Abstract
The phenotype of attenuated mucopolysaccharidosis type II (MPS II), also called Hunter syndrome, has not been previously studied in systematic manner. In contrast to the “severe” phenotype, the “attenuated” phenotype does not present with behavioral or cognitive impairment; however the presence of mild behavior and cognitive impairment that might impact long term functional outcomes is unknown. Previously, significant MRI abnormalities have been found in MPS II. Recent evidence suggests white matter abnormalities in many MPS disorders.
Methods
As the initial cross-sectional analysis of a longitudinal study, we studied the association of brain volumes and somatic disease burden with neuropsychological outcomes, including measures of intelligence, memory and attention in 20 patients with attenuated MPS II with a mean age of 15.8. MRI volumes were compared to 55 normal controls.
Results
While IQ and memory were average, measures of attention were one standard deviation below the average range. Corpus callosum volumes were significantly different from age-matched controls, differing by 22%. Normal age-related volume increases in white matter were not seen in MPS II patients as they were in controls. Somatic disease burden and white matter and corpus callosum volumes were significantly associated with attention deficits. Neither age at evaluation nor age at starting treatment predicted attention outcomes.
Conclusions
Despite average intelligence, attention is compromised in attenuated MPS II. Results confirm an important role of corpus callosum and cortical white matter abnormality in MPS II as well as the somatic disease burden in contributing to attention difficulties. Awareness by the patient and caregivers with appropriate management and symptomatic support will benefit the attenuated MPS II patient.
Introduction
The phenotype of attenuated mucopolysaccharidosis type II (MPS II), also called Hunter syndrome, has not been previously studied in a prospective, systematic manner. We describe the neuropsychological, medical and treatment, and brain imaging characteristics of attenuated MPS II patients and the associations between them.
Mucopolysaccharidosis type II (MPS II) is an X-linked recessive lysosomal storage disease caused by a deficiency of iduronate-2-sulfatase. With a range of severity, patients often appear normal at birth and progressively display symptoms of the disease [1,2]. Age at symptom onset is variable, as is the primary presenting symptom; however severe patients are generally diagnosed earlier in life [3]. Since the approval of enzyme replacement therapy (ERT) with recombinant human idursulfase, it is widely used to treat the entire range of severity of MPS II patients [4].
Two forms of MPS II have been described. Typically, severe MPS II is diagnosed when cognitive impairment and behavioral difficulties develop, and mild or attenuated MPS II has been diagnosed when they do not [5–7]. However, within the attenuated phenotype significant variability in the age of onset, age of diagnosis, somatic disease burden, and rate of progression makes it difficult to accurately predict the course of the disease, and neither genotype nor biomarkers are sufficiently specific. Furthermore, the attenuated form is rarer than the severe, which occurs 3–4 times more frequently [6].
In attenuated mucopolysaccharidosis type I, often compared with attenuated MPS II, cognitive problems have recently been described in some attenuated patients [8], and awareness of white matter abnormalities in both animal and human studies has increased [9–13]. These results have led us to question if such abnormalities are present in attenuated MPS II. It is known that white matter lesions and brain atrophy are common in individuals with MPS I [14]. Studies of severe MPS II patients have described similar findings [15]. We have previously reported that our pilot data indicated that despite normal intelligence, patients with attenuated MPS II may have attention and visual processing problems, along with white matter abnormality[16]. Some studies have shown that despite severe white matter abnormalities, no association has been found with cognitive ability [14,15, 17,18]. In order to understand the natural history of attenuated MPS II, documenting age-related changes with age-matched controls are an essential first step.
We hypothesize that there are abnormalities in attention span and in white matter volumes, especially corpus callosum. Our goal is to describe in detail the attenuated phenotype of MPS II and to define age-related changes and variables that may contribute to long-term cognitive and behavioral outcomes. We can then more accurately inform patients and caregivers regarding potential neurocognitive outcomes and develop more focused treatments.
METHODS
Patients and Controls
26 patients were screened; one was not enrolled due to noncompliance with test procedures, and 3 were severe whose IQs were below 70. A total of 22 attenuated MPS II patients were enrolled in the longitudinal protocol NCT01870375 of the Lysosomal Disease Network (Longitudinal Studies of Brain Structure and Function) at one of five centers. Inclusion criteria were 1) confirmed diagnosis of attenuated MPS II with an IQ > 70, and 2) ability to cooperate with neuropsychological testing. Each institution had an IRB approval of the protocol, and consents and assents were obtained from study participants and caregivers at each local institution, which included permission to upload de-identified data to the RDCRN (Rare Disease Clinical Research Network) Data Monitoring and Coordination Center and with the University of Minnesota for analysis. Two patients had incomplete data and were not included in this study resulting in a final N of 20. All patients were receiving ERT.
Although data was collected annually, cross-sectional data from the first visit at which complete data was available was analyzed for this study beginning in October 2009 and completed in June 2014. Due to lack of control subjects for patients over 25 years of age and the large range of ages, we categorized our subjects in to < 25 years and ≥ 25 years.
