Abstract
Patients with mucopolysaccharidoses (MPS) have accumulation of glycosaminoglycans in multiple tissues which may cause coarse facial features, mental retardation, recurrent ear and nose infections, inguinal and umbilical hernias, hepatosplenomegaly, and skeletal deformities. Clinical features related to bone lesions may include marked short stature, cervical stenosis, pectus carinatum, small lungs, joint rigidity (but laxity for MPS IV), kyphoscoliosis, lumbar gibbus, and genu valgum. Patients with MPS are often wheelchair-bound and physical handicaps increase with age as a result of progressive skeletal dysplasia, abnormal joint mobility, and osteoarthritis, leading to 1) stenosis of the upper cervical region, 2) restrictive small lung, 3) hip dysplasia, 4) restriction of joint movement, and 5) surgical complications. Patients often need multiple orthopedic procedures including cervical decompression and fusion, carpal tunnel release, hip reconstruction and replacement, and femoral or tibial osteotomy through their lifetime. Current measures to intervene in bone disease progression are not perfect and palliative, and improved therapies are urgently required.
Enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), and gene therapy are available or in development for some types of MPS. Delivery of sufficient enzyme to bone, especially avascular cartilage, to prevent or ameliorate the devastating skeletal dysplasias remains an unmet challenge. The use of an anti-inflammatory drug is also under clinical study. Therapies should start at a very early stage prior to irreversible bone lesion, and damage since the severity of skeletal dysplasia is associated with level of activity during daily life.
This review illustrates a current overview of therapies and their impact for bone lesions in MPS including ERT, HSCT, gene therapy, and anti-inflammatory drugs.
Keywords: mucopolysaccharidoses, skeletal dysplasia, enzyme replacement therapy, gene therapy, hematopoietic stem cell transplantation, anti-inflammatory drug
1. Introduction
Mucopolysaccharidoses (MPSs) are a group of lysosomal storage disorders (LSDs) caused by deficiency of a specific lysosomal enzyme, consisting of seven subtypes. In MPSs, the breakdown of the glycosaminoglycans (GAGs), chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS) and/or hyaluronan is disrupted. Accumulation of undegraded GAG(s) is observed in multiple tissues, leading to broad clinical manifestations including mental retardation, skeletal dysplasia, corneal clouding, abnormal facies, coarse hair, hernia, hepatosplenomegaly, respiratory and heart valvular diseases, and abnormal joint mobility. For instance, diagnosis of Hurler syndrome, a severe form of MPS I, is commonly made between 4 and 18 months of age; a combination of skeletal deformities, recurrent ear and nose infections, inguinal and umbilical hernias, coarse facial features, hepatosplenomegaly, and enlarged tongue first prompt medical attention. Clinically, patients with MPS develop a characteristic dysostosis multiplex due to progressive storage of GAGs, especially, CS, DS and/or KS [1].
Patients with MPS appear normal at birth although some types of MPS show excessive growth during the first 2-4 years of age [2,3]. MPS varies from severe systemic bone dysplasia to a lesser form of the disease that includes mild bone involvement, depending upon MPS type and clinical phenotype. MPS patients with skeletal dysplasia (dysostosis multiplex) have deformity of the spine (lumbar gibbus, kyphoscoliosis), deformity of the chest (pectus carinatum, flaring of the rib cage), abnormal joint mobility, abnormal gait, short trunked dwarfism, and/or genu valgum (Fig. 1). Patients may require a series of orthopedic surgeries (cervical decompression and fusion, femoral or tibial osteotomy, hip reconstruction and replacement etc.) throughout their lifetime. These procedures are complicated by anesthetic risks due to airway narrowing, elevated resistance to airflow, and potential pulmonary compromise [4-6].
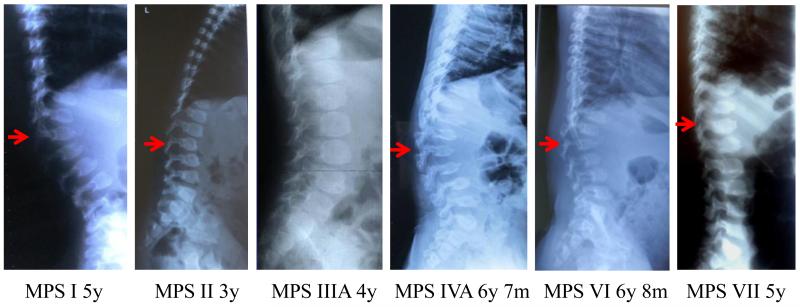
Figure 1.
Dorsolumbar spine X-ray pictures of patients with MPS (arrows show the apex of the kyphosis).
MPS I (severe): A high lumbar kyphosis is seen at L2. The apical ovoid vertebral body has a prominent anteroinferior beaking with hypoplasia of the anterosuperior aspect. The prominent posterior scalloping of the lumbar vertebrae is observed. Hypoplasia of the superior facets is seen. Similar features are seen to a less extent in the levels above and below this vertebra. As a result of these skeletal anomalies, the patient has retrolisthesis between the vertebrae at the apex of the kyphosis.
MPS II (severe): The inferior beaking of ovoid vertebrae at lumbar distinguishes the abnormal condition from MPS IVA. The mild posterior scalloping of the lumbar vertebrae with widening of interpediculate spaces is observed. MPS II is radiologically similar to MPS I (Hurler syndrome); however, the bone deformity is mild with a slower rate of progression.
MPS III (severe): MPS III shows the mildest skeletal deformity among all types of MPS. Ovoid vertebrae at lumbar are seen with mild widening of interpediculate spaces. The bone deformity is mildest with a slower rate of progression among all types of MPS.
MPS IVA (severe): MPS IVA shows the most severe skeletal deformity among all types of MPS. Prominent lumbar kyphosis is seen at L2. Universal platyspondyly shows a central anterior beaking in contrast to the inferior, anterior beaking seen in MPS I (Hurler syndrome). Marked increase of interpediculate spaces (most severe), multiple vertebral subluxations (most severe), and small sacrum are seen. Bone mineral density is low.
MPS VI (severe): Prominent kyphosis is seen at L2. Universal platyspondyly shows a central anterior beak in contrast to the inferior, anterior beaking seen in MPS I (Hurler syndrome). The moderate posterior scalloping of the lumbar vertebrae is observed. Hypoplastic lumbar vertebrae (L1-L5) with characteristic superior notch (L4 and L5), multiple vertebral subluxations, marked increase of interpediculate spaces, and small sacrum are seen. Bone mineral density is low.
MPS VII (severe): Kyphosis is seen at L1. Moderate ovoid vertebrae at lumbar distinguish the abnormal condition from MPS IVA. The lumbar vertebrae with widening of interpediculate spaces are observed. Universal platyspondyly of dorsolumbar vertebrae is observed with a mild central anterior beaking. The patient with MPS VII here shows radiologically less severity compared with MPS IVA.
Supportive measures are often provided. For joint pain, patients are given non-steroidal anti-inflammatory drugs (NSAID), and antibiotics are prescribed for upper respiratory infections. Patients are given anti-inflammatory drugs, steroids, mechanical ventilation, and/or oxygen to treat pulmonary issues. Surgical procedures are often required, including: adenoidectomy, tonsillectomy, cervical decompression and fusion, corrective hip surgery for dysplasia, and knee surgery for genu valgum deformity. Since patients with some types of MPS have extradural collections of GAGs that cause spinal stenosis and odontoid hypoplasia, that in turn lead to atlantoaxial subluxation, physicians recommend cervical spine fusion/decompression surgery. The severity of skeletal dysplasia has a marked impact on activity of daily living (ADL) in patients with MPS.
Currently, several treatments such as enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), and gene therapy are being evaluated. ERT and HSCT are clinically available to prevent or treat the progression of MPS, and gene therapy is under clinical trials for some types of MPS. Substrate reduction therapy (SRT) is also being developed. ERT is approved for use in patients with mucopolysaccharidosis I (MPS I) [7], MPS II [8-9], MPS IVA [10], and MPS VI [11-14]. Patients treated with ERT show clinical improvement of somatic manifestations and improved quality of life (QOL). However, there are several limitations with current ERT: i) limited effect on skeletal symptoms [15-16] ii) rapid clearance from the circulation, and iii) immunological issues (antibody production leads to reduced therapeutic efficacy) [7,17-19]. To resolve the above issues, a long circulating or bone-targeting enzyme was devised to deliver the enzyme to bone [20,21]. The earlier that ERT is performed in animal models and human patients, the better the outcome [22].
HSCT of MPS patients improves their QOL, but the therapeutic effect on bone lesions remains limited [23]. The musculoskeletal manifestations still deteriorate and impact the QOL in most transplanted patients with MPSs [24]. One possibility is due to the limited penetration of the expressed enzyme into musculoskeletal tissues [25]. Although substantial clinical improvements of joint mobility, coarse facial features, and claw hands were reported following transplantation [26], clinical and radiographic musculoskeletal abnormalities still developed. Another possibility is that irreversible bone damage has already occurred prior to the time of the transplant (median age, 16 months). A retrospective analysis demonstrated superior long-term clinical outcome for patients with MPS I when HSCT was performed early in life [27]. HSCT shows some benefits in physical activity and bone mineral density of treated mice, and early interventions provides more benefits [32-34]. ERT and HSCT provide a comparable impact on growth in patients with MPS II [35].
Experimental gene therapies have been tested in animal models and human subjects [28-29]. However, current viral vectors for gene therapy have not delivered enzyme to bone efficiently and targeting the viral vector to bone remains a challenge. Gene therapy using bone-targeting adeno-associated virus (AAV) vectors has been experimentally evaluated in MPS mouse models [4,30,31].
Chronic osteoarthritis is observed in some MPS patients, affecting major joints such as hip, knee, wrist and ankle. To suppress metabolic inflammation caused by GAG accumulation, inhibition of secondary inflammatory processes by anti-inflammatory (or immunosuppressive) agents has been considered. The effect of anti-inflammatory drugs was assessed in MPS VI rats. Early treatment in the presymptomatic period inhibited the elevation of TNF-α, RANKL and other inflammatory factors in the blood, articular chondrocytes and synovial fibroblasts [36]. However, there was no impact on bone growth or mobility since stored GAGs still remained in chondrocytes of the growth plate. The efficacy of ERT alone and combined treatment using ERT and anti-inflammatory drug was also tested [37]. An anti-inflammatory treatment should be evaluated alone or in combined therapy with ERT, HSCT, or future gene therapy for MPSs [38,39].
In this review, we describe potential therapies for bone lesions in MPS, which to date include ERT, HSCT, gene therapy, and anti-inflammatory drugs. These approved and experimental treatments provide a better QOL by ameliorating the underlying disease progression of MPS, leading to prevention of further damage of targeted organs and reduction of risks associated with additional surgical procedures.
2. Therapies for bone
2.1. Initial pathological changes in bone
Most MPS patients have a nearly normal skeletal development at birth, although a few already show signs of skeletal dysplasia such as a gibbus deformity [40,41]. There is postmortem evidence of accumulation of GAGs in articular and epiphyseal cartilage in the fetus or at birth for some forms of MPS. Therefore, the first months of life represent the best window of opportunity for preventing bone deformities in MPS children.
Most skeletal manifestations are progressive and irreversible unless treated before signs and symptoms appear. Recent development of newborn screening programs for patients with MPSs may offer an opportunity to begin therapy in the first weeks of life [42-45].
Prenatal lysosomal GAG storage in chondrocytes has been demonstrated in MPS patients and animal models. Initial clinical signs and symptoms for skeletal dysplasia in newborn patients with MPSs include sacral dimple, gibbus, and abnormal shape of vertebrae in X-ray images [46]. In human, fetuses aged 18-30 weeks gestation (MPS I, II, III, and IVA) have storage vacuoles in chondrocytes as well as other major organs [47,48]. Newborn mice with MPS I, II, IVA, or VII, have storage vacuoles in chondrocytes [49,50]. Among all types of mouse models with MPS, the MPS VII murine model is the most severe [51]. Skeletal abnormalities represent the earliest clinical observations in MPS VII mice. Histological analysis of the growth plate, articular cartilage and cortical bone show early pathology and progressive bone lesion (Fig. 2).
