Abstract
The canonical Wnt/β-catenin (“Wnt”) pathway is an essential signaling cascade in the embryonic central nervous system (CNS) that regulates neuronal differentiation and survival. Loss of Wnt signaling in developing and adult tissue has been implicated in numerous CNS diseases, but the precise role of Wnt in regulating neuronal survival, and how its absence could lead to disease, is not understood. In this study, we investigated the effect of Wnt activation on neuronal survival in the adult retina, and identified cellular and molecular mediators. Pan-retinal Wnt signaling activation using Wnt3a induced functional and morphological rescue of photoreceptor neurons in the rd10 mouse model of retinal degeneration. Furthermore, Wnt activation using constitutively active β-catenin specifically targeted to Muller glia increased photoreceptor survival and reduced markers of glial and neuronal remodeling. Wnt-induced photoreceptor protection was associated with elevated levels of prosurvival protein Stat3, and was reduced by shRNA-mediated knockdown of Stat3, indicating cross-talk between pro-survival pathways. Therefore, these data increase our understanding of the role of Wnt signaling in the retina, and identify radial Muller glia as important cellular mediators of Wnt activity.
Keywords: Neuroprotection, Wnt, Stat3, Retina, Muller glia
1. Introduction
Canonical Wnt signaling is activated by binding of extracellular ligands to the coreceptors Frizzled and LRP5/6, leading to association of β-catenin with nuclear TCF/LEF proteins and induction of target genes. Wnt signaling plays numerous roles in the CNS, ranging from neuronal differentiation (Hunter et al., 2004; Masai et al., 2005; Fuhrmann, 2008), and regeneration (Osakada et al., 2007), to controlling axonal outgrowth and synaptic function (Rosso et al., 2005; Inestrosa and Arenas, 2010). Wnt signaling also regulates neuronal survival in vitro and after acute tissue injuries in vivo (Yi et al., 2007; Lin et al., 2009; Mizukami et al., 2009; Seitz et al., 2010; Fragoso et al., 2011). Furthermore, loss of Wnt signaling is associated with neuronal death in animal models of Alzheimer’s disease (Caricasole et al., 2004). However, the precise cellular and molecular mechanisms that regulate neuroprotective Wnt signaling are poorly understood.
Retinal degenerations, such as age-related macular degeneration and retinitis pigmentosa, are leading causes of vision loss in the developed world, and result from dysfunction and death of the light-sensing photoreceptor neurons. Characterizing molecular signaling pathways that regulate photoreceptor function and viability is an essential step in identifying potential therapeutic targets. Our group and others previously demonstrated that Wnt signaling activation rescued photoreceptors in culture and in vivo from acute toxicity induced by oxidative stress, laser and light injuries, (Yi et al., 2007; Lin et al., 2009; Mizukami et al., 2009; Seitz et al., 2010; Fragoso et al., 2011; Braunger et al., 2013; Liu et al., 2013; Sanges et al., 2013). However, acute toxicity models do not closely mimic inherited human retinal degenerations, leading to the question of what is the effect of Wnt activation in animal models of inherited retinal degenerations, particularly using druggable targets such as Wnt ligands.
The retina is a thin multilayered neuroepithelial tissue that is often used to investigate neuronal survival pathways because it has the advantages of lower complexity and ease of delivering genes and proteins. In the retina, the survival of photoreceptors is influenced by growth factors that are secreted by a radial glial cell type called Muller glia (Chaum, 2003). Endogenous Wnt signaling is upregulated in Muller glia during retinal injury (Yi et al., 2007) and activated Wnt signaling in retinal glia induced expression of prosurvival growth factors, including BDNF and CNTF (Yi et al., 2007; Seitz et al., 2010; Fragoso et al., 2011). However, the role of glial Wnt in photoreceptor survival is unknown. In this study, we characterized the function of canonical Wnt signaling during inherited retinal degeneration in the murine rd10 model, and identified its cellular and molecular mediators. Our results demonstrate that activation of Wnt signaling in Muller glia induced functional and morphological neuroprotection of photoreceptors. Furthermore, Stat3 was shown to be an important contributor to Wnt-induced neuroprotection. Therefore, these studies suggest a model in which the Wnt pathway is stimulated in Muller glia in the presence of neuronal damage, and that over-expressing Wnt activators induces neuronal survival factors and reduces degeneration.