A control group of 55 age-matched healthy, typically developing individuals ages 4–25 was obtained from three separate IRB-approved studies. Inclusion criteria for the control groups were 1) between the ages of 4–25 years, 2) not born prematurely, 3) no history of neurologic disease or developmental delay, 4) has never received special education services, and 5) ability to remain still in the MRI scanner for the duration of scan.
Procedures
Neuropsychological testing
See Table 1 for detailed list of measures used at each center. For controls, only IQ and attention testing was available.
Table 1.
Neurocognitive testing performed.
| Assessments | Age range | Measure |
|---|---|---|
| Mullen Scales of Early Learning19 | <4 years | IQ |
| Wechsler Preschool and Primary Scale of Intelligence-III20 | 4–6 years | IQ |
| Wechsler Abbreviated Scale of Intelligence21 | ≥6 years | IQ |
| Wechsler Intelligence Scale for Children-IV (controls)22 | ≤16 years | IQ |
| Wechsler Adult Intelligence Scale – III (controls)23 | ≥16 years | IQ |
| Test of Variables of Attention24 | ≥6 years | Attention |
| Connors’ Continuous Performance Test (controls)25 | ≥8 years | Attention |
| CANTAB Stockings of Cambridge & Spatial Working Memory26 (Minnesota only) | ≥8 years | Executive Function |
| Kaufman Assessment Battery for Children-II, Atlantis27 | 4–8 years | Verbal Memory |
| Hopkins Verbal Learning Test-Revised28 | ≥8 years | Verbal Memory |
| NEPSY II Memory for Designs29 | 4–8 years | Visual Memory |
| Brief Visuospatial Memory Test–Revised30 | ≥8 years | Visual Memory |
| NEPSY II Narrative Memory29 | 4–6 years | Story Memory |
| Children’s Memory Scale, Stories31 | 6–16 years | Story Memory |
| Wechsler Memory Scale-III, Logical Memory32 | ≥16 years | Story Memory |
| NEPSY II Geometric Puzzles29 | 4–8 years | Spatial Ability |
| Judgment of Line Orientation33 | ≥8 years | Spatial Ability |
| Beery Developmental Test of Visual-Motor Integration34 | <8 years | Visual Motor |
| Rey-Osterreith Complex Figure35 | ≥8 years | Visual Motor |
Medical/Treatment history and status
Patients (if over 18 years) or parents/caregivers completed a detailed report of medical and treatment history (by interview and medical records) and current status was determined for each. Age at symptom onset, age at MPS II diagnosis, and age at the time of ERT initiation were included. Data from these reports were categorized into the MPS Physical Symptom Severity Scale (PSS), which is designed to be a measure of somatic disease burden [36]. PSS summary scores were based on skeletal/orthopedic, vision, hearing, and cardiorespiratory domains of the medical/treatment history report, as well as the number of surgical procedures and the presence of hydrocephalus. Each of the 6 domains can be scored 0 to 3. The range of scores can be from 0 to 18.
Neuroimaging
MRIs were acquired on a 3-T scanner and all examinations used the same protocol of sequences on either a Siemens Trio or Phillips scanner. Magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence was used. Volumetric analysis of collected MRI data was performed using FreeSurfer [37]. FreeSurfer generates an automated parcellation of the brain cortex and subcortical structures. All images were inspected for segmentation failure because enlarged ventricles and abundant dilated perivascular spaces (PVS) caused gray matter/white matter segmentation failure in many patients. Most patients required some adjustment, two subjects required repeated adjustment, and in one patient volumetric analysis was precluded. After manual adjustment for each scan, FreeSurfer was re-run. Volumetric analyses for cortical gray matter (GM), cortical white matter (WM), corpus callosum (CC), and frontal lobes (FL) were analyzed for the purposes of this study.
The majority of patients were scanned in a research facility where patients were able to watch a movie for the duration of the scan. This greatly improved cooperation particularly in younger patients. However, for young patients requiring anesthesia, their clinical scans were utilized with IRB permission. The identical sequence parameters were used.
Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Minnesota [38]. Descriptive statistics were tabulated separately for MPS II and control groups with mean and standard deviation for continuous variables and frequency for categorical variables. Differences in means were evaluated using a t-test with unequal variance and Welch degrees of freedom. Linear regression was used to estimate first order trends (slopes) for unadjusted and adjusted analyses with confidence intervals and P-values based on robust variance estimates. Multiplicative trends were evaluated using a generalized linear model with log link and robust standard error estimation. All analyses were conducted using R v2.15.2 [39].
RESULTS
Neurocognitive
See Table 2 for patient characteristics and cognitive outcomes. Sixteen patients were under age 25 for which we have typically-developing controls. Four patients were older and are described separately, but not included in statistical analyses of comparisons with controls. Differences in means between MPS II < 25 and controls for neurocognitive and neuroimaging measures are described in Table 3.
Table 2.