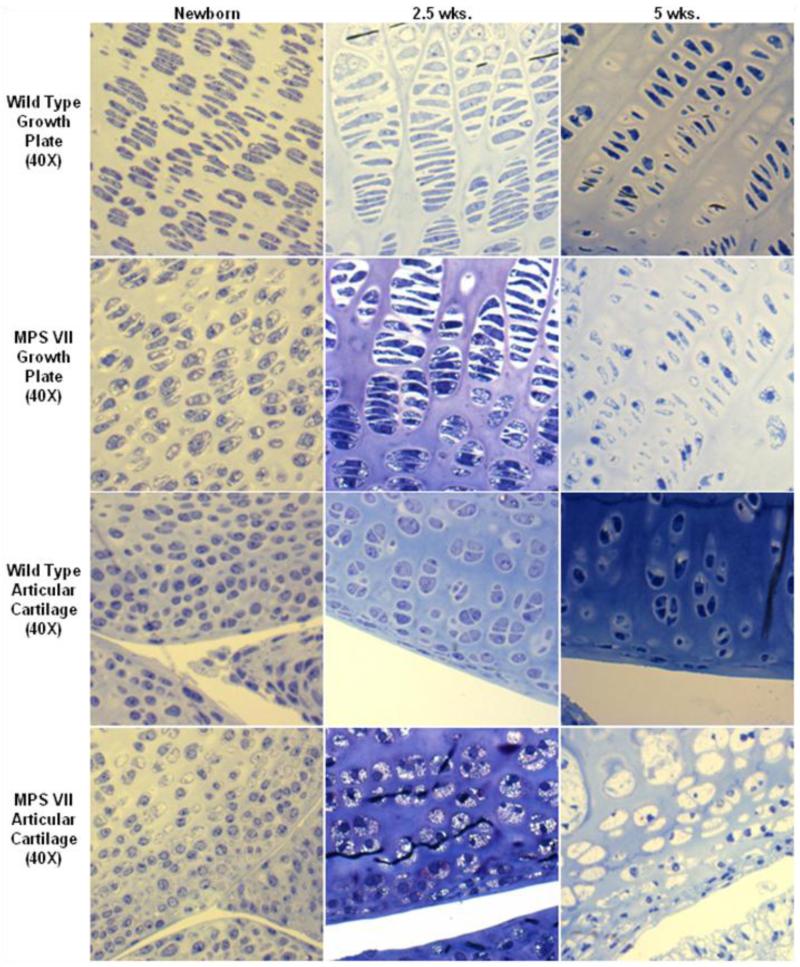
Figure 2.
Age-dependent change of storage vacuoles at an early stage in cartilage of MPS VII mouse model. Left panel: 2-3 days old, middle panel: 2.5 weeks old, right panel: 5 weeks old. Clear vacuoles are observed in most chondrocytes at birth. Vacuoles in chondrocytes increase in number with age and storage materials are fully accumulated at the age of 5 weeks old. Vacuolization and disorganization of the column structure in articular cartilage is more progressive than seen in epiphyseal cartilage (toluidine blue-stained 0.5-μm-thick sections: X 40).
2.1.1. Articular Cartilage
The knee joints of MPS VII mice show noticeable lysosomal storage within the articular cartilage, even at birth. Most articular chondrocytes contain accumulated vacuoles, although the cartilage structure is organized (Fig. 2). Affected mice show marked lysosomal storage within the articular cartilage by 2.5 weeks of age. By 5 weeks of age, the articular cartilage layers (tangential, transitional and radial layers) are abnormally thickened. Chondrocytes are increased in number and ballooned with vacuoles although all three cartilage layers were still distinguishable and organized. Ten-weeks-old affected mice show abnormal proliferation of the meniscal fibro-cartilage with ballooned vacuolated cells. The articular cartilage layers are slightly irregular and hypercellular, and chondrocytes are enlarged and vacuolated. The three layers are thinner compared with those seen at 5 weeks, and their structure is disorganized. The articular cartilage layers at 32 weeks of age show more disorganization, with almost complete loss of the normal arrangement of cells. The surface of articular cartilage is irregular, and few chondrocytes are observed in the tangential layer. The transitional and radial layers show hypercellularity compared with those in the age-matched wild-type mice. There are articular-meniscal-synovial fusions with marked abnormal proliferation of articular, and meniscal cartilage with thickened and vacuolated cells in the meniscus and synovium. The synovial space is markedly diminished. All articular cartilage cells show marked distention, producing a thicker layer. The cells in the periosteum also have marked vacuolar distension.
2.1.2. Epiphyseal cartilage
The growth plate region in 1- or 2-day-old MPS VII mice already has ballooned vacuolated chondrocytes in the resting and proliferative zones. By 2.5 weeks of age, the growth plate is thickened but shows normal resting and proliferative zonal organization (Fig. 2). The cells are swollen with increased fibrillary or vacuolar contents, which are especially prominent in the resting zone. The hypertrophic zone, although hypercellular, shows disorganization with a distorted arrangement of cells. The primary calcification zone is also increased in size. The longitudinal arrangement of the primary trabeculae is abnormal with the trabeculae increased in number and thickness and includes a marked increase of cartilage. Osteoblasts appear to be increased in number, especially in the proximal intertrabecular spaces, and contain numerous vacuoles. At 5 weeks of age, the chondrocytes in all zones are markedly vacuolated and ballooned. At 10 weeks of age, the growth plates are thicker, and their boundaries become irregular. The column structure through all layers of the growth plate is disorganized. The chondrocytes are ballooned with vacuoles. The osteoblasts surrounding diaphyseal bone trabeculae and the cells lining bone marrow sinusoids contain a large amount of clear cytoplasmic vacuoles (data not shown).
At 32 weeks of age, the column structure through all layers of the growth plate is markedly disorganized, and all chondrocytes are prominently ballooned with vacuoles (Fig. 2). The growth plates have a marked decrease in the number of cells in the proliferating zone. The storage is marked, with lysosomal distention in osteoblasts lining the cortical and trabecular bone and in the sinus-lining cells in the bone marrow. Light microscopic views reveal a loss of the parallel order of the bone matrix with loss of the concentric arrangement of lamellae or haversian system formation. The cortex is markedly thickened in affected mice. The osteocytes show clearly increased cytoplasmic volumes filled with vacuoles.
Thus, by 5 weeks of age all chondrocytes in the MPS VII mouse model are vacuolated markedly, leading to characteristic skeletal features.
Other MPS animal models with a severe skeletal form, such as the MPS VI cat and MPS VII dog, have a similar pathology.
Overall, substantial storage materials have already accumulated in chondrocytes at a prenatal (or fetus) period in patients or animal models with a severe form of MPS and fully vacuolated chondrocytes are established at an early stage of the disease.
2.2. Enzyme replacement therapy (ERT)
2.2.1. Conventional and newborn ERT
Native lysosomal enzymes contain mannose-6-phosphate (M6P) residues on their oligosaccharide chains that bind to the M6P receptor which in turn delivers them to lysosomes. By this process, ERT can deliver the deficient enzyme to lysosomes where it can catabolize accumulated GAGs [52,53]. Most infused native enzymes are delivered to the visceral organs such as liver, kidney, and spleen. Lysosomal enzymes have a short half-life in the circulation due to rapid binding to carbohydrate-recognizing receptors such as M6P and uptake into these visceral organs. Only a small fraction of the enzyme is able to reach the bone, and even less enzyme reaches avascular cartilage. Consequently improvement of established bone lesions in patients with MPS I, MPS II, and MPS VI is restricted, even after long-term treatment. An ERT preclinical trial in adult MPS mice resulted in a marked reduction of storage material in the visceral organs but provided a limited effect in hyaline and fibrous cartilage cells in the femur, ligaments, and synovium. The column structure of the growth plate region remained disorganized [54]. While it is well known that adult ERT provides a limited pathological and clinical impact in animal models with several types of MPS, newborn ERT in animal models with MPS are more promising.
Several studies of newborn ERT have been performed in large animal models of MPS I and VI. ERT was started at birth and continued for more than a year in dogs with MPS I [55]. At the end of the study, skeletal abnormalities were reduced in the low dose group of treated dogs and were nearly completely prevented in the high-dose group of treated dogs. Similar results were obtained for MPS VI cats in which ERT was initiated at birth and continued on weekly treatment for 5, 6 or 11 months [56]. Treated cats had improved bone quality, density, and dimensions. Treated cats were heavier than untreated cats and had substantially reduced or no spinal cord compression. Skeletal pathology was improved with normalized bone dimensions, uniform bone density, and regular trabecular pattern visible on radiographs by 5 to 6 months of age. However, no reduction in lysosomal vacuolation was observed in cartilage cells [57].
Newborn ERT for MPS I mice improves visceral organ and brain development [58]; but bone and joints showed only partial or no benefit. The avascular bone and joint were difficult to correct, despite early treatment.
When ERT was started at birth for MPS VII mice, vacuolated epiphyseal and articular cartilage cells remained refractory to therapy. However, more clinical improvements in bone were observed [59-61], including less short femurs and tibias, normal sized nose, and less thickening of cortical bone.
In newborn ERT of MPS IVA mice, clearance of storage in bone was also limited by the avascularity of the growth plate, and the chondrocytes were still vacuolated although the column structure was organized [22].
ERT started at birth for MPS I mice did not show any marked benefits for bone and joint development [58]. These observations suggest that conventional ERT alone, even when started at birth, cannot completely prevent bone pathology in MPS mice.
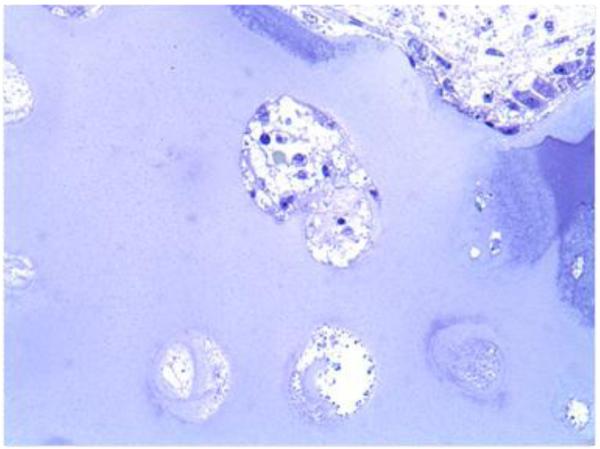
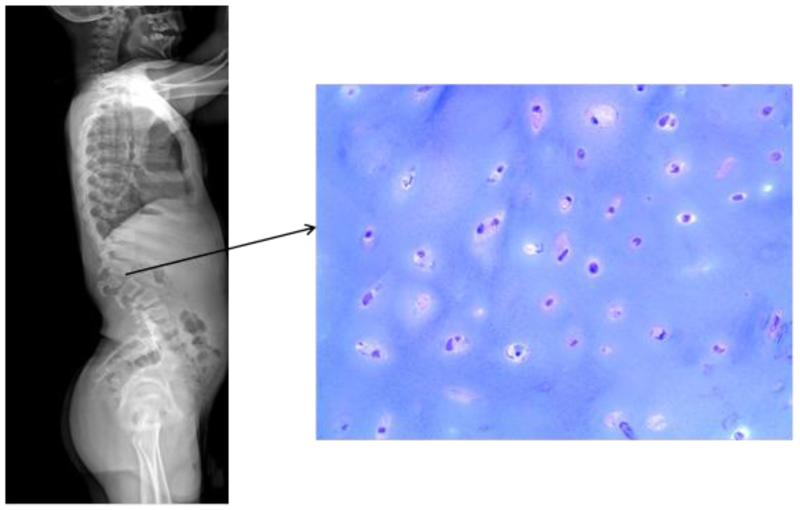
ERT in dogs with MPS I showed some improvement in skeletal disease [55], while studies in patients with MPS suggest that conventional or even newborn ERT is only able to slow the progression of the symptoms [62]. For instance, bone pathology in a patient with MPS IVA, who underwent ERT for over one year, showed that all chondrocytes in iliac crest from surgical remnants were fully vacuolated (Fig. 3). Studies of early ERT on human patients with MPS show the similar findings as those in animal models, demonstrating the benefits of early treatment in patients with MPS; however, skeletal dysplasia continues to progress even if slowly.
Figure 3.
Bone pathology of iliac crest in a 17-year-old patient with MPS IV after clinical trial and 6 months extension study of ERT (toluidine blue-stained 0.5-μm-thick sections: X 100).
In MPS VI patients treated with early ERT (6 weeks to one year), skeletal abnormalities continued to progress although facial dysmorphism and growth were stabilized or improved, and radiographic changes were milder when compared to untreated patients. Early initiation of ERT in MPS VI patients prevents or slows the progression of some skeletal manifestations, but a complete reversal of skeletal pathology has not been observed. Aggregated data from patients who had initiated ERT when less than 5 years of age showed that over 50% of patients were below the 3rd percentile for height, although one girl was above the 97th percentile. Comparing baseline height prior to initiation of ERT to height at the end of the study, 37.5% patients remained on the same percentile growth curve, 9.4% improved to a percentile curve above and 53% fell below their original percentile curve, suggesting that growth is not normalized by ERT [63-65].
Recently more cases of MPS patients treated by ERT at early stages of disease have been reported. A pre-symptomatic boy with attenuated MPS I was started on ERT at 5 months of age. After 5 years of treatment, the boy displayed no signs of coarse facies, joint disease, organomegaly, cardiac valve disease, or dysostosis multiplex [66].
