Abstract
MicroRNAs (miRNAs) are integral molecules in the regulation of numerous physiological cellular processes that have emerged as critical players in cancer initiation and metastatic progression, both by promoting and suppressing metastasis. Recently, cell-free miRNAs shed from cancer cells into circulation have been reported in cancer patients, raising hope for development of novel biomarkers that can be routinely measured in easily accessible samples. In fact, establishing miRNA expression in the circulation likely has advantages over determination in primary tumor tissue, further augmenting the potential applications of miRNA detection in oncological practice. In addition, secretion of miRNAs impacting distant cell signaling or promoting the formation of a niche that sustains a distant tumor microenvironment allows for new treatment approaches to thwart cancer progression.
Keywords: cell-free microRNA, exosomes, body fluids, biomarker, metastasis
1. Introduction
Cancer metastasis is a complex, multi-step process accounting for the vast majority of cancer-related deaths [1]. Each step of the metastatic cascade involves co-evolution of the tumor and its microenvironment [2, 3]. Despite being the driving force of metastasis, cancer cells have to actively recruit stromal components to the primary and metastatic sites in order for tumors to thrive and progress. The tumor microenvironment at primary and secondary sites is thus formed by the combined action of cancer and stromal cells. The establishment of a supportive tumor microenvironment is highly dependent on extracellular signaling between cancer and stromal cells [4], which involves soluble factors, signaling molecules, extracellular matrix (ECM) components, and mechanical cues. In addition, membrane vesicles derived from both cancer and stromal cells play important roles in the promotion of tumor growth and metastasis [4]. For the majority of solid cancers, tumor-secreted factors play crucial roles in orchestrating the establishment and instructing the dynamic evolution of the microenvironment at primary and distant sites [2–4]. For example, in bone metastasis, cancer cells secret factors such as sVACM-1, sICAM-1 and RANKL to activate the differentiation of osteoclasts [5–8], which in turn break down the bone matrix and release growth factors, such as TGFβ, to further enhance expression of bone metastasis genes and promote metastatic tumor growth [9–11]. Understanding mechanisms of tumor-stroma interaction by secreted factors has lead to the development of novel therapeutics such as RANKL blocking antibody denosumab.
While the role of secreted proteins has been intensively investigated during metastatic progression [12–16], cell-free nucleic acids (cfNAs), such as DNA, mRNA and miRNA, have only recently attracted interest as mediators of cancer metastasis. While cfNAs are higher in patients with malignant lesions than in patients without tumors [17–24], increased levels have also been quantified in patients with benign lesions, inflammatory disease and tissue trauma. Although the physiological events leading to the increase of cfNAs during cancer development and progression are not well understood, specific cfNAs present in serum and other body fluids may represent potential biomarkers that could be used as a non-invasive, rapid, sensitive and accurate method of diagnosis and prognosis of cancer and/or metastasis [25–27]. In addition to their potential application as prognostic markers, cfNAs may also guide treatment decisions, as a rise or decline in circulating levels may predict therapy response earlier than conventional evaluation [22, 28]. Furthermore, cfNAs with a functional role in caner or metastatic progression could serve as new therapeutic targets.
Cell-free miRNAs are of particular interest due to their pleiotropic roles in modulating many physiological and pathological processes, including cancer metastasis [14, 29–36]. MiRNAs are small non-coding RNAs with diverse functions that regulate gene expression at the post-translational level by binding to the 3′ untranslated-region (UTR) of their target mRNAs [37–39]. A single miRNA can influence multiple genes thereby altering the expression profile of a cell. Moreover, recent discoveries of secreted miRNAs in virtually all body fluids [40, 41] have provided evidence that miRNAs can influence remote cells and function at a long distance [42], a feature that is critical for metastatic spread of cancer cells. Tumor-secreted miRNAs were first discovered in the serum of patients with diffuse large B-cell lymphoma where high levels of miR-21 correlated with improved relapse-free survival [43]. Using a mouse model, Mitchell et al. demonstrated that tumor-derived miRNAs enter the circulation even when originating from epithelial cancer types [44]. They also showed that circulating miRNA-141 levels are elevated in metastatic prostate cancer compared to healthy controls. Their presence in body fluids, combined with their lower complexity compared to other macromolecules, absence of known post-processing modifications, simple detection and amplification methods, tissue-restricted expression profiles, and sequence conservation between humans and model organisms make extracellular miRNAs ideal candidates for non-invasive biomarkers to reflect and study various physiopathological conditions.
In addition, miRNAs have been identified in tumor-secreted microvesicles called exosomes which can direct intercellular communication under physiological and pathological conditions [4, 45–48]. Accumulating evidence supports that horizontal transfer of exosomal factors can functionally influence stromal cells at distant sites [45–49], thereby facilitating tumor-stroma interactions and promoting the formation of a supporting metastatic niche in distant organs. Here we review the recent discoveries of cell-free miRNAs in metastatic progression as well as their diagnostic, prognostic and therapeutic potential in cancer patients.
2. Metastatic progression
Cancer metastasis is a complex process by which cancer cells spread from a primary tumor to other organs and tissues, forming viable secondary deposits of cancer. Cancer cells often initiate metastasis by breaking away from their neighbor cells and invading adjacent tissues after having undergone an epithelial-mesenchymal transition (EMT) that changes their characteristics to acquire motility and invasiveness. The motile cancer cells then enter into lymphatic and blood vessels. Circulating cancer cells (CTCs) that survive in the vasculature arrest in capillaries distant from the primary tumor site and extravasate into the foreign microenvironment. At this point, the cancer cells are speculated to revert to an epithelial phenotype via a mesenchymal-epithelial transition (MET) and either stay dormant or proliferate into macroscopic secondary tumors [50, 51]. These consecutive steps require close interplay between cancer cells and their microenvironment. Among the multiple factors underlying metastasis, the adaptation of the primary tumor microenvironment and pre-metastatic or metastatic niches by the tumor to facilitate cancer cell dissemination and distant engraftment plays an important pro-metastatic role that is starting to be recognized [3, 8, 15, 52–54]. The recent discovery of miRNAs and their extracellular presence suggest a potential role of these regulatory molecules in defining the metastatic potential of cancer cells and mediating the tumor-stroma communication.
Despite metastasis being the leading cause of mortality in cancer patients [1], the mechanism underlying metastatic spread and growth in secondary sites is poorly understood. Moreover, specific and sensitive markers that can detect metastatic spread at very early stages or can predict which patients are more likely to progress to metastasis are lacking. Therefore, there is a great and urgent need to develop predictive or early diagnostic markers for metastasis and to elucidate the molecular mechanisms of metastasis to allow the development of efficient treatment options.
3. Biogenesis and function of miRNAs
MiRNAs encompass a large family of non-coding small RNA molecules which occur as single-stranded RNAs of 21–23 nucleotides in length and play important roles in regulating gene expression [38]. In fact, miRNAs are estimated to regulate about 50% of all protein-coding genes [55]. The genes coding for miRNAs are mostly located within intergenic regions or introns of genes, and occur individually or within clusters containing other miRNAs [56]. Their genes are transcribed by RNA polymerase II into large hairpin structures called primary miRNA transcripts (pri-miRNAs). Pri-miRNAs initially contain a cap structure at the 5′ end a 3′ poly(A) tail. In the nucleus, pri-miRNAs are cleaved by a complex formed by the RNase III enzyme Drosha and the double stranded RNA-binding protein Pasha to produce precursor miRNAs (pre-miRNAs) [57]. The latter are 70–90 nucleotides in length and have an imperfect stem loop structure. They are exported to the cytoplasm by Exportin 5 and the Ran-GTP cofactor [58], where they are cleaved by the RNase III enzyme Dicer and its binding partner TRBP to generate a short RNA duplex molecule. One strand of the miRNA duplex is usually selected as a mature miRNA, and is assembled into an RNA induced silencing complex (RISC), while the other strand is degraded [59]. RISC interacts with the Argonaute-GW182 protein complex to regulate the function of target mRNA in a sequence-specific manner by recognizing the mRNA target through the 5′-end of the mature miRNA strand (“seed sequence”) [39]. The mechanism of mRNA silencing depends on the degree of complementarity of the seed sequence to the 3′UTR of the target mRNA [60]. When perfect base-paring homology exists between miRNA and mRNA, the RNA-mediated interference pathway is induced, which leads to cleavage of mRNA by Argonaute. More commonly, however, binding is imperfect and the target mRNA is regulated by repression of protein translation. Consequently, proteins are regulated by miRNAs without significantly affecting the corresponding mRNA expression levels in this scenario. In addition, miRNAs can increase the expression of their target gene by acting on gene regulatory sequences [33, 61]. Because most miRNAs recognize their targets with imperfect complementarity, miRNAs are capable of targeting numerous mRNAs; thus, misexpression of one miRNA can disrupt the expression of hundreds of proteins.
Expression of miRNAs is measured using miRNA microarray, miRNA real-time quantitative PCR (qRT-PCR) array, and next-generation sequencing (NGS) technology. qRT-PCR is a popular method for gene expression quantification of miRNA. Furthermore, qRT-PCR arrays can profile large sets of miRNAs simultaneously and for signature-based analyses. The microarray platform also enables simultaneous analysis of all known miRNAs and can be easily redesigned to include newly identified miRNAs. However, both qRT-PCR array and miRNA array analyses are limited in that they profile only known or putative miRNAs, and base sequence data are not always accounted for. NGS has become an increasingly popular method for miRNA profiling [62], which provides quantification of a variety of small RNA species and accurate quantification and differential expression with a wide-dynamic range.
4. Importance of miRNAs in cancer and metastasis
The biogenesis of miRNAs is tightly controlled, and dysregulation of miRNAs is linked to cancer [32, 34, 63, 64]. MiRNAs are thought to play two distinctly different roles in carcinogenesis, functioning both as “oncomirs” and as tumor suppressors [65–68]. In support of this, expression analysis studies have demonstrated that miRNA expression can be up- or down-regulated in tumors compared to normal tissues as well as deregulated during the progression of many solid cancers and hematological malignancies [34, 69, 70]. Additionally, about 50% of miRNA genes are located in cancer-associated genomic regions, or in fragile sites [71], further strengthening the evidence that miRNAs play a crucial role in cancer. Notably, roles of miRNAs are tissue- and tumor-specific; an overexpressed oncomir in one cancer type may be down-regulated in another cancer type where it has tumor-suppressive functions, such as the multifunctional miR-155 which is involved in cancer development, inflammation, immune response and hematopoietic lineage differentiation [72]. MiR-155 is overexpressed in some solid cancers of epithelial origin, in leukemia and in lymphoma [73–78], but down-regulated in some endocrine tumors, melanoma, ovarian and gastric cancer [79–84]. Deregulated miRNA expression can influence a broad range of biological processes that are involved in tumorigenesis such as transcription, signal transduction, cellular proliferation, differentiation, apoptosis and EMT [8, 14, 35, 85–87]. Interestingly, the aberrant regulation of certain miRNAs has been shown to occur across several cancer types. For instance, the oncomir miR-21 is commonly up-regulated in breast [70], colon [88], lung [78], pancreas [78], prostate [78], liver [89], thyroid [90], and ovarian [91] cancers as well as in B-cell lymphoma and chronic lymphocytic leukemia [78].
In addition, metastasis-mediating miRNAs have been discovered and their function as metastasis activators or suppressors have been studied [31, 92]. Notably, several such miRNAs are shown to modulate distinct steps of the metastatic cascade, including migration, invasion, adhesion, EMT, ECM modification and proliferation at the distant site. In particular, expression of miR-21 in cancers, which is shown to correlate with metastasis and poor prognosis, regulates several aspects of the metastatic cascade. MiR-21 promotes cell motility and invasion by targeting inhibitors of the pro-metastatic factor urokinase receptor [88, 93, 94], by modifying the cytoskeletal organization of migrating cancer cells [95, 96], and by remodeling the ECM via indirect up-regulation of metastasis-promoting metalloproteinases (MMPs) [89, 97]. MiR-21 has also been implicated in stimulating tumor growth and suppressing apoptosis [98], two processes that could aid metastatic proliferation at secondary sites. Other miRNAs that contribute to both tumorigenesis and metastasis are the members of the let-7 miRNA family (let-7a-1, 7a-2, 7a-3, 7b, 7c, 7d, 7e, f7-1, 7f-2, 7g, 7i, mir-98, and mir-202) [99] which are down-regulated in a variety of tumors [100]. They exert their effect on tumorigenesis by silencing multiple oncogenes [101–103], and hinder metastasis by inhibition of anchorage-independent growth as well as regulation of stemness and depletion of tumor-initiating cells (or cancer stem cells, CSCs) which are regarded as cells that generate secondary tumors [104, 105].
Further examples of metastasis-promoting miRNAs are miR-10b, miR-373, and miR-520c. MiR-10b down-regulates the metastatic suppressive gene Homeobox D10 (HOXD10) which in turn inhibits the pro-metastasis gene RHOC [106]. MiR-373 and miR-520c have also been classified as pro-metastatic miRNAs [107]. The target of these two miRNAs is CD44 and its down-regulation has been associated with the acquisition of an enhanced migratory potential [107].
Metastasis-suppressive miRNAs include miR-335 and miR-126 which are down-regulated and associated with shorter median time to metastatic relapse in breast cancer. Ectopic expression of these two miRNAs in metastatic breast cancer cell lines reduced both lung and bone metastases [108]. MiR-335 can control ECM deposition and abrogate EMT [108]. On the other hand, miR-126 acts principally to inhibit tumor growth and metastatic initiation [108, 109]. Interestingly, members of the miR-200 family (miR-200a, -200b, -200c, -141, -429) are deregulated in various cancer types [85, 110–112]. Several miRNAs from this family suppress expression of their own repressor, the ZEB family of transcription factors, thereby favoring an epithelial, adhesive phenotype and are down-regulated by cancer cells during EMT [111–114]. On the one hand, their expression has been linked to decreased migration and invasion of cancer cells, and hence their loss of expression is considered an early step of cancer metastasis [14]; on the other hand, they have been associated with inhibition of Sec23-mediated secretion of metastasis-suppressive proteins, such as TINAGL1 and IGFBP4 [115] and increased adhesion at secondary sites though promotion of MET and thus increased colonization [116].