Subject characteristics by group. Values presented are mean (SD) or N (%) where indicated. All scores on neuropsychological tests are standard scores which have a population mean of 100 and a standard deviation of 15).
| Variables | Control (N=55) | MPS II (all) (N=20) | MPS II (<25 years of age) (N=16) | MPS II (>25 years of age (N=4) |
|---|---|---|---|---|
| Site: | ||||
| University of Minnesota | 55 (100.0%) | 16 (80.0%) | 14 (87.5%) | 2 (50.0%) |
| Other sites | 0 (0.0%) | 4 (20.0%) | 2 (12.6%) | 2(50.0%) |
|
| ||||
| Age at evaluation | 12.8 (5.2) | 15.8 (13.2) | 9.6 (3.2) | 40.5 (5.7) |
|
| ||||
| Age at diagnosis | NA | 5.9 (3.9) | 5.1 (2.9) | 9.3 (6.0) |
|
| ||||
| Age at ERT start | NA | 12 (12.4) | 6.3 (3.3) | 35 (5.6) |
|
| ||||
| Time since first ERT | NA | 3.6 (2.2) | 3.2 (2.1) | 5.4 (1.7) |
|
| ||||
| Test Results: | ||||
| IQ | 112 (13.24) | 99.25 (17.85) | 98.00 (18.47) | 104.25 (16.46) |
| Memory | ||||
| Verbal Memory (M=100, SD 15) | NA | 98.16 (18.84) | 98.64 (16.26) | 87.29 (19.35) |
| Story Memory (M=100, SD 15) | NA | 107 (19.81) | 105.02 (18.91) | 108.74 (17.52) |
| Visual Memory (M=100, SD 15) | NA | 86.95 (17.83) | 85.53 (18.84) | 92.28 (14.30) |
| Visual Motor (M=100, SD 15) | NA | 79.59 (24.83) | 76.07 (25.21) | 91.90 (21.91) |
| Spatial Ability (M=100, SD 15) | NA | 88.54 (17.98) | 86.35 (19.71) | 96.78 (3.26) |
| Attention | ||||
| TOVA/CPT Omission errors (M=100, SD 15) | 92.71 (7.17) | 84.69 (23.04) | 78.07 (27.76) | 85.50 (20.98) |
| TOVA/CPT Commission errors (M=100, SD 15) | 98.23 (17.85) | 97.38 (18.86) | 90.29 (19.17) | 113.50 (10.02) |
| TOVA/CPT Variability (VAR) (M=100, SD 15) | 94.61 (18.48) | 81.44 (27.85) | 76.71 (26.57) | 94.50 (32.58) |
| TOVA/CPT Reaction Time (RT) (M=100, SD 15) | 95.73 (15.55) | 88.88 (25.29) | 88.79 (25.53) | 91.25 (22.95) |
| Executive Function | ||||
| SOC: Problems solved (M=100, SD 15) | NA | 94.20 (20.94) | 93.56 (22.00) | 98.05 (18.46) |
| SWM: Errors (M=100, SD 15) | NA | 100.75 (21.08) | 99.09 (22.42) | 110.73 (4.14) |
|
| ||||
| PSS: Physical Symptom Scale | NA | 8.30 (1.87) | 7.88 (1.59) | 10.00 (2.16) |
|
| ||||
| Brain Volumes – absolute values | ||||
| Corpus callosum (mL) | 3.02 (0.51) | 2.57 (0.66) | 2.36 (0.51) | 3.37 (0.57) |
| Cortical white matter (mL) | 454 (61.66) | 474 (58.60) | 458 (47.32) | 534 (63.47) |
| Cortical gray matter (mL) | 591 (52.82) | 583 (78.45) | 611 (60.11) | 478 (38.57) |
| Frontal lobes (mL) | 219 (20.24) | 219 (30.51) | 229 (22.50) | 170 (7.71) |
Table 3.
Differences in means between groups for neurocognitive measures and neuroimaging volumes.
| Neurocognitive Measures | MPS II [Age<25] - Control (95% CI) | P-value |
|---|---|---|
| IQ | −13.83 (−24.25, − 3.41) | 0.012 |
| Errors of omission | −8.29 (−24.07, 7.48) | 0.274 |
| Errors of commission | −6.23 (−19.43, 6.98) | 0.338 |
| Variability (VAR) | −17.52 (−35.32, 0.27) | 0.053 |
| Reaction time (RT) | −7.64 (−25.53, 10.25) | 0.376 |
|
| ||
| Neuroimaging Volumes (mL) | ||
|
| ||
| Corpus callosum (CC) | −0.66 (−0.96, −0.35) | <0.001 |
| Cortical white matter (WM) | 3.66 (−26.67, 33.99) | 0.807 |
| Cortical gray matter (GM) | 19.51 (−16.13, 55.14) | 0.267 |
| Frontal lobes (FL) | 9.71 (−3.67, 23.09) | 0.146 |
The following results can be found in Table 2. IQ for MPS II is within the average range with no significant relationship to age. Attention span as measured by the TOVA (Test of Variables of Attention) shows a trend to be poorer in the MPS II patients compared to healthy controls on the Connors CPT (p= 0.053) and was below the average range compared to the TOVA normative sample. The mean of the memory measures was within one standard deviation of average compared to the normative sample. Within the memory measures, the lowest mean score was found in visual memory, which was a mean standard score of 86.95 (SD= 17.83). No significant relationship with age was found. Spatial ability was found to be slightly low at 88.54 (SD= 17.98), and no significant relationship with age was found. Visual motor skills were below the average range with a standard score of 79.59, but with great variability (SD= 24.83). Executive functioning measures were within the average range (within one standard deviation of the mean) on both SOC (Stockings of Cambridge on the CANTAB; a computerized version of the well-known Tower of London test) and SWM (Spatial Working Memory).