2. Materials and Methods
2.1 Animals and subretinal injections
All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee at the University of Miami. The mouse lines C57Bl/6 and rd10 (B6.CXB1-Pde6brd10/J) were obtained from Jackson Laboratory (Bar Harbor, Maine). Mice of either sex were anesthetized using a ketamine/xylazine cocktail, and the corneas were anesthetized with a drop of 0.5% proparacaine hydrochloride and pupils dilated with 10% phenylephrine ophthalmic solution. The method of injection was as follows: A thin custom-made microsyringe (Hamilton, Reno, NV) was passed through the sclera of the eye into the subretinal space and 1 μl of solution containing Wnt3a, PBS or viral constructs was injected. Successful injection was indicated by a temporary bleb and retinal detachment that quickly resolved, which is typical of this type of injection technique. Mice with unresolved retinal detachments or bleeding were excluded from further analysis. Each injected eye was treated topically post-injection with a drop of Polymyxin B Sulfate and Trimethoprim Ophthalmic Solution USP Sterile (Bausch and Lomb). The fellow eye was uninjected.
2.2 Construction of adenoviral vectors containing Muller glia-specific Wnt signaling regulators
Wnt signaling was modified specifically in Muller glia using the non-secreted Wnt inhibitor β-eng gene (Montross et al., 2000; Tepera et al., 2003) and activator β-catenin-S33A gene (Ouchi et al., 2005), and expression was directed to Muller glia using the 2.2 kb long version of the GFAP promoter (Su et al., 2004; de Leeuw et al., 2006), and packaged into adenovirus (pAdEasy-1 vector, containing adenovirus serotype 5 deleted for the genes E1 and E3) for delivery in vivo. The β-eng and β-catenin-S33A genes were subcloned from their parent vectors into the pcDNA3 vector and the CMV promoter was then removed and replaced with the GFAP promoter, to generate the GFAP-β-eng and GFAP-β-catenin-S33A constructs. A control construct was made using GFP driven by the GFAP promoter. After confirmation of the correct sequences, the GFAP-β-eng, GFAP-β-catenin-S33A and GFAP-GFP plasmids were subcloned into the adenovirus vector pAdEasy-1 and viruses were packaged using the AdEasy system (Agilent, La Jolla, CA) and purified to high titer preps at the Viral Core Facility, Miami Project to Cure Paralysis, University of Miami.
2.3 Wnt reporter luciferase assays
Regulation of Wnt signaling by β-eng and β-catenin-S33A constructs in adenovirus was confirmed by Wnt reporter luciferase assays in primary Muller glia-photoreceptor co-cultures, prepared as described in (Yi et al., 2007). Briefly, retinas from wild-type mice at post-natal day (P) 8 were dissociated in activated papain for 30 min at 37 °C, mixed with Neurobasal medium containing 1x LoOvo plus DNAse I (Invitrogen, Carlsbad, CA) and pelleted by low-speed centrifugation. The cell pellet was washed in neurobasal-LoOvo medium without DNAse and plated in neurobasal medium containing L-glutamine, B27 and antibiotics onto poly-D-lysine/laminin coated 96-well dishes at a density of 2.5×105 cells per well. The cultures were transfected with TOP-FLASH Wnt reporter firefly luciferase (Dr. Randy Moon, UW) and Renilla luciferase plasmid (Promega) using a nucleofector electroporator (Lonza, Wakersville) and then after 24 hr were incubated with the adenoviral constructs GFAP-β-eng, GFAP-β-catenin-S33A and GFAP-GFP, for 3 days. The cells were collected in Reporter lysis buffer (Promega, Madison WI) and luciferase activity assays were performed using the Dual Glo Lucierase System (Promega) in a Lumistar Galaxy luminometer (BMG Labtech Inc, Cary, NC) as described in (Tell et al., 2006). Wnt signaling activity is expressed as firefly luciferase units/Renilla luciferase units. The assays were performed in triplicate wells in four independent experiments.
