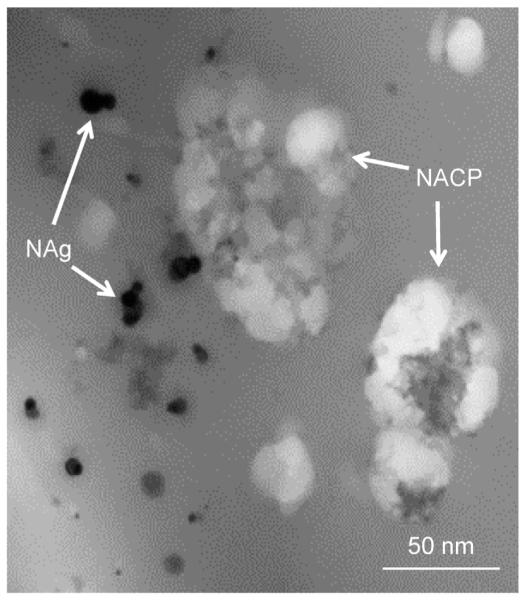
TEM image of amorphous calcium phosphate (NACP) and silver nanoparticles (NAg) incorporated in the adhesive resin.
3. Application of a multiple ion-releasing glass filler
Pioneering work by Wilson and Kent suggests that the release of fluoride from glass-ionomer cements relies on the siliceous hydrogel layers on the surfaces of the glass particles. These layers result from the acid-base reaction between the ionleachable glass fillers and polyalkenoic acids [58]. Based on this theory, a revolutionary pre-reacted glass ionomer (PRG) filler, that are prepared by the acid-base reaction of fluoroboroaluminosilicate glass with polyalkenoic acid and added to resinous materials, has been introduced. Between the two types of fillers prepared, a full reaction type (F-PRG) and a surface reaction type (S-PRG) [59], the latter was found to be more useful because it is fabricated by the reaction limited to the glass surface and the mechanical properties of the core glass are not affected (Fig. 7). A ligand exchange mechanism within the pre-reacted hydrogel endows S-PRG fillers with the ability to release and recharge fluoride ions [60]. In addition, S-PRG fillers release multiple ions such as Sr2+, Na+, BO33−, Al3+, and SiO32− at high concentrations [61] (Fig. 8). Several unique therapeutic effects are expected for resinous materials containing S-PRG fillers owing to the multiple ion-releasing capacity.
Fig. 7.
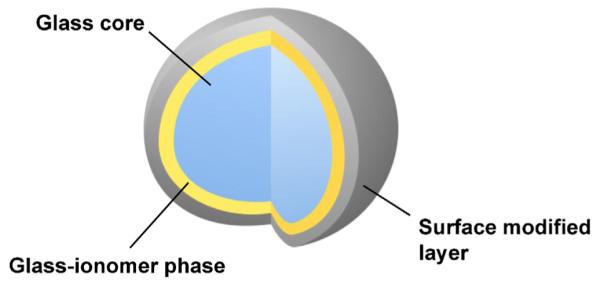
Structure of the surface pre-reacted glass-ionomer (S-PRG) filler.
Fig. 8.
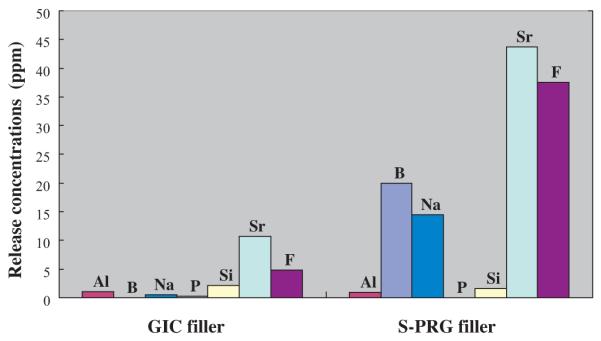
Concentration of ions released from conventional fluoroaluminosilicate glass of glass-ionomer cement (GIC filler) or S-PRG filler into distilled water after 24 h of immersion. Al, B, Na, P, Si, and Sr were detected by inductively coupled plasma atomic emission spectroscopy and F− was measured using a fluoride electrode.
