Abstract
The Merkel cell-neurite complex is a unique vertebrate touch receptor comprising two distinct cell types in the skin. Its presence in touch-sensitive skin areas was recognized more than a century ago, but the functions of each cell type in sensory transduction have been unclear. Three recent studies demonstrate that Merkel cells are mechanosensitive cells that function in touch transduction via Piezo2. One study concludes that Merkel cells rather than sensory neurons are principal sites of mechanotransduction, whereas the other two studies report that both Merkel cells and neurons encode mechanical inputs. Together, these studies settle a longstanding debate on whether Merkel cells are mechanosensory cells, and enable future investigations of how these skin cells communicate with neurons.
Keywords: Mechanosensory cells, Mechanoreceptor, Piezo, Touch dome, Somatosensory
Merkel cell-neurite complexes in skin
We depend on our sense of touch to gather information about the world around us and to accomplish skilled movements. Our ability to experience the richness of our tactile environment relies on touch receptors present in the skin. Touch receptors express mechanically activated (MA) ion channels that detect and convert mechanical stimuli into electrical signals. These electrical signals are then delivered to the central nervous system (CNS), where they are processed and interpreted as touch sensations.
The sensory neurons that initiate touch sensation are called low threshold mechanoreceptors (LTMRs). LTMRs terminate in skin and are classified as Aβ, Aδ, or C fibers based on their degree of myelination and action potential conduction velocities [1–3]. Both hairy and hairless skin areas contain discrete sets of LTMRs, and different types of LTMRs detect specific tactile modalities [4]. For example, lanceolate nerve endings in hair follicles respond to hair movement [5, 6], whereas Pacinian and Meissner’s corpuscles in hairless skin areas respond to vibration of various frequencies [7–9]. The Merkel cell-neurite complex is a LTMR present in both skin types that is thought to be important for mediating gentle touch [3, 10, 11]. Interestingly, the Merkel cell-neurite complex consists of two distinct but closely associated cell types: Aβ sensory neurons and epithelial cells known as Merkel cells.
Merkel cells are a rare population of epithelial cells present in skin of most vertebrates [12]. First identified by Friedrich Sigmund Merkel in 1875, these cells were originally described as Tastzellen (touch cells) because their close association with nerve fibers led Merkel to presume their function to be in touch sensation [11]. Merkel cells are indeed found in touch-sensitive areas of the skin, such as fingertips, lips, and specialized spots in hairy skin called touch domes [10, 11, 13, 14], and they are also found in abundance in mammalian whisker follicles [15]. Among epithelial cells, Merkel cells are unique because they form close contacts with Aβ sensory neurons at the epidermal-dermal junction [10, 15]. The contacts between Merkel cells and afferent terminals are proposed to be anatomically similar to synaptic contacts [16–20].
In 1969, Iggo and Muir provided the first functional evidence to implicate Merkel cellneurite complexes in touch reception. By recording from touch-sensitive neurons in cat hairy skin, they demonstrated that a particular type of slowly adapting (SA) discharge was evoked by mechanical stimulation of touch domes, where Merkel cell-neurite complexes localize [10]. They found that pressure applied to a touch dome produced long-lasting action potential trains characterized by an irregular firing pattern with a large variation in interspike intervals, and they categorized this firing pattern as SA type I (SAI) [10]. SAI afferents are proposed to encode fine details of objects because of their high spatial resolution and sensitivity to object features such as points, edges, and curvature [21].
Based on these findings, Merkel cell-neurite complexes are thought to be the touch receptors that initiate SAI responses of Aβ afferents for tactile discrimination of shapes and textures [10, 22]; however, the precise functions of Merkel cells and Aβ SAI sensory afferents during touch transduction have been debated [4, 15, 22]. A key question is: which cell type is responsible for transducing mechanosensory stimuli into electrical signals? The answer to this question is not immediately obvious because the nervous system has devised two strategies for encoding sensory stimuli into neuronal signals. Sensory transduction can be accomplished either by primary sensory neurons or by epithelial-derived secondary sensory cells. For example, olfactory neurons [23] and most cutaneous LTMRs [4] are primary sensory neurons that both mediate sensory transduction and conduct neuronal impulses to the CNS. In other cases, such as taste receptor cells [24] and mechanosensory hair cells of the inner ear [25, 26], sensory transduction is accomplished by epithelial-derived cells that release neurotransmitters to activate afferent neurons, which then convey sensory information to the CNS.
For the Merkel cell-neurite complex, a case can be made for either primary or secondary sensory cells. Because all other LTMRs are primary sensory neurons, it stands to reason that Aβ SAI afferents might also be mechanosensitive. On the other hand, a number of suggestive anatomical and developmental parallels have been observed between Merkel cells and hair cells of the inner ear. They are both epithelial-derived cells innervated by sensory neurons [27, 28]. Moreover, they express the same developmental transcription factors including atonal homolog 1 (Atoh1), an essential transcription factor for development of both Merkel cells and hair cells [29–32]. Do Merkel cells, like hair cells, also function as mechanosensory cells?
A historical view of Merkel cells
The possibility that Merkel cells are sensory cells has been a subject of debate for decades. Some studies concluded that Merkel cells are mechanosensors, whereas others concluded that SAI afferents are primary sensory receptors, and that Merkel cells are accessory cells that modulate SAI responses [29, 33–38]. For example, phototoxic ablation of Merkel cells caused decreased SAI responses in one study [38], but showed no effect in another report [37]. A third group raised concerns about the effectiveness of this ablation method, as they found that the sensitivity to phototoxic destruction varied among Merkel cells, and this method had an adverse effect on afferent terminals as well [35]. Another study examined SA responses in the neurotrophin receptor p75 knockout mice, in which Merkel cells initially develop but are lost with age [34]. p75 knockout mice showed a normal proportion of SA responses even after losing the majority of epidermal Merkel cells, indicating that Merkel cells are not required for touch-evoked firing in SA afferents [34]. In this study, SAI firing patterns were not analyzed in detail, thus, it is unclear whether Merkel-cell loss might have subtly altered SAI firing properties. Overall, these studies were not sufficient to clarify whether Merkel cells are necessary for SAI firing patterns.