2.4 Western blotting
Subcellular fractionation was performed by lysing the retinas using the Ne-PER Nuclear and Cytoplasmic Extraction Reagent (Pierce), following the manufacturer’s directions and as previously described in (Nakamura and Hackam, 2010). For the whole retina lysates, an isotonic detergent buffer (50 mM Tris pH7.4, 150 mM NaCl, 1% NP40, 0.05% SDS) was used, containing a proteinase and phosphatase inhibitor cocktail (Roche). Protein concentration in the lysates was determined by Lowry assay (BioRad). Twenty micrograms of protein lysates were electrophoresed using 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gels and proteins were transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were probed with the appropriate primary and secondary antibodies (Table 1) with several wash steps in-between, and the membranes were then incubated with the LumiGLO Peroxidase Chemiluminescent Substrate Kit (Kirkegaard & Perry Laboratories, Gaithersburg MD). The bands were detected using a Fuji Film Luminescent Image Analyzer and quantified with ImageJ software (Abramoff et al, 2004). Typically, images were captured over the course of 2–10 minutes, and signals were quantified only from images in which the bands were not saturated. Westerns probed for phospho-Stat3, total Stat3, and β-catenin were normalized to β-actin. Westerns probed for rhodopsin and Gαt2 were normalized to α-tubulin.
Table 1. Antibodies used in this study.
The source and dilutions of antibodies used in this study is shown.
| Antibody | Source | Dilution |
|---|---|---|
| GFAP | Invitrogen (Carlsbad, CA) | 1:200 |
| GFP | Aves Labs (Tigard, OR) | 1:5000 |
| Glutamine Synthetase | Sigma-Aldrich (ST. Louis, MO) | 1:300 |
| Gαt2 | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) | 1:500 |
| Ki67 | Abcam, Inc. (Cambridge, MA) | 1:100 |
| Phospho-Stat3 Tyr705 | Cell Signaling Technology (Danvers, MA) | 1:200 |
| PKC | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) | 1:200 |
| Rhodopsin | Chemicon International (Billerica, MA) | 1:500 |
| Total-Stat3 | Cell Signaling Technology (Danvers, MA) | 1:200 |
| Vimentin | Sigma-Aldrich (ST. Louis, MO) | 1:500 |
| Wnt3a | Abcam, Inc. (Cambridge, MA) | 5 μg/ml |
| α-Tubulin | Cell Signaling Technology (Danvers, MA) | 1:1000 |
| β-Actin | Sigma-Aldrich (ST. Louis, MO) | 1:8000 |
| β-Catenin | BD Transduction Laboratories (San Jose, CA) | 1:1000 |
2.5 Immunohistochemistry
Eyes and attached optic nerves were removed and fixed in 4% fresh paraformaldehyde, incubated in increasing sucrose concentrations (5%–20%) and then embedded in OCT, as described (Tell et al., 2006). Ten micron sections were cut and placed onto glass slides. The slides were blocked in goat serum and incubated with antibody (Table 1) overnight at 4 °C, washed in PBS, and incubated with secondary antibody and then washed again prior to mounting in a DAPI-containing mounting media. Control immunostaining for Wnt3a was performed using the anti-Wnt3a antibody that had been preabsorbed with Wnt3a ligand overnight at 4°C, and controls for the other antibodies included an irrelevant antibody from the same species, or omitted the primary antibody. The immunostained sections were viewed using a Zeiss Axiovert 200 fluorescent microscope or a Leica Microsystems confocal microscope. Image acquisition and analysis software used Axiovision LE (Carl Zeiss) or the Leica Application Suite (Leica), respectively. Photographic and microscopic settings were kept constant among positive and negative control antibody treatments.