As a consequence of the crucial role of miRNAs in cancer initiation and progression, there is a broad range of potential applications of miRNA measurements in oncology. Besides being informative of tumor biology, miRNAs can be used to classify cancers [69, 117] or identify cancer tissue origin for cancers of unknown primary origin [118, 119], outperforming mRNA expression level analyses in those areas [120]. In some instances, deregulated miRNA expression has been established as a useful diagnostic or prognostic marker [98, 120–125]. Furthermore, assessment of miRNA signatures is often more accurate in detecting and prediction prognosis of various types of cancers [78, 81, 126]. MiRNA signatures can also serve as predictive factors of response to systemic therapy [127–131], potential drug targets [132–135] and as pharmacodynamic markers. All of these applications are possible in primary tumors and metastases, but the stability of miRNAs which are more stable than mRNAs - also enables their detection in the circulation. Thus, circulating miRNAs can serve as biomarkers that can be measured repeatedly and non-invasively in a wide array of cancer types.
5. Role of exosomes in cancer and metastasis
Tumor cells often release higher numbers of microvesicles than other cells, a feature that is observable in the often increased numbers of serum exosomes in cancer patients [136]. This might be due to the fact that tumor-derived exosomes have easier access to the vascular system and, thus, may be selectively increased in blood compared with microvesicles from other sources. Smaller microvesicles with speci c molecular surface characteristics may selectively reach the blood and larger microvesicles may remain in the interstitial space and selectively provide autocrine and paracrine signals to stromal, in ammatory, and endothelial cells. However, in several cancer patients, such as melanoma patients, no difference in exosome number or size distribution was observed between healthy individuals and patients with different stages; nevertheless, exosome protein concentrations were higher in Stage IV patients compared to all other stages and normal controls and correlated with poor prognosis [16]. Similarly, exosomal protein concentrations increased with ovarian cancer progression and were the highest in Stage IV cancer patients [137].
Growing evidence indicates that exosomes can direct intercellular communication under physiological and pathological conditions and that exosomal contents play critical roles in inter- and intracellular communication for diverse cell types [4, 45–48]. In particular, exosomes regulate the function of distant cells by releasing their contents far away from the site of origin, influencing processes in recipient cells and establishing favorable environments at potential metastatic sites that aid the survival of neoplastic cells. For example, tumor exosomes confer transformed characteristics to fibroblasts and endothelial cells, trigger the fibroblast-to-myofibroblast transformation [138, 139], induce pro-angiogenic responses in endothelial cells and activate immunosuppressive functions of myeloid-derived suppressor cells [140, 141]. Such long-distance tumor-stroma interactions mediated by exosomes can also educate stromal components towards pro-metastatic phenotypes [16]. The involvement of exosomes and related microvesicles in providing autocrine, paracrine, and endocrine signals has led to the frequent use of the term, signalosomes, being applied to these structures.
Exosomal RNAs are heterogeneous in size but enriched in small RNAs, such as miRNAs. Tumor-secreted exosomes and miRNAs can be internalized by other cell types in the primary tumor microenvironment and pre-metastatic or metastatic niches [16, 142–146]. MiRNAs loaded in these exosomes, which to a certain extent re ect the dysregulated miRNA pro le in cancer cells, can thus be transferred to recipient niche cells to exert genome-wide regulation of gene expression. In addition, cancer-derived exosomal miRNAs may bind as ligands to Toll-like receptors in surrounding immune cells [147]. In light of these results, it is important to understand how exosomes facilitate tumor-stroma interactions and promote formation of a supporting metastatic niche in distant organs.
6. Secretion and uptake of cell-free miRNAs
6.1. Types of cell-free miRNAs
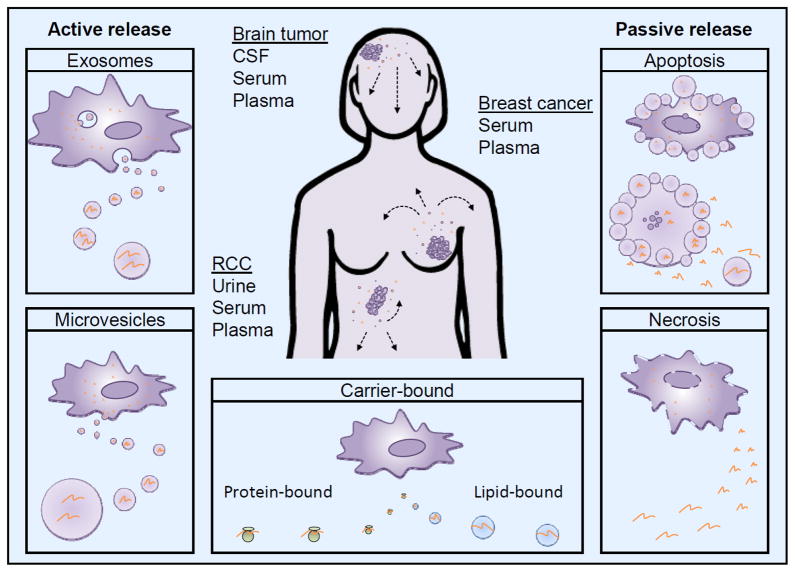
Cells have an array of miRNA packaging and transportation systems. Cell-free miRNAs either bind to protein, such as Argonaute2, or lipid carriers, such as HDL or LDL, [148–150] or are present in membrane encapsulated microvesicles such as exosomes (30 100 nm) [151–153] or larger microparticles (100–1,000 nm) [154] (Figure 1). Microvesicles are released into the extracellular environment by a wide range of cell types, including cancer cells, from where they can readily enter bodily fluids [154–157]. While the origin of exosomes and microvesicles are distinct, the two populations exhibit remarkable similarity so it is unclear whether this distinction between exosomes and shed microvesicles is critical to understand their biologic activities and interactions with target cells of the host [158]. However, this review focuses on the exosome population actively released by viable cells. In addition, miRNAs are present in apoptotic bodies (up to 4,000 nm) as a result of apoptotic death (Figure 1). It has been shown that cell-free miRNAs are stable under harsh conditions such as high temperatures, extreme pH values, repeated freeze-thaw cycles and long-term storage as well as endogenous RNase activity [44, 137, 159–163]. In fact, the remarkable stability of cell-free miRNAs is conferred by the presence of their protein- or lipid-based carriers or their encapsulation into microvesicles as carrier-free miRNAs are easily degraded by RNase and other environmental factors [164]. It is currently unclear whether cell-free miRNAs predominantly exist inside or outside vesicles, with one study detecting most miRNAs in human serum and saliva in exosomes [165], while other studies finding the majority of miRNAs in plasma and serum to be present primarily outside exosomes [148, 149, 166]. One possibility might be that it is dependent on the specific miRNA. In fact, Zhou observed that circulating miR-105 and miR-181a predominantly existed in exosomes isolated from breast cancer patient serum, while miR-375 and miR-422b were detected in both exosomes and exosome-depleted fraction at comparable levels [167]. Furthermore, it is not clear how cells choose between the different ways of secreting miRNA and what stimuli might alter the secretion profile of cells.
Figure 1. Cell-free miRNA secretion pathways.
Tumor-derived cell-free miRNAs can be released into the local microenvironment using multiple pathways from where they can potentially enter any body fluids. The most commonly used body fluids for miRNA detection of brain tumors, breast cancer and renal cell carcinoma (RCC) are illustrated. Exosome and microvesicle secretion are active processes whereby small membrane-enclosed vesicles containing a variety of molecules, including, proteins, DNA, and various RNA species, are released into the extracellular milieu. Cell death by apoptosis and necrosis passively leak cell-free miRNAs into the surrounding. Apoptotic bodies can also enclose miRNAs. Carrier-bound cell-free miRNAs can be bound by protein or lipid carriers; it is not understood how this release is mediated.
Cell-free miRNAs can act in autocrine (local signals between the same cell type, such as cancer cells), paracrine (local signals between different cell types, such as between epithelial cancer cells and stromal cells), and endocrine (distant signals between any types of cells) fashion and have hormone-like effects, which lead to widespread consequences [40]. Additionally, exosomes contain many other molecular constituents of their cell of origin besides miRNAs including proteins, lipids, metabolites and genetic material (mRNAs, DNA) which appear to be selectively recruited and secreted in a regulated manner [49, 138–141, 168–173]. The pool of exosome molecules is both conserved and also cell type-specific, depending on the molecule and the cell from which exosomes are shed. Exosomes mediate cell cell communications via ligand-receptor interaction and transport of intracellular components. The fact that exosomes contain a variety of molecules may allow for their specific uptake by target cells as well as combined delivery of multiple mediator molecules that combined have more profound effects on target cells than one molecule alone. For instance, Argonaute2 and GW182 proteins were found in exosomes secreted by monocytes, dendritic and HeLa cells [174], suggesting that exosomal miRNAs could be primed for gene regulation after arrival in target cells. In addition, the structure of exosomes (lipid and membrane protein content) can also influence distant cell signaling [175].
6.2. Mechanism of miRNA secretion
Although the level of circulating miRNAs may well reflect the expression level of tumor miRNAs, the release mechanism of cell-free protein or lipid-bound miRNAs as an active process is largely unknown. Exosomal miRNA is selectively recruited and actively secreted in a regulated manner [170–172]. During the tightly regulated biogenesis process of exosomes, inward budding from the limiting membrane of multivesicular bodies (MVBs) results in numerous vesicles in the lumen of the latter. The MVBs fuse with the plasma membrane to release the vesicles into the extracellular milieu as exosomes [40, 48]. Rab family proteins have emerged as key regulators of the exosome secretion pathway, in particular Rab27a, Rab27b, and Rab35 [176, 177]. Rab27 has also been shown to be involved in cancer progression and tumor promotion, which provided early indications that components of exosome secretion pathway may have roles in tumor biology [16, 178]. Additionally, Rab35 has been shown to regulate exosome secretion by interacting with GTPase-activating protein TBC1 domain family, member 10A-C (TBC1D10A-C) [177]. Exosome release has been shown to be triggered by various stimuli including ceramide [179] and changes in membrane pH [180]. MiRNA recruitment to MVBs is facilitated by physical and functional coupling of RISC to components of the sorting complex. In particular, GW182, present in GW bodies and congregated to endosomes and MVBs, promotes continued assembly and disassembly of membrane-associated RISC-bound miRNAs [174]. The process by which specific miRNAs are selected for incorporation into MVBs is, however, still not understood. Larger microvesicles, on the other hand, originate from budding of the plasma membrane. Deciphering the molecular mechanism that control miRNA loading into microvesicles and transfer to recipient cells in vitro will provide further evidence for the physiological relevance of vesicle-mediated intercellular communication in vivo. Additionally, miRNAs are enclosed in apoptotic bodies or can be passively leaked or from apoptotic or necrotic cells, which has been shown to occur after heart tissue injury [181, 182].
6.3. Mechanism of cellular miRNA uptake
The process underlying cellular uptake of cell-free miRNAs is poorly understood. Microvesicles are believed to be taken up by target cell via endocytosis [183, 184] or by membrane fusion [180, 185]. Internalization may involve specific interactions between transmembrane proteins on the vesicle and plasmas membrane, but the identity of those proteins is still largely unknown. While the cargo is directly released into the cytoplasm upon uptake by membrane fusion, endocytosis may target it for degradation. Uptake of lipid-bound miRNA, specifically HDL-bound, is mediated by SR-BI (Scavenger receptor class B, type I) [186], and is believed to bypasses the endosomal lysosomal pathway that could otherwise degrade miRNAs.
6.4. Types of body fluids that carry miRNAs
Cell-free miRNAs can be detected in potentially all body fluids, including whole blood, serum, plasma, urine, saliva, pancreatic juice and cyst fluid [41]. However, the total RNA concentration and the total number of distinct miRNAs differ vastly between the various body fluids. Human urine had the lowest RNA concentration and number of detectable miRNA species in a comparison of 12 body fluids [41], followed by cerebrospinal fluid and pleural fluid. In contrast, saliva, breast milk, and seminal fluid had the highest RNA concentrations and the highest numbers of miRNAs. Interestingly, about one-tenth of all identified miRNAs (61 out of a total of 600 different miRNAs) were detectable in all body fluid types while others were enriched in specific fluids. In fact, unsupervised hierarchical clustering of expressed miRNAs revealed groups of body fluids that cluster together, while the miRNA spectrum in plasma differed from that of most other body fluids [41]. On the other hand, comparison of specific miRNA levels between plasma and serum, a more commonly used clinical specimen than plasma, showed that measurements are strongly correlated, indicating that both sample types can be used for blood-based miRNA analyses [44]. Furthermore, peripheral whole blood can also be used for quantification of circulating miRNAs. Even though whole blood is easier to collect and has more RNA content [187], facilitating reliable and accurate global miRNA expression measurements using less clinical material, the majority of miRNAs are derived from red blood cells raising concern about the non-tumor specific miRNA detection.
Fluid-specific miRNAs may have functional roles associated with the surrounding tissue, and hence different body fluids may be more suitable for analyses of different pathologies. As cancer cells secrete cell-free miRNAs into the surrounding microenvironment, these cancer-derived miRNAs can enter body fluids and modify the miRNA spectrum of the latter. Depending on the type of cancer different body fluids have been used for analysis (Figure 1). For instance, blood-based samples are usually used for hematological malignancies and many solid tumors [43, 159], such as breast, prostate, and lung cancer, while urine can be used for urological cancers [188], saliva for oral cancers [189], and cerebrospinal fluid for brain cancers [190].
6.5. Correlation of intracellular and cell-free miRNAs
Similarly to miRNAs found in primary tumors, cell-free miRNAs have been linked to cancer and detection of their altered expression levels in body fluids has opened up new opportunities in cancer diagnosis, prognosis and treatment [43, 44, 137, 191, 192]. While changes in miRNA profiles in the presence of cancer could be due to changes in secretion of stroma-derived miRNAs, many studies have shown that the many circulating miRNAs are directly derived from tumor tissues and that the changes in miRNA profiles are in correlated with biological activity and miRNA expression in tumors. For instance, human xenografts in mice secrete human miR-629 and miR-660, both of which are expressed in the human prostate cancer cells that were inoculated [44]. Furthermore, highly expressed circulating miRNAs from cancer patients have been reported to return to a normal level after tumor resection [193–197], suggesting that the level of circulating miRNAs reflect the expression level of tumor miRNAs to some extent and could be used as pharmacodynamic marker.