More recently, complete ablation of Merkel cells was achieved in the pelage skin of mice by genetically deleting Atoh1 [29]. In these mice, touch domes develop without Merkel cells but are innervated by myelinated afferents [29]. These mice showed a selective and complete loss of SAI firing patterns, which indicates that Merkel cells are essential components for producing SAI responses in sensory afferents [29]. These results are consistent with the hypothesis that Merkel cells are mechanosensory receptor cells; however, two other models can also explain this phenotype. First, developmental deletion of Merkel cells might have adverse effects on Aβ SAI-afferent development [4, 22]. Second, the firing patterns of Aβ SAI afferents could differ in the absence of Merkel cells, leading them to be classified as non-SAI responses.
To qualify as a mechanosensory receptor cell, the candidate cell type should be mechanosensitive. Thus, more direct approaches have been used to ask whether Merkel cells are intrinsically mechanosensitive. In isolated rat whisker hair follicles, direct displacement of Merkel cells using a glass probe elicited robust Ca2+ influx in these cells, and this rise in Ca2+ was suggested to be important for synaptic transmission to the afferent nerve terminals [36]. In this setting, however, SAI afferent terminals were in contact with Merkel cells, so neuronal contribution during mechanotransduction could not be ruled out. Other groups performed similar experiments in dissociated Merkel cells to avoid this problem. In some studies, hypotonicinduced cell swelling was used as an alternative to a displacement stimulus. When dissociated Merkel cells were exposed to hypotonic solutions, a similar increase in intracellular Ca2+ was observed [33, 39]; however, hypotonic-induced Ca2+ influx in Merkel cells might be a consequence of activating volume-regulatory machinery rather than mechanosensory transduction mechanisms [33].
Most LTMRs have mechanosensitive endings that terminate in skin [40, 41]. A lack of definitive proof for mechanosensitivity of Merkel cells led some groups to conclude that SAI sensory neurons are primary mechanoreceptors, and that Merkel cells act as accessory cells [42, 43]. Indeed, it has been argued that the response latency of SAI afferents is too short to involve synaptic transmission from Merkel cells, suggesting that the afferents must be directly mechanosensitive [44].
A third model, which posits two receptors sites, combines elements of the two previous models by proposing that both Merkel cells and Aβ SAI sensory afferents are involved in mechanotransduction [45]. Supported by pharmacological studies that altered synaptic signaling, this model hypothesizes that SAI afferents mediate the initial dynamic phase of touch responses and that Merkel cells transduce the sustained, or static, phase of touch responses [17, 46, 47]. The two-receptor site model can account for both the short latency of SAI firing and the presence of a synapse between Merkel cells and SAI afferents.
Merkel cells are touch-sensitive cells with Piezo2-dependent transduction channels
Recently, important advances have been made to elucidate the function of Merkel cells in touch. Three independent studies report disparate set of experiments and provide direct evidence that Merkel cell are indeed touch-sensitive cells, and that they function as essential components of touch receptors in skin.
Two studies used a combination of mouse genetics and in vitro and intact electrophysiological recordings to examine the role of Merkel cells during touch transduction [48, 49], whereas a third study used an ex vivo rat whisker preparation with pharmacological manipulations to elucidate Merkel-cell function [50]. As an initial step, all three studies independently provided a clear answer to the question of whether Merkel cells are cell-autonomously touch sensitive. When Merkel cells were gently displaced with a glass probe, they produced robust MA currents both in vitro and ex vivo [48–50]. The biophysical properties of these currents resembled those of Piezo2, a MA ion channel expressed in somatosensory neurons [48–51]. Consistent with this observation, all three groups demonstrated that Merkel cells preferentially expressed Piezo2 [48–50]. For the next step, three studies took distinct approaches.