For MPS II, a sibling case report was published in which an affected boy was treated with ERT since 3 months of age [67]. After 3 years of treatment, the only somatic sign of the disease was a mild deformity of one vertebra. No other disease features were found. A more traditional sibling case study was recently published for MPS II, with similar findings. Two Japanese brothers with MPS II caused by a complex rearrangement between the IDS gene and the IDS-2 pseudogene were followed [68]. The older brother began treatment with idursulfase at 3.0 years of age, while the younger sibling started treatment at 4 months of age. At the start of treatment, the older brother showed typical somatic features of MPS II, including skeletal dysplasia with gibbus deformity, joint stiffness, coarse facies, short stature as well as cognitive impairment. After 32 months of ERT (age 3.0 years), the younger brother remained free from most of the somatic features that had already appeared in his brother at the same age. Skeletal manifestations still included mild dysostosis multiplex with slow progression at the age of 5 years (personal communication; Dr. Tajima).
Overall, current conventional ERTs that target enzymes to carbohydrate-recognizing receptors do not function efficiently on established bone and cartilage lesions. The receptor-mediated ERT strategy has been used with substantial success to treat storage in visceral organs in MPS mouse models; however, GAG storage in bone (cartilage) has been resistant to clearance by ERT using conventional doses of enzyme. Newborn or early ERTs prove a better resolution in bone morphology and clearance of storage materials [60-61] although vacuolated materials are still observed in chondrocytes. Discrepancy of therapeutic effect of newborn ERT among species may be related to kinetics and biodistribution of the enzyme [58].
2.2.2. Long circulating ERT
A chemically modified β-glucuronidase (GUS), treated to make it resistant to clearance from circulation by mannose and M6P receptors (PerT-GUS), showed prolonged circulation (half-life over 18 hours) compared with native enzyme (half-life less than 30 min) in an MPS VII mouse model. Long circulating enzyme provided more therapeutic efficacy than the native enzyme at clearing storage from cortical and hippocampal neurons. Higher levels of the enzyme in other tissues suggested improved delivery to other organs as well [69]. The mechanism, by which PerT-GUS enzyme escapes uptake by the mannose and M6P receptors, relies on chemical inactivation of its terminal sugars by treatment with sodium metaperiodate followed by borohydride reduction.
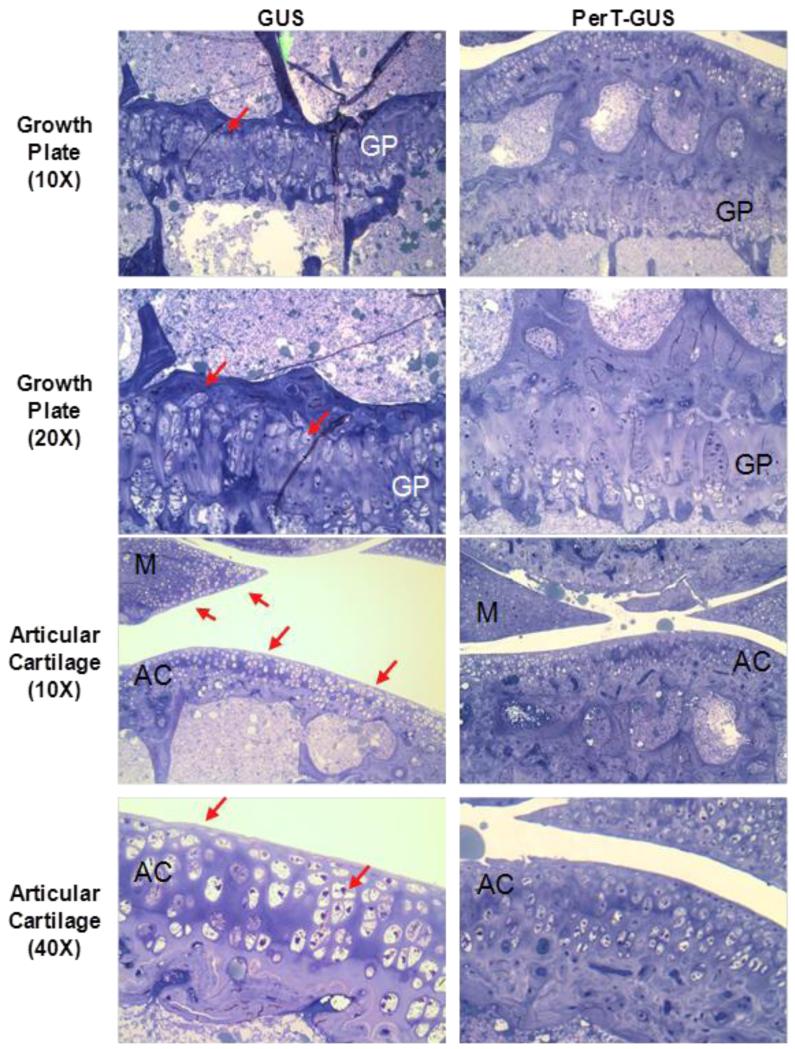
MPS VII mice treated with PerT-GUS showed marked improvements in bone lesions of legs, ribs, and spine of treated mice [21]. Quantitative histopathological assay also showed moderate improvements in GAG storage and morphology of articular and epiphyseal chondrocytes (Figs. 4 and 5). These findings indicate that the PerT-GUS therapy from birth may significantly reduce disability caused by bone dysplasia in MPS, in addition to addressing CNS storage.
Figure 4.
Histopathology of the knee joint of 17 weeks-old IV GUS and PerT-GUS treated MPS VII mice (ERT started at 5 weeks old). Images are of the growth plate and articular cartilage. PerT-GUS treated mouse shows substantial reduced number of vacuolated chondrocytes compared with native GUS treated mouse. Arrows show vacuolated cells in the growth plate, articular cartilage and meniscus area. AC: articular cartilage, GP: growth plate, M: meniscus. Toluidine blue-stained 0.5-μm-thick sections.
Adapted from Rowan DJ, Tomatsu S, Grubb JH, et al. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol Genet Metab 2012 107(1-2):161-72.
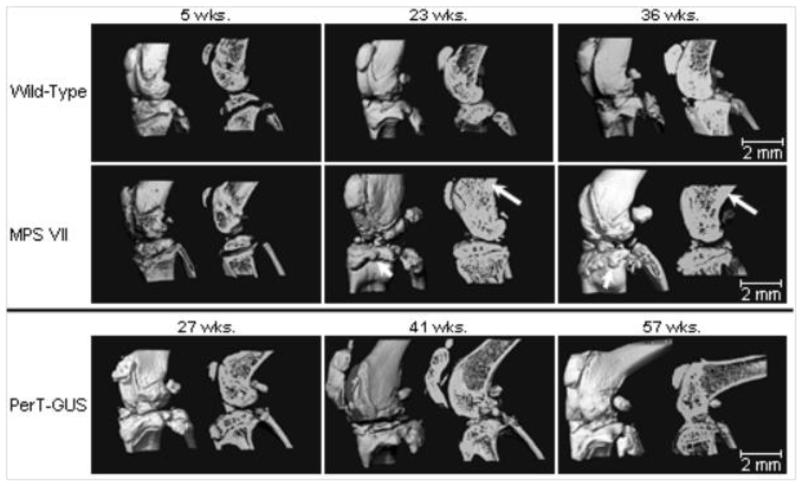
Figure 5.
Three-dimensional micro-CT reconstructions of knee joints of wild-type, untreated MPS VII, and PerT-GUS treated MPS VII mice intraperitoneally (IP) (IP 2 mg/kg ERT started at day 2-3 and continued weekly until the autopsy). Each picture shows unsectioned bone (left side) or sagittal-sectioned bone (right side). Cross sections are sagittal through the midlines. The long arrows identify areas of thickened cortical bone. The short arrows identify abnormal exophytic bone formations on articular surfaces. Ages of wild-type and untreated MPS VII mice are 5, 23, and 36 weeks old. Ages of 2 mg/kg PerT-GUS treated mice are 27, 41, and 57 weeks-old.
Adapted from Rowan DJ, Tomatsu S, Grubb JH, et al. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol Genet Metab 2012 107(1-2):161-72.
The mechanism by which Pert-GUS is taken up by the cells remains unanswered. One possibility is that long-circulating enzyme is slowly delivered to targeted cells by non-specific fluid phase pinocytosis involving no requirements for cell surface binding. The second possibility is that Pert-GUS may bind glucuronide residues of heparan sulfate on the cell surface and be gradually taken up with the internalized membrane during membrane recycling. The third mechanism by which PerT-GUS might correct bone pathology indirectly is by correcting storage in visceral macrophages that elaborate inflammatory cytokines like TNF-α. In support of this explanation, an antibody to TNF-α and an anti-inflammatory drug (pentosan polysulfate) could substantially reduce the skeletal pathology of rats with MPS VI [36,39].
2.2.3. Targeting ERT
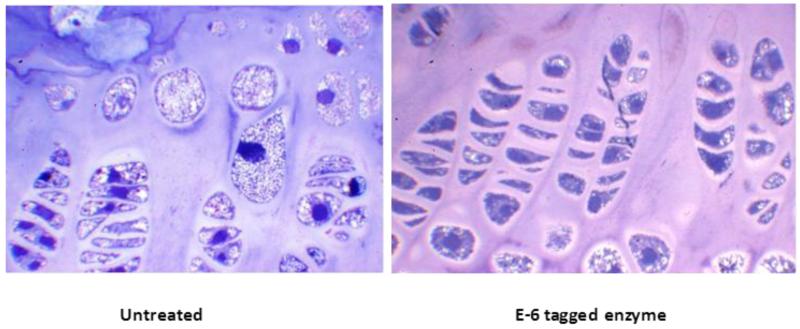
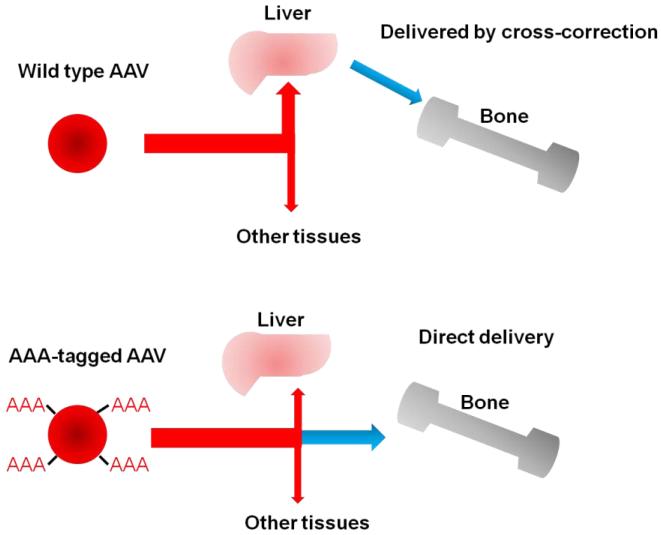
An alternative approach was developed to combine ERT with a bone-targeting strategy. Hydroxyapatite (HA) [Ca10(PO4)6(OH)2] is a positively-charged, major inorganic component of a hard tissue (bone) that is absent in soft tissues. Some bone matrix proteins (osteopontin, bone sialoprotein etc.) that bind to HA have been found to have a repetitive sequence of negatively-charged acidic amino acids (Asp, D or Glu, E), a possible hydroxyapatite-binding site [70,71]. In osteoblastic cell culture, secreted osteopontin and bone sialoprotein rapidly bind to HA [72]. A drug attached to HA is released during bone resorption processes and targeting a drug to HA is a potential strategy for a selective drug delivery to bone [73,74]. Kasugai et al., 2000 showed that they could enhance estradiol uptake by bone and prevent osteoporosis by tagging the hormone to Glu6 (E6) [75,76]. We and others have recently applied this new bone-targeting system to a large molecule, an enzyme (tissue nonspecific alkaline phosphatase), showing that the tagged enzymes are delivered more efficiently to bone [77-79] and that the tagged enzyme improves clinical and pathological effects of a mouse model of a systemic bone disease, hypophosphatasia, better than untagged native enzyme [80]. Human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) and ß-glucuronidase (GUSB) have also been bioengineered to add E6 and D6. These tagged enzymes had markedly reduced rates of clearance from the circulation, increasing blood levels 10-20 times higher than those of native enzymes [20,81]. The bone-targeting enzyme was retained longer in bone, with substantial residual enzyme activity. The pathological features in MPS IVA and VII mice treated with the targeting enzyme showed marked clearance of the storage materials in bone (Fig. 6). These findings suggest that the tagged enzyme enhances delivery and reduces pathological effects in bone.
Figure 6.
Growth plate histology of 8-weeks-old MPS IVA mouse treated with bone-targeting enzyme. Newborn ERT started at day 2, and weekly ERT continued for 8 weeks. Vacuolated storage is substantially reduced in a treated mouse with MPS IVA. Toluidine blue-stained 0.5-μm-thick sections (X 100). Adapted from Tomatsu S, Montaño AM, Oikawa H et al. Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions? Mol Genet Metab Jun 4. pii: S1096-7192(14)00185-1. doi: 10.1016/j.ymgme.2014.05.013. [Epub ahead of print].