Medical symptoms
Age at symptom onset, age of diagnosis associated with specific symptom, and years to diagnosis in those patients not diagnosed due to family history are described in Table 4. Non-specific symptoms such as hearing loss and chronic ear infections led to the longest delay in diagnosis. Facial morphology and joint and skeletal symptoms led to a prompter diagnosis although the children were older when presenting with this symptom. Mean and standard deviation of PSS scores can be found in Table 2. The range of PSS scores was from 5 to 12 out of a possible 18. PSS as a measure of burden of somatic disease was found to be significantly associated with age (P < .001 for < 25 and P < .02 for the entire group including > 25) indicating increased somatic symptoms as the disease progresses.
Table 4.
Initial presenting symptom(s), age in years when symptom(s) first observed and at diagnosis (median and range), and time from each symptom presentation to diagnosis.
| Initial Symptom | Number with symptom* | Age symptom first observed (years) | Age at diagnosis (years) | Time to diagnosis from initial symptom (years) |
|---|---|---|---|---|
| Any | 33 in 15 patients | |||
| Family History | 5 | NA | 6.0 (1–18) | NA |
| Physical appearance | 5 | 5 (1.25–7) | 7.0 (1.25–10) | 1.67 |
| Joint stiffness/ restricted range of motion | 5 | 6 (4–6) | 8.0 (1.25–10) | 1.40 |
| Cardiac symptoms | 3 | 4 (2–6) | 6.0 (4–8) | 2.00 |
| Chronic ear infections | 3 | 1 (0.33–3) | 5.5 (4–6) | 3.72 |
| Respiratory symptoms | 3 | 2 (1–4) | 2.3 (2–5.5) | 0.93 |
| Development delay | 2 | 2.5 (1.5,3.5) | 2.75 (2, 3.5) | 0.25 |
| Hearing loss | 2 | 4 (1,7) | 8.0 (8,8) | 4.00 |
| Skeletal abnormality | 2 | 3.75 (3.5,4) | 3.75 (3.5,4) | 0.00 |
| Organomegaly | 1 | 2 | 2.30 | 0.30 |
| Contractures (fingers) | 1 | 8 | 8.00 | 0.00 |
| Rash | 1 | 4 | 6.00 | 2.00 |
cumulative for 15 patients who were not diagnosed by family history
Neuroimaging results
As thickened dura frequently seen in MPS II [14] can interfere with Intracranial Volume (ICV) reliability and mean ICV volumes were not different between MPS II and controls, we chose not to adjust volumes.
Comparing volumes between MPS II patients < 25 and controls, MPS II patients had significantly smaller CC volumes by 22% (Table 3). No differences were found in GM, WM, and FL volumes between MPS II <25 and controls. While not statistically significantly different, mean GM and FL volumes (in MPS <25) are all larger than controls. Table 5 shows the association of CC, WM, FL, and GM volumes with relevant neuropsychological functions.
Table 5.
Unadjusted association of brain volume with neurocognitive scores among MPS II subjects.
| Score | Volume | Difference in Score (95% CI) | P-value |
|---|---|---|---|
| Errors of omission | Corpus callosum (per mL) | 11.83 (−5.52, 29.17) | 0.181 |
| Errors of commission | Corpus callosum (per mL) | 4.14 (−10.03, 18.30) | 0.567 |
| Reaction time | Corpus callosum (per mL) | 13.53 (4.59, 22.47) | 0.003 |
| Variability | Corpus callosum (per mL) | 21.43 (8.04, 34.81) | 0.002 |
| PSS (total) | Corpus callosum (per mL) | 1.32 (−0.05, 2.70) | 0.060 |
| SOC: Problems solved | Corpus callosum (per mL) | 0.72 (−0.23, 1.66) | 0.137 |
| SWM: Errors | Corpus callosum (per mL) | 0.12 (−0.77, 1.02) | 0.785 |
| Score | Volume | Difference in Score (95% CI) | P-value |
|---|---|---|---|
| Errors of omission | Cortical white matter (per mL) | 0.08 (−0.10, 0.26) | 0.402 |
| Errors of commission | Cortical white matter (per mL) | 0.08 (−0.05, 0.20) | 0.221 |
| Reaction time | Cortical white matter (per mL) | 0.17 (0.00, 0.34) | 0.052 |
| Variability | Cortical white matter (per mL) | 0.26 (0.10, 0.41) | 0.001 |
| PSS (total) | Cortical white matter (per mL) | 0.01 (0.00, 0.03) | 0.148 |
| SOC: Problems solved | Cortical white matter (per mL) | 0.01 (0.00, 0.02) | 0.024 |
| SWM: Errors | Cortical white matter (per mL) | 0.00 (−0.01, 0.01) | 0.464 |
| Score | Volume | Difference in Score (95% CI) | P-value |
|---|---|---|---|
| Errors of omission | Total frontal lobe (per mL) | 0.10 (−0.22, 0.41) | 0.545 |
| Errors of commission | Total frontal lobe (per mL) | −0.14 (−0.39, 0.12) | 0.294 |
| Reaction time | Total frontal lobe (per mL) | 0.14 (−0.18, 0.46) | 0.376 |
| Variability | Total frontal lobe (per mL) | 0.05 (−0.48, 0.57) | 0.858 |
| PSS (total) | Total frontal lobe (per mL) | −0.02 (−0.04, 0.01) | 0.179 |
| SOC: Problems solved | Total frontal lobe (per mL) | 0.04 (0.01, 0.06) | 0.007 |
| SWM: Errors | Total frontal lobe (per mL) | 0.02 (−0.01, 0.05) | 0.230 |
| Score | Volume | Difference in Score (95% CI) | P-value |
|---|---|---|---|
| IQ | Cortical gray matter (per mL) | 0.07 (0.00, 0.15) | 0.055 |
The association with age was not significantly different from controls for GM, CC, and FL volumes. For WM we found a significant difference (Figure 1 and Table 6). While there is notable increase in WM volume with age in controls, such an association was not present in MPS II patients. However, CC volumes appear to show an increase with age similar to the volume increase in controls. (Figure 1 and Table 6).