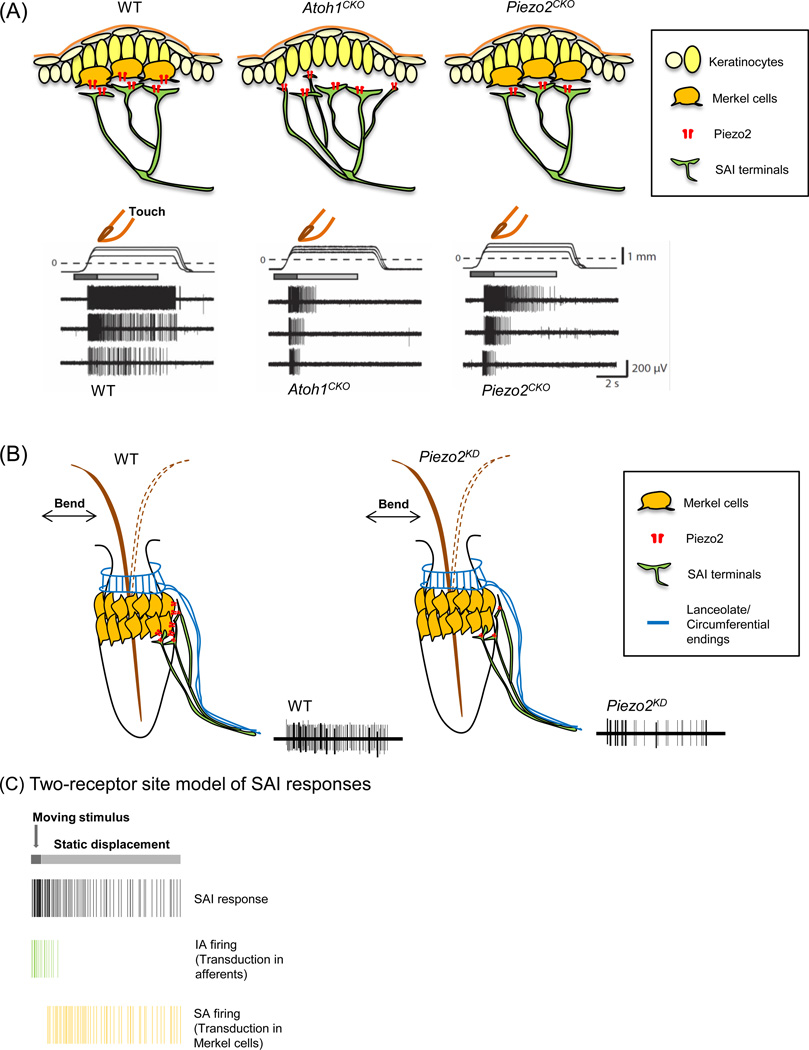
One group showed that Merkel-cell activation alone is sufficient to induce action potential firing in Aβ SAI sensory afferents by directing Channelrhodopsin-2 (ChR2) protein expression in Merkel cells [49]. This optogenetic approach allowed selective Merkel-cell activation without directly exciting associated afferents. Moreover, sustained firing of SAI afferents was acutely and reversibly attenuated by optogenetically silencing Merkel cells expressing the light-gated proton pump ArchT [49]. Together, these experiments show that Merkel-cell depolarization is necessary and sufficient for sustained action potential firing in SAI afferents, which directly demonstrates an excitatory connection between Merkel cells and sensory afferents [49]. Next, they examined the role of Merkel cells in SAI responses by performing ex vivo skin-nerve recordings in skin-specific Atoh1 conditional knockout (Atoh1CKO) mice, which lack Merkel cells in their skin. Targeted electrophysiological recordings from fluorescently labeled Aβ SAI afferents revealed that SAI responses in Atoh1CKO mice are converted to intermediately adapting (IA) firing patterns: the typical sustained firing during the static phase is truncated (Figure 1A, middle) [49]. Interestingly, firing during the dynamic phase is reduced but not completely abolished, confirming that Aβ SAI afferents, like other LTMRs, are also mechanosensitive (Figure 1A, middle) [45, 49]. Together, these results directly demonstrate that Merkel-cell activation is sufficient to produce action potentials in neighboring Aβ fibers, and that Merkel cells are essential to induce sustained neuronal activity in tactile afferents. Importantly, since Aβ SAI afferents lacking Merkel cells showed firing during dynamic stimuli, these data provide direct support for the two-receptor site model discussed above (Figure 1C) [45, 49, 52].
Figure 1. SA firing responses of Merkel cell-neurite complexes in touch domes and whisker follicles.
(A) Mechanically evoked responses from afferents innervating touch domes in wild-type (WT) (left), Atoh1CKO (middle) and Piezo2CKO (right) mice. Displacement applied to a touch dome causes action potential firing in SAI afferents. Moving displacements (dark gray bar) evoke firing in all three genotypes. In wild-type mice, static displacement (light gray bar) evokes slowly adapting (SA) firing; however, firing during static displacement is truncated to intermediately adapting (IA) firing in both Atoh1CKO and Piezo2CKO touch domes. (B) Mechanically evoked responses from sensory afferent bundles innervating WT whisker follicle (left) and the whisker follicle with Piezo2 knockdown (Piezo2KD) (right). Piezo2 knockdown in Merkel cells causes action potentials to be reduced (right). (C) A two-receptor site model of SAI responses. SAI afferents transduce moving stimuli, and Merkel cells mediate sustained firing during static displacement. All traces have been recreated from [48–50].
A different study explored the role of the recently discovered Piezo2 MA ion channel in Merkel-cell mechanotransduction [48]. This report examined whether Piezo2 acts as the principal mechanotransduction molecule in Merkel cells by utilizing skin-specific Piezo2 conditional knockout (Piezo2CKO) mice, in which Merkel cells develop normally but lack Piezo2 ion channels [48]. When whole-cell recordings were performed in dissociated Merkel cells, MA currents were detected only in wild-type Merkel cells, and not in Piezo2-ablated Merkel cells [48]. This result indicates that Piezo2 channel activity is required for intrinsic mechanosensitivity of Merkel cells. Skin-nerve recordings from touch domes of Piezo2CKO mice showed that, although dynamic firing is normal, sustained firing is truncated, mimicking the phenotype of Atoh1CKO mice (Figure 1A, right) [48, 49]. The Piezo2CKO phenotype strongly supports the two-receptor-site model: the intrinsic mechanosensitivity of Aβ SAI afferents is sufficient to account for dynamic firing, whereas Piezo2 activity in Merkel cells is required for SA firing (Figure 1C).
Interestingly, firing during dynamic stimulation differed between the Piezo2 and Atoh1 knockout models [48, 49]. The dynamic phase firing did not differ significantly between Piezo2CKO and wild-type touch domes (Figure 1A, left and right), whereas it was attenuated in Atoh1CKO touch domes (Figure 1A, middle) [48, 49]. This phenotypic difference could be attributed to i) a requirement for Merkel cells in SAI-afferent development, ii) Piezo2-independent functions of Merkel cells in enhancing dynamic firing of SAI afferents, or iii) effects of Merkel cells on touch-dome tissue mechanics [49]. To distinguish between these possibilities, tissue mechanics measurements and controlled ablation of Merkel cells in adult mice using an inducible system are needed.