Overall, newborn, long-circulating or targeting ERT provides the most robust results for improvements of clinical and pathological bone lesions. Combination of newborn ERT with either long-circulating or targeting ERT enhances further improvements.
2.3. Hematopoietic stem cell transplantation (HSCT)
Potential advantages of HSCT for treating MPSs are that marrow-derived donor cells could provide a continuous source of secreted enzyme, and also provide access of enzyme to bone and cartilage that is close to the bone marrow. However, others have proposed that secreted enzyme may not penetrate into the bone after HSCT [26,82-84].
HSCT has successfully corrected the disease course and severity in patients with MPS I or MPS VI [23,85]. The clinical consequence of HSCT depend on 1) the age of the patient at the time of transplantation, 2) the severity of clinical phenotype, 3) the type of donor, and 4) the course of preparative regimen [86]. In patients with MPS I and VI, HSCT results in maintained normal heart function, and hearing is improved. However, skeletal manifestations still developed progressively.
2.3.1. MPS I
Over 500 patients with MPS I have been treated by HSCT. Early hematopoietic stem cell transplantation (HSCT) is the standard of care for patients with a severe phenotype of MPS I. Substantial clinical improvements of joint mobility, coarse facial features, and claw hands were reported following transplantation [26]. However, skeletal disease, including genu valgum, thoracolumbar kyphosis and hip dysplasia, still develops after HSCT and consequently this treatment provides a limited impact to ADL because of pain and loss of ambulation. Skeletal deformity is less responsive to HSCT compared with favorable effects related to other important clinical outcome parameters including CNS involvement [25,87-94]. The skeletal abnormalities lead to a need for orthopedic corrective surgeries. For example, a 15-year-old patient with MPS I had received successful HSCT at the age of 2 years old. His ADL has been kept normal but bone deformity has still developed, resulting in hemiepiphysiodesis of bilateral medial proximal tibia at 12 years old and successive arthrodesis of thoraco-lumbar spine at 13 years old; however, skeletal pathology from surgical remnants showed almost complete clearance of storage materials in chondrocytes with normal level of blood HS and DS (Fig. 7). Thus, even though pathology in chondrocytes appeared to be normalized, there was incomplete correction of the skeletal phenotype by HSCT. The reasons for the continued skeletal phenotype are not known, but it is possible that irreversible bone abnormalities may have already occurred prior to transplantation or that the structure of the extracellular matrix (collagen) remains abnormal.
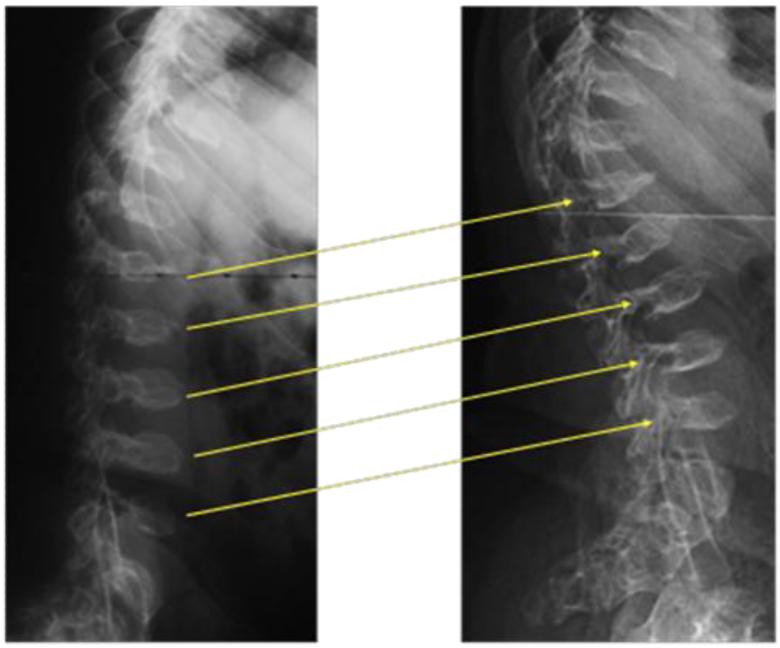
Figure 7.
A 13-years-old Hurler patient: 10 years post-HSCT X-ray of spine and pathology at lumbar spine. Left (X-ray): Severe humpback of L2 is seen. The patient underwent spinal surgery at 13.5 years of age. Mild invagination of the superior and inferior endplates of the lower dorsal vertebrae is observed, and the vertebrae appear flattened. Moderate posterior scalloping of the lumbar vertebrae is observed. There is a mild dextroscoliosis of the lumbar spine. Appearance of the spine is not significantly changed since 6 years of age. Deformity of spine is much milder than that seen in an untreated patient with a severe form. Right (pathology from surgical remnants): no storage vacuoles appear in chondrocytes of lumbar spine at 13.5 years of age and the size and morphology of chondrocytes are normal.
The first months of life represent the best window of opportunity for preventing bone deformities in patients with a severe form of MPS I. A retrospective analysis supports superior long-term clinical outcome of MPS I patients when HSCT is performed early in life [27].
2.3.2. MPS VI
In over 50 MPS VI patients treated with HSCT including 45 cases in Center for International Blood and Marrow Transplant Research (CIBMTR), long-term improvements in facial dysmorphism, hepatosplenomegaly, joint mobility, and cardiac manifestations has been demonstrated [95-98]; however, skeletal disease known as dysostosis multiplex tends to persist or progress despite HSCT [26,82-84].
2.3.3. MPS IVA
There is only one case report of HSCT for MPS IVA [4,30,99,100]. This report shows over 9 years of long-term therapeutic efficacy in a 15-years-old boy with a severe form of MPS IVA, who received successful HSCT. Lumbar bone mineral density increased around 50% one year post-HSCT and remained high. Radiographs showed appearance of figures of trochanter major and minor while epiphyseal dysplasia in the femoral cap remained unchanged. ADL was restored in work/study efficacy, respiratory status, sleep, joint pain, and frequency of infection. After osteotomies of both femurs, he became ambulatory and could walk 400 m unaided although restriction of physical activity due to hyperlaxity of joints remain unsolved. An additional 4 patients with MPS IVA have undergone HSCT in Japan, and are currently under evaluation. All of the treated patients survived the HSCT procedure.
The substantial clinical correction post-HSCT of one MPS IVA patient and the successful HSCT for 4 other cases indicate that HSCT may be a therapeutic option for MPS IVA patients (Fig. 8) [100]. More case reports are needed to prove this concept.
Figure 8.
X-ray photographs with age in a patient with MPS IVA after BMT. Lateral view of thoracolumbar vertebrae a: pre-BMT (left) and three years later post-BMT (right). Platyspondylia and anterior beaking of thoracolumbar vertebra increase slightly in size, and the margin of vertebra becomes clear. One year later post-BMT, BMD at L2-4 increases from 0.372 to 0.548 (g/cm2), and it is maintained at the level of 0.48 ± 0.054 for the following 9 years. Adapted from Chinen Y, Higa T, Tomatsu S, et al. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep 1 (2014) 31-41.
2.3.4. MPS II
In MPS II, there are reports of around 160 patients treated with HSCT. Over 30% of those reported cases are from Japan. Previous reports on the therapeutic effects of HSCT in MPS II patients suggested a limited impact with a high mortality rate; however, most patients had existing neurological symptoms of loss of cognitive function prior to treatment [101]. One reason for the high mortality rate of HSCT during initial attempts between 1980-1990 is that patients who underwent HSCT were already at an advanced or even a terminal stage of disease progression. HSCT will generally not be suitable for patients with an advanced disease stage if they are unable to tolerate the rigorous regimen of HSCT. However, with improved technology and increased awareness of the disease, early diagnosis is becoming more feasible, so that patients with MPS II can receive HSCT when their health condition is favorable at an early stage. Donor-derived cells were detected in the post-mortem brain of a transplanted MPS II patient, indicating the potential of HSCT to treat neurological effects of this MPS [102]. Recent data on Japanese MPS II patients indicate that 1) HSCT 5 year survival rate was 88.5%, 2) HSCT improves brain lesions in magnetic resonance imaging (MRI) and ADL [35], 3) HSCT shows a similar improvement in growth to ERT [103], 4) the average ADL score in HSCT-treated patients is higher than in ERT-treated patients [104], and 5) patients treated with HSCT when under 5 years of age have a better ADL than patients treated later in life [104]. HSCT treatment of patients with MPS II slows appearance of skeletal dysplasia. A patient with an attenuated form of MPS II showed improvements of his skeletal dysplasia 13 years after HSCT, and his current height is over 170 cm at 18 years of age (personal communication; Dr. Yabe).
To compare the impact of HSCT on growth with that of ERT, clinical data were obtained from 44 Japanese male patients with MPS II; 26 had been treated with ERT, 12 had been treated with HSCT and 6 had neither treatment. Patel et al. demonstrated 1) that MPS II patients who had been treated with either ERT or HSCT had increased height and weight when compared to untreated patients, and 2) that HSCT and ERT were equally effective in restoring growth of MPS II patients [35]. Among patients with severe phenotypes, there was an indication that HSCT provides a higher ADL score than early ERT, and there was a significant difference in ADL scores between late ERT and HSCT groups. Early HSCT treatment provided a higher score than late HSCT.
2.3.5. HSCT at birth
A few studies have evaluated the effect of HSCT at birth on skeletal features in MPS animal models [32,33]. Newborn mice with MPS VII receiving ablative BMT lived longer than untreated mice. Treated mice had less severe facial dysmorphism, better mobility, and showed pathological and clinical improvements by clearance of lysosomal storage granules in bones, joints, and visceral organs, even though engraftment achieved was low (15-20%) [32]. Nonablative neonatal BMT showed that twelve months after BMT, several structural features of femurs were more similar to those of normal mice than untreated MPS VII mice. Periosteal circumference and bone cortical thickness were significantly improved, and cortical density did not differ significantly from values in normal mice. Significant reduction of lysosomal GAG storage corresponded with GUS enzyme activity and percentage of histochemically GUS cells in visceral organs and hematopoietic tissues.
We have tested the hypothesis that HSCT at birth can prevent skeletal dysplasia in MPS I mice [105]. Newborn BMT was effective at restoring α-iduronidase (IDUA) activity and preventing elevated glycosaminoglycans in blood and multiple organs. At 37 weeks of age, all bone tissue parameters measured using radiographic, micro-CT, biochemical, and histological analyses were similar to normal mice. The magnitude of improvements correlated with the extent of hematopoietic engraftment. Moreover, improvements in bone parameters correlated with high levels of bone marrow-derived cell engraftment in multiple hematopoietic compartments, suggesting that the early and complete restoration of normal hematopoiesis can have a significant impact on bone development of newborn MPS I mice.
This proof of concept study advocates newborn BMT as a highly effective therapeutic approach for MPS I, demonstrating that an early treatment may further impact the clinical outcome of these patients. Establishment of newborn screening procedures will allow early diagnosis and early treatment of affected children. Patients identified with an attenuated phenotype of MPS would not need treatment with HSCT, but may benefit from early less aggressive treatment and disease progression could be monitored more closely after early diagnosis.
Overall, these findings suggest that if HSCT is performed for MPS patients at an earlier stage, skeletal deformities and impaired growth development for MPS patients can be ameliorated or slowed. Regimens for HSCT have been revised over recent years and well-established institutions with trained staff show the least mortality rates for HSCT. Survival rates should be increased by selecting patients who are healthier and more likely to tolerate HSCT. HSCT would not be ideal for patients who are in poor condition and ERT may be a better therapeutic option at least in the short term.
Since the year 2000, Tokai University has treated over 20 MPS patients by HSCT with no fatalities due to complications of the treatment (personal communication with Dr. Yabe, Tokai University). HSCT is considered in selected cases with careful pre-transplantation counseling, clinical evaluation and systemic longitudinal monitoring of the outcome. Moreover, cord blood from unrelated donors appears to be an excellent source of HSCT for patients with MPS, resulting in full-donor chimerism and normal enzyme levels in almost all transplanted patients [27,106]. Advantages of umbilical cord blood transplantation are 1) easy tissue procurement, 2) no risk to donors, 3) low risk of transmitting infections, 4) immediate availability, and 5) immune tolerance allowing successful transplantation despite human leukocyte antigen (HLA) disparity [107].
The use of ERT before or after HSCT may be considered to improve condition of the patient. Pre-transplant ERT could be critical for patients waiting for suitable donors [26,108] while some patients receive ERT in addition to HSCT [85,109]. As no prospective studies have compared the efficacy of HSCT to ERT, further research is required to support physicians who have to assess the risks and benefits of all therapeutic approaches and define the best regimen for individual patients.
2.4. Gene therapy
Gene therapy has not yet been approved as a therapeutic option but several gene therapy studies on animal models have shown promising results for MPS [110-114], and four human clinical trials have begun to date [112]. Nevertheless, therapeutic efficacy for bone lesion remains unanswered, even after induction of supraphysiological enzyme activity levels in animal models of MPS for several years. Here we summarize the recent achievements for bone lesions in gene therapies of animal models of MPS.