3.1. Promotion of calcification and remineralization
S-PRG fillers act in a quasi-intelligent way such that their release of fluoride is acidity-dependent. Their protective effects are then proportional to the threat being encountered [60]. The S-PRG filler can also achieve a sustained fluoride release owing to its fluoride recharging capacity [60,62–64]. Along with the release of multiple ions, S-PRG filler can modulate the pH of the surrounding medium, shifting the pH to neutral and weak alkaline regions [61]. Because of the released fluoride and silica, the eluate of resins filled with S-PRG fillers remarkably enhances the formation of apatite on phosvitinimmobilized agarose beads in the presence of a mineralizing solution [65]. It is well known that fluoride can improve the acid resistance of enamel and dentin by promoting the conversion of hydroxyapatite to fluoroapatite.
Sr released from S-PRG fillers may also enhance the acid resistance of teeth by converting hydroxyapatite to strontiumapatite [66,67]. In vitro studies have demonstrated that ions released from the S-PRG filler-containing adhesive [68] or endodontic sealer [69] can be taken up by the enamel and dentin adjacent to the material, and the corresponding areas showed decreased demineralization following acid exposure. Similar results have also been reported for other S-PRG filler-containing materials, including proprietary materials such as orthodontic adhesive [70], fissure sealants [71], coating material [72], resinous vanish [73] and denture base resin [74].
3.2. Antibacterial effects/anti-plaque effects
There is increasing interest in the biological functions of the multiple ions released from S-PRG fillers, including the inhibitory effects on bacterial viability or activity. It was found that the S-PRG filler-containing resins, compared with conventional composites, significantly reduced the growth of S. mutans on their surfaces (unpublished data from Imazato’s group). It was also reported that eluate from the S-PRG filler can suppress the adherence of S. mutans [75], and S-PRG filler-containing proprietary composites (Beautifil II) inhibited their adherence in the presence of saliva [76]. Further in vivo studies demonstrated that, after 8 h of intraoral exposure, a considerably lower quantity of dental plaque accumulated on the surface of Beautifil II [76]. Although the exact mechanism for the anti-plaque effects of the S-PRG filler is unknown, the release of multiple ions is believed to be related to this phenomenon.
Besides anti-plaque effects, S-PRG filler-containing proprietary resinous material inhibited bacteria-induced pH drop on the material surface, possibly due to the release of multiple ions [72]. Eluate from the S-PRG fillers exhibited inhibiting effects on the protease and gelatinase activities of Porphyromonas gingivalis. It also prevented the coaggregation between P. gingivalis and Fusobacterium nucleatum [75], indicating that these fillers may also be effective in combating periodontitis.
4. Addition of growth factors
Recently, in addition to conventional restorative treatments, resin adhesives have been attempted to be used for the adhesion of fractured roots, root-end filling, or sealing of perforations because they can provide a hermetic seal that prevents re-infections. In particular, several clinical studies reported the successful reconstruction of fractured roots with 4-META/MMA-based adhesive resin [77–79], which showed good bonding ability in a wet environment and high compatibility with osteoblasts or mesenchymal precursor cells [80,81]. However, none of the present adhesives available on the market promote tissue healing. Successful results cannot be expected when large bone defects exist adjacent to the sites that are repaired with adhesives.
To increase the success rate of these new treatment options and expand the use of resin adhesives, it is valuable to add the capacity to promote tissue regeneration. An effective, simple way to provide tissue regeneration abilities is to release growth factors from the adhesives. A number of studies on the local delivery of growth factors from dental implants to enhance osseointegration are available. For these studies, the use of fibroblast growth factor-2 (FGF-2) has been well documented. Utilization of polymer-based particle as a carrier to deliver FGF-2, such as poly(lactide-coglycolide) microspheres reported for titanium implant [82], may be applied for resin-based restoratives. Few trials have been reported regarding the delivery of growth factors from dental resins. Attempts to fabricate FGF-2-releasing adhesives using drug carrier polymers are currently under investigation.