In parallel, another group investigated the function of Piezo2 in Merkel-cell mechanotransduction in isolated rat whisker follicles containing Merkel cells and associated sensory afferent bundles [50]. This report demonstrates that Merkel cells are touch-sensitive cells with rapidly inactivating MA currents and slow, regenerative calcium action potentials by patch-clamp recordings of Merkel cells in situ [50]. After recording in the presence of a Piezo2 antibody or Piezo2 shRNA lentiviral particles that acutely inhibited the Piezo2 activity, the authors showed that mechanosensitivity of Merkel cells is mediated via Piezo2 [50].
Merkel cells in whisker follicles are activated by hair deformation [50]. Thus, the study reported compound action potentials from sensory afferent bundles of the whisker follicle during whisker movement in the presence of various pharmacological inhibitors or Piezo2 knockdown (Figure 1B) [50]. shRNA-mediated knockdown of Piezo2 in situ reduced, but did not fully abolish, compound action potentials (Figure 1B) [50]. Based on these data, this study concluded that Merkel cells, rather than their associated Aβ-afferent nerve endings, are primary sites of tactile transduction [50]. However, the presence of action potentials following Piezo2 knockdown in the whisker follicle is consistent with observations in mouse touch domes that support the two-receptor site model (Figure 1C).
Although some differences in action potential firing properties of tactile afferents in semi-intact systems were observed across these studies, these discrepancies can be attributed to differences in experimental approaches and model systems utilized by each group. For instance, the two mouse studies used genetic manipulations to specifically ablate either Merkel cells or Piezo2 protein in Merkel cells, whereas the rat whisker study used biochemical and pharmacological manipulations to block Piezo2 channel function in situ [48–50]. In the latter setting, a local application of these reagents to Merkel cells can impact the sensory nerve terminals as well. Piezo2 is expressed in sensory afferents in addition to Merkel cells [48, 53, 54]; therefore, Piezo2 knockdown by shRNA molecules might occur in the associated afferents, although the study reported that Piezo2 knockdown is specific to Merkel cells [50]. On the other hand, gene knockout studies using mouse models can also introduce compensation for constituitive deletion of genes during development, compared to the acute ablation of proteins by pharmacological reagents. Another possibility is that SA afferents in different anatomical locations might utilize distinct transduction mechanisms.
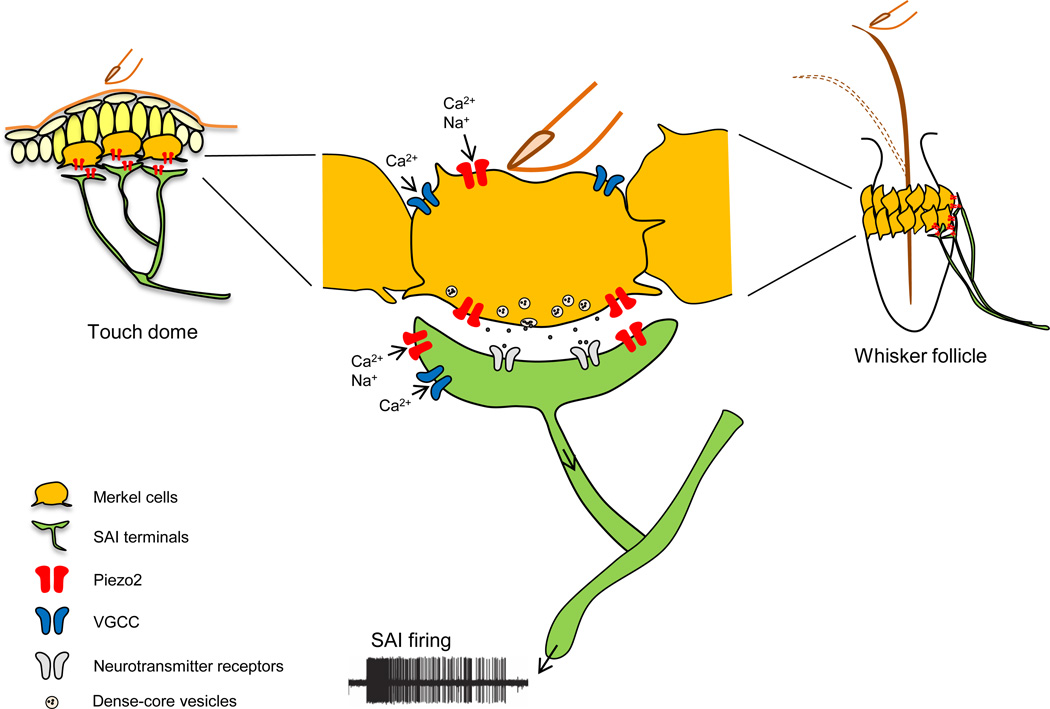
Collectively, three studies convincingly showed that Merkel cells play an instructive role in mechanosensitivity of Merkel cell-neurite complexes: Merkel cells transduce mechanical stimuli into electrical signals through Piezo2, and consequently induce action potentials in SAI afferents through activation of voltage-gated Ca2+ channels (Figure 2) [48–50]. Moreover, these studies indicate that both Merkel cells and SAI afferents act as sensors: sensory afferents respond to moving mechanical stimuli, followed by Merkel cells that confer sustained responses during static indentation of the skin or deformation of whisker hairs (Figure 2) [48–50].
Figure 2. A model of touch transduction in the Merkel cell-neurite complex.