2.4.1. MPS I
Gene therapy approaches for MPS I have used plasmids [115,116], sleeping beauty transposon [117,118], gamma-retrovirus [58,119-121], lentivectors [122], and adeno-associated virus (AAV) vectors [123,124]. Non-viral vectors (i.e. plasmids and transposons) have shown improvements in some manifestations of the disease, but the low-level and short-term expression limits the impact of these vectors in bone lesions. The sleeping beauty transposon system resulted in a significant increase in IDUA activity in several tissues, normalization of GAG storage in those tissues, and reduction of hepatomegaly [118]. In addition, sleeping beauty-based therapy corrected thickening of the bone of the zygomatic arch and growth plate abnormalities in femur and tibia; but consistent positive effects were not observed in cortical thickness or diameters of mid-diaphyseal bone areas [118]. AAV vectors have been evaluated only to correct the neurological alterations of the disease in mice, showing significant improvement in accumulation of GAGs and neurocognitive dysfunction [123,124].
The use of gamma-retroviral vectors (RV) in MPS I mice and dogs has allowed long-term expression, and enzyme activity in serum ranging from normal to supraphysiological levels. Adult MPS I mice treated with the γ-retroviral vector intravenously resulted in relative low IDUA activity, although a complete correction of hearing and vision abnormalities, and partial correction in femur diameters and bone mineral density was observed [120,121]. Neonatal treatment of MPS I dogs led to an increase in serum IDUA activity that was up to 28-fold greater than wild-type levels and was maintained for 1.8 years [119]. These supraphysiological enzyme levels improved survival, normal facial appearance, and reduction or elimination of umbilical hernias, chest deformities, joint disease (effusion, laxity, crepitus on manipulation, and/or deformity of the normal joint angle of the elbows, carpi, phalanges, and/or stifles), corneal clouding, and heart disease [119]. After two years of follow-up of treated dogs, skeletal disease evaluation showed no improvement in lengths of cervical or lumbar vertebral bodies, reduction of vertebral fusion, modest improvement in widening, beaking, and tipping in cervical spine, reduction in vertebral space, and reduction in severity of stifle joint effusions. In summary, neonatal gene therapy in MPS I dogs ameliorates, but does not prevent, skeletal disease even with supraphysiological enzyme activity levels in serum and several organs [120,121].
Lentivirus has been used in MPS I gene therapy to transduce hematopoietic stem cells (HSC) followed by transplantation of the modified cells. Mice treated with HSC-gene therapy showed supraphysiological enzyme activity levels in serum and normalization of GAG storage in urine and tissues [122]. Treated mice showed normalization of neurologic and skeletal (width of the skull and of the humerus and femur, length of the femur, and volume of the zygomatic bones) disease, which contrast with the results observed after the infusion of wild-type HSC, which showed only partial correction of skeletal abnormalities [122].
2.4.2. MPS IVA
Gamma-retroviral and AAV vectors have been used in MPS IVA gene therapy studies. Gamma-retroviral vector was used in several cell lines showing a significant increase in GALNS activity and reduction of GAG storage [125]. In-vivo experiments in mice have been carried out with AAV2 vectors [126]. Twelve weeks after a single intravenous administration of the AAV vector, plasma enzyme activity levels were restored to 19% of wild-type levels, while enzyme activity in peripheral tissues increased up to 22% of wild-type levels. However, activity was not restored in bone. Interestingly, co-administration of AAV-GALNS with AAV-SUMF1 resulted in a significant increase in GALNS activity in several tissues, including bone, where activity was 33% of wild-type levels [126,127]. Nevertheless, vector biodistribution analysis showed delivery to bone was poorer than to other tissues [126,127]. To enhance vector delivery to bone, the vector capsid was modified by insertion of multiple copies of a short acidic amino acid peptide [126]. Inclusion of short acidic amino acid peptide modification conferred affinity of the vector for hydroxyapatite (HA), the major constituent of bone matrix, which resulted in higher vector genome copies in bone cells, and enzyme activity was 42% of wild-type levels [111,127] (Fig. 9). Recently, a new set of AAV vectors carrying both GALNS and sulfatase-modifying factor 1 (SUMF1) genes were constructed using internal ribosome entry site (IRES), which induced higher enzyme activity than that produced using AAV-GALNS: AAV-SUMF1 co-transduction [128]. Furthermore, GALNS transduction mediated by the lentiviral vectors allowed up to a 100-fold increase in enzyme activity in comparison to the levels observed with the AAV vectors. In Morquio A fibroblasts, lentiviral vectors normalized GAG, β-hexosaminidase and β-galactosidase levels [128].
Figure 9.
Mechanism of multiple-AAA targeting system. Viral capsid in the right panel has multiple copies of D8 integrated into capsid proteins, showing the retargeting of gene vector to bone (hydroxyapatite in the mineral region) schematically.
2.4.3. MPS VI
A couple of gene therapies have been evaluated for correction of bone disease in a MPS VI cat. AAV2/8 vectors were administered intravenously to MPS VI cats at 5 or 50 days of age and resulted in a reduction of GAG storage in urine and tissues, improvement of femur and humeri length, reduction of heart valve thickness, and improvement in spontaneous mobility [129]. Although improvement in cervical spine and joint abnormalities was limited regardless of the vector dose and the age of treatment, clearance of GAG storage in cortical bone osteocytes and improvement of the growth plate resting zone thickness and were observed only in MPS VI cats receiving high vector doses; while reduction of GAG storage was not observed in articular cartilage regardless of the vector doses. [129]. Furthermore, the presence of neutralizing antibodies was associated with a significant reduction of the therapeutic efficacy measured by GAG normalization and femur length improvement [130].
Neonatal gene therapy on MPS VI cats using a gamma-retroviral vector carrying feline ARSB gene, showed that 8 years post-infusion supraphysiological enzyme activity levels were maintained in serum and tissues [131]. Treated animals showed normalization of GAG in urine and tissues, less pronounced facial dysmorphism, increased body weight and improvement in appendicular skeleton lengths, articular cartilage erosion, and cervical vertebral articular process widening, and mobility. Nevertheless, there was limited effect in cervical vertebrae, cervical vertebral fusion, and intervertebral disc degeneration [131].These results showed that an early treatment with a long-term supraphysiological enzyme activity levels was not able to completely resolve all skeletal abnormalities.
2.4.4. MPS VII
Gene therapy reports for MPS VII have used plasmid [132] sleeping beauty transposon [117], gamma-retroviral [120,121,132,133], lentiviral [134], and AAV [135,136] vectors. Favorable results for bone lesions were reported in MPS VII dogs by neonatal gene therapy using a gamma-retroviral vector [133]. Treated MPS VII dogs were followed for up to 11 years, which showed maintained therapeutic levels of enzyme activity associated with a reduction of GAG levels in urine and tissues. Treated MPS VII dogs showed a significant correction for most bone deformities and could walk throughout their lives, while untreated MPS VII dogs could not stand beyond 6 months and were dead by 2 years. Luxation of the coxofemoral joint and the patella, dysplasia of the acetabulum and supracondylar ridge, deep erosions of the distal femur, and synovial hyperplasia were reduced, and the quality of articular bone was improved in treated dogs [133]. Nevertheless, treated dogs continued to have osteophyte formation, cartilage abnormalities, calcification of the ventral epiphysis of the vertebral bodies, and intervertebral disk degeneration, resulting in an abnormal gait. Thus, neonatal gene therapy reduces some skeletal abnormalities in MPS VII dogs and dramatically improves their life span, but clinically-relevant abnormalities in bone remain due to the inability of GUSB to diffuse into spine tissues, suggesting that ERT will probably have similar limitations long-term [120,121,132,133].
Neonatal and adult gene therapy in MPS VII mice by the use of lentiviral vectors showed improvement in parameters of bone mass and architecture as well as biochemical and enzymatic correction [134]. However, growth plate chondrocytes were not responsive to treatment, as evidenced by the lack of improvement in vertebral and femoral bone length and growth plate height.
In summary, gene therapies for MPS animal models have shown a favorable safety profile with long-term expression periods over 10 years for the viral vectors, and promising results for non-viral vectors. Both vectors lead to substantial impact in bone lesion in animal models, when supraphysiological enzyme activity levels are maintained. However, spine deformities especially on large animal models remain challenging to correct, even with neonatal therapy and supraphysiological levels of the enzyme activity. The results for MPS IVA show the potential to treat the bone lesion by using a novel system of AAV vectors with a bone-targeting system. Further studies should shed light on the long-term evaluation of gene therapy with both viral and non-viral vectors, which will show a great potential for the treatment of bone disease in MPS.
2.5. Anti-inflammatory drugs
In patients with MPS, chronic osteoarthritis associated with skeletal dysplasia can happen in any major joints such as shoulder, wrist, hip, knee, and ankle. In the last decade, there have been several key reports showing that inflammatory responses exacerbate MPS symptoms. Accumulated GAGs (keratan sulfate; KS, chondroitin-6-sulfate; C6S) in bone, cartilage, and extracellular matrix (ECM) induce pro-inflammatory factors (e.g. TNF-α, RANTES, TIMP-1, MIP-1α IL-1, 2, 5 etc.), that lead to cartilage degradation by degradative proteases (e.g. MMPs), and subsequently chronic osteoarthritis and spondyloepiphyseal dysplasia. Chondrocytes and ECM in patients with MPS are markedly vacuolated and tissues are affected with appearance of foam cells, macrophages, and T-cells (Fig. 10), suggesting that inflammation plays a key role of skeletal dysplasia [137].
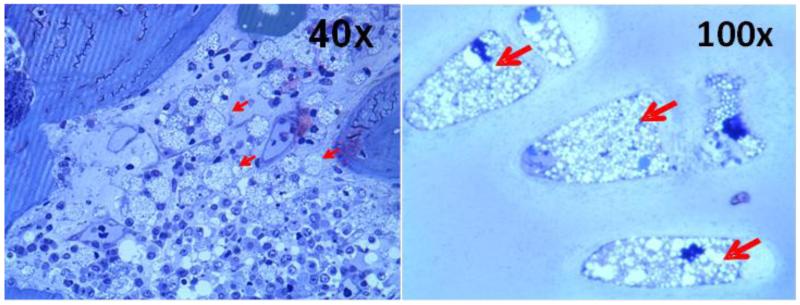
Figure 10.
Appearance of foam cells/macrophages/vacuolated cells in tissues in autopsied specimens from a 20-year-old male MPS IVA patient1. Left; Bone marrow in the vertebrae shows foam cells and vacuolated osteoblasts (40x), right; Trachea shows ballooned vacuolated chondrocytes (100x). Stained with toluidine blue (0.5 μm; light microscopy).
To suppress metabolic inflammation caused by GAG accumulation, two treatments are available: one is to reduce the causative factor (reduce GAGs by ERT, gene therapy, SRT, HSCT etc.), while the other is to inhibit secondary inflammatory processes using anti-inflammatory (or immunosuppressive) agents. These anti-inflammatory agents have distinct mechanisms of action, including inhibition of the action of cytokines, blocking cell-cell interactions, and depleting certain cell types. TNF-α is a dominant proinflammatory cytokine in the pathophysiology of MPS, and several biologic agents are approved to target this cytokine to treat autoimmune diseases such as rheumatoid arthritis (RA).
The effect of anti-TNF-α (infliximab) therapy was assessed in MPS VI rats. Early treatment in the presymptomatic period inhibited the elevation of TNF-α, RANKL and other inflammatory factors in the blood, articular chondrocytes and synovial fibroblasts [36]. The number of apoptotic articular chondrocytes was reduced, and there was no difference from healthy control rats. However, there was no impact on bone growth or mobility since stored GAGs still remained in chondrocytes of the growth plate. The efficacy of ERT alone and combined treatment using ERT and anti-TNF-α drug (specific monoclonal antibody against TNF-α: CNTO1081) was also tested [37]. Both treatments markedly reduced serum levels of TNF-α and RANKL, although only the combined treatment reduced TNF-α in the articular cartilage. Analysis of cultured articular chondrocytes showed that combination therapy restored collagen IIA1 expression and reduced expression of apoptotic markers. Only the combined therapy suppressed hyperplasia of synovial cells into underlying bone and clinical effects on other organs that are not accessible to the enzyme (e.g. cartilage) [37]. However, these therapies do have adverse effects.