5. Concluding remarks
The approaches to achieve therapeutic polymers described here have the potential to contribute to successful restorative treatments. However, several points remain to be clarified to make these new types of materials clinically available. Researchers must show quantitative evidence that they have achieved appropriate kinetic control such that the bioactive elements will provide real benefits under in vivo conditions. For example, it is important to prove that the bioactive components are delivered at a rate and concentration to exhibit therapeutic effects regardless of shifts in environmental condition such as ionic concentrations, pH, or temperature. There also have been concerns about the potential toxicity of newly synthesized compounds. While the biocompatibility of the QAC-based monomer MDPB incorporated into the commercially available adhesive has been well documented [83], information to warrant the safety of other new compounds for clinical use needs to be collected. Since much attention has been paid on toxic effects of nanoparticles including those of silver [84,85] rigorous investigations on the risk of adverse health effects by usage of nanoparticles are required. Obviously, clinical evidence can be a key for commercialization of new technologies in this category.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (Nos. 23390434 and 24659846) from the JSPS.
REFERENCES
- [1].Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. International Journal of Antimicrobial Agents. 2012;39(5):381–9. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- [2].Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RRB. Incorporation of bacterial inhibitor into resin composite. Journal of Dental Research. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- [3].Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RRB, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. Journal of Dental Research. 1997;76:768–72. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- [4].Imazato S, Torii Y, Takatsuka T, Inoue K, Ebi N, Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. Journal of Oral Rehabilitation. 2001;28:314–9. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- [5].Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dental Materials. 2006;22:527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- [6].Schmalz G, Ergücü Z, Hiller KA. Effect of dentin on the antibacterial activity of dentin bonding agents. Journal of Endodontics. 2004;30:352–8. doi: 10.1097/00004770-200405000-00011. [DOI] [PubMed] [Google Scholar]
- [7].Imazato S, Walls AWG, Kuramoto A, Ebisu S. Penetration of an antibacterial dentine-bonding system into demineralized human root dentine in vitro. European Journal of Oral Sciences. 2002;110:168–74. doi: 10.1034/j.1600-0722.2002.11221.x. [DOI] [PubMed] [Google Scholar]
- [8].Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Operative Dentistry. 2004;29:369–75. [PubMed] [Google Scholar]
- [9].Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentin primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- [10].Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- [11].Kuramoto A, Imazato S, Walls AWG, Ebisu S. Inhibition of progression of root caries by an antibacterial adhesive. Journal of Dental Research. 2005;84:89–93. doi: 10.1177/154405910508400116. [DOI] [PubMed] [Google Scholar]
- [12].Tziafas D, Koliniotou-Koumpia E, Tziafa C, Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. International Endodontic Journal. 2007;40:58–66. doi: 10.1111/j.1365-2591.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- [13].Xiao YH, Ma S, Chen JH, Chai ZG, Li F, Wang YJ. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90:813–7. doi: 10.1002/jbm.b.31350. [DOI] [PubMed] [Google Scholar]
- [14].Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. Anti-biofilm effect of dental adhesive with cationic monomer. Journal of Dental Research. 2009;88:372–6. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- [15].Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, et al. Antibacterial activity and cytotoxicity of two novel crosslinking antibacterial monomers on oral pathogens. Archives of Oral Biology. 2011;56:367–73. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- [16].Huang L, Sun X, Xiao YH, Dong Y, Tong ZC, Xing XD, et al. Antibacterial effect of a resin incorporating a novel polymerizable quaternary ammonium salt MAE-DB against Streptococcus mutans. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1353–8. doi: 10.1002/jbm.b.32703. [DOI] [PubMed] [Google Scholar]