1) Gentle pressure on the skin or hair deformation of the whisker opens mechanotransduction channels, hypothesized to be Piezo2, in SAI afferents to initiate SAI action potential firing. 2) Simultaneously, it opens Piezo2 channels in Merkel cells, which causes Merkel cell depolarization. 3) Voltage-gated calcium channels (VGCC) in Merkel cells are subsequently activated, and 4) neurotransmitters are released as a result and contribute to SAI firing. Adapted from [49, 50, 74].
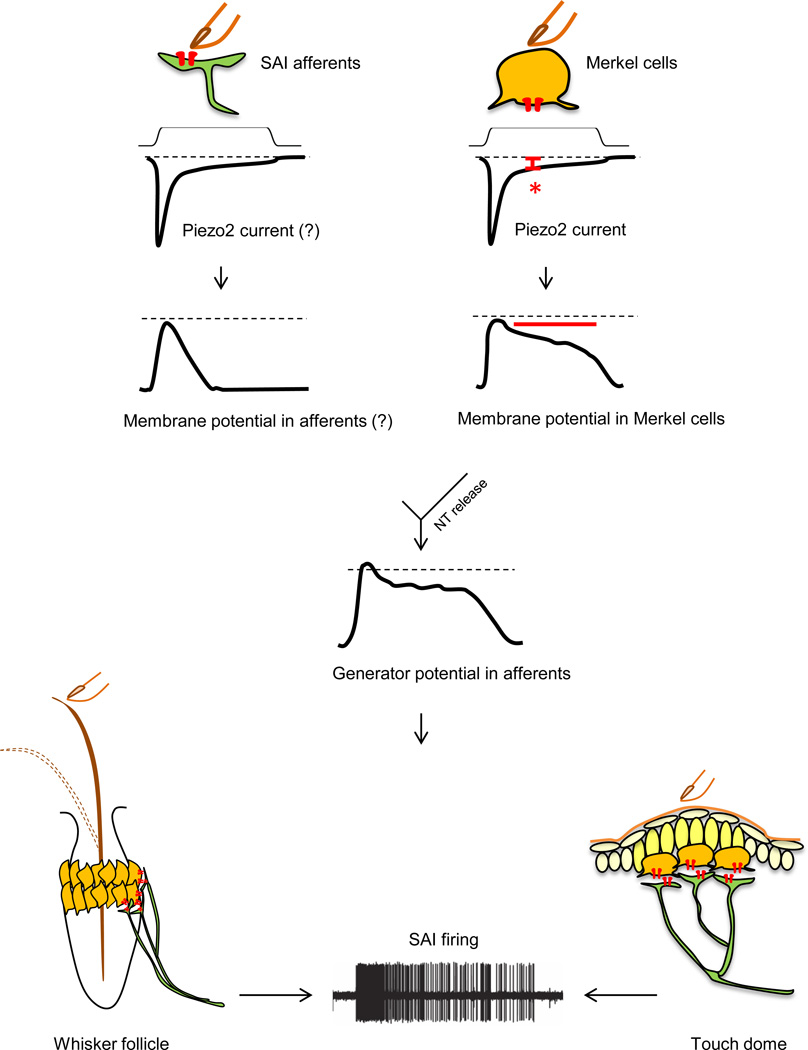
Interestingly, these studies concurrently offer an explanation for the obvious question: how can a rapidly adapting Piezo2 MA channel in Merkel cells give rise to SA firing in tactile afferents? Although Piezo2 channel activity inactivates within a few milliseconds, a small steady-state current is observed during mechanical stimulation of Merkel cells (Figure 3, right) [48]. Merkel cells have a high membrane resistance; therefore, a small, long-lasting Piezo2-dependent current produces a large sustained depolarization in Merkel cells (Figure 3, right) [48–50, 55]. This prolonged depolarization of Merkel cells can consequently contribute to slowly adapting firing in SAI afferents (Figure 3). Indeed, Merkel cells’ ability to produce sustained depolarization via Piezo2 could be one of the reasons why a two-receptor-site mechanism has evolved: an elegant way to utilize a rapidly inactivating MA ion channel to generate SA firing of a LTMR.
Figure 3. A model of mechanotransduction in each component of the Merkel cell-neurite complex.
Left: Skin indentation by touch opens rapidly inactivating mechanotransduction channels, hypothesized to be Piezo2, in SAI afferents and causes a rapid depolarization. Right: Simultaneously, it opens Piezo2 channels in Merkel cells. A Piezo2-dependent small, long-lasting current (red asterisk) induces a sustained depolarization in Merkel cells (red bar) due to the high membrane resistance of Merkel cells. Merkel cell depolarization causes consequent neurotransmitter (NT) release from Merkel cells to SAI afferents. Combined generator potential changes from the afferents and Merkel cells contribute to slowly adapting action potential firing in SAI afferents.
Now that the function of Merkel cells during touch transduction is clear, it will be informative to reveal the contribution of sensory afferents during mechanotransduction. Indeed, a recent study reports that the ablation of Piezo2 in both Merkel cells and sensory neurons leads to a profound loss of touch sensation in mice, suggesting that Piezo2 is the major transducer of cutaneous LTMRs [54].
How do Merkel cells communicate with SAI sensory neurons?
To understand mechanosensory signaling in Merkel cell-neurite complexes, the next important question to address is: how do Merkel cells excite SAI afferents? Ultrastructural studies and molecular profiling suggest that Merkel cells are presynaptic cells that communicate with afferent nerve terminals through synaptic transmission [10, 20, 30, 56, 57]. Microarray analysis of purified Merkel cells from mouse skin has identified presynaptic active-zone molecules, synaptic vesicle proteins, and molecules involved in neuropeptide production and excitatory glutamate release [30]. Ultrastructural studies have identified the accumulation of dense-core vesicles in Merkel cell cytoplasm near the nerve contact site [10, 20, 56, 57]. These vesicles have been reported to contain both classic neurotransmitters (e.g. serotonin (5-HT)) and neuropeptides (e.g. vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), substance P, met-enkephalin, and cholecystokinin octapeptide (CCK8)) [20, 30, 58–64]. Functional evidence also supports a role for glutamatergic signaling between Merkel cells and their afferent terminals [16, 17, 30, 65, 66]. Our current model suggests that Merkel cells transduce the static phase of the SAI response in addition to shaping the dynamic response either directly or indirectly [48–50]. It will be important to determine how Merkel cells regulate the release of neurotransmitters during touch-evoked responses and what roles these molecules play during the two phases of SAI responses.