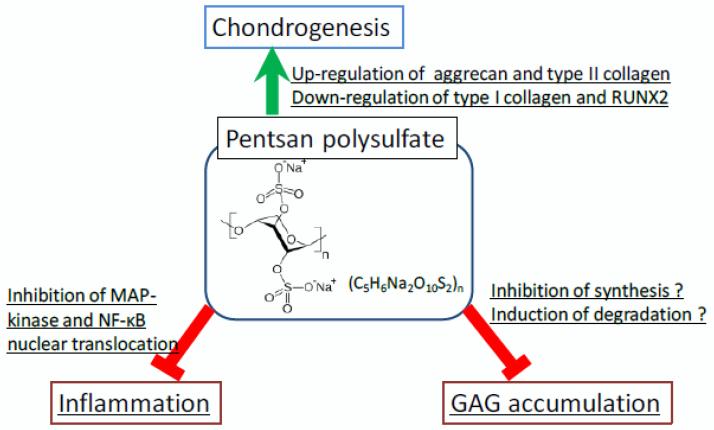
Pentosan polysulfate (PPS) has potent anti-inflammatory effects and is an FDA-approved drug used for patients with interstitial cystitis and has also been used for thrombosis prophylaxis and to treat phlebitis in Europe for several decades. Successively, clinical trials of PPS in patients with knee osteoarthritis provided significant reduction in pain [138]. Schuchman et al. also reported that oral and subcutaneous administration of PPS reduced inflammation and improved skeletal pathology, bone mineral density, and mobility of joints in MPS VI rats [39,139,140]. Ghosh et al. reported that PPS promotes proliferation, and chondrogenic differentiation of adult human bone marrow-derived mesenchymal precursor cells [139]. In our preliminary experiment, PPS suppressed GAG accumulation in fibroblasts of several types of MPS patients. Osteoarthritis is one of the primary concerns in patients with MPS. Progressive bone and joint disorders in MPS cause severe pain, resulting in disability of walking and poor ADL. Since PPS reduced pain in interstitial cystitis and osteoarthritis patients [138], anti-inflammatory effects of PPS could provide improvements of ADL and QOL in patients with MPS. PPS will provide suppression of inflammation, reduction of GAG accumulation, and/or promotion of chondrogenesis in chondrocytes of the patients with MPS (Fig. 11).
Figure 11.
Hypothesis for role of PPS on reduction of GAGs, suppression of inflammation, and promotion of chondrogenesis in chondrocytes RUNX2: runt-related transcription factor 2, MAP: Mitogen-activated protein, NF-κB: nuclear factor-kappa B.
A clinical study and a trial of PPS for patients with MPS I and II started in 2014 and adverse effects and therapeutic efficacy are under investigation.
3. Conclusion
Resolution of bone and cartilage issues remains an unmet challenge for patients with MPS. Patients with MPS have severe progressive skeletal dysplasia that leads to significant morbidity and handicap with poor ADL. Management requires multidisciplinary approaches for the patient, particularly for those who have serious issues such as spinal cord compression, ambulatory problems, and restrictive and obstructive lung issues. A comprehensive assessment of individual patient at initial diagnosis is required, and continued follow-up by primary care clinicians. Supportive management, physiotherapy, and appreciation of possible complications can also improve the QOL of MPS patients and their families. Families of the patients should be offered tailor-made management including genetic counseling, choice of ERT, HSCT, gene therapy (if it becomes available), anti-inflammatory drugs, supportive therapies, physiotherapies and orthopedic interventions. Physicians who take care of MPS patients should be familiar with the most common complications, diagnosis of the disease, and locations of expert centers as well as available therapies. Metabolic and transplant doctors as well as genetic counselors should cooperatively examine the range of therapeutic options to provide the optimal outcome for individual patients. Hopefully, this will lead to earlier diagnosis for patients, resulting in better comprehensive therapy and avoidance of progression to irreversible damage. ERT, HSCT, gene therapy, and anti-inflammatory drug are therapies that could be offered before or after onset of the disease. Although the current treatments will not cure the disease, they provide the potential to rescue most patients from consequences of the disease and to improve the QOL. It seems that therapy outcomes are better if treatment is started at an early stage, which should drive support for policies that advocate newborn screening for these diseases. Established systemic bone dysplasia remains a serious challenge, and robust, innovative approaches such as bone targeting should be considered. Longitudinal observation of MPS patients under current therapies provides more precise and valuable information regarding the appropriate assessment, including physical activity, supportive treatment, efficacy of therapy, and the clinical endpoints.
Highlights.
ERT, HSCT, and gene therapy are compared for therapies in bone lesions of MPS.
Delivery of sufficient enzyme to bone, especially avascular cartilage, remains an unmet challenge.
Use of anti-inflammatory drug is also under clinical study.
Therapies should start at a very early stage prior to irreversible bone lesion.
The severity of skeletal dysplasia is associated with level of activity during daily life.
Acknowledgement
This work was supported by grants from Austrian MPS Society, National MPS Society, and International Morquio Organization (Carol Ann Foundation). S.T. and R.W.M. were supported by National Institutes of Health grant P20GM103464. F.K. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico from Brazil (CNPQ). The content of the article has not been influenced by the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
All the authors contributed to the Review Article and had no conflict of interest with any other party. Shunji Tomatsu, Carlos J. Alméciga-Díaz, Adriana M. Montaño, Hiromasa Yabe, Akemi Tanaka, Vu Chi Dung, Roberto Giugliani, Francyne Kubaski, Robert W. Mason, Eriko Yasuda, Kazuki Sawamoto, William Mackenzie, Yasuyuki Suzuki, Kenji E. Orii, Luis A. Barrera, William S. Sly, and Tadao Orii declare that they have no conflict of interests. Part of the content was presented at 13th International Symposium on Mucopolysaccharidoses and Related Diseases (Bahia, Brazil; August 13-17, 2014).
Contributions to the project:
Shunji Tomatsu is a Principal Investigator for this review article and has contributed to the concept and planning of the article, collection of data, and reporting of the work described.
Carlos J. Alméciga-Díaz contributed to the planning of the article, collection of data on gene therapy, and reporting of the work described.
Adriana M. Montaño contributed to the planning of the article, collection of data on ERT and gene therapy, and reporting of the work described.
Hiromasa Yabe contributed to the planning of the article, collection of data on HSCT, and reporting of the work described.
Akemi Tanaka contributed to the planning of the article, collection of data on ERT and HSCT, and reporting of the work described.
Vu Chi Dung contributed to the planning of the article, collection of data on ERT and mouse pathology, X-ray pictures, and reporting of the work described.
Roberto Giugliani contributed to the planning of the article, collection of data on ERT and X-ray pictures, and reporting of the work described.
Francyne Kubaski contributed to the planning of the article, collection of data in HSCT, and reporting of the work described.
Robert W. Mason contributed to the planning of the article, collection of data, and reporting of the work described.
Eriko Yasuda contributed to the planning of the article, collection of data in pathology, and reporting of the work described.
Kazuki Sawamoto contributed to the planning of the article, collection of published data in ERT, gene therapy and HSCT, and reporting of the work described.
William Mackenzie contributed to the planning of the article, collection of data on surgical specimen, and reporting of the work described.
Yasuyuki Suzuki contributed to the planning of the article, collection of data on ERT and HSCT, and reporting of the work described.
Kenji E. Orii contributed to the planning of the article, collection of data on ERT and HSCT, and reporting of the work described.
Luis A. Barrera contributed to the planning of the article, collection of data on gene therapy, and reporting of the work described.
William S. Sly contributed to the planning of the article, collection of data on ERT, and reporting of the work described.
Tadao Orii is a Principal Investigator for this review article and has contributed to the concept of the manuscript, planning of the article, collection of data, and reporting of the work described.
References
- 1.Neufeld E, Muenzer J. The mucopolysaccharidoses. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2001. pp. 3421–3452. [Google Scholar]
- 2.Tomatsu S, Montaño A, Oikawa H, Giugliani R, Harmatz P, Smith M, Orii T. Impairment of Body Growth in Mucopolysaccharidoses, In Handbook of Growth and Growth Monitoring in Health and Disease 1. Springer; New York: 2012. pp. 2091–2117. [Google Scholar]
- 3.Patel P, Suzuki Y, Maeda M, Yasuda E, Shimada T, Orii KE, Orii T, Tomatsu S. Growth charts for patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:5–18. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomatsu S, Montaño AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez M, Miller TL, Mackenzie WG, Ditro C, Chidekel AS, Shaffer TH. Characteristics of Impulse Oscillometry and Thoracoabdominal Motion in Children with Thoracic Cage Disorders. Pediatr. Pulmonol. 2010;45:679–686. doi: 10.1002/ppul.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr. Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 7.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 8.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsayas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 9.Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta. Paediatr. Suppl. 2002;91:98–99. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 10.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin SP, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, STRIVE Investigators. Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014 May 9; doi: 10.1007/s10545-014-9715-6. [Epub ahead of print] [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmatz P, Whitley CB, Waber L, Pais R, Steiner R, Plecko B, Kaplan P, Simon J, Butensky E, Hopwood JJ. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J. Pediatr. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Harmatz P, Ketteridge D, Giugliani R, Guffon N, Teles EL, Miranda MC, Yu ZF, Swiedler SJ, Hopwood JJ. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–689. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- 13.Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Ketteridge D, Hopwood JJ, Plecko B, Steiner R, Whitley CB, Kaplan P, Yu ZF, Swiedler SJ, Deck C. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol. Genet. Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, Moore D. A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type I. Health Technol. Assess. 2006;10:1–6. doi: 10.3310/hta10200. [DOI] [PubMed] [Google Scholar]
- 16.Rohrbach M, Clarke JT. Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs. 2007;67:2697–2716. doi: 10.2165/00003495-200767180-00005. [DOI] [PubMed] [Google Scholar]
- 17.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat. Biotechnol. 2004;22:1383–1391. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 18.Parveen S, Sahoo SK. Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs. Clin. Pharmacokinet. 2006;45:965–988. doi: 10.2165/00003088-200645100-00002. [DOI] [PubMed] [Google Scholar]
- 19.Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomatsu S, Montaño AM, Dung VC, Ohashi A, Oikawa H, Oguma T, Orii T, Barrera L, Sly WS. Enhancement of drug delivery: enzyme replacement therapy for murine Morquio A syndrome. Mol. Ther. 2012;18:1094–1102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowan DJ, Tomatsu S, Grubb JH, Haupt B, Montaño AM, Oikawa H, Sosa AC, Chen A, Sly WS. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol. Genet. Metab. 2012;107:161–172. doi: 10.1016/j.ymgme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomatsu S, Montaño AM, Oikawa H, Dung VC, Hashimoto A, Oguma T, Gutiérrez ML, Takahashi T, Shimada T, Orii T, Sly WS. Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions? Mol. Genet. Metab. 2014 Jun 4; doi: 10.1016/j.ymgme.2014.05.013. [pii: S1096-7192(14)00185-1] [Epub ahead of print] [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith JE. Long-term follow-up following bone marrow transplantation for Hunter disease. J. Inherit. Metab. Dis. 1999;22:638–648. doi: 10.1023/a:1005525931994. [DOI] [PubMed] [Google Scholar]
- 24.Aldenhoven M, Boelens JJ, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol. Blood Marrow Transplant. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Field RE, Buchanan JA, Copplemans MG, Aichroth PM. Bone-marrow transplantation in Hurler’s syndrome. Effect on skeletal development. J. Bone Joint Surg. Br. 1994;76:975–981. [PubMed] [Google Scholar]
- 26.Rovelli AM. The controversial and changing role of haematopoietic cell transplantation for lysosomal storage disorders: an update. Bone Marrow Transplant. 2008;41(Suppl. 2):S87–89. doi: 10.1038/bmt.2008.62. [DOI] [PubMed] [Google Scholar]