2.6 Electroretinography (ERG)
All ERG recordings were performed at approximately the same time of day and the experimenters were masked to the animal treatments. Dark-adapted rod and light-adapted cone responses were measured by flash ERG using an UTAS system (LKC Technologies, Gaithersburg, MD), based on the methods described in (Li et al., 2010; Patel and Hackam, 2014). The mice were placed on a heating pad maintained at 37 °C, anesthetized with ketamine/xylazine, and pupils were dilated with 10% phenylephrine. All manipulations were performed under dim red light. A thin silver wire loop was placed onto the corneal surface, with care taken to maintain contact and position. A needle electrode was placed subcutaneously in the scalp, and a ground electrode was placed in the base of the tail. Simultaneous recordings from both eyes were recorded at increasing stimulus intensities of −2.0 to 2.0 log cd-s/m2, elicited by 250 μs flashes generated by white LEDs in the Ganzfeld sphere. Each recording was an average of 10 flash responses with 5 second intervals between each flash. For cone ERGs, the mice were light-adapted in white light then were stimulated with a green flash on a low green background light. Amplitudes of a- and b-waves were quantified.
2.7 Statistical analysis
Statistical analyses were performed by Student’s t-test or analysis of variance (ANOVA) using GraphPad Prizm, and comparisons with a p<0.05 were considered statistically significant.
3. Results
3.1 Wnt3a activates Wnt signaling in the retina
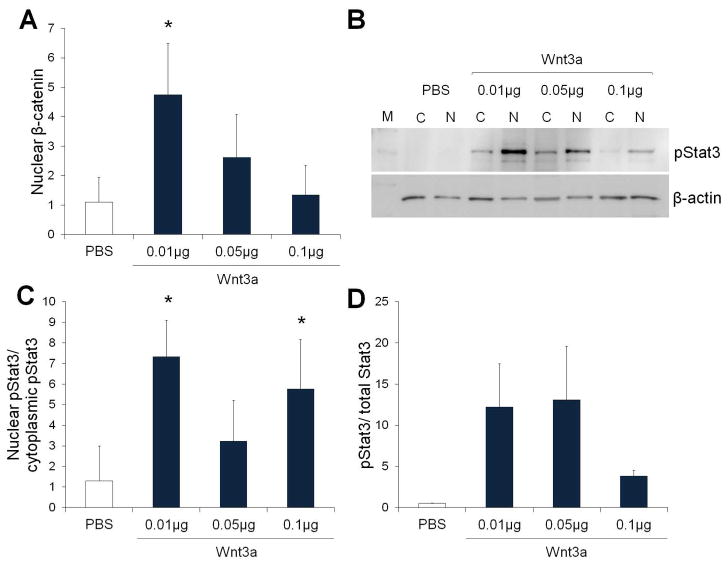
To identify the role of canonical Wnt signaling during retinal degeneration, our approach was to first induce Wnt signaling throughout the retina (pan-retinal activation) using the Wnt3a ligand, followed by identification of the cellular mediator using a cell-restricted Wnt activator, β-cateninS33A. The Wnt3a ligand was used because it protected primary photoreceptor cultures from acute oxidative stress injury, and it induced robust Wnt signaling and neurotrophin secretion in cultured Muller glia (Yi et al., 2007; Yi et al., 2012). To determine the optimal Wnt3a dose for Wnt activation, increasing amounts of Wnt3a were injected subretinally into wildtype mice, and the levels of nuclear β-catenin, used as a marker of induced Wnt signaling, were measured. As shown in Fig. 1, subretinal injection of Wnt3a induced up to a 5-fold increase in nuclear β-catenin, indicating that Wnt3a activates Wnt signaling in the retina (Fig. 1A). The highest level of nuclear β-catenin was obtained using the lowest dose (0.01 μg), which is consistent with reported stronger effects of growth factors at lower concentrations (Albuquerque et al., 2002).
Fig. 1.