To elucidate mechanisms of crosstalk between Merkel cells and tactile afferents, two studies applied pharmacological inhibitors such as voltage-gated calcium channel (VGCC) blockers in situ to disrupt Merkel-cell depolarization during touch transduction [50, 67]. They found that VGCC blockers inhibited responses in sensory afferents. However, VGCCs are also expressed in sensory afferents, so it is possible that these blockers have effects on both Merkel cells and sensory afferents. To avoid this uncertainty, genetic manipulations, such as a genetic inhibition of the neurotransmitter release from Merkel cells by disrupting a synaptic vesicle formation or release, or an ablation of VGLUTs in Merkel cells to block glutamate release, will be useful to elucidate the nature of Merkel cell-neurite crosstalk [22].
What is the role of Merkel cells in touch-evoked behaviors?
Different classes of LTMRs are tuned to selectively respond to specific mechanical stimuli (e.g. vibration, hair deflection, static pressure) [4, 40]. What is special about the information that SAI afferents send to our brain that it warrants a dedicated cell type in the epidermis? First, SAI afferents densely innervate the skin (about 100 per cm2 in our fingertips) [68]. Second, they have high sensitivity to points, edges and curvature [21]. Third, they have high spatial resolution as individual SAI afferents resolve spatial detail of 0.5 mm, which is much smaller than their receptive field diameter of 2–3 mm [21]. Thus, SAI afferents have long been postulated to encode curvature, edges, and textures [21]. Their sustained electrophysiological responses also indicate that they convey information about static mechanical stimuli [10, 21].
Until recently, it was difficult to determine whether SAI responses are necessary for either pressure detection or shape and texture discrimination in vivo. The advent of skin-specific Atoh1 and Piezo2 genetic silencing provides an opportunity to test the requirement for Merkel cells in touch-driven behaviors. It is not straightforward to test the sole contribution of Merkel cell-neurite complexes to gentle touch responses at the behavioral level because other nearby LTMRs can be simultaneously activated by a given mechanical stimuli. Despite this challenge, recent studies describe behavioral assays to elucidate the role of Merkel cells.
One study subjected skin-specific Atoh1 knockout mice (Atoh1CKO), in which Merkel cells are depleted in skin, to texture discrimination tasks [69]. Female Atoh1CKO mice showed a lack of preference for textured surfaces that wild-type mice display, thus this result implicates the role of Merkel cells in texture discrimination [69]. A second study tested skin-specific Piezo2 knockout mice (Piezo2CKO) for a wide range of behavioral assays that examined both innocuous and noxious touch responses [48]. Piezo2CKO mice containing Merkel cells that lack Piezo2 channels showed normal responses to most behavioral assays [48]. However, in a paw withdrawal test using von Frey filaments, these mice showed a deficit in sensing gentle touch stimuli [48]. These mice displayed decreased paw withdrawal responses to low force von Frey filaments, whereas they responded normally to filaments with higher force [48]. This data suggests that Merkel cells function in sensing gentle pressure on skin. Lastly, another group induced sensitization of the face of a rat for easier interpretation of behavioral responses [50]. Capsaicin was injected into the whisker pad to provoke tactile allodynia, a condition in which innocuous mechanical stimuli are perceived as painful [50, 52]. When the whisker hairs were gently bent to activate Merkel cell-neurite complexes in capsaicin-injected rats, animals showed nocifensive behavior that was blocked by Piezo2 knockdown in vivo [50]. These findings suggest that understanding the molecular mechanism of Merkel cell-neurite mechanotransduction could potentially lead to therapeutic benefits to treat pain. With appropriate animal models in hand that specifically suppress Merkel-cell function, we expect future studies to identify additional sensory behaviors that depend on Merkel cells.
Do Merkel cells shape responses of other cutaneous afferents?
Recent studies have suggested that touch domes are innervated by other fiber types in addition to Aβ afferents. Human touch domes are innervated by thickly myelinated, thinly myelinated and unmyelinated afferents that likely correspond to Aβ, Aδ and C fibers, respectively [70]. In mouse skin, some touch domes have been reported to contain both thickly myelinated Aβ afferents and thinly myelinated, likely Aδ, afferents that contact Merkel cells [55]. Moreover, in neonatal touch domes, Merkel cells are innervated by two types of afferents: NFH+ Aβ fibers and Ret+/TrkA+ unmeylinated or thinly myelinated fibers [71]. The existence of other fiber types innervating Merkel cells in touch domes strongly suggests additional functions for Merkel cell-neurite complexes. Indeed, modulation of SAI responses by nociceptive C fiber activation within touch domes has been observed [72]. Whether Merkel cells confer slowly adapting firing or otherwise shape firing properties of other afferent types needs to be elucidated.