As tumor-secreted miRNAs can reach distant sites and regulate various cellular components of the pre-metastatic and metastatic microenvironments, they might be of particular importance in understanding, detecting and targeting metastatic progression. Furthermore, the presence of miRNAs that are associated with the process of metastasis or EMT might identify those patients that already have distant micrometastases too small to diagnose otherwise. Hence, cell-free miRNAs might be superior prognostic markers and superior targets for therapeutic intervention than miRNAs in primary tumors.
7. Cell-free miRNAs in metastasis
Multiple studies have found miRNA signatures as well as individual cell-free miRNAs whose circulating levels are markedly altered in metastasis patients relative to patients with localized disease or differentially secreted from cancer cells with varying metastatic abilities (Table 1). For instance in breast cancer patients, circulating serum total RNA [198] or circulating miR-195 and let-7a predict presence of primary tumors [199], while increased levels of miR-10b and miR-34a, and decreased levels of miR-155 were shown to correlate with presence of overt metastases [198]. Global miRNA signatures may also be able to identifying patients with metastases or with increased risk of metastasis as they were shown to not only diagnose prostate cancer but also segregate them with different risk for progression [200]. Here, we outline how signatures and individual cell-free miRNAs correlate with metastatic progression and review the current knowledge on the validated or potential roles in metastasis of cell-free miRNAs.
Table 1.
Altered cell-free miRNA levels in metastatic cancer patients compared to localized cancer patients and healthy controls
| miRNA | Cancer | Source | Methods | Patient cohorts | Metastases | Reference |
|---|---|---|---|---|---|---|
| let7 family | ||||||
| let-7a | CRC | Serum | qRT-PCR | Metastatic vs localized | DM (not LiM) | [201] |
| let7-c | BC | Serum | miR array | LN positive vs negative | LN | [202] |
| let-7e | PTC | Serum | NGS, qRT-PCR | LN positive vs negative | LN | [203] |
| miR-9-79 family | ||||||
| miR-9 | MM | Serum | qRT-PCR | Metastatic vs localized | DM | [204] |
| miR-10-99-100-125 family | ||||||
| mir-10a, miR-10b | BC | Serum | miR array | LN positive vs negative | LN | [202] |
| miR-10b | BC | Serum | qRT-PCR | Metastatic vs localized | VM | [198] |
| miR-10b | BC | Plasma | qRT-PCR | LN positive vs negative | LN | [205] |
| miR-10b | NSCLC | Serum | qRT-PCR | LN positive vs negative | LN | [17] |
| miR-125b | MM | Plasma | qRT-PCR | Metastatic vs localized/HCs | DM | [206] |
| miR-126/miR-125b | BCa | Urine | qRT-PCR | Mets/high grade vs low grade | DM | [207] |
| miR-15-16-195 family | ||||||
| miR-16 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-17/92 cluster | ||||||
| miR-17 | BC | Serum | qRT-PCR | Metastatic vs localized | VM | [209] |
| miR-17* | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-20a | MM | Plasma | qRT-PCR | Metastatic vs localized | DM | [206] |
| miR-20a | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211, 212] |
| miR-20a* | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-92 | OvC | Serum | qRT-PCR | Stage III/IV vs I/II | LN & DM | [213] |
| miR-21 | ||||||
| miR-21 | BC | Serum | qRT-PCR | Metastatic vs localized | VM | [162] |
| miR-21 | BC | Serum | qRT-PCR | LN positive vs negative | LN | [214] |
| miR-21 | CRC | Serum | qRT-PCR | Metastatic vs localized | DM | [201] |
| miR-21 | EOC | Serum | qRT-PCR | Stage III/IV vs I/II | LN & DM | [215] |
| miR-21 | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-21 | GC | Plasma | qRT-PCR | LN positive vs negative, post-op | LN | [216] |
| miR-21 | NSCLC | Serum | qRT-PCR | LN positive vs negative | LN | [217, 218] |
| miR-21 | OSA | Plasma | qRT-PCR | Metastatic vs localized | DM | [219] |
| miR-21 | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | BM | [220, 221] |
| miR-21 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-23a/24-2/27a cluster | ||||||
| miR-23a* | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-24 | PCa | Serum | qRT-PCR | Metastatic vs localized | LN | [200] |
| miR-27a | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-25/93/106b cluster | ||||||
| miR-93 | PCa | Serum | qRT-PCR | Metastatic vs localized/HCs | LN | [200] |
| miR-106b | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-28/151 cluster | ||||||
| miR-151-3p | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-151-5p | PTC | Serum | NGS, qRT-PCR | LN positive vs negative | LN | [203] |
| miR-29 family | ||||||
| miR-29a | BC | Serum | qRT-PCR | LN positive vs negative | LN | [214] |
| miR-29a | CRC | Serum | qRT-PCR | Metastatic vs localized | LiM | [222] |
| miR-29b-1 | BC | Serum | miR array | LN positive vs negative | LN | [202] |
| miR-29c | MM | Serum | qRT-PCR | Metastatic vs localized | DM | [223] |
| miR-34 family | ||||||
| miR-34a | BC | Serum | qRT-PCR | Metastatic vs localized | VM | [198] |
| miR-105 | ||||||
| miR-105 | BC | Serum | NGS, qRT-pCR | Stage II-III, followed up | DM | [212] |
| miR-106a/363 cluster | ||||||
| miR-106a | PCa | Serum | qRT-PCR | Metastatic vs localized/HCs | LN | [200] |
| miR-122/3591 cluster | ||||||
| miR-122 | BC | Serum | NGS | Stage II-III, followed up | DM | [224] |
| miR-122 | GC | Plasma | qRT-PCR | Metastatic vs localized | DM | [225] |
| miR-126 | ||||||
| miR-126/miR-125b | BCa | Urine | qRT-PCR | Mets/high grade vs low grade | DM | [207] |
| miR-126 | CRC | Serum | qRT-PCR | Metastatic vs localized | VM | [201] |
| miR-126 | NSCLS | Serum | qRT-PCR | Stage IV vs Stage I/II | DM | [226] |
| miR-126 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-130 family | ||||||
| miR-130b | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-143/145 cluster | ||||||
| miR-143 | OSA | Plasma | qRT-PCR | Metastatic vs localized | DM | [219] |
| miR-146 family | ||||||
| miR-146a | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-146a | MM | Plasma | qRT-PCR | Metastatic vs localized | DM | [206] |
| miR-148-152 family | ||||||
| miR-148a | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-152 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-154 family | ||||||
| miR-409-3p | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | DM | [227] |
| miR-155 | ||||||
| miR-155 | BC | Serum | qRT-PCR | Metastatic vs localized | VM | [198, 209] |
| miR-155 | CRC | Serum | qRT-PCR | Metastasis/recurrence vs disease-free post-op/chemotherapy | VM | [228] |
| miR-155 | EnC | Serum | qRT-PCR | Metastatic vs localized | LN & DM | [229] |
| miR-155 | LC | Plasma | qRT-PCR | Stage IV vs stage I | DM | [163] |
| miR-155 | MM | Plasma | qRT-PCR | Metastatic vs localized | DM | [206] |
| miR-181 family | ||||||
| miR-181 | MM | Plasma | qRT-PCR | Metastatic vs localized | DM | [206] |
| miR-182/96/183 cluster | ||||||
| miR-183 | NSCLS | Serum | qRT-PCR | Stage IV vs Stage I/II | DM | [226] |
| miR-192-215 family | ||||||
| miR-192 | GC | Plasma | qRT-PCR | Metastatic vs localized | DM | [225] |
| miR-215 | BC | Serum | qRT-PCR | Metastatic vs HCs | DM | [187] |
| miR-197 | ||||||
| miR-197 | LC | Plasma | qRT-PCR | Stage IV vs stage I | DM | [163] |
| miR-198 | ||||||
| miR-198 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-199 family | ||||||
| miR-199a-3p | GC | Plasma | miR array, qRT-PCR | Metastatic vs localized | LN & DM | [230] |
| miR-200 family | ||||||
| miR-141, miR-200a, miR-200b, miR-200c | BC | Plasma | miR array, qRT-PCR | CTC-positive vs CTC-negative | DM | [231] |
| miR-141, miR-200a, miR-200b, miR-200c | BC, LC | CSF | qRT-PCR | BrM vs primary brain cancer | BrM | [190] |
| miR-141 | CRC | Serum | qRT-PCR | Metastatic vs localized | DM | [201] |
| miR-141 | CRC | Plasma | qRT-PCR | Stage IV vs Stage I/II | DM (not LN) | [232] |
| miR-141 | PCa | Serum | qRT-PCR | Metastatic vs localized | BM | [220, 233, 234] |
| miR-141 | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | LN & DM | [227, 235] |
| miR-141 | PCa | Plasma | qRT-PCR | Metastatic vs localized | DM | [44] |
| miR-141 (exo) | PCa | Plasma | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-141, miR-200c | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-141, miR-200a, miR-200c | PCa | Serum | miR array, qRT-PCR | Metastatic vs HCs | DM | [236] |
| miR-200b | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-200c | CRC | Serum | qRT-PCR | Metastasis/recurrence vs disease-free post-op/chemotherapy | VM | [228] |
| miR-200c | CRC | Serum | qRT-PCR | Metastatic vs localized | LN & DM | [237] |
| miR-200c | GC | Blood | qRT-PCR | Stage IV vs I-III | LN & DM | [238] |
| miR-203 family | ||||||
| miR-203 | BC | Plasma | miR array, qRT-PCR | CTC-positive vs CTC-negative | DM | [231] |
| miR-205 | ||||||
| miR-205 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-210 | ||||||
| miR-210 | BC | Plasma | miR array, qRT-PCR | CTC-positive vs CTC-negative | DM | [231] |
| miR-210 | BC | Plasma | qRT-PCR | LN positive vs negative | LN | [239] |
| miR-210 | CRC | Serum | qRT-PCR | Metastasis/recurrence vs disease-free post-op/chemotherapy | VM | [228] |
| miR-210 | PCa | Serum | miR array, qRT-PCR | Metastatic vs HCs | DM | [236] |
| miR-214/3120/199a-2 cluster | ||||||
| miR-214 | BC | Serum | qRT-PCR | LN positive vs negative | LN | [240] |
| miR-218 family | ||||||
| miR-218 | CCa | Serum | qRT-PCR | Stage III/IV vs I/II | LN | [241] |
| miR-221/222 cluster | ||||||
| miR-221 | PaC | Plasma | qRT-PCR | Metastatic vs localized | DM (not LN) | [242] |
| miR-221 | PCa | Plasma | qRT-PCR | Metastatic vs localized | BM | [220] |
| miR-221 | RCC | Plasma | qRT-PCR | Metastatic vs localized | DM | [243] |
| miR-222 | PTC | Serum | NGS, qRT-PCR | LN positive vs negative | LN | [203] |
| miR-223 | ||||||
| miR-223 | GC | Serum | qRT-PCR | GC stages | LN | [193] |
| miR-223 | MM | Plasma | qRT-PCR | Metastatic vs localized | DM | [206] |
| miR-296/298 cluster | ||||||
| miR-298 | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | LN & DM | [235] |
| miR-299/379/411 (belong to miR-379/miR-656 cluster) | ||||||
| miR-299, miR-411 | BC | Serum | qRT-PCR | Metastatic vs HCs | DM | [187] |
| miR-379 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-324 | ||||||
| miR-324-3p | MM | Serum | qRT-PCR | Metastatic vs localized | DM | [223] |
| miR-346 | ||||||
| miR-346 | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | LN & DM | [235] |
| miR-371/372/373 cluster | ||||||
| miR-373 | BC | Plasma | qRT-PCR | LN positive vs negative | LN | [205] |
| miR-375 | ||||||
| miR-375 | BC | Plasma | miR array, qRT-PCR | CTC-positive vs CTC-negative | DM | [231] |
| miR-375 | NSCLC | Plasma | qRT-PCR | Metastatic vs localized | DM | [244] |
| miR-375 | PCa | Serum | qRT-PCR | Metastatic vs localized | BM | [210, 233] |
| miR-375 | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | LN & DM | [227, 235, 236] |
| miR-375 | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-375 (exo) | PCa | Plasma | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-378 family | ||||||
| miR-378* | PCa | Serum | miR array, qRT-PCR | Metastatic vs localized | DM | [227] |
| miR-423/3184 cluster | ||||||
| miR-423-3p | PCa | Plasma | qRT-PCR | Metastatic vs localized | LN & DM | [208] |
| miR-451/144/4732 cluster | ||||||
| miR-451 | PCa | Serum | qRT-PCR | Metastatic vs localized/HC | LN | [200] |
| miR-506 family | ||||||
| miR-513a-5p | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-572 | ||||||
| miR-572 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-577 | ||||||
| miR-577 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-582 | ||||||
| miR-582-3p | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-609 | ||||||
| miR-609 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-619 | ||||||
| miR-619 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-624 | ||||||
| miR-624* | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-1236 | ||||||
| miR-1236 | PCa | Serum | qRT-PCR | Metastatic vs localized | DM | [210] |
| miR-1246 | ||||||
| miR-1246 | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211] |
| miR-2392 | ||||||
| miR-2392 | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211] |
| miR-3147 (provisional) | ||||||
| miR-3147 | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211] |
| miR-3162 (provisional) | ||||||
| miR-3162-5p | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211] |
| miR-4484 | ||||||
| miR-4484 | CCa | Serum | miR array, qRT-PCR | LN positive vs negative | LN | [211] |
Red boxes: higher levels in metastatic patients; green boxes: lower levels in metastatic patients.
indicates a product of the opposite arm of the precursor miRNA.