Figure 1.
Brain volumetric data plotted against age for MPS II patients < 25.
Table 6.
Fold difference of volumes across age. For all regions control volumes significantly increased with age while they did not increase significantly in MPS II patients.
| Volume | Group | Fold Difference (95% CI) | P-value |
|---|---|---|---|
| Corpus callosum (per mL) | Control (age per 10 years) | 1.15 (1.07, 1.25) | <0.001 |
| MPS II (age per 10 years) | 1.29 (0.75, 2.21) | 0.356 | |
|
| |||
| Cortical WM (per mL) | Control (age per 10 years) | 1.20 (1.15, 1.26) | <0.001 |
| MPS II (age per 10 years) | 1.02 (0.85, 1.23) | 0.793 | |
|
| |||
| Cortical GM (per mL) | Control (age per 10 years) | 1.04 (1.00, 1.09) | 0.048 |
| MPS II (age per 10 years) | 0.98 (0.85, 1.12) | 0.721 | |
|
| |||
| Total frontal lobe (per mL) | Control (age per 10 years) | 1.05 (1.01, 1.10) | 0.023 |
| MPS II (age per 10 years) | 1.02 (0.86, 1.20) | 0.839 | |
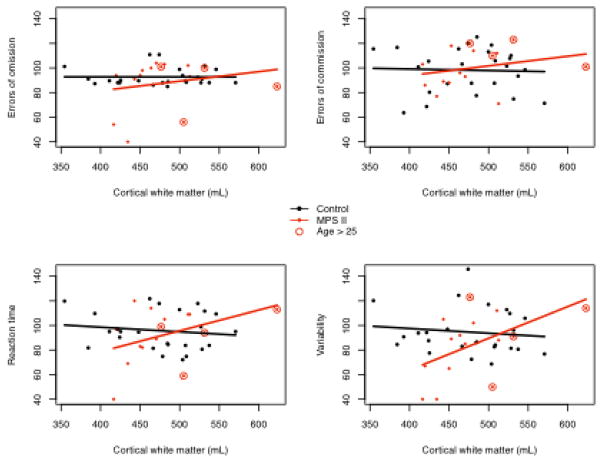
The relationship of attention variables assessed by the TOVA to cortical WM volumes are demonstrated in Figure 2. Higher TOVA scores represent better performance. VAR differs in its association with WM in the MPS II compared to controls. Table 5 shows the significant association of attentional variables (RT and VAR) to corpus callosum and white matter volumes. SOC scores, the measure of executive functions, were positively associated with both WM volumes (p= 0.024) and with total frontal lobe volumes (p= 0.007).
Figure 2.
TOVA standard scores and their association with cortical WM (mL).
Predictors of attention
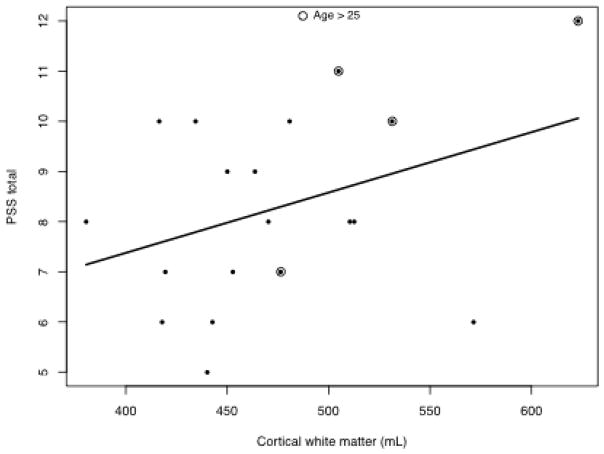
Two models used variability as the dependent variable, one examined cortical WM and the other CC. These models also included somatic disease burden (PSS), age of subject and age at first treatment (Table 7). WM has the largest association with attention followed by PSS with adjustment for age and age at first treatment (see Figure 3). For every PSS point, TOVA variability is lower by 12 points; for every mL of WM, TOVA variability is lower by 0.48 points. Age at evaluation and age at first treatment were not significantly associated with attention after adjusting for PSS and WM (or CC) volumes. PSS and WM volumes were found to be associated (Figure 3).
