Abstract
Background
Trimethylamine-N-oxide (TMAO) has been linked to increased cardiovascular risk. We aim to determine the prognostic value of TMAO and its dietary precursors, choline and betaine, in heart failure (HF).
Methods and Results
In 112 patients with chronic systolic HF with comprehensive echocardiographic evaluation, we measured plasma TMAO, choline, and betaine by mass spectrometry. Median TMAO levels, choline, and betaine levels were 5.8 [3.6, 12.1] μM, 10.9 [8.4, 14.0] μM, 43.8 [37.1, 53.0] μM, respectively, and were correlated with each other (all p<0.0001 for both). TMAO levels were significantly higher in patients with diabetes mellitus (9.4 [4.9, 13.2] vs 4.8 [3.4, 9.8] μM, p=0.005) and in subjects with New York Heart Association (NYHA) class III or greater (7.0 [4.7, 14.8] vs 4.7 [3.4, 11.3] μM, p=0.02). Elevated TMAO, choline, and betaine levels were each associated with higher plasma NT-proBNP levels and more advanced left ventricular diastolic dysfunction, but not systolic dysfunction or inflammatory and endothelial biomarkers. Higher choline (Hazard ratio (HR) 1.64 [95% CI: 1.22 2.20], p=0.001), betaine (HR 1.51 [1.10–2.08], p=0.01), and TMAO (HR 1.48 [1.10–1.96], p=0.01) predicted increased risk for 5-year adverse clinical events (death/transplant). Only higher TMAO levels predicted incident adverse clinical events independent of age, eGFR, mitral E/septal Ea, and NT-proBNP levels (HR 1.46 [1.03 2.14], p=0.03).
Conclusion
Elevated plasma TMAO, choline and betaine levels are each associated with more advanced left ventricular diastolic dysfunction and portend poorer long-term adverse clinical outcomes in chronic systolic HF. However, only higher plasma TMAO levels was associated with poor prognosis after adjustment for cardio-renal indices.
Keywords: Intestinal microbiota, trimethylamine N-oxide, diastolic dysfunction, heart failure
INTRODUCTION
Intestinal microbiota are implicated in the development of metabolic phenotypes such as obesity and insulin resistance[1]. Using an unbiased metabolomics approach, our group recently identified three metabolites of the dietary lipid phosphatidylcholine – choline, betaine and the gut-microbiota generated metabolite trimethylamine-N-oxide (TMAO) – that are associated to atherosclerotic cardiovascular disease[2]. We have recently validated these findings in a larger scale clinical cohort whereby elevated plasma TMAO levels portend greater risk of major adverse cardiac events[3], and showed the mechanistic link between TMAO and macrophage activation[2] as well as alterations in cholesterol metabolism and transport[4]. Since choline and betaine are substrates in the formation of TMAO by intestinal microbiota, we have further demonstrated their prognostic values in predicting future major adverse cardiac events being largely driven by the presentation of elevated TMAO levels[5].
Heart failure is a frequent adverse complication of atherosclerotic cardiovascular disease, which may present either as myocardial ischemia, vascular dysfunction, and fibrosis leading to progressive diastolic dysfunction or progressive myocyte damage and cardiac remodeling leading to systolic dysfunction. We have recently reported the association between TMAO and long-term mortality risk in a large cohort of patients with a history of chronic HF, independent of renal insufficiency or natriuretic peptide levels[6]. However, the relationship between TMAO, and its dietary precursors, choline and betaine, to myocardial and inflammatory indices, markers of endothelial dysfunction, and their relative prognostic values in patients with chronic systolic HF, have not yet been carefully explored. Herein, our objective was to investigate the relationship between the three phosphatidylcholine metabolites TMAO, choline and betaine with myocardial indices, inflammatory and endothelial biomarkers, and long-term clinical prognosis in subjects with chronic systolic HF.
