Abstract
On-demand postsynaptic synthesis and release of endocannabinoid lipids and subsequent binding to presynaptic CB1 receptors (CB1Rs) mediates short and long-term depression (LTD) of excitatory transmission in many brain regions. However, mechanisms involved in the synthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG) by diacylglycerol lipase α (DGLα) are poorly understood. Since Gq-coupled receptor activation can stimulate production of a major DGL substrate 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG) by PLCβ, we sought to determine if 2-AG biosynthesis was limited only by a lack of substrate availability, or if other pathways, such as Ca2+ signaling, also need to be simultaneously engaged. To address this question, we loaded medium spiny neurons of the dorsolateral striatum with SAG while monitoring excitatory synaptic inputs. SAG-loading had no significant effect on evoked excitatory synaptic currents when cells were voltage-clamped at −80 mV. However, depolarization of MSNs to −50 mV revealed a SAG-loading dependent decrease in the amplitude of excitatory currents that was accompanied by an increase in paired pulse ratio, consistent with decreased glutamate release. Both effects of loading SAG at −50 mV were blocked by chelation of postsynaptic Ca2+ using BAPTA or by bath application of tetrahydrolipstatin (THL), a DGL inhibitor. Loading of SAG into glutamatergic pyramidal neurons of the amygdala similarly inhibited excitatory synaptic inputs and increased the PPR. SAG-induced depression was absent in both regions from mice lacking CB1Rs. These data show that increasing substrate availability alone is insufficient to drive 2-AG mobilization and that DGL-dependent synaptic depression via CB1R activation requires postsynaptic Ca2+ signals.
Keywords: endocannabinoids, diacylglycerol lipase, 2-arachidonoylglycerol
Introduction
Endocannabinoids are important retrograde modulators of both excitatory and inhibitory synapses throughout the brain (Gerdeman et al., 2002; Hashimotodani et al., 2007; Kreitzer and Regehr, 2001; Robbe et al., 2001; Stella et al., 1997). Accordingly, multiple behavioral studies have demonstrated the importance of endocannabinoid signaling in a variety of physiological functions (Fernandez-Ruiz and Gonzales, 2005; Marsicano et al., 2002; Orio et al., 2009; Patel et al., 2009; Varvel and Lichtman, 2002). On-demand, postsynaptic synthesis and release of endocannabinoids can induce short (Kreitzer and Regehr, 2001; Shonesy et al., 2013) or long-term depression of transmitter release (Gerdeman et al., 2002; Lerner et al., 2010; Lerner and Kreitzer, 2012) through activation of presynaptic type 1 cannabinoid receptors (CB1Rs) (El Manira and Kyriakatos, 2010; Heifets and Castillo, 2009).
There remains a significant gap in our understanding of how endocannabinoid synthesis is regulated at a molecular level. The most well studied eCBs in the brain are anandamide (AEA) (Devane and Axelrod, 1994) and 2-arachidonylglycerol (2-AG) (Sugiura et al., 1995). 2-AG is the most abundant endocannabinoid in the CNS (Bisogno et al., 1999) and striatum (Ade and Lovinger, 2007), and is thought to be synthesized from diacylglycerol predominantly by sn1-diacylglycerol lipase-α (DGLα) (Gao et al., 2010; Tanimura et al., 2010; Yoshino et al., 2011).
Identifying the rate-limiting step(s) and determining the mechanisms underlying activity-dependent 2-AG synthesis is essential to understanding the role of endocannabinoids in modulating synaptic function and behavior. 2-AG-dependent synaptic depression can be initiated by either Ca2+ influx (Kreitzer and Regehr, 2002; Wilson and Nicoll, 2001), or by the activation of Gq/11-protein-coupled receptors, most prominently Group I metabotropic glutamate receptors (mGluR1/5) (Maejima et al., 2001; Varma et al., 2001). Canonically, mGluR1/5 receptors activate phospholipase C β (PLCβ) to generate diacylglycerol from membrane phosphoinositides, thereby enhancing substrate supply to DGLα, and mobilizing intracellular Ca2+ stores. Previous studies support the importance of PLCβ in mGluR-dependent 2-AG signaling (Hashimotodani et al., 2005), but it is unclear whether additional mechanisms are required to stimulate 2-AG synthesis following mGluR activation. Specifically, PLCβ-mediated 2-AG release can be blocked by calcium chelators (Hashimotodani et al., 2005), suggesting that both calcium and mGluR activation are required. Based on the similar Ca2+-dependence of PLCβ and 2-AG release and the fact that Ca2+ is known to bind PLCβ, Ca2+ has been suggested to drive 2-AG synthesis by enhancing PLCβ activity. However, chelating calcium does not block mGluR-dependent striatal 2-AG LTD (Lerner and Kreitzer, 2012). Similarly, other studies have identified Gq-coupled receptor dependent forms of 2-AG release that are Ca2+-independent (Kim et al., 2002). Thus, there remains some controversy as to 1) whether or not Ca2+ is required for Gq-driven 2-AG release, and 2) if required, whether it is required for the activity of PLCβ or DGLα, or both enzymes.