Table 7.
Adjusted associations with differences in IQ and Variability (attention) MPS II <25 years
| Score | Covariate | Difference in Score (95% CI) | P-value |
|---|---|---|---|
| IQ | Cortical GM (per mL) | 0.20 (0.07, 0.32) | 0.002 |
| PSS | −0.52 (−4.34, 3.30) | 0.791 | |
| Age (per yr) | −0.84 (−4.84, 3.15) | 0.679 | |
| Age at treatment start (per yr) | 2.01 (−2.17, 6.20) | 0.346 | |
|
| |||
| Variability | Cortical WM (per mL) | 0.48 (0.34, 0.62) | <0.001 |
| PSS | −12.20 (−17.51, −6.88) | <0.001 | |
| Age (per yr) | −0.62 (−3.79, 2.56) | 0.704 | |
| Age at treatment start (per yr) | 0.53 (−2.85, 3.90) | 0.760 | |
|
| |||
| Variability | Corpus callosum (per mL) | 26.60 (12.04, 41.16) | <0.001 |
| PSS | −7.41 (−13.71, −1.11) | 0.021 | |
| Age (per yr) | −1.64 (−7.07, 3.80) | 0.555 | |
| Age at treatment start (per yr) | 1.71 (−4.09, 7.51) | 0.564 | |
Figure 3.
PSS scores and cortical WM volumes
DISCUSSION
We have systematically described aspects of the attenuated MPS II phenotype, including cognitive ability, medical, and brain volume data of this rarely studied group. Previous studies seeking to describe the MPS II profile have not examined these features solely within the attenuated group. Prospective studies to delineate disease progression have been attempted but are restricted to either familial history reports or clinical observations of mostly severe patients, or contain a combination of mild and severe phenotypes.
Our cohort of attenuated MPS II patients were diagnosed between the ages of 1–18 years of age, based on a variety of presenting symptoms. Cardiovascular, respiratory, gastrointestinal, skeletal, and ear-nose-throat symptoms appear in all MPS II patients, however, the symptom that distinguishes between mild and severe phenotypes is the presence of neurocognitive decline in the severe form along with behavioral difficulties; note that is not a presenting complaint in our sample [1,7]. One younger patient, aged 6 years, is observed to have behavioral problems and differed from the other attenuated patients in having a low IQ despite initially presenting as an attenuated patient. Since neurocognitive decline remains the single distinguishing factor between the two severities, IQ is an important factor when examining phenotypes depending on the age of the patient. In addition, MPS II has a spectrum of cognitive ability and intermediate forms likely exist. Furthermore, in the literature the upper limit in age that cognitive decline begins to occur is not clear [1,3].
Not unlike other reports [7,16, 40] we found that the IQ of attenuated patients are in the average range, however with great variability. IQ was associated with GM volume, but no other variable. We also found that two measures of executive function, SOC and SWM, were within the average range as were measures of memory. Visual motor skills were poor, but that is not surprising given their carpal tunnel syndrome and other orthopedic problems.
Measures of attention, notably the measure of variability on the TOVA, were found to be significantly poorer in the <25 MPS II group compared to controls. Previous studies using the TOVA note that increased variability has been found to differentiate children with ADHD from controls [41], possibly indicating an attention deficit in MPS II individuals. However, it is important to note that increased variability could also be a result of fatigue and/or carpal tunnel syndrome, as the TOVA requires the subject to press a button periodically over a period of 22 minutes. However, as discussed below corpus callosum and white matter volumes are associated with both PSS and TOVA variability suggesting brain involvement.
Reaction time and variability on the TOVA were positively associated with both corpus callosum and white matter volumes. In our study, faster reaction times and decreased variability on the TOVA were associated with larger white matter volumes. These relationships do not appear with our healthy controls, suggesting a distinct difference in white matter in MPS II. In addition, faster reaction time and less variability were associated with larger CC volumes.
While CC volumes appear to be developing at a similar rate to controls, white matter does not seem to be developing at the same rate (approximately 85% of the rate in controls) as can be seen in Table 6. For this reason, it is critical that longitudinal studies become the next step in order to determine if there is a within subject similar pattern over time, and to begin to explore the pathological basis for this difference in attenuated MPS II patients.
In the multivariate analysis (Table 7), IQ was predicted only by GM volumes. For TOVA VAR two analyses were run. In the first, WM and PSS were both significant predictors of VAR and in the second, CC and PSS. Neither age at evaluation nor age at starting treatment predicted VAR. WM abnormalities have been documented in MPS I, but we document here both cortical WM and CC differences from controls in MPS II. PSS is an important variable in MPS II; it increases with age as the patient has additional medical symptoms and surgeries. It is an important predictor of VAR; it may be secondary to fatigue or carpal tunnel syndrome although range of motion is likely not a factor as the motion required is minimal to press the button. Note that PSS is also associated with white matter volumes. Overall, inability to rapidly process visual information on this test, and in real life, may have an impact on long-term success academically.