METHOD
Study Population
This is a single-center, prospective cohort study approved by the Cleveland Clinic Institutional Review Board, and all subjects provided written informed consent. We enrolled 112 ambulatory subjects, ≥18 years of age, with stable but symptomatic, chronic systolic heart failure (left ventricular [LV] ejection fraction ≤35%;), who underwent comprehensive echocardiographic evaluation as part of a research study at the Cleveland Clinic. Subjects were excluded if they had significant primary valvular abnormalities. Comprehensive transthoracic echocardiographic evaluation of systolic and diastolic myocardial performance was assessed as previously described[7]. The composite endpoint of adverse clinical events (all-cause mortality and cardiac transplantation) was prospectively tracked for 5 years by telephone follow-up and medical chart review.
TMAO, Choline, and Betaine Assay
Quantification of TMAO, choline and betaine was performed utilizing stable isotope dilution liquid chromatography with tandem mass spectrometry (LC/MS/MS) with the use of a stable-isotope-dilution assay and high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry on an AB SCIEX QTRAP 5500 mass spectrometer as previously described[8]. Arginine metabolites (asymmetric dimethylarginine [ADMA], symmetric dimethylarginine [SDMA], L-arginine, L-ornithine, L-citrulline) were quantified using stable isotope dilution LC/electrospray ionization (ESI)/MS/MS assays using an upgraded ABI 365 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, California) with Ionics EP 10+ redesigned source (Concord, Ontario, Canada) and ESI needle connected to an Aria LX4 series multiplexed high-performance LC system with Flux pumps (Cohesive Technologies, Franklin, Massachusetts), as previously described[7]. Global arginine bioavailability ratio (GABR) was calculated as the ratio between the substrates (L-arginine) and the products (L-ornithine plus L-citrulline) of nitric oxide production[9].
Aminoterminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured by the Elecsys platform (Roche Diagnostics, Indianapolis IN)[10]. Plasma myeloperoxidase (MPO) and high-sensitivity C-reactive protein (hsCRP) levels were measured by CardioMPO assay(Cleveland Heart Labs, Cleveland OH) [11] as well as N latex Cystatin C CardioPhase assay (Siemens/Dade Behring, Deerfield IL)[12, 13] as previously described
Statistical Analysis
Continuous variables were summarized as mean ± standard deviation if normally distributed and median [IQR] if non-normally distributed. Normality was assessed by the Shapiro-Wilk W-test. Spearman’s rank correlation was used as non-parametric measure of association between metabolites and dependent variables. Plasma levels of TMAO, choline, and betaine were compared across categorical variables using the Wilcoxon rank-sum or Kruskal-Wallis test. The optimal receiver operating characteristic (ROC) curve cutoff value for plamsa levels of TMAO, choline, and betaine in predicting adverse clinical events was chosen as the value maximizing sensitivity plus specificity. Kaplan-Meier survival plots were calculated from baseline to time of adverse event and compared using the log-rank test. Cox proportional hazards analysis were used to assess the clinical risks associated with higher TMAO, choline, and/or betaine levels, in which the proportional hazards assumption was verified with log (time) vs. log [-log (survival)] plots. A two-sided p value of <0.05 was considered statistically significant. All analyses were performed by JMP 10 Pro (SAS, Cary NC).
RESULTS
Table 1 illustrates the baseline characteristics of the study population. Overall, the cohort is representative of a stable outpatient cohort of patients with systolic heart failure, with 40 (36%) experiencing at least NYHA class III symptoms. Overall, TMAO correlated with choline (r= 0.40, p<0.0001) but not betaine (r=0.08, p=0.43), and choline correlated with betaine (r= 0.46, p<0.0001). Older patients were more likely to have higher choline levels, but not betaine or TMAO. Meanwhile, men have higher choline and betaine levels than women (p<0.001), yet levels were similar between those with versus without diabetes. As expected, and as previously described[2, 3], those with history of diabetes mellitus or renal insufficiency witnessed higher levels of TMAO, choline, and betaine compared to those without. Interestingly, there were no significant differences between TMAO, choline, and betaine in those with ischemic versus non-ischemic etiologies, nor across MPO or hsCRP levels (Table 2). Also, those receiving loop diuretics have higher TMAO but similar choline/betaine levels compared to those not on loop diuretics (p<0.05).