To better understand mechanisms underlying synaptic 2-AG signaling in situ, we optimized a method to detect synaptic effects of loading diacylglycerol into neurons in acutely isolated brain slices. We show that at synaptic inputs to striatal medium spiny neurons (MSN) and basolateral amygdala (BLA) pyramidal neurons, diacylglycerolinduced synaptic depression requires a postsynaptic Ca2+ signal in the form of subthreshold membrane depolarization. Since this approach bypasses Gq-coupled receptor signaling and PLC activity, these data suggest a novel model in which increased substrate availability and Ca2+-dependent activation of DGLα are required for receptor-driven endocannabinoid release.
Methods
Animals
C57BL/6J WT animals used in SAG loading experiments were generated by crossing WT mice bred in-house. A previously characterized CB1R knockout mouse line on an ICR strain background (generously provided by Dr. C.J. Hillard, Medical Collge of Wisconsin) (Sanhueza et al., 2011) was maintained by crossing homozygous knockout animals. Control WT ICR mice were also bred in house. All mice were housed on a 12 hr light-dark cycle with food and water ad libitum. Experiments were performed at postnatal days 30–40, and were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Brain Slice Preparation
Mice were allowed to rest for a minimum of 1 h in sound- and light-attenuating chambers prior to sacrifice. Mice were then anesthetized using isoflurane and moved to a separate room for decapitation and slicing. Brains were hemisected, and coronal slices (300 µM) were made using a Leica VT1000S vibratome (Leica Microsystems) in oxygenated (95% v/v O2, 5% v/v CO2), high-sucrose, low-Na+ ACSF (in mM: 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, 26 NaHCO3) maintained at 1–4 °C.
Whole-Cell Recordings
Slices were allowed to recover for at least 1 hour in oxygenated (95% v/v O2, 5% v/v CO2) ACSF (in mM: 124 NaCl, 2.5 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, 26 NaHCO3) at 28°C. Following this incubation period, slices were moved to the recording chamber, where they were continuously perfused (2 ml/min) with oxygenated ACSF (28°C) containing picrotoxin (50 µM). Patch electrodes (2–4 MΩ) were pulled with a Flaming-Brown puller (Sutter Instruments) and were filled with pipette solution consisting of (in mM): 120 CsMeSO3, 5 NaCl, 10 TEA-Cl, 10 HEPES, 5 QX-314, 1.1 EGTA, 4 Mg-ATP, and 0.3 Na-GTP, with pH set at 7.2 with CsOH.
MSNs were voltage clamped at −50 mV or −80 mV. To evoke EPSCs, paired stimuli (200 ms duration; 50 ms interpulse interval) were delivered at 0.05 Hz through a bipolar platinum/irridium electrode (FHC) placed in the overlying white matter. Stimulus intensity (10–40 µA) was adjusted to evoke EPSC amplitudes of 200–400 pA. We chose to load SAG at a concentration of 200 µM in the patch pipette solution, after initial pilot studies found that loading 50 or 100 µM SAG at −50 mV had little effect. In previous reports, the intracellular application of 50–100 µ M 2-AG or anandamide was necessary for synaptic depression in striatal MSNs (Adermark and Lovinger, 2007b; Gerdeman et al., 2002; Ronesi et al., 2004). A cyclodextrin was also included in the solution to improve lipid solubility. In all cells, recordings were initiated immediately upon rupturing the membrane to gain whole-cell access. Series and input resistances were monitored throughout the experiment and recordings were discarded if series resistance changed by >20%. All cells demonstrated an initial increase in EPSC amplitude that stabilized by ~2 min after membrane rupture. Importantly, this ~1.5 fold increase was not significantly different between any of the experimental groups studied here (data not shown). Therefore, for each cell the baseline current (100%) was set as the average of 7 responses collected from 2 to 4 min after membrane rupture. For clarity, average responses of cells in each group are graphed with time=0 being set to 2 min after break-in when responses were stabilized and the baseline recording period was initiated. Experimental groups were compared to each other using a 2-way repeated measures ANOVA with Sidak’s post-hoc testing of individual time points, as indicated in figure legends. The effect of treatment in each cell was also determined as the average of 7 normalized response amplitudes collected 18 to 20 min following break-in, which was then compared to the theoretical value of 100% (no change) using a one-sample t-test. Results from one-sample t-test are reported in the figure legends. The paired-pulse ratio (PPR) for each cell over the baseline and post-treatment time periods indicated above was calculated by averaging the amplitudes of the first and second eEPSC, and then dividing the average of the second pulse by the average of the first. The means of baseline and post-treatment PPRs across all cells in each condition were then compared by a paired Student’s t-test. Potential effects of treatments on the kinetics of synaptic responses were compared based on averages of the 10%–90% rise and 90%–10% decay times during the baseline and post-treatment periods.