Concluding remarks
Nearly 50-year-old mysteries of Merkel cell-neurite complexes are now at least partly solved: Merkel cells are touch-sensitive cells that transduce mechanical stimuli through Piezo2 MA cation channels, and they are required for proper output of SAI responses in tactile afferents. Moreover, the Merkel cell-neurite complex is a unique cutaneous sensory receptor containing two receptor cell types that mediate different aspects of touch-induced responses. The next important question to solve is the nature of the crosstalk between Merkel cells and tactile afferents to elucidate the molecular mechanisms used by Merkel cells for their subsequent effect on SAI responses. Moreover, analysis of neural circuits that receive SAI inputs [73] is needed to define how SAI afferents and the curious epidermal cells they innervate impact sensory coding. The field is now poised to answer these questions.
Highlights.
Merkel cells are touch-sensitive cells that transduce touch via Piezo2 channels.
The Merkel cell-neurite complex contains two sensory receptor cell types.
Merkel cells and neurons together mediate different aspects of touch responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rice FL, Albrecht PJ. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin. In: Kaas JH, Gardner EP, editors. The Senses: A Comprehensive Reference. Academic Press; 2008. pp. 1–31. [Google Scholar]
- 2.Gardner EP, Martin JH, Jessell TM. The bodily senses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. McGraw-Hill; 2000. pp. 430–449. [Google Scholar]
- 3.Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. The Journal of physiology. 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi-Iwanaga H. Three-dimensional microanatomy of longitudinal lanceolate endings in rat vibrissae. The Journal of comparative neurology. 2000;426:259–269. doi: 10.1002/1096-9861(20001016)426:2<259::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Halata Z. Sensory innervation of the hairy skin (light- and electronmicroscopic study. The Journal of investigative dermatology. 1993;101:75S–81S. doi: 10.1111/1523-1747.ep12362877. [DOI] [PubMed] [Google Scholar]
- 7.Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Human neurobiology. 1984;3:3–14. [PubMed] [Google Scholar]
- 8.Knibestol M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. The Journal of physiology. 1973;232:427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot WH, et al. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. Journal of neurophysiology. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 10.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. The Journal of physiology. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkel F. Tastzellen und Tastkörperchen bei den Hausthieren und beim Menschen. Archiv für Mikroskopische Anatomie. 1875;11:636–652. [Google Scholar]
- 12.Moll I, et al. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. The Journal of investigative dermatology. 1996;106:281–286. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- 13.Boot PM, et al. The distribution of Merkel cells in human fetal and adult skin. The American Journal of dermatopathology. 1992;14:391–396. doi: 10.1097/00000372-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lacour JP, et al. Anatomical mapping of Merkel cells in normal human adult epidermis. The British journal of dermatology. 1991;125:535–542. doi: 10.1111/j.1365-2133.1991.tb14790.x. [DOI] [PubMed] [Google Scholar]
- 15.Halata Z, et al. Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;271:225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock IS, et al. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neuroscience letters. 2004;362:196–199. doi: 10.1016/j.neulet.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 17.Fagan BM, Cahusac PM. Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. Neuroreport. 2001;12:341–347. doi: 10.1097/00001756-200102120-00032. [DOI] [PubMed] [Google Scholar]
- 18.Hartschuh W, et al. Electron microscopic immunogold cytochemistry reveals chromogranin A confined to secretory granules of porcine Merkel cells. Neuroscience letters. 1990;116:245–249. doi: 10.1016/0304-3940(90)90081-j. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, et al. Neuron-specific enolase in the Merkel cells of mammalian skin. The use of specific antibody as a simple and reliable histologic marker. The American journal of pathology. 1981;104:63–68. [PMC free article] [PubMed] [Google Scholar]
- 20.Hartschuh W, Weihe E. Fine structural analysis of the synaptic junction of Merkel cell-axon-complexes. The Journal of investigative dermatology. 1980;75:159–165. doi: 10.1111/1523-1747.ep12522555. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Current opinion in neurobiology. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 22.Maksimovic S, et al. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Annals of the New York Academy of Sciences. 2013;1279:13–21. doi: 10.1111/nyas.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancet D. Vertebrate olfactory reception. Annual review of neuroscience. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar J, et al. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs PA, et al. The afferent synapse of cochlear hair cells. Current opinion in neurobiology. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 26.Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. The Journal of physiology. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. The Journal of cell biology. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison KM, et al. Mammalian Merkel cells are descended from the epidermal lineage. Developmental biology. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maricich SM, et al. Merkel Cells Are Essential for Light-Touch Responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haeberle H, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Arie N, et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- 32.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 33.Haeberle H, et al. Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PloS one. 2008;3:e1750. doi: 10.1371/journal.pone.0001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinkelin I, et al. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. The European journal of neuroscience. 1999;11:3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 35.Senok SS, et al. Selective phototoxic destruction of quinacrine-loaded Merkel cells is neither selective nor complete. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1996;110:325–334. doi: 10.1007/BF00229133. [DOI] [PubMed] [Google Scholar]
- 36.Chan E, et al. Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1996;108:357–366. doi: 10.1007/BF00227259. [DOI] [PubMed] [Google Scholar]