Subretinal injection of recombinant Wnt3a protein induces Wnt and Stat3 signaling in C57Bl/6 mice. (A) Subretinal injection of Wnt3a ligand (μg) activated canonical Wnt signaling, as measured by elevated levels of nuclear β-catenin compared with PBS control injections (n=3, * p<0.05). (B–D) Analysis of Stat3, a downstream target of Wnt, by Western blotting. (B) Representative Western blot showing nuclear and cytoplasmic levels of pStat3 in retinas injected with increasing amounts of Wnt3a ligand. (C) Wnt3a increased the ratio of nuclear (N) to cytoplasmic (C) levels of phosphorylated Stat3 (pStat3) in Wnt3a injected retinas, compared with PBS injected retinas (n=3, * p<0.05). (D) Injection of Wnt3a increased the ratio of activated phosphorylated Stat3 to total Stat3.
Quantification of Wnt3a-induced Stat3 activation was used as a downstream marker of Wnt signaling, based on findings that Wnt3a increased Stat3 signaling in a retinal pigment epithelium cell line (Fragoso et al., 2012) and increased CNTF (Seitz et al., 2010), an upstream activator of Stat3. Stat3 is a transcription factor that induces pro-survival genes in the retina (Rhee et al., 2007; Ueki et al., 2008; Burgi et al., 2009). Western blotting on nuclear fractions using an antibody against phosphorylated Stat3 demonstrated that subretinal injection of 0.01 μg Wnt3a induced the highest nuclear phosphorylated Stat3 levels and highest pStat3/tStat3 ratios (Fig. 1B–D). Injection of 0.1 μg also increased pStat3/tStat3, but the 0.05 μg injection did not. Therefore, the 0.01 μg Wnt3a dose was used to test the effect of Wnt3a on photoreceptor survival.
3.2 Expression of endogenous Wnt3a in Muller glia and photoreceptors
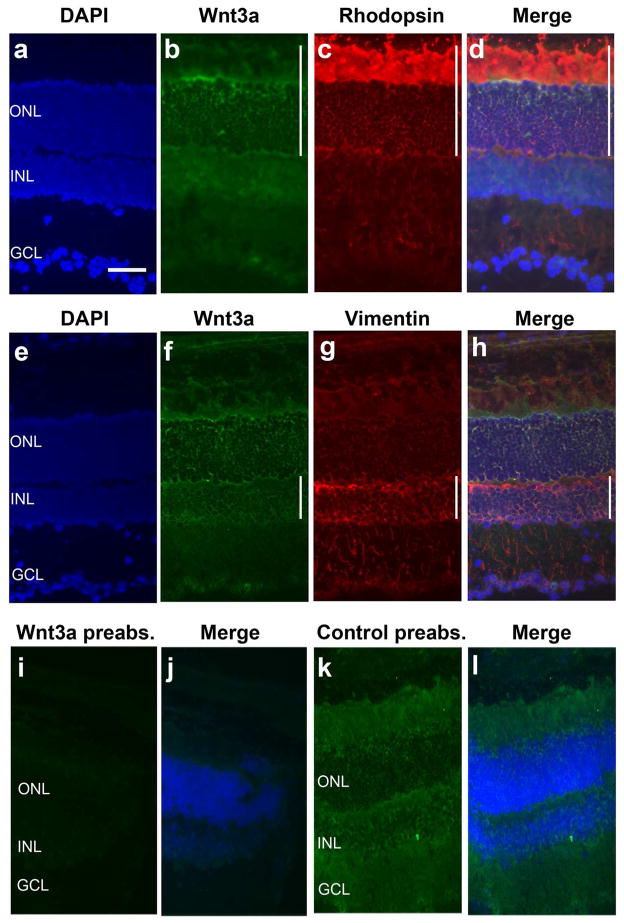
To confirm the relevance of Wnt3a to the retina, the distribution of endogenous Wnt3a was analyzed by immunohistochemistry. Wnt3a was detected in the photoreceptor layer, in a region that corresponds to the inner and outer segments and nuclei (Fig. 2). Detection of Wnt3a overlapped with detection of the rod photoreceptor marker rhodopsin, which confirmed expression in photoreceptors (Fig. 2A–D). Wnt3a was also detected in the inner leaflet of the inner nuclear layer (INL) and overlapped with the glial marker vimentin, consistent with localization to Muller glia (Fig. 2E–H). There was also detection of Wnt3a in cells in the GCL, which are most likely RGCs (Liu et al., 2003; Yi et al., 2007). The specificity of the Wnt3a antibody was confirmed, shown in Fig. 2I–K. Therefore, endogenous Wnt3a is localized to photoreceptors and Muller glia, which are the two main cell types involved in retinal degenerations.