BC, Breast cancer; BCa, Bladder cancer; BM, Bone metastasis; BrM, Brain metastasis; CCa, Cervical cancer; CRC, Colorectal cancer; CSF, Cerebrospinal fluid; DM, distant metastasis; EnC, Endometrial cancer; EOC, Epithelial ovarian cancer; GC, Gastric cancer; HC, healthy control; LC, Lung cancer; LiM, Liver metastasis, LN, lymph node; Mets, Metastases; miR array, microRNA microarray; MM, Malignant melanoma; NSCLC, Non-small cell lung cancer; NGS, Next-generation sequencing; OSA, Osteosarcoma; OvC, Ovarian cancer; PaC, pancreatic cancer; PCa, prostate cancer; post-op, post-operative; PTC, Papillary thyroid carcinomas; qRT-PCR, quantitative real-time PCR; RCC, Renal cell carcinoma; VM, visceral metastasis
7.1. Total cell-free miRNA levels in metastatic patients and cancer cells
While increased RNA and miRNA concentrations have been observed in serum of cancer patients compared to healthy controls [245], only a few studies have looked at their levels in metastatic relative to non-metastatic cancer patients. Initially, Roth et al. reported total serum RNA levels to be increased in localized breast cancer patients compared to control and metastatic patients [198]. However, a follow-up study reported total serum RNA concentrations to be highest in women with metastatic breast cancer, followed by non-metastatic patients and finally healthy controls [209]. These findings indicate a progressive rise of serum RNA from healthy women to patients with primary breast cancer to patients with metastatic disease which could be used for staging of breast cancer patients. The discrepancy between the two studies could be explained by the different patient populations, as the first study included patients with metastatic disease at the end of chemotherapy, whereas the later analyzed patients with metastatic disease who had not received chemotherapy. This suggests that chemotherapy could impact total serum RNA concentrations, in a similar way that highly expressed circulating miRNAs return to normal levels after tumor resection. Likewise, Brase et al. specifically quantified total serum miRNA levels and found higher levels in patients with metastatic compared to localized prostate cancer [233].
Similarly, the RNA levels in exosomes secreted by metastatic cancer cells have been compared to those secreted by non-metastatic cancer and normal cells. The metastatic gastric cancer cell line AZ-P7a secreted exosomes had over 10 times more RNA than the exosomes from the parental, poorly metastatic AZ-521 cancer cell line [246].
7.2. Regulation of the metastatic cascade by cell-free miRNAs
Circulating levels of specific cell-free miRNAs have been shown to change during cancer progression, especially with the onset of metastasis. While many cell-free miRNAs have been reported to be suitable prognostic or diagnostic markers of the presence of lymph node or distant metastases (Table 1), their specific role in the circulation and the molecular mechanism by which they promote metastasis have not been addressed for the majority of identified circulating miRNAs. In fact, elucidating whether deregulated miRNAs are causes or consequences of metastatic progression requires detailed functional characterization using in vitro and in vivo models of cancer metastasis, and is essential for development of targeted therapeutic strategies. While this has been done only for a few miRNAs, there is evidence for other miRNAs to promote metastasis based on correlation analyses and previous functional studies in tumor tissues (Figure 2, Table 2).
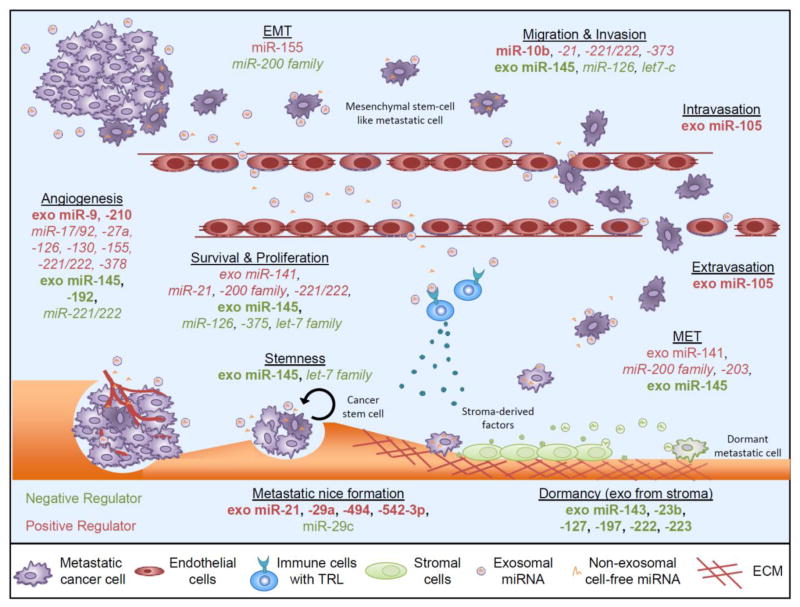
Figure 2. Cell-free miRNA involvement in regulating metastasis.
Cell-free miRNA can influence each step of the metastatic cascade by either promoting (onocmirs, red) or inhibiting (tumor suppressive miRNAs, green) metastatic progression. Cancer cells disseminate from the primary sites after acquiring an invasive phenotype, in part through the EMT, and enter the blood and lymphatic systems. Once in circulation, circulating cancer cells extravasate into distant organs such as the lung and the bone, where they actively grow into metastatic tumors or stay dormant before resuming expansion into overt metastasis. While a number of tumor miRNAs which have been identified in circulation of metastasis patients have been implicated in influencing particular metastatic steps, their role as cell-fee miRNAs has not been validated (miRNAs in italics). Nevertheless, the function of several cell-free miRNAs has been studied during metastatic progression (miRNAs in bold).
Table 2.
Validated and potential mechanisms of metastasis mediating cell-free miRNAs
| Invasion & Migration | Activity | Targets | Target cell | Reference |
|---|---|---|---|---|
| miR-10b | Enhances invasion/migration, metastasis in vivo | HOXD10† | TC | [106, 247, 248] |
| miR-21† | Enhances invasion/migration, metastasis in vivo | SERPINB5, TMP1, PDCD4, MARCKS | TC | [88, 93, 95–97] |
| miR-126† | Decreases migration/invasion, enhances cell adhesion | CRK1 | TC | [108, 109] |
| miR-145 (exo‡) | Decreases invasion/migration, metastasis in vivo | AKT3 | TC | [249] |
| miR-155† | Promotes EMT | RHOA | TC | [250] |
| miR-221/222† | Enhance invasion/migration in vitro | PTEN, TIMP3 | TC | [251] |
| miR-373† | Enhances invasion/migration, metastasis in vivo | CD44 | TC | [107, 252, 253] |
| let-7c† | Decreases invasion/migration, metastasis in vivo | MMP11, PBX3 | TC | [254] |
| Intra-& Extravasation | Activity | Targets | Target cell | Reference |
|
| ||||
| miR-105 (exo) | Inhibits tight junctions, enhances vascular leakiness, metastasis in vivo | ZO-1 | EC | [167] |
| MET | Activity | Targets | Target cell | Reference |
|
| ||||
| miR-145 (exo‡) | Decreases E-cadherin expression in vitro | AKT3 | TC | [249] |
| miR-200 family† (miR-141 also exo) | Promote MET, enhance metastasis in vivo | ZEB1, ZEB2 | TC | [115, 116, 210] |
| miR-203† | Promotes MET | ZEB2, SMAD4 | TC | [255] |
| ECM & niche | Activity | Targets | Target cell | Reference |
|
| ||||
| miR-9 (exo) | Promotes angiogenesis and tumor growth in vivo | SOCS5 | EC | [173] |
| miR-17/92† | Promotes angiogenesis | TSP1, E2F1, CTGK | EC, TC | [256–259] |
| miR-21† | Establishes pro-metastatic niche | RECK, TIMP3, PTEN | [89, 97] | |
| miR-21, miR-29a (exo) | Induce pro-metastatic inflammatory response, enhance metastasis in vivo | TLR 7, TLR8 | IC | [147] |
| miR-27a† | Promotes EC-mediated angiogenesis | ZBTB10, SEMA6A | EC | [260, 261] |
| miR-29c† | Inhibits ECM (laminin & collagen) deposition | Collagens, LAMC1 | [262, 263] | |
| miR-126† | Promotes EC-mediated angiogenesis | SPRED1, PIK3R2, VEGFA, PIK3R2 | EC | [264–266] |
| miR-130† | Promotes EC-mediated angiogenesis | GAX, HOXA5 | EC | [267] |
| miR-145 (exo‡) | Decreases VEGF secretion and angiogenesis in vitro & in vivo | AKT3 | [249] | |
| miR-155† | Promotes angiogenesis | AT1R | EC | [268, 269] |
| miR-192 (exo) | Inhibits angiogenesis and metastasis in vivo | ICAM-1, IL-8, CXCL1 | EC | [270] |
| miR-210 (exo) | Promotes EC-mediated angiogenesis in vitro | EFNA3 | EC | [271] |
| miR-221/222† | Reduce EC-mediated angiogenesis | c-kit, eNOS* | EC | [272, 273] |
| miR-221/222† | Promote tumor induced angiogenesis | P27/KIP1 | TC | [274] |
| miR-378† | Promotes tumor induced angiogenesis | Sufu, Fus-1 | TC | [275] |
| miR-494, miR-542-3p (exo) | Establish pro-metastatic niche, enhance metastasis in vivo | Mal, Cdh17, Traf4 | LN stroma, lung fibroblasts | [276] |
| Renal CSC-derived exosomes | Promote EC-mediated angiogenesis | EC | [277] | |
| Stemness & growth | Activity | Targets | Target cell | Reference |
|
| ||||
| miR-21† | Promotes survival | BCL2 | TC | [98] |
| miR-21† | Promotes tumor growth | PTEN, BTG2, SPR2, P12/CDK2AP1 | TC | [132, 278, 279] |
| miR-126† | Decreases cell proliferation | CRK1 | TC | [264, 265] |
| miR-145 (exo‡) | Decreases cell proliferation, stemness in vitro | AKT3 | TC | [249] |
| miR-200 family (miR-141 also exo)† | Promotes survival and metastasis | Sec23a | TC | [115, 116, 210] |
| miR-221/222† | Promotes cell proliferation and tumor growth | p27/KIP1 | TC | [274, 280] |
| miR-375† | Decreases cell proliferation | SEC23A | TC | [210] |
| let-7 family† | Decrease cell proliferation, inhibit stemness | RAS, HMGA2, MYC | TC | [101–105] |
Studies entirely done on tumor miRNAs. Activity and targets potentially similar of cell-free miRNAs.
Effects of miRNA were not tested in exosomes, but by overexpression/knockdown in tumor cells
EC, endothelial cell; EMT, epithelial-mesenchymal transition; exo, exosomal; IC, immune cell; LN, lymph node; TC, tumor cell
7.2.1. Invasion, migration and ECM remodeling at primary site
Elevated levels of circulating miR-21, an oncomir shown to promote tumor growth, invasion and migration in many cancer types [281] and the first circulating miRNA discovered [43], have been detected in patients with metastatic disease from different tumors as compared to patients with localized disease, including breast [162, 214], lung [217, 218], prostate [208, 220, 221], colorectal cancer [201] and gastric cancer [193]. In fact, serum miR-21 levels can distinguish breast cancer patients from healthy controls, and further distinguish patients with distant metastasis from those with locoregional disease [162], suggesting that this particular miRNA could be used to stage breast cancer patients. Furthermore, a decrease in miR-21 levels is seen in post-operative plasma of gastric cancer patients that corresponds to the degree of differentiation of the primary tumor and lymph node metastasis status of the patient [216]; patients with lymph node metastasis exhibit a smaller decrease in plasma miR-21 levels than those without, indicating that miR-21 may be useful for monitoring response to treatment. While a number of studies reported high miR-21 levels to correlate with advanced clinical stage, metastasis, and poor prognosis in cancer patients [218, 282], a few studies have failed to validate this correlation [283, 284], possibly due to differences in patient cohorts (number, ethnicity etc.) or preparation and analysis methods. Various in vitro and in vivo experiments have established a role for tumor miR-21 in multiple steps of tumor progression, including tumor growth at primary and potentially secondary sites, invasion and migration, ECM modification and survival. Tumor miR-21 promotes tumor growth by repression of a number of tumor suppressor genes, including PTEN [89], P12/CDK2AP1 [285], BTG2 [278], and hinders apoptosis through inhibition of BCL2 [286], two features that aid metastatic outgrowth at the secondary site. Cell motility and invasion are increased by specific targeting of regulators of the metastasis-promoting factor urokinase receptor, namely SERPINB5 (Maspin) [93] and PDCD4 (programmed cell death 4) [88, 93, 94]. MiR-21 also modifies cytoskeletal organization by inhibiting TMP1 [95] (tropomyosin 1), an acting-binding protein that prohibits anchorage-independent growth, and MARCKS [96] (myristoylated alanine-rich protein kinase c substrate), a regulator of actin filament formation and a modulator of the metastatic phenotype [287]. Additionally, miR-21 remodels the ECM by indirectly up-regulating metastasis-promoting metalloproteinases (MMPs) via repression of MMP inhibitors [97], RECK (Revision-induced-cysteine-rich protein with kazal motifs) and TIMP3 (tissue inhibitor of metalloproteinases 3). MMP2 and MMP9 expression are also increased due to miR-21-induced loss of PTEN (phosphatase and tensin homolog) expression [89]. Treatment of metastatic cancer cells with anti-sense oligonucleotide against the miR-21 inhibits their metastatic ability, as gauged by in vitro invasion and migration assays [88, 97], in vivo tail-vein lung metastasis assay [93] and a chick-embryo chorioallantoic membrane metastasis assay [88]. However, the effect of systemic inhibition of miR-21 on metastatic progression has not been tested yet.
MiR-10b is another metastasis-promoting miRNA facilitating migration and invasion whose elevated levels in many primary and metastatic tumors as well as metastatic cell lines correlate with tumor progression [106]. Similarly, circulating miR-10b levels also correlate with metastatic breast cancer progression, as the levels in blood increase from healthy controls, to patients with localized disease and are highest in patients with visceral [198] or lymph node metastasis [205]. Hence, miR-10b expression is likely induced at a relatively late stage of the multistep tumorigenesis process and plays a role specifically in metastatic progression but not in primary tumor formation. Even though one study reported correlation of low levels of circulating miR-10b and its variant miR-10a with lymph node metastasis [202], these results were not further validated by qRT-PCR. Knock-down of miR-10b in metastatic cell lines decreased their migratory and invasive capabilities [106, 247], while overexpression in poorly metastatic cancer cells induced invasion and migration in vitro [106, 248] and increased metastasis in vivo [106]. Furthermore, systemic treatment of tumor-bearing mice with miR-10b antagomirs suppressed breast cancer metastasis [247]. Tumor miR-10b is transcriptionally activated by the pro-metastatic transcription factor TWIST1 and is essential for TWIST1-induced EMT [106]. Tumor miR-10b exerts its metastasis-promoting function by directly down-regulating the metastatic suppressive gene Homeobox D10 (HOXD10) which in turn inhibits the pro-metastatic gene RHOC [106], although this target has not been confirmed for cell-free miR-10b.