1-stearoyl-2-arachidonoyl-sn-glycerol (SAG; Cayman Chemical) was stored in 100% acetonitrile stocks at 100 mM and was diluted into the pipette solution so that the final concentrations of SAG and acetonitrile were 200 µM and 0.2% (v/v) respectively. (2-Hydroxypropyl)-β-cyclodextrin (0.006% w/v; Sigma-Aldrich) was included in the pipette solution to increase the solubility of the lipids. The SAG Vehicle control experiments included the same concentrations of acetonitrile and β-cyclodextrans in the pipette solution.
Results
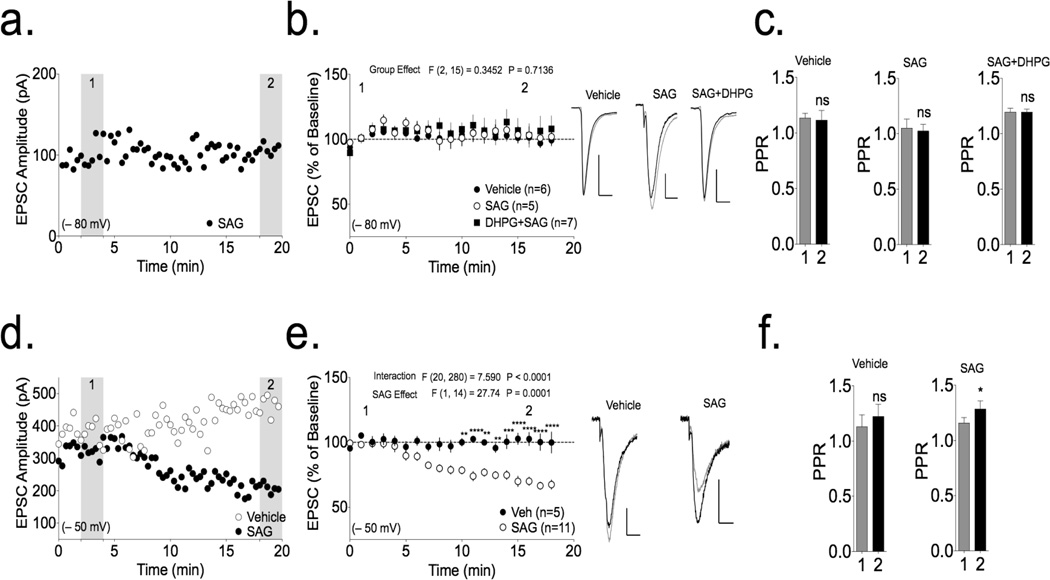
While it is clear that 2-AG mobilization can be triggered through either Ca2+ or mGluR signaling, a full understanding of the mechanism underlying these pathways has remained elusive. One possible scenario is that DGL is active under basal conditions, and that 2-AG synthesis is initiated by increasing substrate availability, presumably through PLC-mediated cleavage of phosphatidylinositol-4,5-bisphosphate (PIP2) to generate diacylglycerols. If enhanced substrate delivery alone is sufficient for 2-AG mobilization, one would predict that loading diacylglycerol into the postsynaptic neuron would enhance 2-AG synthesis to act as a retrograde neurotransmitter and inhibit presynaptic glutamate release, depressing synaptic transmission. Therefore, we included 1-steroyl-2-arachidonoylglycerol (SAG; 200 µM), a preferred substrate for DGL (Bisogno et al., 2003; Jung et al., 2007), in the pipette solution during patch clamp recordings from medium spiny neurons (MSNs) of the dorsolateral striatum held at resting membrane potential (approx. −80 mV (Cepeda et al., 2008)). However, we failed to detect significant changes in the amplitude of evoked excitatory postsynaptic currents (eEPSCs; Fig. 1a,b; p>0.05 by one-sample t-test). Similarly, the ratio of current amplitudes evoked by each pair of stimuli (the paired pulse ratio; PPR) was not consistently altered during the 20 min recording period (Fig. 1c). Moreover, SAG loaded cells were not significantly different from vehicle loaded cells (Fig. 1b p>0.05 by 2-way RM ANOVA). Combining intracellular SAG application with activation of group I mGluR by DHPG (10 µM) at −80 mV also failed to induce depression or a change in PPR (Fig. 1b,c p>0.05 by one-sample t-test). This concentration of DHPG is able to augment 2-AG mobilization when combined with brief postsynaptic depolarization (Shonesy et al., 2013). These data suggest that SAG is not converted to an active synaptic pool of 2-AG under these basal conditions and that the main role of mGluR activation is to stimulate production of DAG.