Table 1.
Baseline Characteristics
| Variable | Value |
|---|---|
|
| |
| Age, years | 57 ± 14 |
|
| |
| Male, n (%) | 84 (75%) |
|
| |
| Ischemic etiology, n (%) | 48 (43%) |
|
| |
| History of diabetes mellitus (%) | 32 (29%) |
|
| |
| Echocardiographic indices: | |
| Left ventricular end-diastolic volume, index (mL/m2) | 111± 36 |
| Left ventricular ejection fraction (%) | 26 ± 6 |
| Diastolic stage III, n (%) | 44 (40%) |
| Mitral Regurgitation ≥3+ | 11 (10%) |
| Right ventricular systolic pressure (mmHg) | 34 ± 13 |
| Right ventricular systolic dysfunction ≥3+ | 31 (28%) |
| Left atrial volume index (mL/m2) | 42 ± 15 |
| Mitral septal E/Ea | 19 ± 12 |
|
| |
| Medications: | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blockers, n (%) | 103 (94%) |
| Beta-blocker, n (%) | 66 (60%) |
| Spironolactone, n (%) | 31 (30%) |
| Loop diuretics, n (%) | 87 (78%) |
|
| |
| Laboratory data: | |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 70 ± 26 |
| Aminoterminal pro-B-type natriuretic peptide (pg/mL) | 1473 [543, 3438] |
| Myeloperoxidase (pg/mL) | 312 [263, 427] |
| High-sensitivity C-reactive protein (mg/L) | 3.33 [1.55, 6.81] |
| Cystatin C (mg/dL) | 1.23 [1.03, 1.66] |
| Aysmmetric dimethylarginine (μM) | 0.43 [0.36, 0.55] |
| Symmetric dimethylarginine (μM) | 0.29 [0.23, 0.39] |
| Global arginine bioavailability ratio | 0.76 [0.55, 1.03] |
| Choline (μM) | 10.9 [8.4, 14.0] |
| Betaine (μM) | 43.8 [37.1, 53.0] |
| Trimethylamine N-oxide (μM) | 5.8 [3.6, 12.1] |
Table 2.
Univariate Correlations between Gut-Flora-Dependent Phosphatidylcholine Metabolites and Clinical and Echocardiographic Indices
| Variable | Choline | Betaine | TMAO |
|---|---|---|---|
| Age | 0.26 ** | 0.09 | 0.12 |
| Left ventricular end-diastolic volume, indexed | 0.12 | 0.09 | 0.06 |
| Left ventricular ejection fraction | - 0.10 | - 0.19 | 0.09 |
| Mitral régurgitation | 0.20* | 0.13 | 0.05 |
| Right ventricular systolic pressure | 0.34** | 0.28** | 0.14 |
| Right ventricular systolic dysfunction | 0.29** | 0.34*** | 0.05 |
| Mitral septal E/Ea | 0.33 ** | 0.29 * | 0.29 ** |
| Left atrial volume index | 0.45 ** | 0.22 * | 0.29 ** |
| Aminoterminal pro-B-type natriuretic peptide | 0.45 ** | 0.27 ** | 0.26 ** |
| Myeloperoxidase | -0.03 | -0.04 | -0.11 |
| High sensitivity C-reactive protein | 0.01 | 0.14 | 0.19 |
| Cystatin C | 0.54**** | 0.24 * | 0.40 **** |
| Estimated glomerular filtration rate | -0.39**** | -0.13 | -0.36 *** |
| Asymmetric dimethylarginine | 0.33*** | 0.20* | 0.11 |
| Symmetric dimethylarginine | 0.39**** | 0.13 | 0.18 |
| Global arginine bioavailability ratio | -0.54**** | -0.37**** | -0.23* |
p<0.05,
p<0.01,
p<0.001,
p<0.0001.