- 37.Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. Journal of neurocytology. 1995;24:117–134. doi: 10.1007/BF01181555. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda I, et al. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. The Journal of physiology. 1994;479(Pt 2):247–256. doi: 10.1113/jphysiol.1994.sp020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tazaki M, Suzuki T. Calcium inflow of hamster Merkel cells in response to hyposmotic stimulation indicate a stretch activated ion channel. Neuroscience letters. 1998;243:69–72. doi: 10.1016/s0304-3940(98)00066-4. [DOI] [PubMed] [Google Scholar]
- 40.Delmas P, et al. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nature reviews. Neuroscience. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 41.Lumpkin EA, et al. The cell biology of touch. The Journal of cell biology. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tachibana T, Nawa T. Recent progress in studies on Merkel cell biology. Anatomical science international. 2002;77:26–33. doi: 10.1046/j.0022-7722.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 43.Pasche F, et al. Relationship between Merkel cells and nerve endings during embryogenesis in the mouse epidermis. The Journal of investigative dermatology. 1990;95:247–251. doi: 10.1111/1523-1747.ep12484847. [DOI] [PubMed] [Google Scholar]
- 44.Gottschaldt KM, Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981;214:183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita Y, Ogawa H. Slowly adapting cutaneous mechanoreceptor afferent units associated with Merkel cells in frogs and effects of direct currents. Somatosensory & motor research. 1991;8:87–95. doi: 10.3109/08990229109144732. [DOI] [PubMed] [Google Scholar]
- 46.Press D, et al. Evidence of fast serotonin transmission in frog slowly adapting type 1 responses. Somatosensory & motor research. 2010;27:174–185. doi: 10.3109/08990220.2010.516670. [DOI] [PubMed] [Google Scholar]
- 47.Cahusac PM, Mavulati SC. Non-competitive metabotropic glutamate 1 receptor antagonists block activity of slowly adapting type I mechanoreceptor units in the rat sinus hair follicle. Neuroscience. 2009;163:933–941. doi: 10.1016/j.neuroscience.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Woo SH, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maksimovic S, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda R, et al. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasquez V, et al. Sensory Biology: It Takes Piezo2 to Tango. Current biology : CB. 2014;24:R566–R569. doi: 10.1016/j.cub.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lou S, et al. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranade S, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014 doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesniak DR, et al. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. eLife. 2014;3:e01488. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mihara M, et al. The specialized junctions between Merkel cell and neurite: an electron microscopic study. The Journal of investigative dermatology. 1979;73:325–334. doi: 10.1111/1523-1747.ep12550322. [DOI] [PubMed] [Google Scholar]
- 57.Breathnach AS, Robins J. Ultrastructural observations on Merkel cells in human foetal skin. Journal of anatomy. 1970;106:411. [PubMed] [Google Scholar]
- 58.Tachibana T, et al. Immunohistochemical expressions of mGluR5, P2Y2 receptor, PLC-beta1, and IP3R-I and -II in Merkel cells in rat sinus hair follicles. Histochemistry and cell biology. 2003;120:13–21. doi: 10.1007/s00418-003-0540-5. [DOI] [PubMed] [Google Scholar]
- 59.English KB, et al. Serotonin-like immunoreactivity in Merkel cells and their afferent neurons in touch domes from the hairy skin of rats. The Anatomical record. 1992;232:112–120. doi: 10.1002/ar.1092320112. [DOI] [PubMed] [Google Scholar]
- 60.Toyoshima K, Shimamura A. Uranaffin reaction of Merkel corpuscles in the lingual mucosa of the finch, Lonchula striata var. domestica. Journal of anatomy. 1991;179:197–201. [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Caballero T, et al. Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry. 1989;92:127–132. doi: 10.1007/BF00490231. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez FJ, et al. Immunocytochemical analysis of calcitonin gene-related peptide and vasoactive intestinal polypeptide in Merkel cells and cutaneous free nerve endings of cats. Cell and tissue research. 1988;254:429–437. doi: 10.1007/BF00225816. [DOI] [PubMed] [Google Scholar]
- 63.Hartschuh W, et al. Immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in Merkel cells of various mammals: evidence for a neuromodulator function of the Merkel cell. The Journal of investigative dermatology. 1983;81:361–364. doi: 10.1111/1523-1747.ep12519966. [DOI] [PubMed] [Google Scholar]
- 64.Hartschuh W, et al. Met enkephalin-like immunoreactivity in Merkel cells. Cell and tissue research. 1979;201:343–348. doi: 10.1007/BF00236994. [DOI] [PubMed] [Google Scholar]
- 65.Nunzi MG, et al. Merkel cells, corpuscular nerve endings and free nerve endings in the mouse palatine mucosa express three subtypes of vesicular glutamate transporters. Journal of neurocytology. 2004;33:359–376. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- 66.Morimoto R, et al. Co-expression of vesicular glutamate transporters (VGLUT1 and VGLUT2) and their association with synaptic-like microvesicles in rat pinealocytes. Journal of neurochemistry. 2003;84:382–391. doi: 10.1046/j.1471-4159.2003.01532.x. [DOI] [PubMed] [Google Scholar]
- 67.Pacitti EG, Findlater GS. Calcium channel blockers and Merkel cells. Progress in brain research. 1988;74:37–42. doi: 10.1016/s0079-6123(08)62995-7. [DOI] [PubMed] [Google Scholar]
- 68.Johnson KO, et al. Tactile functions of mechanoreceptive afferents innervating the hand. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2000;17:539–558. doi: 10.1097/00004691-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Maricich SM, et al. Rodents rely on Merkel cells for texture discrimination tasks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3296–3300. doi: 10.1523/JNEUROSCI.5307-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Annals of neurology. 2005;58:88–95. doi: 10.1002/ana.20527. [DOI] [PubMed] [Google Scholar]
- 71.Niu J, et al. Dual innervation of neonatal Merkel cells in mouse touch domes. PloS one. 2014;9:e92027. doi: 10.1371/journal.pone.0092027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, et al. C-fiber modulation of the rat type I slowly adapting mechanoreceptor. Neuroscience. 2002;115:797–804. doi: 10.1016/s0306-4522(02)00505-5. [DOI] [PubMed] [Google Scholar]
- 73.Sakurai K, et al. The organization of submodality-specific touch afferent inputs in the vibrissa column. Cell reports. 2013;5:87–98. doi: 10.1016/j.celrep.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakatani M, et al. Mechanotransduction in epidermal Merkel cells. Pflugers Archiv : European journal of physiology. 2014 doi: 10.1007/s00424-014-1569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]