Figure 1. Depression of excitatory transmission to striatal MSNs by postsynaptic loading of the 2-AG precursor SAG.
(a) EPSC amplitudes are plotted from a representative individual cell loaded with SAG. The shaded regions labeled as 1 and 2 correspond to the baseline and post-treatment periods respectively. (b) Compilation of data from cells loaded with Vehicle (100±9% baseline), SAG (102±6% baseline) and SAG loaded in the presence of DHPG (10 µM; 132±19% baseline) at −80 mV showing no depression in any group (p>0.05 by one-sample t-test comparing to 100%). There is no significant difference between groups as determined by 2-way repeated measures ANOVA. Representative traces averaged over the 1st and 2nd periods shown in the shaded regions of panel (a) are shown (1: black trace; 2: gray trace, scale bars 100 pA, 10 ms). (c) SAG loading did not affect PPR in MSNs voltage-clamped at −80 mV (p>0.05 by paired t-test). (d) EPSC amplitudes are plotted from representative cells loaded with SAG or vehicle at −50 mV. (e) Compilation of data from cells loaded with SAG at −50 mV (SAG) showing a progressive and significant depression of EPSC amplitudes (68±3% baseline; p<0.0001 by one-sample t-test), whereas vehicle-loaded cells were unaffected (102±6% baseline). Analysis using a 2-way repeated measures ANOVA revealed a significant interaction between SAG and Vehicle loaded cells with Sidak’s Posthoc showing significantly different normalized amplitudes as indicated (** p<0.01, *** p<0.001, **** p<0.0001). (f) SAG loading significantly increased PPR at −50 mV (* p<0.05 by paired t-test).
Previous work showed that eCB synthesis/release can be stimulated by depolarization, and depolarization can be essential or sufficient for some forms of endocannabinoid-dependent synaptic depression (Bisogno et al., 1997; Jung et al., 2005; Stella et al., 1997),(Adermark and Lovinger, 2007a; Kreitzer and Malenka, 2005). Indeed, the loading of SAG to MSNs held at a sub-threshold depolarizing membrane potential (−50 mV) induced a progressive depression of eEPSC amplitude (Fig. 1d,e; p<0.0001 by one-sample t-test), whereas eEPSCs were stable in vehicle-loaded cells under the same conditions. This SAG-induced depression of eEPSC amplitude at −50 mV was accompanied by a significant increase in the paired pulse ratio (PPR; p<0.05, by paired t-test) that was not evident in vehicle-loaded cells (Fig. 1f). However, the kinetics of synaptic responses were unaffected by SAG loading at −50 mV, as measured by the average rise (10–90%) and decay (90–10%) times in the baseline and post-treatment periods (data not shown). In combination, these data show that synaptic depression induced by loading SAG was dependent on postsynaptic membrane depolarization and suggest that this was due to presynaptic inhibition of glutamate release.