Several other miRNAs that were previously identified as enhancers of migration and invasion have been detected at high levels in body fluids of metastatic patients and correlated with lymph node and/or distant metastasis, including circulating miR-373 and miR-221/222, raising the possibility that these cell-free miRNAs could be mediating metastasis. Tumor miR-373 and miR-520c have been classified as pro-metastasis miRNAs as they increase fibrosarcoma, breast and prostate cancer cell migration, invasion and eventual metastasis in vitro and in vivo [107, 252, 253]. Both miRNAs have a similar seed sequence and inhibit CD44 expression which has been associated with the acquisition of an enhanced migratory potential [107]. However, only circulating miR-373 has been detected in blood of breast cancer patients and higher levels were correlated with lymph node metastasis, but its role in the circulation has not yet been unraveled. MiR-221 and miR-222 are expressed in the same cluster. Increased circulating miR-221 has been detected in patients with metastatic disease arising from renal cell carcinoma [243], pancreatic [242] and prostate cancer [220]. In all cases, increased plasma levels of miR-221 were correlated with distant metastasis. On the other hand, increased serum levels of cell-free miR-222 in papillary thyroid carcinoma patients were associated with presence of lymph node metastasis [203]. In lung cancer cells, tumor miR-221 was shown to enhance migratory and invasive ability by down-regulating tumor suppressor genes PTEN and TIMP3 as well as to stimulate resistance of TRAIL-induced apoptosis [251].
7.2.2. Entry into, survival in and exit from the circulation
Even though leakiness of the vasculature caused by cancer-derived exosomes has been observed previously [16], only recently has it become clear that this phenomena may be at least in part due to exosomal miRNAs, such as exosomal miR-105 [167]. MiR-105, which is characteristically expressed and secreted by metastatic breast cancer cells, is a potent regulator of the tight junction protein ZO-1 and efficiently destroys tight junctions on endothelial cells and thus endothelial barriers at the primary and secondary sites [167]. Therefore, exosomal miR-105 not only promotes intravasation by creating gaps within the endothelial layer that lines blood and lymphatic vessels, but also extravasation and pre-metastatic niche formation in secondary organs. Overexpression of miR-105 in non-metastatic cancer cells induced metastasis and vascular permeability in distant organs, whereas inhibition in highly metastatic tumors alleviated the effects. Cell-free miR-105 could be detected in serum at the pre-metastatic stage, and high levels were associated with low ZO-1 expression and metastatic progression in early stage breast cancer [167].
7.2.3. Mesenchymal-Epithelial Transition (MET)
Interestingly, several studies have shown high levels of circulating miR-200 family members (miR-200a, -200b, -200c, -141, -429) in metastasis patients suggesting a role as markers of progression and survival. For instance, miR-141 was found elevated in serum and plasma of metastatic prostate cancer patients and correlated with lymph node and distant metastasis and poor survival [44, 208, 220, 227, 233–236]. In colorectal cancer patients, high levels of miR-141 were associated with distant, but not lymph node metastasis [201, 232]. Furthermore, increased cell-free miR-141 levels were measured in serum of patients with various secondary lesions [201], indicating that this miRNA might be correlated with general metastatic spread to distant organs. All miR-200 family members except miR-429, were found higher in plasma of breast cancer metastasis patients [231] and could also be detected in the cerebrospinal fluid of breast and lung cancer patients suffering from brain metastasis [190]. Furthermore, the miR-200 family may also be a good indicator of the CTC status in breast cancer [231], a feature that is correlated with increased risk of metastasis. Elevated levels of circulating miR-200c were found in blood samples of metastatic prostate [208, 236], colorectal [228] and gastric cancer [238] patients, and correlated with lymph node and distant metastasis. Furthermore, higher cell-free miR-200b and exosomal miR-141 levels were identified in patients with metastatic relative to localized prostate cancer [210]. These findings are consistent with experimental observation in animal models showing a role of the miR-200 family in promoting metastatic colonization by inducing MET, adhesion to secondary sites, and inhibition of Sec23-mediated secretion of metastasis-suppressive proteins, such as TINAGL1 and IGFBP4 [115, 116]. The miR-200 family exerts its effects by directly targeting ZEB1/ZEB2 gene expression in tumor cells, both of which are transcriptional repressors of E-cadherin and inducers of EMT. Whether this function is also excreted by cell-free mi-200 family members still needs to be validated.
MiR-203 is another EMT repressor that targets the 3′UTR of ZEB2 and SMAD4 [255], whose increased expression has been discerned in a number of cancers [73, 91, 123]. Similar to the miR-200 family, higher levels of circulating miR-203 have been detected in plasma of breast cancer patients with CTCs compared to those without and correlated with poor prognosis [231], but its role in the circulation during metastatic progression has not been addressed yet.
7.2.4. ECM remodeling at secondary sites and metastatic niche formation
Metastatic cancer cells have been shown to modify the site of metastasis before and after arrival to the foreign microenvironment by actively modifying the ECM as well as by recruiting and activating various stromal components to the metastatic niche [3, 4]. Such education toward a pro-metastatic phenotype has, for instance, been observed with exosomes isolated from the metastatic rat adenocarcinmoa cell line ASML which can modulate draining lymph node and lung tissue to support settlement of the poorly metastatic ASML-CD44v-KD cell line that lacks expression of the metastasis-promoting CD44v molecule [288]. The pre-metastatic education was later attributed to exosomal miRNAs that perturb the stromal compartment at these distant sites [276]. Specific uptake of tumor cell-secreted exosomes by lymph node stromal cells and lung fibroblasts impacted the expression of target genes in these two cell lines leading to the establishment of the pre-metastatic niche [276]. Specifically, exosomal miR-494 and miR-542-3p have been implicated in ECM remodeling at these remote sites as they regulate expression of Mal, Cdh17, and Traf4 genes and lead release of MMP2, MMP3 and MMP14 into the extracellular milieu [276].
Other stromal cells important for the evolution of the metastatic niche are various types of immune cells which are often educated by cancer cells. Notably, Fabbri et al. showed that tumor-secreted exosomal miR-21 and miR-29a are capable of inducting a pro-metastatic inflammatory response in the lung by binding to the Toll-like receptor (TLR) family, murine TLR7 and human TLR8, in immune cells. This binding activates the NF-κB pathway in the latter leading to secretion of pro-metastatic inflammatory cytokines [147]. Thus, by acting as paracrine agonists of TLRs, the two secreted miRNAs regulate the tumor microenvironment via tumor-immune system communication. Although the Lewis lung carcinoma model used in the study is not a model of lung cancer metastasis, the mechanism by which miRNAs act as agonists of TLRs may be involved in tumor-stroma interactions during metastasis. While high circulating miR-21 levels are found in metastatic patients (reviewed above), circulating miR-29a levels in metastatic cancer patients are not as consistent, indicating that its role in metastasis might be cancer type dependent. For instance, serum levels of cell-free miR-29a were increased in the presence of colorectal liver metastasis [222], but decreased in lymph node positive breast cancer patients [214].
Other stromal cells recruited to the secondary site are endothelial cells that can be activated by miRNAs to form new blood vessels in support of metastatic outgrowth [289, 290]. Several of these previously identified angiogenesis-promoting miRNAs have been reported at higher levels in body fluids of metastatic cancer patients relative to non-metastatic patients or healthy controls, including cell-free miR-17/92 [210, 213], miR-27a [193], miR-126 [207, 208], miR-130 [210], miR-155 [163, 206, 228, 229], miR-221/222 [203, 220, 242, 243], and miR-378 [227]. Some of these pro-angiogenic miRNAs have been shown to inhibit anti-angiogenic factors such as TSP1 (thrombospondin 1), SPRED1 (sprouty-related, EVH1 domain containing 1), PIK3R2 (phosphoinositide 3-kinase regulatory subunit 2), and ZBTB10 (zinc finger and BTB domain containing 10) often leading to an indirect up-regulation of VEGF (vascular endothelial growth factor) [31]. Whether all these circulating miRNAs promote angiogenesis at secondary sites is, however, not known. Moreover, high levels of circulating miR-210 have also been measured in blood of breast [231, 239], colorectal [228] and prostate cancer [236] where they correlated with presence of lymph node and distal metastasis. Specific release of exosomal miR-210 from metastatic breast cancer cell lines increased in vitro angiogenesis through the inhibition of EFNA3 (ephrin-A3) [271], indicating that some if not all of identified miRNAs could modify the metastatic microenvironment by inducing angiogenesis. Other tumor-derived miRNAs found to influence angiogenesis in vitro and in vivo by specifically targeting endothelial cells are exosomal miR-9 [173] and exosomal miR-192 [270]. MiR-9 targets SOCS5 in endothelial cells after they take up exosomes derived from a number of cancer types (melanoma, glioblastoma, lung, colorectal, and pancreatic cancers) leading to activation of the JAK-STAT pathway, increased tumor growth and angiogenesis [173]. Treatment with miR-9 antagomirs abolishes pro-proliferative and pro-angiogenic effects of tumor-derived exosomes. The anti-angiogenic miR-192, on the other hand, is reduced in bone metastatic lung cancer cells and its transfer via exosomes to bone stromal cells markedly diminishes bone metastasis in vivo and impairs tumor-induced angiogenesis in vitro and in vivo [270]. Upon uptake of the pleiotropic exosomal miR-192 endothelial cells repress the expression of pro-angiogenic factors such as ICAM-1, IL-8 and CXCL1. Conversely, cell-free miR-192 was detected at elevated levels in plasma of metastatic gastric cancer patients [225], while cell-free miR-9 was found to decrease in serum of melanoma patients with distant metastatic disease [291]. These discrepancies between exosomal total cell-free miRNA levels could be due to selective packaging of miR-9 into and exclusion of miR-192 from exosomes leading to a change in non-exosomal secretion into the circulation in metastatic cancer patients.
In addition, exosomes isolated from the CD105-positive renal CSC population have been shown to trigger angiogenesis in vitro and in vivo, and to induce the formation of a pre-metastatic niche in the lung that enhanced lung metastasis formation [277]. This pro-metastatic property was lost when exosomes were treated with RNase indicating that RNAs play a key role in the formation of the pre-metastatic niche. Comparison of the miRNA expression profiles of exosomes released from CSCs and differentiated tumor cells revealed the presence of 24 significantly up-regulated miRNAs and 33 down-regulated miRNAs in CSC-derived exosomes. Among the miRNAs shuttled by CSC-derived exosomes were angiogenesis-promoting miRNAs miR-92, miR-130 and miR-151 [277]. Additionally, these exosomes contained metastasis-mediating miRNAs such as miR-29a, miR-141 and miR-200c. However, the identity of the CSC-secreted miRNA responsible for the observed pro-angiogenic and pro-metastatic phenotypes remains unknown.
7.2.5. Stemness and growth into macrometastases
A similar set of specific miRNAs fundamental for the maintenance of CSCs in the primary tumor is thought to also support the survival and stemness of these cells at secondary microenvironments where they are regarded as cells that generate metastases [292]. In particular, as cancer cells that have undergone EMT acquire stem cell-like properties [293], it might be of particular importance to support the CSC state at distant sites after cell go through MET by miRNA-mediated stemness pathways that bypass cellular senescence and maintain self-renewal capacity of tumor cells.
MiRNA family (let-7a-1, 7a-2, 7a-3, 7b, 7c, 7d, 7e, f7-1, 7f-2, 7g, 7i, mir-98, and mir-202), which are down-regulated in a variety of tumors [100], exert their effect on tumorigenesis by silencing multiple oncogenes, including RAS, HMGA2 (high mobility group A2), and MYC [101–103]. They also hinder metastasis by inhibition of anchorage-independent growth as well as regulation of stemness and depletion of CSCs [104, 105]. Reduced let-7 in breast CSCs increases in vivo tumorigenic and metastatic capabilities when inoculated into mice [104]. In support of this anti-metastatic role, circulating let-7c was decreased in serum of lymph node positive relative to lymph node negative breast cancer patients [202], although this result was not validated by qRT-PCR. On the contrary, circulating let-7a in colorectal cancer was higher in metastatic patients than those with localized disease. The high let-7 serum levels correlated with distant, but not lymph node metastasis [201]. High levels of circulating let-7e detected in papillary thyroid carcinomas patients with lymph node metastasis correlated with the presence of lymph node metastasis [203]. Furthermore, let-7 family members are enriched in exosomes and selectively secreted from metastatic but not poorly metastatic gastric cancer cells, although the functional role of the differences has not been elucidated [246]. In exosomes isolated from ovarian cancer cells, let-7a through let-7f were found at higher concentrations in exosomes shed from invasive SKOV-3 relative to non-invasive OVCAR-3 cells [170]. This indicates that these up-regulated miRNAs may have a different pro-metastatic role or may target the stemness of normal stem cells to simplify the competitive landscape.
Several studies have shown increased levels of circulating and exosomal miR-375 in plasma and serum of metastatic prostate cancer patients [208, 210, 227, 233, 235, 236]. Circulating miR-375 was also found elevated in plasma of breast cancer patients [231]. In both cancer types, high levels of miR-375 correlated with lymph node and distant metastasis. On the contrary, circulating miR-375 levels were found to decrease in lung cancer patients with metastatic compared to localized disease [244]. MiRNA-375 has been shown to regulate a cluster of genes responsible for cellular growth and proliferation in the pancreas and its function is essential for glucose-induced insulin secretion [294, 295], suggesting it may have a role in promoting cell growth. Similarly to the miR-200 family in breast cancer, miR-375 down-regulates SEC23A in prostate cancer cell lines, resulting in enhanced proliferation [296]. However, if and how cell-free miR-375 enhances metastatic proliferation in prostate and breast cancer as well as the function of the decrease in circulating miR-375 levels in lung cancer metastasis is unknown.