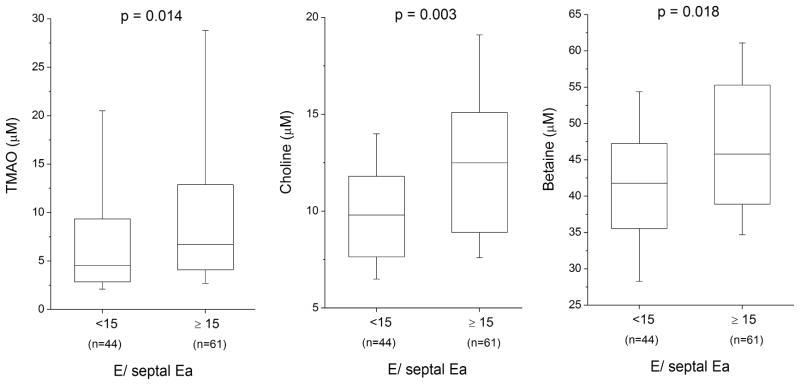
The relationship between TMAO, choline, and betaine levels with clinical, laboratory, and echocardiographic parameters are presented in Table 2. Interestingly, there were statistically significant correlations between all the metabolites with multiple echocardiographic indices. Moreover, we observed consistent positive correlations between TMAO and indices of diastolic dysfunction, especially with mitral E/septal Ea and LA volume index (Table 2). In contrast, there were no statistically significant correlations noted between TMAO, choline, and betaine in LV ejection fraction or LV dimensions. Figure 1 demonstrates the relationship between TMAO, choline, and betaine levels stratified according to mitral septal E/Ea cut-offs at 15. Meanwhile there was strong positive correlation between methylated arginine metabolites with choline and to a lesser extent betaine, while an inverse correlation between both choline and betaine was observed with GABR. In contrast, TMAO did not correlate with any methylated arginine metabolites, and only modestly with GABR. In multivariate linear regression analysis with all variables, choline was independently associated only with cystatin C (Std β 0.40, p<0.0001) and GABR (Std β −0.39, p<0.0001), betaine only with GABR (Std β −0.31, p=0.002) and RV systolic dysfunction class (Std β 0.21, p=0.030), and TMAO only with NTproBNP (Std β 0.34, p=0.003) and cystatin C (Std β 0.75, p<0.0001).
Figure 1.
Relationship between Intestinal Microflora-Dependent Phosphatidylcholine Metabolites And Diastolic Dysfunction in Chronic Systolic Heart Failure.
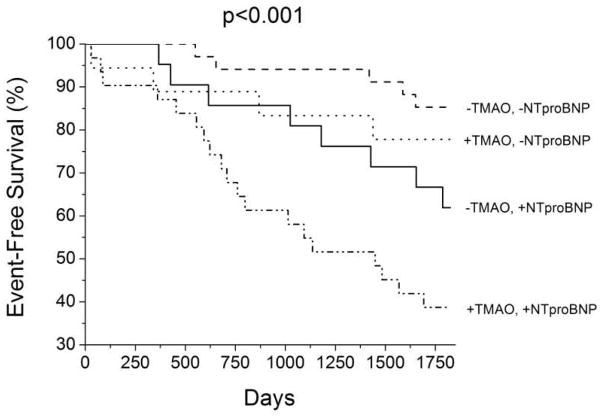
Table 3 illustrates the Cox proportional hazard models of TMAO, choline, and betaine for long-term adverse clinical outcomes. There was a total of 40 events over 5 years of follow up. All three measures (choline, betaine, and TMAO) were predictive of the composite endpoint of 5-year all-cause mortality plus cardiac transplantation. However, only the prognostic value of TMAO remained statistically significant following adjustments with age, eGFR, mitral E/septal Ea, and NT-proBNP levels (adjusted HR 1.46, 95% confidence interval 1.03–2.14, p=0.031). When stratified according to the optimal ROC cut-off value of 6.1 μM for TMAO and median NT-proBNP (1,473 pg/mL) for this cohort, the subset of patients with both elevated TMAO and NT-proBNP demonstrated the greatest risk of future adverse cardiac events (Figure 2).