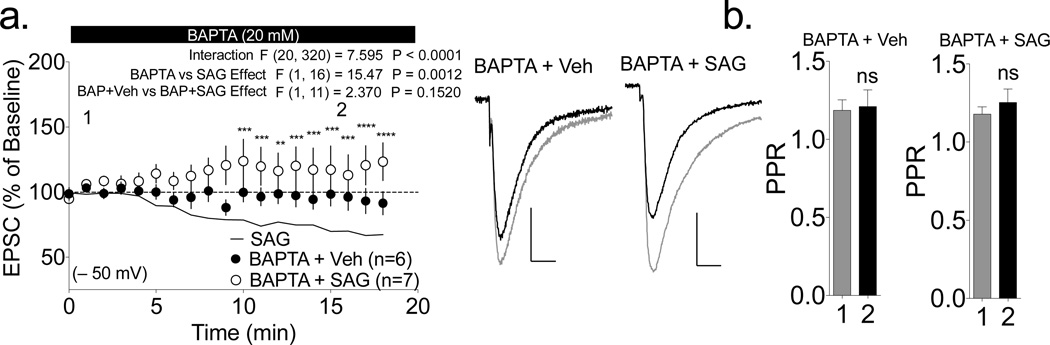
To test if SAG-induced depression at −50 mV was dependent on Ca2+, we loaded the postsynaptic neuron with SAG and BAPTA to chelate Ca2+. Cells co-loaded with BAPTA and SAG exhibited a trend for a slight increase in the final eEPSC amplitudes that was not significantly different from eEPSC amplitudes in cells loaded with BAPTA and vehicle (Fig. 2a), but that was significantly different from the reduced eEPSCs observed in cells loaded with SAG alone (p<0.001 by 2-way RM ANOVA). Moreover, we failed to detect the SAG-induced increase in PPR in cells co-loaded with BAPTA (Fig. 2b). Thus, the synaptic depression induced by loading SAG at −50 mV was Ca2+-dependent.
Figure 2. SAG-induced depression is dependent on Ca2+.
(a) SAG-induced depression at −50 mV was blocked by simultaneous loading of BAPTA (SAG+BAPTA) A 2-way repeated measures ANOVA revealed significant interaction and a significant difference between SAG alone and SAG+BAPTA. Individual SAG and SAG+BAPTA time points were compared using Sidak’s post-hoc analysis; ** p<0.01, ***p<0.001, ***p<0.0001). Neither BAPTA+SAG (113±15% baseline) nor BAPTA alone (94±9% baseline) showed a significant change from baseline (P>0.05 by one-sample ttest) and were not significantly different from each other (p>0.05 by 2-way repeated measures ANOVA). Representative traces for each condition (1: black trace, 2: gray line) are shown to the right (scale bars 100 pA, 10 ms). (b) The SAG-induced increase in PPR was also blocked by co-loading BAPTA (p>0.05 by paired t-test).
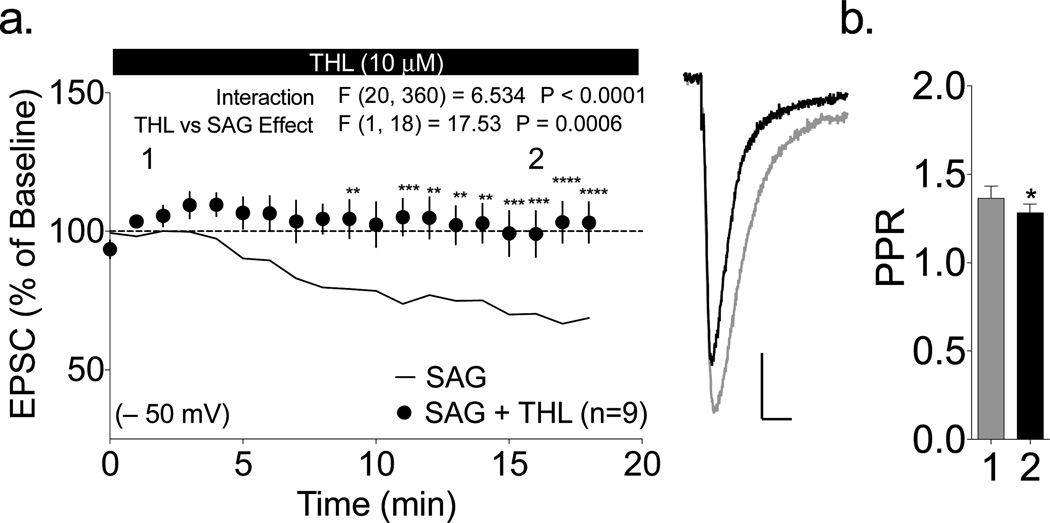
The production of striatal 2-AG is largely dependent on DGLα activity (Tanimura et al., 2010). To determine whether DGL activity is required for SAG-induced synaptic depression at −50 mV, we investigated the effect of pharmacological inhibition of DGL by bath application of tetrahydrolipstatin (THL; 10 µM). THL prevented both SAG-induced depression (Fig. 3a; p>0.05 by one-sample t-test), and the increase in PPR (Fig. 3b; in fact, SAG loading in the presence of THL slightly, but significantly, reduced the PPR (p<0.05 by paired t-test). These data suggest that DGL activity is required for SAG-induced depression.