- 27.Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, Wraith E, Cavazzana-Calvo M, Rovelli A, Fischer A, Tolar J, Prasad VK, Escolar M, Gluckman E, O’Meara A, Orchand PJ, Veys P, Eapen M, Kurtzberg J, Rocha V. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. J. Gene Med. 2004;6:481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 29.Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr. Gene Ther. 2006;6:227–241. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- 30.Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montaño AM, Rowan D, Sly W, Alméciga-Díaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T. Current and emerging treatments and surgical interventions for Morquio A Syndrome: A review. Res. Rep. Endocr. Disord. 2012;2012:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alméciga-Díaz CJ, Montaño AM, Tomatsu S, Barrera LA. Adeno-associated virus gene transfer in Morquio A disease: effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010;277:3608–3619. doi: 10.1111/j.1742-4658.2010.07769.x. [DOI] [PubMed] [Google Scholar]
- 32.Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N, Sly WS, Birkenmeier E. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab. Invest. 1993;68:676–686. [PubMed] [Google Scholar]
- 33.Soper BW, Lessard MD, Vogler CA, Levy B, Beamer WG, Sly WS, Barker JE. Nonablative neonatal marrow transplantation attenuates functional and physical defects of beta-glucuronidase deficiency. Blood. 2001;97:1498–1504. doi: 10.1182/blood.v97.5.1498. [DOI] [PubMed] [Google Scholar]
- 34.Lau AA, Shamsani NJ, Winner LK, Hassiotis S, King BM, Hopwood JJ, Hemsley KM. Neonatal Bone Marrow Transplantation in MPS IIIA Mice. JIMD Rep. 2013;8:121–132. doi: 10.1007/8904_2012_169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel P, Suzuki Y, Tanaka A, Yabe H, Kato S, Shimada T, Mason RW, Orii KE, Fukao T, Orii T, Tomatsu S. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:184–196. doi: 10.1016/j.ymgmr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonaro CM, Ge Y, Eliyahu E, He X, Jepsen KJ, Schuchman EH. Involvement of the Toll-like receptor 4 pathway and use of TNF-alpha antagonists for treatment of the mucopolysaccharidoses. Proc. Nat. Acad. Sci. U.S.A. 2010;107:222–227. doi: 10.1073/pnas.0912937107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliyahu E, Wolfson T, Ge Y, Jepsen KJ, Schuchman EH, Simonaro CM. Anti-TNF-alpha therapy enhances the effects of enzyme replacement therapy in rats with mucopolysaccharidosis type VI. PloS One. 2011;6:1–11. doi: 10.1371/journal.pone.0022447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuchman EH, Ge Y, Lai A, Borisoy Y, Faillace M, Eliyahu E, He X, Iatridis J, Vlassara H, Striker G, Simonaro CM. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0054459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frohbergh M, Ge Y, Meng F, Karabul N, Solyom A, Lai A, Iatridis J, Schuchman EH, Simonaro CM. Dose responsive effects of subcutaneous pentosan polysulfate injection in mucopolysaccharidosis type VI rats and comparison to oral treatment. PLoS One. 2014;9:e 100882. doi: 10.1371/journal.pone.0100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleary MA, Wraith JE. The presenting features of mucopolysaccharidosis type IH (Hurler syndrome) Acta. Paediatr. 1995;84:337–339. doi: 10.1111/j.1651-2227.1995.tb13640.x. [DOI] [PubMed] [Google Scholar]
- 41.Chakrapani A, Cleary MA, Wraith JE. Detection of inborn errors of metabolism in the newborn. Arch. Dis. Child. Fetal. Neonatal. Ed. 2001;84:F205–210. doi: 10.1136/fn.84.3.F205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paciotti S, Persichetti E, Pagliardini S, Deganuto M, Rosano C, Baducci C, Codini M, Filocamo M, Menghini AR, Pagliardini V, Pasqui S, Bembi B, Dardis A, Beccari T. First pilot newborn screening for four lysosomal storage diseases in an Italian region: identification and analysis of a putative causative mutation in the GBA gene. Clin. Chim. Acta. 2012;413:1827–1831. doi: 10.1016/j.cca.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, Gelb MH, Turecek F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SP, Lin HY, Wang TJ, Chang CY, Lin CH, Huang SF, Tsai CC, Liu HL, Keutzer J, Chuang CK. A pilot newborn screening program for Mucopolysaccharidosis type I in Taiwan. Orphanet. J. Rare Dis. 2013;8:1–8. doi: 10.1186/1750-1172-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, Shibata Y, Futatsumori H, Montaño AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta. Paediatr. 2009;98:768–769. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 47.Beck M, Braun S, Coerdt W, Merz E, Young E, Sewell AC. Fetal presentation of Morquio disease type A. Prenat. Diagn. 1992;12:1019–1029. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- 48.Martin JJ, Ceuterick C. Prenatal pathology in mucopolysaccharidoses: a comparison with postnatal cases. Clin. Neuropathol. 1983;2:122–127. [PubMed] [Google Scholar]
- 49.Tomatsu S, Orii KO, Vogler C, Nakayama J, Levy B, Grubb JH, Gutierrez MA, Shim S, Yamaguchi S, Nishioka T, Montaño AM, Noguchi A, Orii T, Kondo N, Sly WS. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 50.Wiesmann UN, Spycher MA, Meier C, Liebaers I, Herschkowitz N. Prenatal mucopolysaccharidosis II (Hunter): a pathogenetic study. Pediatr. Res. 1980;14:749–756. doi: 10.1203/00006450-198005000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Rowan DJ, Tomatsu S, Grubb JH, Montaño AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013;36:235–46. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–77. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okuyama T, Tanaka A, Suzuki Y, Ida H, Tanaka T, Cox GF, Eto Y, Orii T. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, Dung VC, Nishiota T, Orii T, Sly WS. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 55.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:1–8. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J. Clin. Invest. 1997;99:651–662. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol. Genet. Metab. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 58.Baldo G, Mayer FQ, Martinelli BZ, de Carvalho TG, Meyer FS, de Oliveira PG, Meurer L, Tavares A, Matte U, Giugliani R. Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice. Mol. Genet. Metab. 2013;109:33–40. doi: 10.1016/j.ymgme.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Sands MS, Vogler C, Kyle JW, Grubb JH, Levy B, Galvin N, Sly WS, Birkenmeier EH. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J. Clin. Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS. Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type VII: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival. Pediatr. Res. 1996;39:1050–1054. doi: 10.1203/00006450-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 61.Vogler C, Sands MS, Galvin N, Levy B, Thorpe C, Barker J, Sly WS. Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J. Inherit. Metab. Dis. 1998;21:575–586. doi: 10.1023/a:1005423222927. [DOI] [PubMed] [Google Scholar]
- 62.Tylki-Szymanska A, Marucha J, Jurecka A, Syczewska M, Czartoryska B. Efficacy of recombinant human alpha-L-iduronidase (laronidase) on restricted range of motion of upper extremities in mucopolysaccharidosis type I patients. J. Inherit. Metab. Dis. 2010;33:151–157. doi: 10.1007/s10545-010-9059-9. [DOI] [PubMed] [Google Scholar]
- 63.Harmatz PR, Garcia P, Guffon N, Randolph LM, Shediac R, Braunlin E, Lachman RS, Decker C. Galsulfase (Naglazyme®) therapy in infants with mucopolysaccharidosis VI. J. Inherit. Metab. Dis. 2014;37:277–287. doi: 10.1007/s10545-013-9654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furujo M, Kubo T, Kosuga M, Okuyama T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI. Mol. Genet. Metab. 2011;104:597–602. doi: 10.1016/j.ymgme.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 65.McGill JJ, Inwood AC, Coman DJ, Lipke ML, de Lore D, Swiendler SJ, Hopwood JJ. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age-a sibling control study. Clin. Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 66.Gabrielli O, Clarke LA, Bruni S, Coppa GV. Enzyme-replacement therapy in a5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2010;125:e183–e187. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- 67.Tylki-Szymanska A, Jurecka A, Zuber Z, Rozdzynska A, Marucha J, Czartoryska B. Enzyme replacement therapy for mucopolysaccharidosis II from 3 months of age: a 3-year follow-up. Acta. Paediatr. 2012;101:e42–e47. doi: 10.1111/j.1651-2227.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 68.Tajima G, Sakura N, Kosuga M, Okuyama T, Kobayashi M. Effects of idursulfase enzyme replacement therapy forMucopolysaccharidosis type II when started in early infancy: comparison in two siblings. Mol. Genet. Metab. 2013;108:172–177. doi: 10.1016/j.ymgme.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Grubb JH, Vogler C, Levy B, Galvin N, Tan Y, Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oldberg A, Franzen A, Heinegard D. The primary structure of a cell-binding bone sialoprotein. J. Biol. Chem. 1988;263:19430–19432. [PubMed] [Google Scholar]
- 71.Kasugai S, Fujisawa R, Waki Y, Miyamoto K, Ohya K. Selective drug delivery system to bone: Small peptide (Asp)6 conjugation. J. Bone Miner. Res. 2000;15:936–943. doi: 10.1359/jbmr.2000.15.5.936. [DOI] [PubMed] [Google Scholar]
- 72.Nagata T, Bellows CG, Kasugai S, Butler WT, Sodek J. Biosysnthesis of bone proteins [SPP-1 (secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin) in association with mineralized-tissue formation by fetal-rat calvarian cells in culture. Biochem. J. 1991;274:513–520. doi: 10.1042/bj2740513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler WT. The nature and significance of osteopontin. Connect. Tissue Res. 1989;23:123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 74.Fujisaki J, Tokunaga Y, Takahashi T, Shimojo F, Kimura S, Hata T. Osteotropic drug delivery system (ODDS) based on biphosphonic prodrug. I.v. effects of osteotropic estradiol on bone mineral density and uterine weight in ovariectomized rats. J. Drug. Target. 1998;5:129–138. doi: 10.3109/10611869808995866. [DOI] [PubMed] [Google Scholar]
- 75.Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, Sawanishi H, Morito K, Masamune Y, Kasugai S, Yokogawa K, Miyamoto K. Novel drug delivery system to bone using acidic oligopeptide: pharmacokinetic characteristics and pharmacological potential. J. Drug Target. 2001;9:111–121. doi: 10.3109/10611860108997922. [DOI] [PubMed] [Google Scholar]
- 76.Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M, Fujisawa R, Morito K, Masamune Y, Waki YY, Kasugai S, Miyamoto K. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–1233. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- 77.Nishioka T, Tomatsu S, Gutierrez MA, Trandafirescu GG, Lopez P, Grubb JH, Kanai R, Kobayashi H, Yamaguchi S, Gottesman GS, Cahill R, Noguchi A, Sly WS. Targeted Drug Delivery to Bone: Characterization of Human Tissue-nonspecific Alkaline Phosphatase Tagged with an Acidic Oligopeptide. Mol. Genet. Metab. 2006;88:244–255. doi: 10.1016/j.ymgme.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, Van Sickle BJ, Simmons JH, Edgar TS, Bauer ML, Hamdan MA, Bishop N, Lutz RE, McGinn M, Craig S, Moore JN, Taylor JW, Cleveland RH, Cranley WR, Lim R, Thacher TD, Mayhew JE, Downs M, Millán JL, Skrinar AM, Crine P, Landy H. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 2012;8:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 79.Oikawa H, Tomatsu S, Haupt B, Montaño AM, Shimada T, Sly WS. Enzyme replacement therapy on hypophosphatasia mouse model. J. Inherit. Metab. Dis. 2014;37:309–317. doi: 10.1007/s10545-013-9646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Millán JL, Narisawa S, Lemire I, Loisel TP, Boileau G, Leonard P, Gramatikova S, Terkeltaub R, Camacho NP, McKee MD, Crine P, Whyte MP. Enzyme replacement therapy for murine hypophosphatasia. J. Bone Miner. Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montaño AM, Oikawa H, Tomatsu S, Nishioka T, Vogler C, Gutierrez MA, Oguma T, Tan Y, Grubb JH, Dung VC, Ohashi A, Miyamoto K, Orii T, Yoneda Y, Sly WS. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol. Genet. Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Wang C, Hwu W, Lin K. Long-term follow-up of a girl with Maroteaux-Lamy syndrome after bone marrow transplantation. World J. Pediatr. 2008;4:152–154. doi: 10.1007/s12519-008-0031-9. [DOI] [PubMed] [Google Scholar]
- 83.Boelens J. Trends in haematopoietic cell transplantation for inborn errors of metabolism. J. Inherit. Metab. Dis. 2006;29:413–420. doi: 10.1007/s10545-005-0258-8. [DOI] [PubMed] [Google Scholar]
- 84.Orchard P, Blazar B, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J. Pediatr. 2007;151:340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 85.Tolar J, Grewal SS, Bjoraker KJ, Whitley CB, Shapiro EG, Charnas L, Orchard PJ. Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome. Bone Marrow Transplant. 2008;41:531–535. doi: 10.1038/sj.bmt.1705934. [DOI] [PubMed] [Google Scholar]
- 86.Khanna G, Van Heest AE, Agel J, Bjoraker K, Grewal S, Abel S, Krivit W, Peters C, Orchard PJ. Analysis of factors affecting development of carpal tunnel syndrome in patients with Hurler syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;39:331–334. doi: 10.1038/sj.bmt.1705586. [DOI] [PubMed] [Google Scholar]