SOC is a spatial planning and executive function test that has been associated with frontal lobe function. In this study, our MPS II patients had SOC scores within the average range and with a strong association with FL volumes. Their performance suggests normal frontal lobe functions. Attention problems in MPS II appear to be associated with CC and WM but not FL volumes.
Neuropsychological scores are somewhat higher for patients over 25. This may be an ascertainment bias in that those older patients who were able to manage to travel as participants in this study could do so because they had more attenuated disease.
There are some limitations to this study. Cross-sectional data was used and we did not include patients who were not able to complete cognitive testing and MRI; possibly associated with their degree of impairment. Since the severity of MPS II is a spectrum and we show here that cognitive functioning is quite variable, there is a possibility that some attenuated patients were misdiagnosed as “severe” and were therefore excluded from this study. Longitudinal study of changes over time in younger patients might reveal important information about this spectrum of severity. Future studies would be strengthened by an effort to associate phenotypic severity with genotype and biomarker information as well as white matter measurements such as diffusion tensor imaging. Collection and analysis of such longitudinal data is in progress.
In conclusion, we have determined that attention difficulties and decreased corpus callosum volumes are present in MPS II attenuated patients under the age of 25, despite normal IQ and gray matter volumes. Increasing white matter volumes with age in controls was not found in MPS II patients. Both CC and WM volumes were associated with attention difficulties. Somatic burden of disease is also significantly associated with attention problems. This latter finding suggests that despite treatment with ERT, physical symptoms exact a heavy toll on attention, which presumes an inability to process information efficiently and easy fatigue. Inefficient processing of visual information which is the consequence of poor ability to manage attention can lead to lack of success academically and in the real world. Awareness by the patient and caregivers with appropriate management and symptomatic support will benefit the attenuated MPS II patient.
Highlights.
MPS II attenuated patients have normal IQ, memory, and executive function
They show decreased attention span relative to population norms and controls
Their corpus callosum (CC) volume is decreased compared to controls
They have less volume increase with age in white matter (WM) than controls
Attention is associated with CC and WM volumes and somatic disease burden
Acknowledgments
Support and acknowledgments:
The Lysosomal Disease Network supported this study through “Longitudinal Studies of Brain Structure and Function in the Mucopolysaccharidoses” (E. Shapiro, P.I.). The Lysosomal Disease Network (U54NS065768) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH (C. Whitley P.I.).
Shire supported this study as an investigator initiated research grant (E Shapiro, P.I.).
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant number UL1 TR000004 (P. Harmatz) and UMN-CTSI grant number UL1 TR000114 (K. Rudser). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
-
Support for Imaging Controls:
NIH 5K12RR023247, J Wozniak, P.I.
NIH 5P41RR008079, 5R01MH060662, P30-NS057091, & M01-RR00400 K Lim, P.I.
-
Brain Structure and Function in Developmentally Normal Children Ages 4–7:” supported by:
Shire, I Nestrasil, P.I.
The National MPS Society, E Shapiro, P.I.
Ryan Foundation for Orphan Diseases, E Shapiro, P.I.
We are very grateful for the cooperation of our participants. We thank Brenda Diethelm-Okita, David Erickson, and Evelyn Redtree in the Lysosomal Disease Network office at the University of Minnesota for administrative assistance. We thank James Provenzale, M.D. (Duke University) and Bryon Mueller, Ph.D. (University of Minnesota) for neuroimaging consultation. We are also grateful to the Center for Neurobehavioral Development, the Center for Magnetic Resonance Research, and the Minnesota Supercomputer Center for the provision of infrastructure for this research.
Footnotes
Clinical Trials Number: NCT01870375
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lampe C, Atherton A, Burton BK, Descartes M, Giugliani R, Horovitz DD, Kyosen SO, Magalhães TS, Martins AM, Mendelsohn NJ, Muenzer J, Smith LD. Enzyme Replacement Therapy in Mucopolysaccharidosis II Patients Under 1 Year of Age. JIMD Reports. 2014 doi: 10.1007/8904_2013_289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin R, Beck M, Eng C, Giugliani R, Harmatz P, Muñoz V, Muenzer J. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121(2) doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 4.Muenzer Joseph, Wraith James E, Beck Michael, Giugliani Roberto, Harmatz Paul, Eng Christine M, Vellodi Ashok, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genetics in Medicine. 2006;8(8):465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 5.Young ID, Harper PS. Incidence of Hunter’s syndrome. Hum Genet. 1982;60(4):391–392. doi: 10.1007/BF00569230. [DOI] [PubMed] [Google Scholar]