Figure 3. SAG-depression is dependent on the 2-AG synthetic enzyme DGL.
(a) The effect of SAG at −50 mV was blocked by bath application of the DGL inhibitor THL (SAG+THL, 101±8% baseline; p>0.05 by one-sample t-test). The 2-way repeated measures ANOVA showed significant interaction between SAG alone and SAG+THL, and a significant effect of THL. Sidak’s post hoc testing revealed the indicated significant differences between individual time; ** p<0.01, ***p<0.001, ***p<0.0001). Representative traces for each condition (1: black trace, 2: gray line) are shown to the right (scale bars 100 pA, 10 ms). (b) SAG loading in the presence of THL significantly decreased, rather than increased, PPR (* p<0.05 by paired t-test).
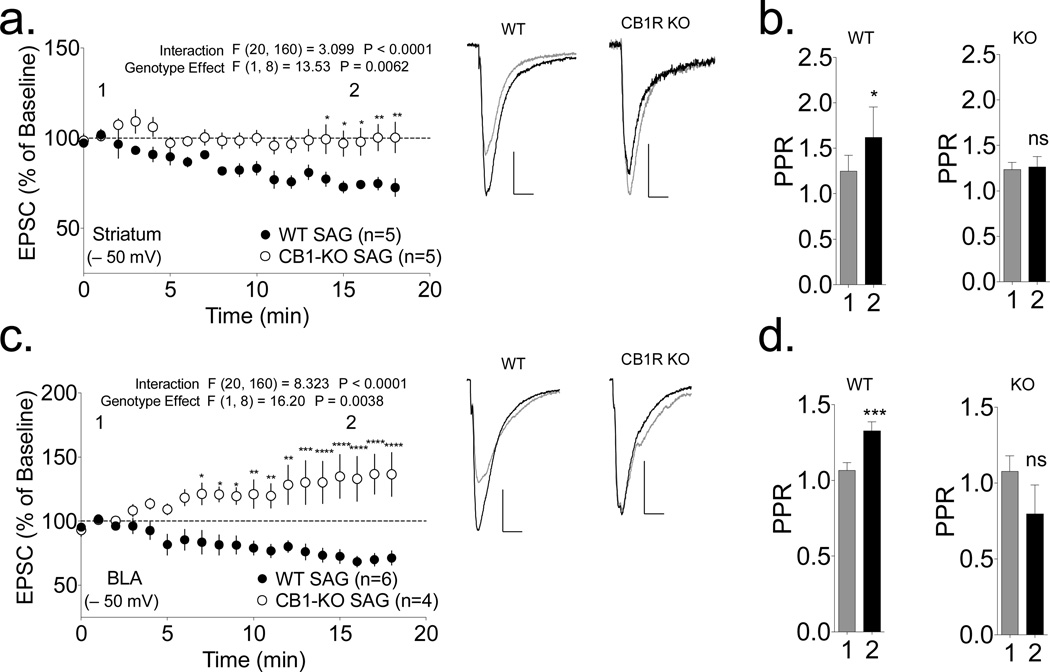
Endocannabinoid-dependent synaptic depression is caused by the activation of presynaptic CB1Rs (Gerdeman and Lovinger, 2001; Gerdeman et al., 2002). To determine the role of CB1Rs in SAG-induced depression and the changes in PPR, we investigated the effects of loading SAG into striatal MSNs from CB1R-KO mice, which were maintained on a different genetic background. In MSNs from WT ICR mice, the loading of SAG at −50 mV induced significant synaptic depression (Fig. 4a; p<0.001 by one-sample t-test) and a significant increase in PPR (Fig. 4b; p<0.05 by paired t-test). These effects were not significantly different from those of SAG in MSNs from WT C57BL/6J mice (p>0.05 by 2-way RM ANOVA). However, SAG loading at −50 mV had no effect on MSNs from CB1R-KO mice (Fig. 4a; p>0.05 by one-sample t-test). Thus, the depression induced by loading SAG into postsynaptic MSNs at −50 mV requires presynaptic CB1Rs.
Figure 4. SAG loading initiates CB1R-dependent depression of synaptic transmission in both the striatum and basolateral amygdala.