Table 3.
Cox Proportional Hazard Analyses of Adverse Clinical Outcomes
| Variable | Hazard Ratio (95% confidence interval) | P value |
|---|---|---|
|
| ||
| Choline | ||
| Unadjusted | 1.64 (1.22 – 2.20) | 0.001 |
| Adjusted | 1.22 (0.82 – 1.85) | 0.333 |
|
| ||
| Betaine | ||
| Unadjusted | 1.51 (1.10 – 2.08) | 0.011 |
| Adjusted | 1.33 (0.93 – 1.89) | 0.115 |
|
| ||
| Trimethylamine N-oxide | ||
| Unadjusted | 1.48 (1.10 – 1.96) | 0.010 |
| Adjusted | 1.46 (1.03 – 2.14) | 0.031 |
Adjusted for age, estimated glomerular filtration rate, mitral septal E/Ea, and aminoterminal pro-B-type natriuretic peptide
Hazard ratios per 1 standard deviation (Ln Choline = 0.38 μM; Ln Betaine = 0.27 μM; Ln TMAO = 0.99 μM).
Figure 2.
Kaplan-Meier Survival Curve Stratified According to Levels of Trimethylamine N-oxide (TMAO) and NT-proBNP Levels. −/+ TMAO = below versus above 6.1 μM; −/+ NTproBNP = below versus above 1473 pg/mL (median); TMAO = trimethylamine N-oxide; NT-proBNP = amino-terminal pro-B-type natriuretic peptide
DISCUSSION
We have recently reported the association between elevated TMAO levels and increased 5-year mortality risk independent of cardio-renal indices in a large cohort of patients with a history of HF[6]. In this study, we now validate our findings in an independent cohort of ambulatory patients with chronic systolic HF, with the added insights into the relationship between all three phosphatidylcholine metabolites, TMAO, choline and betaine, with both echocardiographic determinants, as well as renal and inflammatory biomarker associations. There are several notable findings. First, we observed a stronger prognostic value in TMAO than choline and betaine in patients with chronic systolic heart failure, which was independent of cardio-renal indices. Second, we observed correlations between all three metabolites with LV diastolic dysfunction rather than LV systolic dysfunction. Third, the relatively lack of correlations between TMAO, choline, and betaine in several well-known inflammatory biomarkers and differential relationships with markers of endothelial dysfunction also suggested an independent pathophysiological pathway. Of note, the higher levels of TMAO found in subjects with renal insufficiency or diabetes mellitus points to an underlying metabolic defect relevant to those disease states rather than a systemic inflammatory response. None-the-less, the association between elevated TMAO and both HF severity and incident adverse outcomes independent of other cardio-renal indices argues for a potential pathogenic mechanistic link between the gut microbiota pathway generating TMAO and HF development and/or progression. Of note, this is a cohort of ambulatory stable heart failure patients with left ventricular systolic dysfunction, with an annualized mortality of 7.1% (assuming transplant as equivalent as death) that was not too far off from that reported clinical trials. Taken together, these findings provided validation of the clinical significance of TMAO levels in heart failure[6] and suggest more studies are warranted to understand the mechanistic underpinnings of the association.