- [17].Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He J, Söderling E, Vallittu PK, Lassila LV. Preparation and evaluation of dental resin with antibacterial and radio-opaque functions. International Journal of Molecular Sciences. 2013;14:5445–60. doi: 10.3390/ijms14035445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He J, Söderling E, Lassila LV, Vallittu PK. Incorporation of an antibacterial and radiopaque monomer into dental resin system. Dental Materials. 2012;28:e110–7. doi: 10.1016/j.dental.2012.04.026. [DOI] [PubMed] [Google Scholar]
- [20].Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- [21].Carrilho MRO, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of hybrid layer by chlorhexidine. Journal of Dental Research. 2007;68:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- [22].Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou J, Tan J, Yang X, Xu X, Li D, Chen L. MMP-inhibitory effect of chlorhexidine applied in a self-etching adhesive. Journal of Adhesive Dentistry. 2011;13:111–5. doi: 10.3290/j.jad.a18783. [DOI] [PubMed] [Google Scholar]
- [24].Yiu CK, Hiraishi N, Tay FR, King NM. Effect of chlorhexidine incorporation into dental adhesive resin on durability of resin–dentin bond. Journal of Adhesive Dentistry. 2012;14:355–62. doi: 10.3290/j.jad.a25674. [DOI] [PubMed] [Google Scholar]
- [25].Zhou J, Yang X, Chen L, Liu X, Ma L, Tan J. Pre-treatment of radicular dentin by self-etch primer containing chlorhexidine can improve fiber post bond durability. Dental Materials Journal. 2013;32:248–55. doi: 10.4012/dmj.2012-134. [DOI] [PubMed] [Google Scholar]
- [26].Tezvergil-Mutluay A, Mutluay MM, Gu LS, Zhang K, Agee KA, Carvalho RM, et al. The anti-MMP activity of benzalkonium chloride. Journal of Dentistry. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. Journal of Dental Research. 2005;84:355–69. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- [29].Nakajima M, Okuda M, Ogata M, Pereira PN, Tagami J, Pashley DH. The durability of a fluoride-releasing resin adhesive system to dentin. Operative Dentistry. 2003;28:186–92. [PubMed] [Google Scholar]
- [30].Hoskins JS, Karanfil T. Removal and sequestration of iodide using silver-impregnated activated carbon. Environmental Science and Technology. 2002;36:784–9. doi: 10.1021/es010972m. [DOI] [PubMed] [Google Scholar]
- [31].Damm C, Munsted H, Rosch A. Long-term antimicrobial polyamide 6/silver-nanocomposites. Journal of Materials Science. 2007;42:6067–73. [Google Scholar]
- [32].Kassaee MZ, Akhavan A, Sheikh N, Sodagar A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. Journal of Applied Polymer Science. 2008;110:1699–703. [Google Scholar]
- [33].Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, et al. In situ formation of silver nanoparticles in photocrosslinking polymers. Journal of Biomedical Materials Research Part B. 2011;97:124–31. doi: 10.1002/jbm.b.31793. [DOI] [PubMed] [Google Scholar]
- [34].Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dental Materials. 2013;29:450–61. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HH. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dental Materials. 2013;29:462–72. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:345–55. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, et al. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. Journal of Dentistry. 2013;41:464–74. doi: 10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang K, Li F, Imazato S, Cheng L, Liu H, Arola DD, et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013 doi: 10.1002/jbm.b.32898. http://dx.doi.org/10.1002/jbm.b.32898 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dental Materials. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- [42].Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. Journal of Dental Research. 1996;75:1679–86. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- [43].Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. Journal of Biomedical Materials Research. 2000;53:381–91. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [44].Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dental Materials. 2003;19:558–66. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- [45].Xu HH, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano DCPA-whisker composites with high strength and Ca and PO4 release. Journal of Dental Research. 2006;85:722–7. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu HH, Weir MD, Sun L, Takagi S, Chow LC. Effects of calcium phosphate nanoparticles on Ca-PO4 composite. Journal of Dental Research. 2007;86:378–83. doi: 10.1177/154405910708600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xu HH, Weir MD, Sun L. Nanocomposites with Ca-PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dental Materials. 2007;23:1482–91. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xu HH, Weir MD, Sun L, Ngai S, Takagi S, Chow LC. Effect of filler level and particle size on dental caries-inhibiting Ca-PO(4) composite. Journal of Materials Science Materials in Medicine. 2009;20:1771–9. doi: 10.1007/s10856-009-3740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. Strong nanocomposites with Ca, PO(4), and F release for caries inhibition. Journal of Dental Research. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Materials. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Moreau JL, Weir MD, Giuseppetti AA, Chow LC, Antonucci JM, Xu HH. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1264–73. doi: 10.1002/jbm.b.32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. Journal of Dental Research. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cheng L, Weir MD, Xu HH, Antonucci JM, Lin NJ, Lin-Gibson S, et al. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1378–86. doi: 10.1002/jbm.b.32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.03.011. http://dx.doi.org/10.1016/j.jdent.2013.03.011 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013;101:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dental Materials. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wilson AD, Kent BE. A new translucent cement for dentistry. British Dental Journal. 1972;132:133–5. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- [59].Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dental Materials Journal. 2008;27:315–39. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- [60].Han L, Edward CV, Li M, Niwano K, Neamat AB, Okamoto A, et al. Effect of fluoride mouth rinse on fluoride releasing and recharging from aesthetic dental materials. Dental Materials Journal. 2002;21:285–95. doi: 10.4012/dmj.21.285. [DOI] [PubMed] [Google Scholar]
- [61].Fujimoto Y, Iwasa M, Murayama R, Miyazaki M, Nagafuji A, Nakatsuka T. Detection of ions released from S-PRG fillers and their modulation effect. Dental Materials Journal. 2010;29:392–7. doi: 10.4012/dmj.2010-015. [DOI] [PubMed] [Google Scholar]
- [62].Itota T, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release and recharge in giomer, compomer and resin composite. Dental Materials. 2004;20:789–95. doi: 10.1016/j.dental.2003.11.009. [DOI] [PubMed] [Google Scholar]
- [63].Itota T, Al-Naimi OT, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release from aged resin composites containing fluoridated glass filler. Dental Materials. 2005;21:1033–8. doi: 10.1016/j.dental.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [64].Shimazu K, Ogata K, Karibe H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dental Materials Journal. 2011;30:923–7. doi: 10.4012/dmj.2011-124. [DOI] [PubMed] [Google Scholar]
- [65].Ito S, Iijima M, Hashimoto M, Tsukamoto N, Mizoguchi I, Saito T. Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. Journal of Dentistry. 2011;39:72–9. doi: 10.1016/j.jdent.2010.10.011. [DOI] [PubMed] [Google Scholar]
- [66].Featherstone JDB, Shields CP, Khademazad B, Oldershaw MD. Acid reactivity of carbonated apatite with strontium and fluoride substitutions. Journal of Dental Research. 1983;62:1049–53. doi: 10.1177/00220345830620100801. [DOI] [PubMed] [Google Scholar]
- [67].Dedhiya MG, Young F, Higuchi WI. Mechanism for the retardation of the acid dissolution rate of hydroxyapatite by strontium. Journal of Dental Research. 1973;52:1097–109. doi: 10.1177/00220345730520051901. [DOI] [PubMed] [Google Scholar]