- 87.Stoop FJ, Kruyt MC, van der Linden MH, Sakkers RJ, van Hasselt PM, Castelein RMC. Prevalence and development of orthopaedic symptoms in the dutch hurler patient population after haematopoietic stem cell transplantation. JIMD Rep. 2013;9:17–29. doi: 10.1007/8904_2012_175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vellodi A, Young EP, Cooper A, Wraith JE, Winchester B, Meaney C, Ramaswami U U, Will A. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch. Dis. Child. 1997;76:92–99. doi: 10.1136/adc.76.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, Galambrun C, Bertrand Y, Froissart R, Dorche C, Gebuhrer L, Garin C, Berard J, Guibaud P. Outcome of 27 patients with hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 90.Masterson EL, Murphy PG, O’Meara A, Moore DP, Dowling FE, Fogarty EE. Hip dysplasia in hurler’s syndrome: orthopaedic management after bone marrow transplantation. J. Pediatr. Orthop. 1996;16:731–733. doi: 10.1097/00004694-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Taylor C, Brady P, O’Meara A, Moore D, Dowling F, Fogarty E. Mobility in hurler syndrome. J. Pediatr. Orthop. 2008;28:163–168. doi: 10.1097/BPO.0b013e3181649e25. [DOI] [PubMed] [Google Scholar]
- 92.Weisstein JS, Delgado E, Steinbach LS, Hart K, Packman S. Musculoskeletal manifestations of hurler syndrome: long-term follow-up after bone marrow transplantation. J. Pediatr. Orthop. 2004;24:97–101. doi: 10.1097/00004694-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 93.Aldenhoven M, Sakkers RJ, Boelens J, de Koning TJ, Wulffraat NM. Musculoskeletal manifestations of lysosomal storage disorders. Ann. Rheum. Dis. 2009;68:1659–1665. doi: 10.1136/ard.2008.095315. [DOI] [PubMed] [Google Scholar]
- 94.Langereis EJ, Borgo A, Crushell E, Harmatz PR, van Hasselt PM, Jones SA, Kelly PM, Lampe C, van der Lee JH, Odent T, Sakkers R, Scarpa M, Schafroth MU, Struijs PA, Valayannopoulos V, White KK, Wijburg FA. Treatment of hip dysplasia in patients with mucopolysaccharidosis type I after hematopoietic stem cell transplantation: results of an international consensus procedure. Orphanet. J. Rare Dis. 2013;8:1–9. doi: 10.1186/1750-1172-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herskhovitz E, Young E, Rainer J, Hall C, Lidchi V, Chong K, Vellodi A. Bone marrow transplantation for Maroteaux-Lamy syndrome (MPS VI): long-term follow-up. J. Inherit. Metab. Dis. 1999;22:50–62. doi: 10.1023/a:1005447232027. [DOI] [PubMed] [Google Scholar]
- 96.Malatack J, Consolini D, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr. Neurol. 2003;29:391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum. Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 98.Turbeville S, Nicely H, Rizzo JD, Pedersen TL, Orchard PJ, Horwitz ME, Horwitz EM, Veys P, Bonfim C, Al-Seraihy A. Clinical outcomes following hematopoietic stem cell transplantation for the treatment of mucopolysaccharidosis VI. Mol. Genet. Metab. 2011;102:111–115. doi: 10.1016/j.ymgme.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tomatsu S, Yasuda E, Patel P, Ruhnke K, Shimada T, Mackenzie WG, Mason RW, Thacker MM, Theroux M, Montaño AM, Alméciga-Díaz CJ, Barrera LA, Chinen Y, Sly WS, Rowan D, Suzuki Y, Orii T. Morquio A syndrome: diagnosis and current and future therapies. Pediatr. Endocrinol. Rev. in press. [PMC free article] [PubMed] [Google Scholar]
- 100.Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol. Genet. Metab. Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R. Bone marrow transplantation in children with Hunter syndrome: Outcome after 7 to 17 years. J. Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Araya K, Sakai N, Mohri I, Kagitani-Shimono K, Okinaga T, Hashii Y, Ohta H, Nakamichi I, Aozasa K, Taniike M, Ozono K. Localized donor cells in brain of a Hunter disease patient after cord blood stem cell transplantation. Mol. Genet. Metab. 2009;98:255–263. doi: 10.1016/j.ymgme.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, Tanaka T, Otomo T, Ohashi T, Ishige-Wada M, Yabe H, Ohura T, Suzuki N, Kato K, Adachi S, Kobayashi R, Mugishima H, Kato S. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Tanjuakio J, Suzuki Y, Patel P, Yasuda E, Kubaski F, Tanaka A, Yabe H, Mason RW, Montaño AM, Orii KE, Orii KO, Fukao T, Orii T, Tomatsu S. Activities of Daily Living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol. Genet. Metab. doi: 10.1016/j.ymgme.2014.11.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pievani A, Azario I, Antolini L, Shimada T, Patel P, Remoli C, Rambaldi B, Valsecchi MG, Riminucci M, Biondi A, Tomatsu S, Serafini M. Neonatal bone marrow transplantation prevents bone pathology in a mouse model of mucopolysaccharidosis type I. Blood. doi: 10.1182/blood-2014-06-581207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N. Engl. J. Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 107.Tse W, Laughlin MJ. Umbilical Cord Blood Transplantation: A New Alternative Option. Hematology Am Soc Hematol Educ Program. 2005:377–383. doi: 10.1182/asheducation-2005.1.377. [DOI] [PubMed] [Google Scholar]
- 108.Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin. Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 109.Grewal SS, Wynn R, Abdenur JE, Burton BK, Gharib M, Haase C, Hayashi RJ, Shenoy S, Sillence D, Tiller GE, Dudek ME, van Royen-Kerkhof A, Wraith JE, Woodard P, Young GA, Wulffraat N, Whitley CB, Peters C. Safety and efficacy of enzyme replacement therapy in combination with hematopoietic stem cell transplantation in Hurler syndrome. Genet. Med. 2005;7:143–146. doi: 10.1097/01.gim.0000154299.22120.6a. [DOI] [PubMed] [Google Scholar]
- 110.Seregin SS, Amalfitano A. Gene therapy for lysosomal storage diseases: progress, challenges and future prospects. Curr. Pharm. Des. 2011;17:2558–2574. doi: 10.2174/138161211797247578. [DOI] [PubMed] [Google Scholar]
- 111.Ponder K, Haskins M. Gene therapy for mucopolysaccharidosis. Expert Opin. Biol. Ther. 2007;7:1333–1345. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomatsu S, Alméciga-Díaz CJ, Barbosa H, Montaño AM, Barrera LA, Shimada T, Yasuda E, Mackenzie WG, Mason RW, Suzuki Y, Orii KE, Orii T. Therapies of Mucopolysaccharidosis IVA (Morquio A Syndrome) Expert Opin. Orphan Drugs. 2013;1:805–818. doi: 10.1517/21678707.2013.846853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. http://clinicaltrials.gov/
- 114.Cheng SH. Gene therapy for the neurological manifestations in lysosomal storage disorders. J. Lipid Res. 2014;55:1827–1838. doi: 10.1194/jlr.R047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osborn MJ, McElmurry RT, Peacock B, Tolar J, Blazar BR. Targeting of the CNS in MPS-IH using a nonviral transferrin-alpha-L-iduronidase fusion gene product. Mol. Ther. 2008;16:1459–1466. doi: 10.1038/mt.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Osborn MJ, McElmurry RT, Lees CJ, DeFeo AP, Chen ZY, Kay MA, Naldini L, Freeman G, Tolar J, Blazar BR. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Mol. Ther. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aronovich EL, Bell JB, Belur LR, Gunther R, Koniar B, Erickson DC, Schachern PA, Matise I, McIvor RS, Whitley CB, Hackett PB. Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J. Gene Med. 2007;9:403–415. doi: 10.1002/jgm.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aronovich EL, Bell JB, Khan SA, Belur LR, Gunther R, Koniar B, Schachern PA, Parker JB, Carlson CS, Whitley CB, McIvor RS, Gupta P, Hacket PB. Systemic correction of storage disease in MPS I NOD/SCID mice using the sleeping beauty transposon system. Mol. Ther. 2009;17:1136–1144. doi: 10.1038/mt.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O’donnell P, Sleeper MM, Vite C, Herati R, Aquirre GD, Haskins M, Ponder KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol. Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 120.Herati RS, Ma X, Tittiger M, Ohlemiller KK, Kovacs A, Ponder KP. Improved retroviral vector design results in sustained expression after adult gene therapy in mucopolysaccharidosis I mice. J. Gene Med. 2008;10:972–982. doi: 10.1002/jgm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herati RS, Knox VW, O’Donnell P, D’Angelo M, Haskins ME, Ponder KP. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol. Genet. Metab. 2008;95:142–151. doi: 10.1016/j.ymgme.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Visigalli I, Delai S, Politi LS, Di Domenico C, Cerri F, Mrak E, D’Isa R, Ungaro D, Stok M, Sanvito F, Mariani E, Staszewsky L, Godi C, Russo I, Cecere F, Del Carro U, Ruvinacci A, Brambilla R, Quattrini A, Di Natale P, Ponder K, Naldini L, Biffi A. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;9:5130–5139. doi: 10.1182/blood-2010-04-278234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK, Snella EM, Mohammed EE, Thomson CB, Raoul S, Joussemet B, Roux F, Chérel Y, Lajat Y, Piraud M, Benchaouir R, Hermening S, Petry H, Froissart R, Tardieu M, Ciron C, Moullier P, Parkes J, Kline KL, Maire I, Vanier MT, Heard JM, Colle MA. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol. Ther. 2011;19:251–259. doi: 10.1038/mt.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolf DA, Lenander AW, Nan Z, et al. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol. Dis. 2011;43:123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Toietta G, Severini G, Traversari C, et al. Various cells retrovirally transduced with N-acetylgalactosoamine-6-sulfate sulfatase correct Morquio skin fibroblasts in vitro. Hum. Gene Ther. 2001;12:2007–2016. doi: 10.1089/104303401753204571. [DOI] [PubMed] [Google Scholar]
- 126.Alméciga-Díaz C, Montaño AM, Tomatsu S. Adeno-associated virus mediated gene therapy in a murine model of Morquio syndrome type A. Mol. Genet. Metab. 2009;96:S13. [Google Scholar]
- 127.Alméciga-Díaz C, Montaño A, Tomatsu S. Contribución colombiana al conocimiento de la enfermedad de Morquio. Medicina (Mex) 2012;34:221–241. [Google Scholar]
- 128.Alméciga-Díaz C, Herrera J, Barbosa H. New viral vectors for Morquio syndrome type A gene therapy. Mol. Genet. Metab. 2013;108:S19. [Google Scholar]
- 129.Cotugno G, Annunziata P, Tessitore A, O1Malley T, Capalbo A, Faella A, Bartolomeo R, O’Donnell P, Wang P, Russo F, Sleeper MM, Knox VW, Fernandez S, Levanduski L, Hopwood J, De Leonibus E, Haskins M, Auricchio A. Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer. Mol. Ther. 2011;19:461–469. doi: 10.1038/mt.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferla R, O’Malley T, Calcedo R, O’Donnell P, Wang P, Cotugno G, Claudiani p., Wilson JM, Haskins M, Auricchio A. Gene therapy for mucopolysaccharidosis type VI is effective in cats without pre-existing immunity to AAV8. Hum. Gene Ther. 2013;24:163–169. doi: 10.1089/hum.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ponder KP, O’Malley TM, Wang P, O’Donnell PA, Traas AM, Knox VW, Aquirre GA, Ellinwood NM, Metcalf JA, Wang B, Parkinson-Lawrence EJ, Sleeper MM, Brooks DA, Hopwood JJ, Haskins ME. Neonatal gene therapy with a gamma retroviral vector in mucopolysaccharidosis VI cats. Mol. Ther. 2012;20:898–907. doi: 10.1038/mt.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith LJ, Martin JT, O’Donnell P, Wang P, Elliot DM, Haskins ME, Ponder KP. Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol. Genet. Metab. 2012;107:145–152. doi: 10.1016/j.ymgme.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xing EM, Knox VW, O’Donnell PA, Sikura T, Liu Y, Wu S, Casal ML, Haskins ME, Ponder KP. The effect of neonatal gene therapy on skeletal manifestations in mucopolysaccharidosis VII dogs after a decade. Mol. Genet. Metab. 2013;109:183–193. doi: 10.1016/j.ymgme.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Macsai CE, Derrick-Roberts AL, Ding X, Zarrinkalam KH, McIntyre C, Anderson PH, Anson DS, Byers S. Skeletal response to lentiviral mediated gene therapy in a mouse model of MPS VII. Mol. Genet. Metab. 2012;106:202–213. doi: 10.1016/j.ymgme.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 135.Chen YH, Claflin K, Geoghegan JC, Davidson BL. Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol. Ther. 2012;20:1393–1399. doi: 10.1038/mt.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Husain T, Passini MA, Parente MK, Fraser NW, Wolfe JH. Long-term AAV vector gene and protein expression in mouse brain from a small pan-cellular promoter is similar to neural cell promoters. Gene Ther. 2009;16:927–932. doi: 10.1038/gt.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yasuda E, Fushimi K, Suzuki S, Shimizuki K, Takami T, Zustin J, Patel P, Ruhnke K, Shimada T, Boyce B, Kokas T, Barone C, Theroux M, Mackenzie W, Nagel B, Ryerse JS, Orii KE, Iida H, Orii T, Tomatsu S. Pathogenesis of Morquio A syndrome: na autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 138.Kumagai K, Shirabe S, Miyata N, Murata M, Yamauchi A, Kataoka Y, Niwa M. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis--an open clinical trial. BMC Clin. Pharmacol. 2010;10:7. doi: 10.1186/1472-6904-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schuchman EH, Ge Y, Lai A, Borisov Y, Faillace M, Eliyahu E, He X, Iatridis J, Vlassara H, Striker G, Simonaro CM. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. Plos One. 2013;8:e54459. doi: 10.1371/journal.pone.0054459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simonaro C, Frohbergh M, Ge Y, Meng F, Schuchman E. A63: treatment of arthritis in animal models of the mucopolysaccharidoses using a novel anti-inflammatory drug, pentosan polysulfate. Arthritis. Rheumatol. 2014;66(Suppl. 11):S93. [Google Scholar]