Intracellular loading of SAG at −50 mV depressed EPSCs in both (a) striatal MSNs (74±3% baseline; p<0.001 by one-sample t-test) and (c) basolateral amygdala (BLA) pyramidal neurons (70±4% baseline; p<0.001 by one-sample t-test) in WT ICR mice, but failed to induce depression in MSNs from CB1R-KO mice (striatum: 100±8% baseline; BLA: 134±17% baseline; p>0.05 by one-sample t-test for both). The 2-way repeated measures ANOVA revealed a significant genotype×treatment interaction and a significant genotype effect as indicated. Sidak’s post hoc testing revealed the indicated significant differences between individual time points; ** p<0.01, ***p<0.001, ***p<0.0001). PPR was significantly increased by loading SAG at −50 mV in both striatal MSNs (b) and BLA neurons (d) of WT ICR mice, but not in neurons from CB1R-KO mice on the same background (* p<0.05, *** p<0.001 by paired t-test). Representative traces for each condition (1: black trace, 2: gray line) are shown to the right (scale bars 100 pA, 10 ms).
In order to determine whether similar mechanisms are involved in synaptic 2-AG signaling in other types of neuron, we examined the effects of loading SAG into pyramidal neurons in the basolateral amygdala (BLA) of WT and CB1R-KO mice. Loading of SAG at −50 mV induced a significant decrease in evoked EPSCs in BLA neurons (Fig. 4c; p<0.001 by one-sample t-test), accompanied by a significant increase in PPR (Fig. 4d; p<0.001 by paired t-test). However, SAG loading had no significant effects in BLA neurons from CB1R-KO mice (p>0.05 by one-sample t-test for depression or paired t-test for PPR). Thus, 2-AG signaling at excitatory synapses appears to be induced by similar mechanisms in GABAergic MSNs in the striatum and glutamatergic pyramidal neurons in the BLA.
Discussion
The striatum contains substantial basal levels of 2-AG. However, a major fraction of the basal amount of 2-AG is likely within a general metabolic pool, rather than an active synaptic signaling pool (Buczynski and Parsons, 2010; Wettschureck et al., 2007). Therefore, measurement of bulk tissue 2-AG turnover may provide little insight into the activity-dependent regulation of synaptic pools of this endocannabinoid. Furthermore, this approach cannot distinguish between 2-AG signaling at excitatory versus inhibitory synapses. Here, we report a novel electrophysiological approach that allows us to directly investigate the regulation of DGL-dependent synaptic 2-AG signaling.
The active synaptic signaling pool of endocannabinoids is generally considered to be synthesized “on demand” in response to restricted patterns of synaptic stimulation. While DGLα is thought to be the predominant enzyme for activity-dependent 2-AG synthesis, pharmacological and gene knockout studies have implicated an array of upstream signaling mechanisms in the control of 2-AG signaling. There is considerable evidence that group 1 mGluR and L-type calcium channel (LTCC) activation are both necessary and/or sufficient to initiate 2-AG mobilization depending on the cell type and conditions (Lerner and Kreitzer, 2012; Puente et al., 2011). PLCβ activation increases not only the production of the diacylglycerol substrate for DGL, but also the release of Ca2+ from intracellular stores. Moreover, its been reported that both PLCβ and DGLα are Ca2+-sensitive (Bisogno et al., 2003; Stella et al., 1997). Thus, mGluR1/5 activation alone could increase the supply of diacylglycerol in addition to activating downstream signaling to enhance DGLα activity. Significantly, although PLCβ-1 and -4 have been shown to be required for mGluR1/5-stimulated 2-AG release (Hashimotodani et al., 2005; Maejima et al., 2005), the synaptic roles of isoforms such as PLCδ, which can be stimulated by Ca2+ alone (Rebecchi and Pentyala, 2000), has not been tested. Thus, Ca2+ signals initiated by activation of LTCCs could also have dual effects both to increase the supply of diacylglycerol substrate and to enhance DGLα activity.
The approach reported here clearly differentiates whether Ca2+ is required for PLC activation versus DGL activation in that synaptic 2-AG signaling cannot be initiated by increasing the supply of diacylglycerol substrate alone or by the increase of Ca2+ caused by depolarization to −50 mV alone. Postsynaptic loading of the 2-AG precursor SAG into striatal MSNs had no effect at resting membrane potential (−80 mV), showing that the availability of diacylglycerols is not rate limiting for 2-AG synthesis under these “basal” conditions. Furthermore, activation of group I mGluRs was unable to facilitate the SAG-induced depression at −80 mV, further highlighting the need for Ca2+ influx rather than mobilization of intracellular Ca2+ stores. These findings strongly suggest that the major role for mGluR signaling in mGluR-assisted Ca2+-induced 2-AG mobilization is to stimulate the PLC-mediated production of DAG. However, our studies are unable to test the role of mGluR activation in pure-mGluR forms of 2-AG release, which typically require afferent stimulation protocols or high concentrations of mGluR agonists. More robust mGluR activation may be able to stimulate Ca2+ or other DGL activating signaling pathways, the elucidation of which should be the focus of further investigation. Moreover, evoked EPSCs were stable when MSNs were held at a subthreshold depolarizing potential (−50 mV) intended to mimic striatal upstate transitions that occur in vivo (Wilson and Kawaguchi, 1996), enhancing Ca2+ influx into MSN dendritic spines (Carter and Sabatini, 2004). Significantly, we show here that the combination of subtheshold depolarization to −50 mV with loading SAG induced a DGL- and CB1R-dependent suppression synaptic transmission. Although we cannot exclude the possibility that synaptic 2-AG signaling cannot be activated by depolarization alone due to wash-out of SAG or other cellular factors during these whole-cell recordings, our findings that the effect of SAG loading was blocked by intracellular calcium chelation strongly indicate that both increased substrate supply and Ca2+ influx are essential for synaptic depression under these conditions.