- [68].Han L, Okamoto A, Fukushima M, Okiji T. Evaluation of a new fluoride-releasing one-step adhesive. Dental Materials Journal. 2006;25:509–15. doi: 10.4012/dmj.25.509. [DOI] [PubMed] [Google Scholar]
- [69].Han L, Okiji T. Evaluation of the ions release/incorporation of the prototype S-PRG filler-containing endodontic sealer. Dental Materials Journal. 2011;30:898–903. doi: 10.4012/dmj.2011-101. [DOI] [PubMed] [Google Scholar]
- [70].Horiuchi S, Kaneko K, Mori H, Kawakami E, Tsukahara T, Yamamoto K, et al. Enamel bonding of self-etching and phosphoric acid-etching orthodontic adhesives in simulating clinical conditions: debonding force and enamel surface. Dental Materials Journal. 2009;28:419–25. doi: 10.4012/dmj.28.419. [DOI] [PubMed] [Google Scholar]
- [71].Shimazu K, Ogata K, Karibe H. Caries-preventive effect of fissure sealant containing surface reaction-type pre-reacted glass ionomer filler and bonded by self-etching primer. Journal of Clinical Pediatric Dentistry. 2012;36:343–7. doi: 10.17796/jcpd.36.4.n444r730r773un53. [DOI] [PubMed] [Google Scholar]
- [72].Ma S, Imazato S, Chen JH, Mayanagi G, Takahashi N, Ishimoto T, et al. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dental Materials Journal. 2012;31:909–15. doi: 10.4012/dmj.2012-061. [DOI] [PubMed] [Google Scholar]
- [73].Shiiya T, Mukai Y, Tomiyama K, Teranaka T. Anti-demineralization effect of a novel fluoride-releasing varnish on dentin. American Journal of Dentistry. 2012;25:347–50. [PubMed] [Google Scholar]
- [74].Mukai Y, Kamijo K, Fujino F, Hirata Y, Teranaka T, ten Cate JM. Effect of denture base-resin with prereacted glass-ionomer filler on dentin demineralization. European Journal of Oral Sciences. 2009;117:750–4. doi: 10.1111/j.1600-0722.2009.00678.x. [DOI] [PubMed] [Google Scholar]
- [75].Yoneda M, Suzuki N, Masuo Y, Fujimoto A, Iha K, Yamada K, et al. Effect of S-PRG eluate on biofilm formation and enzyme activity of oral bacteria. International Journal of Dentistry. 2012;2012:814913. doi: 10.1155/2012/814913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saku S, Kotake H, Scougall-Vilchis RJ, Ohashi S, Hotta M, Horiuchi S, et al. Antibacterial activity of composite resin with glass-ionomer filler particles. Dental Materials Journal. 2010;29:193–8. doi: 10.4012/dmj.2009-050. [DOI] [PubMed] [Google Scholar]
- [77].Sugaya T, Kawanami M, Noguchi H, Kato H, Masaka N. Periodontal healing after bonding treatment of vertical root fracture. Dental Traumatology. 2001;17:174–9. doi: 10.1034/j.1600-9657.2001.170407.x. [DOI] [PubMed] [Google Scholar]
- [78].Hayashi M, Kinomoto Y, Takeshige F, Ebisu S. Prognosis of intentional replantation of vertically fractured roots reconstructed with dentin-bonded resin. Journal of Endodontics. 2004;30:145–8. doi: 10.1097/00004770-200403000-00005. [DOI] [PubMed] [Google Scholar]
- [79].Unver S, Onay EO, Ungor M. Intentional re-plantation of a vertically fractured tooth repaired with an adhesive resin. International Endodontic Journal. 2011;44:1069–78. doi: 10.1111/j.1365-2591.2011.01922.x. [DOI] [PubMed] [Google Scholar]
- [80].Imazato S, Horikawa D, Ogata K, Kinomoto Y, Ebisu S. Responses of MC3T3-E1 cells to three dental resin-based restorative materials. Journal of Biomedical Materials Research Part A. 2006;76A:765–72. doi: 10.1002/jbm.a.30422. [DOI] [PubMed] [Google Scholar]
- [81].Imazato S, Horikawa D, Takeda K, Kiba W, Izutani N, Yoshikawa R, et al. Proliferation and differentiation potential of pluripotent mesenchymal precursor C2C12 cells on resin-based restorative materials. Dental Materials Journal. 2010;29:341–6. doi: 10.4012/dmj.2009-082. [DOI] [PubMed] [Google Scholar]
- [82].Zou GK, Song YL, Zhou W, Yu M, Liang LH, Sun DC, et al. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. 2012;114:284–9. doi: 10.1016/j.tripleo.2011.07.006. [DOI] [PubMed] [Google Scholar]
- [83].Imazato S, Chen J-H, Ma S, Izutani N, Li F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Japanese Dental Science Review. 2012;48:115–25. [Google Scholar]
- [84].AshaRani PV, Mun GLK, Hande MP, Valiyavettil S. Cytotoxicity and genotoxicity of sliver nanoparticles in human cells. ACS Nano. 2009;3:279–90. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- [85].Gil PR, Oberdorster G, Elder A, Puntes V, Parak WJ. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano. 2010;4:5527–31. doi: 10.1021/nn1025687. [DOI] [PubMed] [Google Scholar]