- 6.Young ID, Harper PS. The natural history of the severe form of Hunter’s syndrome: a study based on 52 cases. Dev Med Child Neurol. 1983;25:481–489. doi: 10.1111/j.1469-8749.1983.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. III. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 8.Ahmed A, Whitley CB, Cooksley R, Rudser K, Cagle S, Ali N, Delaney K, Yund B, Shapiro E. Neurocognitive and neuropsychiatric phenotypes associated with the mutation L238Q of the α-L-iduronidase gene in Hurler-Scheie syndrome. Molecular Genetics and Metabolism. 2014;111(2):123–127. doi: 10.1016/j.ymgme.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrielli O, Polonara G, Regnicolo L, et al. Correlation between cerebral MRI abnormalities and mental retardation in patients with mucopolysaccharidoses. Am J Med Genet A. 2004;125A(3):224–231. doi: 10.1002/ajmg.a.20515. [DOI] [PubMed] [Google Scholar]
- 10.Vedolin L, Schwartz IV, Komlos M, et al. Correlation of MR imaging and MR spectroscopy findings with cognitive impairment in mucopolysaccharidosis II. AJNR Am J Neuroradiol. 2007;28(6):1029–1033. doi: 10.3174/ajnr.A0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C, Dineen TE, Brack M, Kirsch JE, Runge VM. The mucopolysaccharidoses: characterization by cranial MR imaging. AJNR Am J Neuroradiol. 1993;14(6):1285–1292. [PMC free article] [PubMed] [Google Scholar]
- 12.Vite C, Nestrasil I, Mlikotic A, et al. Features of Brain MRI in Dogs with Treated and Untreated Mucopolysaccharidosis Type I. Compar Med. 2013;63:163–173. [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson P, McEntee M, Vogler C, et al. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matheus MG, Castillo M, Smith JK, Armao D, Towle D, Muenzer J. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;13:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang RY, Cambray-Forker EJ, Ohanian K, Karlin DS, Covault KK, Schwartz PH, Abdenur JE. Treatment reduces or stabilizes brain imaging abnormalities in patients with MPS I and II. Mol Genet Metab. 2009;98:406–411. doi: 10.1016/j.ymgme.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Yund B, Rudser K, Kovac V, Nestrasil I, Ahmed A, Delaney K, Whitley C, Shapiro E. White Matter Structure and Function in Attenuated MPS II. Molecular Genetics and Metabolism. 2014;111:S116–117. [Google Scholar]
- 17.Parsons VJ, Hughes DG, Wraith JE. Magnetic Resonance Imaging of the Brain, Neck and Cervical Spine in Mild Hunter’s Syndrome (Mucopolysaccharidoses Type II) Clin Radiol. 1996:719–723. doi: 10.1016/s0009-9260(96)80246-7. [DOI] [PubMed] [Google Scholar]
- 18.Shimoda-Matsubayashi S, Kuru Y, Sumie H, Ito T, Hattori N, Okuma Y, Mizuno Y. MRI findings in the mild type of mucopolysaccharidosis II (Hunter’s syndrome) Neuroradiology. 1990;32(4):328–30. doi: 10.1007/BF00593056. [DOI] [PubMed] [Google Scholar]
- 19.Mullen EM. Mullen Scales of Early Learning. Circle Pines MN: American Guidance Service; 1995. [Google Scholar]
- 20.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio TX: Psychological Corporation; 2002. [Google Scholar]
- 21.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio TX: Psychological Corporation; 1999. [Google Scholar]
- 22.Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio TX: Psychological Corportation; 2003. [Google Scholar]
- 23.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 24.Greenberg LM. The Test of Variables of Attention (Version 7.3) [Computer software] Los Alamitos: The TOVA Company; 2007. [Google Scholar]
- 25.Conners CK. Conners’ Continuous Performance Test II. Toronto CA: Multi-Health Systems; 2000. [Google Scholar]
- 26.Luciana M, Nelson CA. Assessment of neuropsychological function in children through the Cambridge Neuropsychological Testing Automated Battery (CANTAB): Normative performance in 4 to 12 year-olds. Developmental Neuropsychology. 2002;22(3):595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. 2. Circle Pines, MN: American Guidance Service; 2004. Manual. [Google Scholar]
- 28.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12:1, 43–55. [Google Scholar]
- 29.Korkman M, Kirk U, Kemp S. NEPSY. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 30.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial MemoryTest: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8:145–153. [Google Scholar]
- 31.Cohen MJ. The children’s memory scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 32.Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 33.Benton AL. Contributions to neuropsychological assessment: A clinical manual. Oxford University Press; 1994. [Google Scholar]
- 34.Beery KE, Buktenica NA, Beery NA. The Beery-Buktenica developmental test of visual-motor integration: Administration, scoring and teaching manual. Minneapolis, MN: NCS Pearson; 2004. [Google Scholar]
- 35.Waber DP, Holmes JM. Assessing children’s copy production of the Rey- Osterrieth Complex Figure. Journal of Clinical and Experimental Neuropsychology. 1985;7:264–280. doi: 10.1080/01688638508401259. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed A, Kunin-Batson A, Redtree E, Whitley CB, Shapiro E. MPS (mucopolysaccharidosis) specific physical symptom score-development, reliability and validity. Molecular Genetics and Metabolism. 2014;111(2):S17–S18. [Google Scholar]
- 37.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. URL http://www.R-project.org/ [Google Scholar]
- 40.Young ID, Harper PS. Mild form of Hunter’s syndrome: clinical delineation based on 31 cases. Arch Dis Child. 1982;57(11):828–836. doi: 10.1136/adc.57.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schatz AM, Ballantyne AO, Trauner DA. Sensitivity and specificity of a computerized test of attention in the diagnosis of Attention-Deficit/Hyperactivity Disorder. Assessment. 2001;8(4):357–65. doi: 10.1177/107319110100800401. [DOI] [PubMed] [Google Scholar]