While miR-126 acts principally to inhibit tumor growth at distant sites, endothelial activation, and metastatic initiation [108, 109], its role as a secreted miRNA during metastatic development is not easily apparent. MiR-126 has been described as a metastasis suppressor whose expression in tumor tissue is down-regulated during breast cancer progression [108], mainly because of its inhibition of tumor cell proliferation, actin remodeling and cell adhesion by CRK1 repression [31]. Accordingly, circulating miR-126 has been reported to be reduced in serum of colorectal [201] and lung cancer [226] patients suffering from metastatic lesions at distant sites, but the importance of this down-regulation has not been addressed yet. In contrast, increased levels of cell-free miR-126 were detected in urine and plasma of metastatic bladder [207] and prostate [208] cancer patients, respectively. This increase in circulating miR-126 could be due to secretion by cancer cells that target endothelial cells or by endothelial cells themselves as miR-126 in the latter has been shown to promote angiogenesis by inhibition of SPRED1 and PIK3R2 leading to up-regulation of VEGF and FGF2 [264, 265].
7.2.6. Cell-free miRNAs secreted by stromal cells
MicroRNAs transfer between cancer cells and the genetically normal niche cells is bidirectional. In addition to the cancer-derived adaptation of niche cells, stromal cells also secrete and transfer miRNAs to cancer cells that can promote or inhibit tumor progression (Table 3). Exosomal miR-143 is shuttled by normal prostate cells to cancer cells as a potential strategy to maintain tissue homeostasis at an early stage in cancer formation [297]. Specifically, cell-free miR-143 was shown to inhibit tumor cell proliferation in vitro and tumor growth in vivo. This raises the possibility that this miRNA may also function at distant sites to thwart proliferation of metastatic cells into full-blown metastases. Similarly, four miRNAs (miR-127, miR-197, miR-222, and miR-223) are transferred to breast cancer cell through gap-junctions and to a smaller extent via exosomes from bone marrow stromal cells [298]. These miRNAs inhibit proliferation though inhibition of CXCL12 and arrest the metastatic cells in a dormant state in which it can survive high-dose chemotherapy, making them interesting targets for combination treatment with conventional therapy in which dormancy is an obstacle. Moreover, exosomal miR-23b has also recently been shown to induce dormancy in breast cancer cells after being released by bone marrow mesenchymal stem cells [299]. Furthermore, transfer of these stromal exosomes containing miR-23b was shown to inhibit cell cycling and mobility by targeting MARCS gene expression in the cancer cells.
Table 3.
Cell-free miRNAs secreted by stromal cells
| miRNA | Cell of origin | Activity | Targets | Reference |
|---|---|---|---|---|
| miR-143 (exo) | PCa cells | Inhibits cell proliferation in vitro and tumor growth in vivo | [271] | |
| miR-127, miR-197, miR-222, miR-223 (exo) | BMSC | Induce dormancy in metastatic cells | CXCL12 | [298] |
| miR-223 (exo) | TAMs | Enhances invasion, migration, metastasis in vivo | MF2C | [300] |
| miR-16, miR-378 | Unknown (OCs?) | Unknown | [8] | |
| miR-23b (exo) | BM-MSCs | Inhibit proliferation in vitro, tumor growth in vivo, induce dormancy | MARCS | [299] |
BMSC, Bone marrow stem cell; BM-MSC, Bone marrow mesenchymal stem cell; exo, exosomal; OC, osteoclast; PCa, prostate cancer; TAM, tumor-associated macrophage
Stromal cells that are recruited to the tumor site can also secrete cell-free miRNAs. For instance, tumor-associated macrophages (TAMs) have been shown to secrete exosomal miR-223 which increased the invasive capabilities of breast cancer cells in vivo by targeting MF2C and regulating the Mf2c-β-catenin pathway [300]. The exosome-mediated effect on invasion could be reversed by treatment of breast cancer cells with miR-223 anti-sense oligonucleotides [300].
Interestingly, serum miRNAs shown to be up-regulated during osteoclast differentiation, an important process promoting osteolytic bone metastasis, could be used as an indicator of osseous metastases [8]. Both miR-16 and miR-378 are specifically elevated in metastatic, but not primary tumor samples and cell-free miR-16 and miR-378 are detectable at elevated levels in serum of bone metastatic breast cancer patients compared to healthy controls, revealing the presence of osseous metastases [8]. Hence, these miRNAs could be promoting the differentiation process at remote sites through secretion by either cancer or stromal cells.
8. MiRNA Targeting and Therapy
Characterization of tumor-secreted messengers and effectors, such as miR-21 or miR-105, will enable the selection of patients for appropriate targeted therapies and lead to use of combination therapies that simultaneously target multiple secretory miRNAs and/or proteins. Such patient selection may be achieved by a quantitative diagnostic test for specific circulating or exosomal miRNAs whose levels correlate with metastasis in cancer patients. This would identify breast cancer patients with high risk for metastasis who would greatly benefit from a preventive treatment that targets the miRNA effectors.
8.1. Direct targeting of cell-free miRNAs
The evidence that some cell-free miRNAs are de-regulated during metastasis and may have functional effects on metastatic progression indicates that they can serve as novel therapeutic targets. For oncomirs, such as miR-10b, miR-21, miR-221 and miR-222, potential therapies include siRNA- or antisense oligonucleotides, miRNA sponges, miRNA masking, and small molecule inhibitors, which should be more effective on circulating cell-free miRNAs as compared to tumor miRNAs. Antisense oligonucleotides targeting tumor miR-21 has been successfully used in vitro and in vivo in a breast cancer model [132]. Depleting oncomirs using miRNA sponges involves cloning multiple copies of the complementary sequence to a selected miRNA which leads to competition with the natural target for miRNA binding. Using this technique, Ma et al. demonstrated reduction of invasiveness of a breast cancer cell line using a sponge trapping tumor miR-9 [301].
Furthermore, exosomes can be used to efficiently deliver siRNA, antisense oligonucleotides or other inhibitors in a tissue-specific manner, either using endogenous or synthetic exosomes [302, 303]. Using exosomes isolated from dendritic cells to reduce immunogenicity, Alvarez-Erviti et al. loaded exosomes with exogenous siRNA by electroporation and fused specific targeting peptides to the exosomal membrane Lamp2b protein. Intravenously injection of these exosomes delivered siRNA specifically to the target tissues, resulting in a specific gene knockdown, while non-specific uptake in other tissues was not observed [304]. A similar methodology could be used to specifically target miRNA knockdown, though identification of specific targeting peptides might limit the number of tissues available for such targeting. Furthermore, exosomes derived from cancer patients are complex structures secreted by multiple cell types with many molecular effectors and may induce adverse effects if used for treatment. Synthetic exosomes (liposomes) loaded with specific proteins for targeting and inhibitors of miRNA function may be a viable alternative [302].
Since a number of miRNAs shuttled by exosomes are pro-metastatic, approaches by which exosomes could be specifically removed from circulation in cancer patients could greatly reduce metastatic spread and outgrowth. Several pharmacological strategies have been proposed for targeting exosomal malignant activities [141, 179], but risks of toxicity and drug interactions may limit their use in the clinic. Interestingly, a novel device strategy has also been proposed which involves extracorporeal hemofiltration of exosomes from the entire circulatory system using an affinity plasmapheresis platform [305]. This technology allows affinity agents, including exosome-binding lectins and antibodies, to be immobilized in the outer-capillary space of plasma filtration membranes leading to selective retention of target particles < 200 nm from the entire circulatory system.
On the other hand, tumor-suppressor miRNAs are usually down-regulated in cancer and administration of synthetic miRNAs or forced expression of endogenous miRNAs may be a useful therapeutic option to restore their expression. To this end, the tumor suppressor let-7 expressing vectors have been injected intra-tumor in a mouse model of lung cancer leading to reduction of tumor size [306]. Additionally, a miRNA-based formulation (MRX34), a liposomal nanoparticle loaded with a synthetic mimic of the tumor suppressor miR-34, has entered in a clinical trial for hepatocellular carcinoma treatment [307]. In preclinical animal models, systemic administration of MRX34 was shown to inhibit the growth of primary tumors, to block metastasis, and to extend survival [308]. Similarly, systemic inoculation of pre-miRNA oligonucleotides for miR-141 or miR-219 was sufficient to inhibit bone metastasis of human breast cancer cells in a xenograft mouse model [8]. The two miRNAs reduce bone metastasis by targeting osteoclast genes and inhibiting osteoclast differentiation, a process that leads to degradation of the bone and subsequent release of bone matrix-embedded tumor-promoting growth factors such as TGFβ [7, 309, 310]. For exosomal tumor-suppressor miRNAs which are downregulated during metastasis a similar approach of restoration of miRNA expression could be used. Endogenous exosomes could be loaded with the specific miRNA by electroporation or synthetic exosomes could be produced from liposomes.
A big advantage of targeting cell-free miRNAs is the ability to monitor their expression in the circulation. When treatment is started, miRNA expression levels can be monitored at various time points, and their levels can predict treatment resistance and warrant a switch in therapeutic regimen. Already, it was shown that the administration of intravenous anti-miR-16, anti-miR-122, anti-miR-192 and anti-miR-194 caused a decrease in the levels of the corresponding miRNAs across all organs in mice [311]. In this regard, circulating miRNAs could serve as combined drug targets and pharmacodynamic markers.
8.2. Targeting miRNA secretion
While the secretion process of non-exosomal cell-free miRNAs is currently not well understood, a selective export mechanism is likely to exist as many studies have reported similar, but distinct miRNA profiles derived from tumors and body fluids [202]. Understanding the mechanism underlying this specific secretion and the key regulators involved will potentially identify new drug targets whose manipulation could modify circulating miRNA levels. On the other hand, while the process of exosome secretion is better characterized, targeting exosome secretion as a potential means of blocking cancer-host crosstalk requires the identi cation of cancer-speci c molecules or pathways that control tumor exosome production due to the fact that exosomes secreted by normal cells mediate important functions. Rab27A is highly expressed in cancer and its interference reduces exosome production, providing a way to specifically target tumor exosome secretion. In fact, reduction of Rab27a in a murine melanoma cell line dramatically reduced lung metastasis [16] by preventing education of bone marrow progenitors and reducing vascular leakiness. In addition, as the exosomal secretion of miRNAs exhibits a highly selective pattern that differs between cancer and normal cells [312], understanding the cellular selection mechanism for miRNA secretion, which may involve RNA-binding proteins that recognize the primary or secondary structures of miRNA, and its dysregulation in cancer and metastasis may reveal unique strategies to block cancer-speci c miRNA secretion.
9. Conclusion
Cell-free miRNAs constitute a novel class of dysregulated molecules that could provide new avenues for non-invasive diagnosis, classification and treatment of tumor-specific malignancies but also different phases of cancer development from tumor initiation to growth, invasion and metastasis. The diagnostic and prognostic potential of circulating miRNAs as biomarkers is based mainly on their non-invasive detection in body fluids and on their high resistance and stability under various conditions that could degrade the majority of RNAs, such as extended storage, frequent freeze-thaw cycles, extreme pH variations, boiling, preservation in archived human blood samples for several years, and transport. In addition, several methodologies are available for establishing miRNA expression levels and signatures (qRT-PCR, miRNA microarrays, NGS) with only a few limitations regarding their cross-comparison. Although the recent data are almost exclusively pre-clinical evidence, the application of cell-free miRNAs in metastasis therapy as adjuvant tools or targets appears very promising. A better understanding of the complex network of genes and cellular signaling transduction pathways regulated by miRNAs would enrich our knowledge of metastatic progression and hence improve the therapeutic outcome of cancer patients.
Combinations of markers either multiple cell-free miRNAs or miRNAs in combination with other known makers, such as circulating proteins or tumor cells (CTCs) to enhance sensitivity and specificity may be of potential use in the clinic and need to be further evaluated. For instance, in a comparison of the prognostic value of miR-200b, identified in a study as the most promising prognostic marker for progression-free and overall survival, with that of circulating tumor cells (CTCs), the miRNA level had a better prognostic value in breast cancer patients than CTC numbers, while the combination of miRNA and CTCs performed a bit better than the former [231]. Similarly, combination of detection of elevated levels of cell-free miR-141 and carcinoembryonic antigen, a widely used marker for colorectal cancer, improved the accuracy of detection as compared to any one marker alone [225].
Nonetheless, crucial issues need to be resolved before establishing extracellular miRNAs as biomarkers and therapeutic tools for metastasis. The lack of larger prospective clinical trials with robust and standardized analyzing methods, uncertainty of the target cell, lack of clarity regarding the origin of identified circulating miRNA species as some of them may be by-products of normal tissues or dead cells, and the validation of well-characterized metastasis-specific cell-free miRNA signatures of different primary cancers are important limitations that need to be overcome when bringing miRNAs from bench to bedside. Moreover, studies of miRNAs present in biological fluids have generated contrasting results, possibly due to the different sensitivity and specificity of the detection approaches, different patient numbers, compositions and/or treatment regimens in the study cohorts. Furthermore, most studies fail to assay for the presence of mature miRNAs, as opposed to miRNA passenger strands or miRNA degradation products, which could explain some of the discrepancies between different studies. Also, the choice of appropriate endogenous controls for data normalization is a relevant limit. The use of synthetic spike-in controls from C. elegans, such as cel-miR-39, cel-miR-43, cel-miR-54, and cel-miR-238 has been proposed as an alternative reference instead of ubiquitary expressed miRNAs such as miR-16 or miR-21 [211, 232, 313]. Nevertheless, the studies to-date have shown promising results in detecting and inhibiting metastatic progression by the use of cell-free miRNAs, suggesting the potential utility of this therapeutic strategy in clinical practice. Taken together, the therapeutic potential of cell-free miRNA treatments has the potential to transform clinical paradigms for cancer metastasis.
Highlights.
Cell-free miRNAs have been detected in various body fluids of cancer patients
Levels of specific miRNAs change in metastatic compared non-metastatic patients
These could be used for diagnosis, prognosis and treatment of metastasis
The functional role of several cell-free miRNAs in metastasis has been investigated
Acknowledgments
Research in our laboratory was supported by grants from the Komen for the Cure (KG110464), Department of Defense (BC123187), Brewster Foundation, and the National Institutes of Health (R01CA134519 and R01CA141062) to Y.K.