A mechanistic link between altered intestinal microbiota and myocardial function has previously been suggested to result largely in the setting of responses to circulatory insult[14, 15]. Dysbiosis (abnormal changes in intestinal microbiota composition) has also been reported in rodent models of myocardial infarction11. Interestingly, supplementation with a probiotic was reported to be associated with improved cardioprotection in a surgical model of myocardial infarction in a recent study[16]. The present analysis is the first human study to our knowledge to report the relationship between the intestinal microbiota-dependent analyte TMAO and its dietary precursors, choline and betaine, with echocardiographic indices in the setting of chronic systolic heart failure. Of note, there was no demonstrable relationship between TMAO, choline, or betaine with left ventricular systolic function or degree of cardiac remodeling. Rather, the stronger correlations observed were between all three metabolites and LV diastolic functional indices (Table 2). These observations suggest that a greater degree of “backward failure” (congestion, with either scarring or ischemia) rather than “forward failure” (or impaired perfusion) may be associated with the primary metabolic defect driving the observed associations. Consistent with this, associations with renal functional indices were noted for choline and TMAO, but again, the association between TMAO and adverse events among subjects remained even following adjustments for renal function.
Another possibility for the association with elevated TMAO levels among subjects with poorer prognosis may arise due to the greater degree of intestinal congestion from right-sided HF. Indeed, it is tempting to speculate that such congestion and attendant intestinal edema could lead to alterations in intestinal microbiota composition that adversely impacts (enhances) TMAO production[17]. Prior investigations have observed a reduction in active carrier-mediated intestinal transport processes in decompensated HF leading to epithelial dysfunction, possibly as a consequence of intestinal ischemia[18]. As intestinal microbiota shift rapidly from ischemia to reperfusion[19], it is conceivable that the balance and amount of intestinal commensal versus pathobiont bacteria may also be affected by venous congestion, thereby leading to altered metabolic milieu and downstream metabolic derangements for the host. Hence, how heart failure itself impacts intestinal microbial composition and function, and conversely, how intestinal microbiota directly or indirectly affect the HF phenotype and pathophysiology, should be further explored.
The lack of relationship between TMAO and its dietary precursors, choline and betaine, and inflammatory biomarkers is also somewhat unexpected, as prior studies have implied that the lack of intestinal microbiota may be associated with a state of active interleukin-10-mediated inflammatory hyporesponsiveness[20]. That being said, our previous studies in a broad population of cardiac patients also demonstrated prognostic value of TMAO being independent of plasma MPO levels[3]. This is perhaps not too surprising in our cohort of ambulatory chronic systolic HF patients, where organ perfusion remains largely preserved. Hence, our current findings may imply concepts beyond the traditional gut hypothesis, whereby reduced circulatory efficiency in the setting HF leads to impaired barrier function of the intestinal epithelia and heightened inflammatory responses to endotoxins and peptidoglycans[21]. The dissociation between TMAO and choline/betaine in terms of relationships with markers of endothelial dysfunction further suggests that the metabolic defect associated with elevated TMAO may even be independent of traditional measures of vascular dysfunction that has known to impact diastolic dysfunction[7, 9].
What are the implications of these findings? First, understanding why patient with advancing systolic HF have increased levels of TMAO will be important. While increased production (from increased dietary sources or microbial/host enzymes) or reduced clearance (renal insufficiency) may contribute, there is further need to determine if the underlying cardiac insufficiency or whether metabolic defects associated to cardiac dysfunction may contribute to such a phenomenon. The independence of the association between elevated TMAO levels and poor outcomes following adjustments for cardio-renal indices like eGFR and NT-BNP argues for additional underlying mechanistic links. Second, neither dietary nor intestinal microbiota influences on heart failure pathophysiology have been well studied, even though the “gut hypothesis” of heart failure has been well established in the literature. Further studies are needed to determine if manipulation of gut microbiota composition or dietary modifications (similar to a renal or diabetic diet) may offer novel therapeutic approaches in the setting of elevated TMAO levels in patients with HF. Indeed, a low protein diet, which is typical for patients with chronic kidney disease, is anticipated to be low in dietary nutrients like carnitine, choline, and phosphatidylcholine that can generate TMAO[4, 5]. Nutritional and microbiota-associated metabolic factors that can lead to the causation, development, and progression of myocardial dysfunction may therefore provide important therapeutic targets in a disease with grave morbidity and mortality that has largely focused on hemodynamic augmentation and neurohormonal suppression.