Postsynaptic Ca2+ might function at several levels to initiate synaptic 2-AG signaling, including direct effects on DGLα and/or PLCβ (see above). Since our experimental paradigm bypasses PLCβ activity, the requirement for calcium clearly indicates an interaction between calcium and other non-PLCβ targets in the generation of synaptic 2-AG. Although the most obvious interpretation is that the Ca2+ signal is required for direct activation of DGLα, some findings argue against this possibility. Ca2+ was reported to activate DGLα in a membrane fraction of HEK293 cells (Bisogno et al., 2003), but we could not detect Ca2+-dependent activation of 2-AG synthesis in a striatal membrane fraction (unpublished observations) and there is no evidence to support direct binding of Ca2+ to DGLα. In fact, our recent findings identified a Ca2+-dependent mechanism to restrain synaptic 2-AG signaling mediated by CaMKII inhibition of DGLα (Shonesy et al., 2013). Thus, a molecular mechanism to explain DGLα activation by Ca2+ remains elusive. Importantly, our data also do not exclude as of yet unrecognized Ca2+-dependent mechanisms for stimulating synaptic 2-AG signaling. While the current study mainly focused on the striatum, we were able to replicate the key finding that SAG loading causes a CB1-dependent synaptic depression in pyramidal neurons of the BLA. This suggests that some common mechanisms may exist between different types of neurons and brain circuits. Future studies should be aimed at understanding similarities and differences between DGL regulatory pathways across different brain regions.
In summary, it is becoming increasingly clear that a variety of mechanisms are involved in the synthesis and release of 2-AG. These mechanisms may be variably engaged in response to different stimuli to achieve different outcomes, including both short- and long-term synaptic depression. Now that many of the enzymes involved in 2-AG metabolism have been identified, identifying the full network of the DGLα signalosome and how it functions in the CNS should be the focus of future studies. The methodology reported here may provide a useful approach to address these issues.
Highlights.
Neurons were loaded with a diacylglycerol precursor of 2-AG, a major endocannabinoid.
This caused retrograde depression of excitatory inputs at −50 mV but not −80 mV.
Synaptic depression at −50 mV was prevented by chelating postsynaptic calcium.
Synaptic depression required diacylglycerol lipase (DGL) activity and CB1 receptors.
Acknowledgments
Supported by grants from NIH (T32-NS07491 and T32-MH065215 to BCS; K08-MH090412 and R21-MH103515 to SP; R01-NS078291 to RJC), and the Luton Society (S.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007a;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc Natl Acad Sci U S A. 2007b;104:20564–20569. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322(Pt 2):671–677. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol. 2010;160:423–442. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994;91:6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A. The role of endocannabinoid signaling in motor control. Physiology (Bethesda) 2010;25:230–238. doi: 10.1152/physiol.00007.2010. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Gonzales S. Cannabinoid control of motor function at the basal ganglia. Handb Exp Pharmacol. 2005:479–507. doi: 10.1007/3-540-26573-2_16. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to Parkinsonian motor deficits. Neuron. 2012;73:347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Wang X, Rose KL, Ramikie TS, Cavener VS, Rentz T, Baucum AJ, 2nd, Jalan-Sakrikar N, Mackie K, Winder DG, Patel S, Colbran RJ. CaMKII regulates diacylglycerol lipase-alpha and striatal endocannabinoid signaling. Nat Neurosci. 2013;16:456–463. doi: 10.1038/nn.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+ -sensing receptor. Mol Endocrinol. 2007;21:274–280. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, Gonzalez-Burgos G. Postsynaptic diacylglycerol lipase alpha mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011 doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]