Abbreviations
- cfNA
Cell-free nucleic acid
- CSC
Cancer stem cell
- CTC
Circulating tumor cell
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- MET
Mesenchymal-epithelial transition
- miR
MicroRNA
- miRNA
MicroRNA
- NGS
Next-generation sequencing
- RISC
RNA-induced silencing complex
- qRT-PCR
quantitative real-time polymerase chain reaction
- TAM
Tumor-associated macrophage
- UTR
Untranslated region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21(2):139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20(6):701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ell B, Kang Y. SnapShot: Bone Metastasis. Cell. 2012;151(3):690–690.e1. doi: 10.1016/j.cell.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Ell B, et al. Tumor-Induced Osteoclast miRNA Changes as Regulators and Biomarkers of Osteolytic Bone Metastasis. Cancer Cell. 2013;24(4):542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 10.Guise TA, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6216. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 11.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48(1):23–29. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Blanco MA, et al. Global secretome analysis identifies novel mediators of bone metastasis. Cell Res. 2012;22(9):1339–55. doi: 10.1038/cr.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17(7):867–74. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korpal M, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth C, et al. Screening for circulating nucleic acids and caspase activity in the peripheral blood as potential diagnostic tools in lung cancer. Mol Oncol. 2011;5(3):281–91. doi: 10.1016/j.molonc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosse SA, et al. Circulating cell-free cancer-testis MAGE-A RNA, BORIS RNA, let-7b and miR-202 in the blood of patients with breast cancer and benign breast diseases. Br J Cancer. 2014 doi: 10.1038/bjc.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhavan D, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat. 2014;146(1):163–74. doi: 10.1007/s10549-014-2946-2. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzenbach H, et al. Genomic profiling of cell-free DNA in blood and bone marrow of prostate cancer patients. J Cancer Res Clin Oncol. 2011;137(5):811–9. doi: 10.1007/s00432-010-0941-5. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzenbach H, et al. Detection and monitoring of cell-free DNA in blood of patients with colorectal cancer. Ann N Y Acad Sci. 2008;1137:190–6. doi: 10.1196/annals.1448.025. [DOI] [PubMed] [Google Scholar]
- 22.Tokuhisa Y, et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Brit J Cancer. 2007;97(10):1399–1403. doi: 10.1038/sj.bjc.6604034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolesnikova EV, et al. Circulating DNA in the blood of gastric cancer patients. Ann NY Acad Sci. 2008;1137:226–231. doi: 10.1196/annals.1448.009. [DOI] [PubMed] [Google Scholar]
- 24.Madhavan D, et al. Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clin Cancer Res. 2012;18(21):5972–82. doi: 10.1158/1078-0432.CCR-12-1407. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 26.Mostert B, et al. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn. 2011;11(3):259–275. doi: 10.1586/erm.11.11. [DOI] [PubMed] [Google Scholar]
- 27.Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384–8. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 28.Agostini M, et al. Circulating cell-free DNA: A promising marker of pathologic tumor response in rectal cancer patients receiving preoperative chemoradiotherapy. Ann Surg Oncol. 2011;18(9):2461–2468. doi: 10.1245/s10434-011-1638-y. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, et al. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 30.He L, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicoloso MS, et al. MicroRNAs - The micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9(4):293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 32.Yong SL, Dutta A. MicroRNAs in cancer. Annu Rev Pathol-Mech. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiemer EAC. The role of microRNAs in cancer: No small matter. Eur J Cancer. 2007;43(10):1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 35.Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet. 2008;74(4):296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 36.Mann M, et al. miRNA-based mechanism for the commitment of multipotent progenitors to a single cellular fate. Proc Natl Acad Sci U S A. 2010;107(36):15804–9. doi: 10.1073/pnas.0915022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 38.Mattick JS, I, Makunin V. Non-coding RNA. Hum Mol Genet. 2006;15(Spec No 1):R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 39.Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 40.Cortez MA, et al. MicroRNAs in body fluids-the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber JA, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56 (11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanemaru H, et al. The circulating microRNA-221 level in patients with malignant melanoma as a new tumor marker. J Dermatol Sci. 2011;61(3):187–193. doi: 10.1016/j.jdermsci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Lawrie CH, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Brit J Haematol. 2008;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Roberson CD, et al. Tumor-derived exosomes as mediators of disease and potential diagnostic biomarkers. Cancer Biomark. 2010;8(4–5):281–291. doi: 10.3233/CBM-2011-0211. [DOI] [PubMed] [Google Scholar]
- 47.Février B, Raposo G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr Opin Chem Biol. 2004;16(4):415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 49.Aliotta JM, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38(3):233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 51.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 52.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 53.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321(5897):1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sethi N, Kang Y. Unravelling the complexity of metastasis-molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11(10):735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 56.Lee Y, et al. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO Journal. 2002;21(17):4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han J, et al. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yi R, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denli AM, et al. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 60.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18(5):504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 62.Hafner M, et al. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44(1):3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meltzer PS. Cancer genomics: Small RNAs with big impacts. Nature. 2005;435(7043):745–746. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 64.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 65.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26(34):5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 66.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu S, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70(14):6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 68.Esquela-Kerscher A, Slack FJ. Oncomirs - MicroRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 69.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 70.Iorio MV, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 71.Calin GA, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faraoni I, et al. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792(6):497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Greither T, et al. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126(1):73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 74.Jiang S, et al. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70(8):3119–27. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 75.Kluiver J, et al. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207(2):243–9. doi: 10.1002/path.1825. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen IM, et al. Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol Med. 2009;1(5):288–95. doi: 10.1002/emmm.200900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryu JK, et al. Aberrant MicroRNA-155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology. 2010;10 (1):66–73. doi: 10.1159/000231984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. 0027-8424 (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roldo C, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24(29):4677–84. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 80.Li CL, et al. microRNA-155 is downregulated in gastric cancer cells and involved in cell metastasis. Oncol Rep. 2012;27(6):1960–6. doi: 10.3892/or.2012.1719. [DOI] [PubMed] [Google Scholar]
- 81.Rokah OH, et al. Downregulation of miR-31, miR-155, and miR-564 in chronic myeloid leukemia cells. PLoS One. 2012;7(4):e35501. doi: 10.1371/journal.pone.0035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahiya N, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3(6):e2436. doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levati L, et al. Altered expression of selected microRNAs in melanoma: antiproliferative and proapoptotic activity of miRNA-155. Int J Oncol. 2009;35(2):393–400. [PubMed] [Google Scholar]
- 84.Levati L, et al. MicroRNA-155 targets the SKI gene in human melanoma cell lines. Pigment Cell Melanoma Res. 2011;24(3):538–50. doi: 10.1111/j.1755-148X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 85.Ceppi P, et al. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res. 2010;8(9):1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 86.Eulalio A, et al. Deadenylation is a widespread effect of miRNA regulation. RNA. 2009;15(1):21–32. doi: 10.1261/rna.1399509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lundstrom K. Micro-RNA in disease and gene therapy. Curr Drug Discovery Technol. 2011;8(2):76–86. doi: 10.2174/157016311795563857. [DOI] [PubMed] [Google Scholar]
- 88.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 89.Meng F, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tetzlaff MT, et al. Differential expression of miRNAs in papillary thyroid carcinoma compared to multinodular goiter using formalin fixed paraffin embedded tissues. Endocr Pathol. 2007;18(3):163–173. doi: 10.1007/s12022-007-0023-7. [DOI] [PubMed] [Google Scholar]
- 91.Iorio MV, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67(18):8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 92.Ma L, Weinberg RA. Micromanagers of malignancy: role of microRNAs in regulating metastasis. Trends Genet. 2008;24(9):448–456. doi: 10.1016/j.tig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18(3):350–9. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 94.Zhu Q, et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol Rep. 2012;27(5):1660–1668. doi: 10.3892/or.2012.1682. [DOI] [PubMed] [Google Scholar]
- 95.Zhu S, et al. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282(19):14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 96.Li T, et al. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383(3):280–285. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 97.Gabriely G, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Bio. 2008;28(17):5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15(12):3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 99.Ruby JG, et al. Large-Scale Sequencing Reveals 21U-RNAs and Additional MicroRNAs and Endogenous siRNAs in C. elegans. Cell. 2006;127(6):1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 100.Takamizawa J, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 101.Johnson SM, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 102.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315(5818):1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koscianska E, et al. Prediction and preliminary validation of oncogene regulation by miRNAs. BMC Mol Biol. 2007;8:79. doi: 10.1186/1471-2199-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu F, et al. let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 105.Ibarra I, et al. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21(24):3238–43. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 107.Huang Q, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10(2):202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 108.Tavazoie SF, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meister J, Schmidt MH. miR-126 and miR-126*: new players in cancer. Scientific World J. 2010;10:2090–100. doi: 10.1100/tsw.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li A, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70(13):5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 112.Park SM, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hurteau GJ, et al. Overexpression of the MicroRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007;67(17):7972–7976. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 114.Burk U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Korpal M, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17(9):1101–8. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dykxhoorn DM, et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sempere LF, et al. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67(24):11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 118.Rosenwald S, et al. Validation of a microRNA-based qRT-PCR test for accurate identification of tumor tissue origin. Modern Pathol. 2010;23(6):814–823. doi: 10.1038/modpathol.2010.57. [DOI] [PubMed] [Google Scholar]
- 119.Rosenfeld N, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26(4):462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 120.Ferracin M, Veronese A, Negrini M. Micromarkers: MiRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10(3):297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 121.Gallardo E, et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30(11):1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 122.Markou A, et al. Prognostic value of mature MicroRNA-21 and MicroRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54(10):1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 123.Schetter AJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA - J Am Med Assoc. 2008;299(4):425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ota D, et al. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 2011;38(4):955–962. doi: 10.3892/ijo.2011.926. [DOI] [PubMed] [Google Scholar]
- 125.Li W, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123(7):1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 126.Calin GA, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101(32):11755–60. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang H, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68(2):425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 128.Weiss GJ, et al. EGFR regulation by microRNA in lung cancer: Correlation with clinical response and survival to gefitinib and EGFR expression in cell lines. Ann Oncol. 2008;19(6):1053–1059. doi: 10.1093/annonc/mdn006. [DOI] [PubMed] [Google Scholar]
- 129.Budhu A, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47(3):897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 130.Giovannetti E, et al. MicroRNA-21 in pancreatic cancer: Correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 131.Rodríguez-González FG, et al. MicroRNA-30c expression level is an independent predictor of clinical benefit of endocrine therapy in advanced estrogen receptor positive breast cancer. Breast Cancer Res Tr. 2011;127(1):43–51. doi: 10.1007/s10549-010-0940-x. [DOI] [PubMed] [Google Scholar]
- 132.Si ML, et al. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 133.Meng F, et al. Involvement of Human Micro-RNA in Growth and Response to Chemotherapy in Human Cholangiocarcinoma Cell Lines. Gastroenterology. 2006;130(7):2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 134.Iorio MV, et al. MicroRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69(6):2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- 135.Kota J, et al. Therapeutic microRNA Delivery Suppresses Tumorigenesis in a Murine Liver Cancer Model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 138.Antonyak MA, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108(12):4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Webber J, et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 140.Yu S, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 141.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hood JL, San Roman S, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 143.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10 (12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan A, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE. 2009;4(3) doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang H, et al. MicroRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol Cancer Therapeut. 2012;11(7):1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y, et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol Cell. 2010;39(1):133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 147.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turchinovich A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91–97. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 152.Redis RS, et al. Cell-to-cell miRNA transfer: From body homeostasis to therapy. Pharmacol Therapeut. 2012;136(2):169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119(3):646–648. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 154.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107(9):1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 155.Caby MP, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 156.Almqvist N, et al. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125(1):21–27. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen CY, Hogan MC, Ward CJ. Purification of exosome-like vesicles from urine. Methods Enzymol. 2013;524:225–241. doi: 10.1016/B978-0-12-397945-2.00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33(5):441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 159.Chen X, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 160.Gilad S, et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3(9) doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brase JC, et al. Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer. 2010;9 doi: 10.1186/1476-4598-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Asaga S, et al. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91. doi: 10.1373/clinchem.2010.151845. [DOI] [PubMed] [Google Scholar]
- 163.Zheng D, et al. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol. 2011;4(6):575–86. [PMC free article] [PubMed] [Google Scholar]
- 164.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallo A, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang K, et al. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou W, et al. Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nazarenko I, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70 (4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 169.Al-Nedawi K, et al. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kobayashi M, et al. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J Transl Med. 2014;12(1):4. doi: 10.1186/1479-5876-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li C, et al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10 (8):1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhuang G, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO Journal. 2012;31 (17):3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gibbings DJ, et al. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 175.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–42. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 177.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bobrie A, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 179.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 180.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ji X, et al. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55(11):1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 182.El-Hefnawy T, et al. Characterization of Amplifiable, Circulating RNA in Plasma and Its Potential as a Tool for Cancer Diagnostics. Clin Chem. 2004;50(3):564–573. doi: 10.1373/clinchem.2003.028506. [DOI] [PubMed] [Google Scholar]