There are several limitations in our single-center, tertiary referral center experience. Our prior studies have largely confined to measurements of fasting samples of phosphatidylcholine metabolites, while samples in the present study were collected without undergoing an overnight fast. Nevertheless, data from our prior studies suggested that the overall plasma TMAO, choline, and betaine levels, albeit fluctuates shortly after feeding, appears to maintain within a confined range[3]. Furthermore, we do not have any information or measurements regarding the dietary habits (especially dietary phosphatidylcholine amounts ingested) of our subjects, nor do we have any quantification of intestinal microbiota composition, intestinal barrier function, or commensal versus pathobiont bacterial growth. We also do not have independently adjudicated NYHA class (majority of patients are symptomatic in NYHA 2–3), physical exam findings, or electrocardiographic data at the time of evaluation for the study, and the relatively low number of adverse events (n=40) may have limited our multivariate analysis.
Conclusion
Elevated plasma TMAO, choline and betaine levels are each associated with more advanced left ventricular diastolic dysfunction and portend poorer long-term adverse clinical outcomes in chronic systolic HF. However, only higher plasma TMAO levels was associated with poor prognosis after adjustment for cardio-renal indices.
Highlights.
Stronger prognostic value in TMAO than choline/betaine in chronic systolic HF
Prognostic value of TMAO was independent of cardio-renal indices
Correlations between metabolites with LV diastolic but not systolic dysfunction
No correlations between metabolites and inflammatory biomarkers
Acknowledgments
Funding
This research was supported by National Institutes of Health grants P01HL076491, P01HL103453, P01HL098055, R01HL103866 (with Office of Dietary Supplements), R01HL103931, P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). The main ADEPT study was supported in part by grant funding from American Society of Echocardiography, GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc. Mass spectrometry studies were performed within a Mass Spectrometry Core facility that is supported in part by a Center of Innovation Award by AB SCIEX.
Footnotes
Disclosure
Drs. Hazen and Wang report being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., Pfizer Inc., and Procter & Gamble. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., Pfizer Inc., Procter & Gamble and Takeda Pharmaceuticals. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens. Dr. Hazen is also partially supported by a gift from the Leonard Krieger endowment and by the Foundaton LeDucq. All other authors have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite, Trimethylamine-N-oxide (TMAO), in Patients with Heart Failure: Refining the Gut Hypothesis. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.02.617. Accepted, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–13. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455C:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–8. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang WH, Shrestha K, Troughton RW, Borowski AG, Klein AL. Integrating plasma high-sensitivity C-reactive protein and myeloperoxidase for risk prediction in chronic systolic heart failure. Congest Heart Fail. 2011;17:105–9. doi: 10.1111/j.1751-7133.2011.00221.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–70. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, et al. Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol. 2008;101:370–3. doi: 10.1016/j.amjcard.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Tang WH, Van Lente F, Shrestha K, Troughton RW, Francis GS, Tong W, et al. Impact of myocardial function on cystatin C measurements in chronic systolic heart failure. J Card Fail. 2008;14:394–9. doi: 10.1016/j.cardfail.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–74. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 15.Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014;35:426–30. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 16.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–9. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 17.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–5. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Li Q, He Q, Geng Y, Tang C, Wang C, et al. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock. 2013;39:96–103. doi: 10.1097/SHK.0b013e318279265f. [DOI] [PubMed] [Google Scholar]
- 20.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–46. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 21.Sandek A, Rauchhaus M, Anker SD, von Haehling S. The emerging role of the gut in chronic heart failure. Curr Opin Clin Nutr Metab Care. 2008;11:632–9. doi: 10.1097/MCO.0b013e32830a4c6e. [DOI] [PubMed] [Google Scholar]