- 183.Morelli AE, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 184.Tian T, et al. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 185.Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vickers KC, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Schooneveld E, et al. Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res. 2012;14(1):R34. doi: 10.1186/bcr3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mlcochova H, et al. Urine microRNAs as potential noninvasive biomarkers in urologic cancers. Urol Oncol. 2014;32(1):41.e1–9. doi: 10.1016/j.urolonc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 189.Park NJ, et al. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15(17):5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teplyuk NM, et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012;14(6):689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rabinowits G, et al. Exosomal microRNA: A diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 192.Keller A, et al. MiRNAs in lung cancer - Studying complex fingerprints in patient’s blood cells by microarray experiments. BMC Cancer. 2009;9:353. doi: 10.1186/1471-2407-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim SY, et al. Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J Mol Diagn. 2013;15(5):661–669. doi: 10.1016/j.jmoldx.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 194.Yamamoto Y, et al. MicroRNA-500 as a potential diagnostic marker for hepatocellular carcinoma. Biomarkers. 2009;14(7):529–38. doi: 10.3109/13547500903150771. [DOI] [PubMed] [Google Scholar]
- 195.Heneghan HM, et al. Circulating micrornas as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251(3):499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 196.Yamada Y, et al. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: Correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 2011;102(3):522–529. doi: 10.1111/j.1349-7006.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 197.Wong TS, et al. Mature miR-184 and squamous cell carcinoma of the tongue. Scientific World J. 2009;9:130–132. doi: 10.1100/tsw.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roth C, et al. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 2010;12(6) doi: 10.1186/bcr2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Heneghan HM, Miller N, Kerin MJ. Circulating microRNAs: promising breast cancer Biomarkers. Breast Cancer Res. 2011;13(1):402–402. doi: 10.1186/bcr2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Moltzahn F, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011;71(2):550–560. doi: 10.1158/0008-5472.CAN-10-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yin J, et al. Differential expression of serum miR-126, miR-141 and miR-21 as novel biomarkers for early detection of liver metastasis in colorectal cancer. Chin J Cancer Res. 2014;26(1):95–103. doi: 10.3978/j.issn.1000-9604.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chan M, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–87. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 203.Yu S, et al. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(6):2084–92. doi: 10.1210/jc.2011-3059. [DOI] [PubMed] [Google Scholar]
- 204.Huang V, et al. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012;40(4):1695–707. doi: 10.1093/nar/gkr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen W, et al. The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34(1):455–62. doi: 10.1007/s13277-012-0570-5. [DOI] [PubMed] [Google Scholar]
- 206.Achberger S, et al. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol Immunol. 2014;58(2):182–6. doi: 10.1016/j.molimm.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Snowdon J, et al. A pilot study of urinary microRNA as a biomarker for urothelial cancer. Can Urol Assoc J. 2012:1–5. doi: 10.5489/cuaj.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Watahiki A, et al. Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer. Int J Mol Sci. 2013;14(4):7757–70. doi: 10.3390/ijms14047757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eichelser C, et al. Deregulated serum concentrations of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin Chem. 2013;59(10):1489–1496. doi: 10.1373/clinchem.2013.205161. [DOI] [PubMed] [Google Scholar]
- 210.Bryant RJ, et al. Changes in circulating microRNA levels associated with prostate cancer. Brit J Cancer. 2012;106(4):768–774. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen J, et al. Serum microRNA expression levels can predict lymph node metastasis in patients with early-stage cervical squamous cell carcinoma. Int J Mol Med. 2013;32(3):557–67. doi: 10.3892/ijmm.2013.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao S, et al. Circulating miRNA-20a and miRNA-203 for screening lymph node metastasis in early stage cervical cancer. Genet Test Mol Biomarkers. 2013;17(8):631–6. doi: 10.1089/gtmb.2013.0085. [DOI] [PubMed] [Google Scholar]
- 213.Guo F, et al. Serum microRNA-92 expression in patients with ovarian epithelial carcinoma. J Int Med Res. 2013;41(5):1456–61. doi: 10.1177/0300060513487652. [DOI] [PubMed] [Google Scholar]
- 214.Si H, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2012 doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu YZ, et al. Identification of serum microrna-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac J Cancer Prev. 2013;14(2):1057–60. doi: 10.7314/apjcp.2013.14.2.1057. [DOI] [PubMed] [Google Scholar]
- 216.Ma GJ, et al. Plasma post-operative miR-21 expression in the prognosis of gastric cancers. Asian Pac J Cancer Prev. 2013;14(12):7551–4. doi: 10.7314/apjcp.2013.14.12.7551. [DOI] [PubMed] [Google Scholar]
- 217.Wang ZX, et al. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol. 2011;104(7):847–851. doi: 10.1002/jso.22008. [DOI] [PubMed] [Google Scholar]
- 218.Liu XG, et al. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29 (2):618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 219.Ouyang L, et al. A three-plasma miRNA signature serves as novel biomarkers for osteosarcoma. Med Oncol. 2013;30(1):340. doi: 10.1007/s12032-012-0340-7. [DOI] [PubMed] [Google Scholar]
- 220.Yaman Agaoglu F, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011;32(3):583–8. doi: 10.1007/s13277-011-0154-9. [DOI] [PubMed] [Google Scholar]
- 221.Zhang HL, et al. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate. 2011;71(3):326–331. doi: 10.1002/pros.21246. [DOI] [PubMed] [Google Scholar]
- 222.Wang LG, Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012;36(1):e61–7. doi: 10.1016/j.canep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 223.Greenberg E, et al. A comparative analysis of total serum miRNA profiles identifies novel signature that is highly indicative of metastatic melanoma: a pilot study. Biomarkers. 2013;18(6):502–8. doi: 10.3109/1354750X.2013.816777. [DOI] [PubMed] [Google Scholar]
- 224.Wu X, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. Journal of Translational Medicine. 2012;10(1) doi: 10.1186/1479-5876-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen Q, et al. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep. 2014;31 (4):1863–70. doi: 10.3892/or.2014.3004. [DOI] [PubMed] [Google Scholar]
- 226.Lin Q, et al. A cluster of specified microRNAs in peripheral blood as biomarkers for metastatic non-small-cell lung cancer by stem-loop RT-PCR. J Cancer Res Clin. 2012;138(1):85–93. doi: 10.1007/s00432-011-1068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nguyen HCN, et al. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate. 2013;73(4):346–354. doi: 10.1002/pros.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen J, et al. Predicting distant metastasis and chemoresistance using plasma miRNAs. Med Oncol. 2014;31(1):799. doi: 10.1007/s12032-013-0799-x. [DOI] [PubMed] [Google Scholar]
- 229.Tan ZQ, et al. Expression and its clinical significance of hsa-miR-155 in serum of endometrial cancer. Zhonghua Fu Chan Ke Za Zhi. 2010;45(10):772–4. [PubMed] [Google Scholar]
- 230.Li C, et al. miRNA-199a-3p in plasma as a potential diagnostic biomarker for gastric cancer. Ann Surg Oncol. 2013;20(Suppl 3):S397–405. doi: 10.1245/s10434-012-2600-3. [DOI] [PubMed] [Google Scholar]
- 231.Madhavan D, et al. Circulating miRNAs as Surrogate Markers for Circulating Tumor Cells and Prognostic Markers in Metastatic Breast Cancer. Clin Cancer Res. 2012;18(21):5972–5982. doi: 10.1158/1078-0432.CCR-12-1407. [DOI] [PubMed] [Google Scholar]
- 232.Cheng H, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6(3):e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brase JC, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2011;128(3):608–616. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 234.Zhang HL, et al. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J Androl. 2013;15(2):231–5. doi: 10.1038/aja.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Selth LA, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int J Cancer. 2012;131(3):652–661. doi: 10.1002/ijc.26405. [DOI] [PubMed] [Google Scholar]
- 236.Cheng HH, et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One. 2013;8(7):e69239. doi: 10.1371/journal.pone.0069239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Toiyama Y, et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014;259(4):735–43. doi: 10.1097/SLA.0b013e3182a6909d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Valladares-Ayerbes M, et al. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jung EJ, et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118(10):2603–14. doi: 10.1002/cncr.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schwarzenbach H, et al. Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res Treat. 2012;134(3):933–41. doi: 10.1007/s10549-012-1988-6. [DOI] [PubMed] [Google Scholar]
- 241.Yu J, et al. Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J Cancer Res Clin Oncol. 2012;138(4):671–4. doi: 10.1007/s00432-012-1147-9. [DOI] [PubMed] [Google Scholar]
- 242.Kawaguchi T, et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108(2):361–9. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Teixeira AL, et al. Higher circulating expression levels of miR-221 associated with poor overall survival in renal cell carcinoma patients. Tumour Biol. 2014;35 (5):4057–66. doi: 10.1007/s13277-013-1531-3. [DOI] [PubMed] [Google Scholar]
- 244.Yu H, et al. Decreased circulating miR-375: A potential biomarker for patients with non-small-cell lung cancer. Gene. 2013 [PubMed] [Google Scholar]
- 245.Lodes MJ, et al. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ohshima K, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ma L, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28(4):341–7. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Moriarty CH, Pursell B, Mercurio AM. miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem. 2010;285(27):20541–6. doi: 10.1074/jbc.M110.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Boufraqech M, et al. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21(4):517–31. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kong W, et al. MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Molecular and Cellular Biology. 2008;28(22):6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Garofalo M, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16 (6):498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 252.Liu P, Wilson MJ. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-kappaB factor in human fibrosarcoma cells. J Cell Physiol. 2012;227(2):867–76. doi: 10.1002/jcp.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yang K, Handorean AM, Iczkowski KA. MicroRNAs 373 and 520c are downregulated in prostate cancer, suppress CD44 translation and enhance invasion of prostate cancer cells in vitro. Int J Clin Exp Pathol. 2009;2(4):361–9. [PMC free article] [PubMed] [Google Scholar]
- 254.Han HB, et al. Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. Journal of Pathology. 2012;226(3):544–555. doi: 10.1002/path.3014. [DOI] [PubMed] [Google Scholar]
- 255.Saini S, et al. Regulatory Role of mir-203 in Prostate Cancer Progression and Metastasis. Clin Cancer Res. 2011;17(16):5287–98. doi: 10.1158/1078-0432.CCR-10-2619. [DOI] [PubMed] [Google Scholar]
- 256.Suarez Y, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105(37):14082–7. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.O’Donnell KA, et al. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 258.Aguda BD, et al. MicroRNA regulation of a cancer network: Consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19678–19683. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bonauer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in Mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 260.Tang W, et al. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene. 2014;33(20):2629–38. doi: 10.1038/onc.2013.214. [DOI] [PubMed] [Google Scholar]
- 261.Urbich C, et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119(6):1607–16. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 262.Jiang L, et al. Sp1 mediates microRNA-29c-regulated type I collagen production in renal tubular epithelial cells. Exp Cell Res. 2013;319(14):2254–65. doi: 10.1016/j.yexcr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 263.Yang T, et al. miR-29 mediates TGFbeta1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J Cell Biochem. 2013;114(6):1336–42. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 264.Fish JE, et al. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang S, et al. The Endothelial-Specific MicroRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kuehbacher A, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 267.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111(3):1217–26. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Martin MM, et al. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J Biol Chem. 2007;282(33):24262–9. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 269.Martin MM, et al. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006;281(27):18277–84. doi: 10.1074/jbc.M601496200. [DOI] [PubMed] [Google Scholar]
- 270.Valencia K, et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol. 2014;8(3):689–703. doi: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kosaka N, et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849–59. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Poliseno L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 273.Suarez Y, et al. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 274.Le Sage C, et al. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO Journal. 2007;26(15):3699–3708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lee DY, et al. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia (United States) 2013;15(3):281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 278.Liu M, et al. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009;19(7):828–37. doi: 10.1038/cr.2009.72. [DOI] [PubMed] [Google Scholar]
- 279.Sayed D, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Molecular Biology of the Cell. 2008;19(8):3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Galardi S, et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282(32):23716–24. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- 281.Krichevsky AM, Gabriely G. miR-21: A small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Toiyama Y, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105(12):849–59. doi: 10.1093/jnci/djt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138(10):1659–66. doi: 10.1007/s00432-012-1244-9. [DOI] [PubMed] [Google Scholar]
- 284.Wei J, et al. Identification of plasma microRNA-21 as a biomarker for early detection and chemosensitivity of non-small cell lung cancer. Chin J Cancer. 2011;30(6):407–14. doi: 10.5732/cjc.010.10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zheng J, et al. miR-21 downregulates the tumor suppressor P12 CDK2AP1 and stimulates cell proliferation and invasion. J Cell Biochem. 2011;112(3):872–80. doi: 10.1002/jcb.22995. [DOI] [PubMed] [Google Scholar]
- 286.Shi L, et al. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–64. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 287.Rombouts K, et al. Myristoylated Alanine-Rich protein Kinase C Substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett. 2013;333(2):244–52. doi: 10.1016/j.canlet.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 288.Jung T, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11(10):1093–105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Finn NA, Searles CD. Intracellular and Extracellular miRNAs in Regulation of Angiogenesis Signaling. Curr Angiogenes. 2012;4(102):299–307. doi: 10.2174/2211552811201040299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104(4):442–54. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shiiyama R, et al. Sensitive detection of melanoma metastasis using circulating microRNA expression profiles. Melanoma Res. 2013 doi: 10.1097/CMR.0b013e328363e485. [DOI] [PubMed] [Google Scholar]
- 292.Guo W. Concise Review: Breast Cancer Stem Cells: Regulatory Networks, Stem Cell Niches, and Disease Relevance. Stem Cells Transl Med. 2014 doi: 10.5966/sctm.2014-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Poy MN, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. :1476–4687. doi: 10.1038/nature03076. (Electronic)) [DOI] [PubMed] [Google Scholar]
- 295.Poy MN, et al. miR-375 maintains normal pancreatic α- and β-cell mass. Proc Natl Acad Sci U S A. 2009;106(14):5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Szczyrba J, et al. Downregulation of Sec23A protein by miRNA-375 in prostate carcinoma. Mol Cancer Res. 2011;9(6):791–800. doi: 10.1158/1541-7786.MCR-10-0573. [DOI] [PubMed] [Google Scholar]
- 297.Kosaka N, et al. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287(2):1397–1405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lim PK, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–60. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 299.Ono M, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 300.Yang M, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ma L, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–56. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kooijmans SAA, et al. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lakhal S, Wood MJ. Exosome nanotechnology: An emerging paradigm shift in drug delivery: Exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 304.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 305.Marleau AM, et al. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10(1) doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Esquela-Kerscher A, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7(6):759–64. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 307.Kelnar K, et al. Quantification of therapeutic miRNA mimics in whole blood from nonhuman primates. Anal Chem. 2014;86(3):1534–42. doi: 10.1021/ac403044t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Korpal M, et al. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med. 2009;15(8):960–6. doi: 10.1038/nm.1943. [DOI] [PubMed] [Google Scholar]
- 310.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 312.Pigati L, et al. Selective release of MicroRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Redova M, et al. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med. 2012;10:55. doi: 10.1186/1479-5876-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]