Fig. 2.
Wnt3a is expressed in the retina. Immunodetection using anti-Wnt3a antibodies demonstrates prominent localization of Wnt3a (vertical line) in the outer segments and nuclear layer of photoreceptors (B–D), shown by codetection with rhodopsin (D). Wnt3a was also detected in cells in the inner nuclear layer (INL) (vertical line), most likely Muller glia based on codetection with vimentin (H). DAPI was used to stain the nuclear layers. The specificity of the anti-Wnt3a antibody was verified by preabsorbing with Wnt3a ligand (I–J), which blocked immunodetection. Immunodetction was not blocked when the antibody was preabsorbed with non-Wnt3a containing solution (K–L). Scale bar, 50 μm
3.3 Wnt3a signaling protects photoreceptors in a mouse model of inherited retinal degeneration
The rd10 mouse contains a spontaneous missense point mutation in the rod cGMP phosphodiesterase 6B beta polypeptide (Pde6b) gene and is a well-established naturally occurring model for studying photoreceptor death in inherited retinal degeneration (Gargini et al., 2007; Barhoum et al., 2008). rd10 is an ideal model because the timing of photoreceptor death is well characterized and begins after retinal development is complete. To test the effect of Wnt signaling on photoreceptors, the rd10 mice were subretinally injected with recombinant Wnt3a protein prior to onset of retinal degeneration, and the phenotype was assessed at 7 and 14 days after injection.
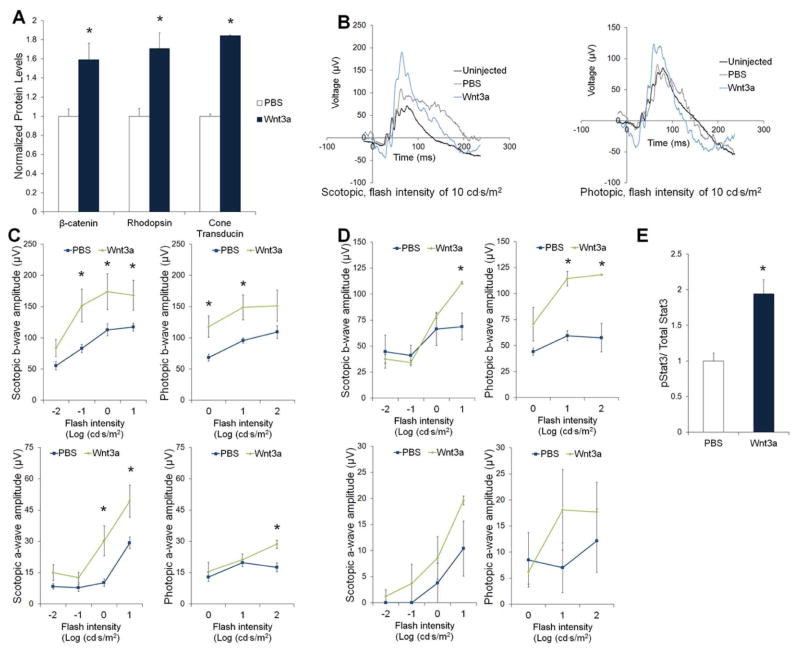
Mouse retinas injected with Wnt3a showed significant increases in β-catenin compared with PBS, validating that our treatment activates Wnt signaling (Fig. 3). The quantification of light-stimulated retinal responses by ERGs is often used as a measurement of photoreceptor viability. As shown in Fig 3, mice treated with Wnt3a showed significant increases in the amplitude of a-waves (primarily photoreceptor-derived) and b-waves (photoreceptor-stimulated bipolar cell response) of rod and cone photoreceptors at 7 days and at 14 days post-injection, compared with PBS injection controls, which demonstrates functional rescue of the retina (Fig. 3B–D). Furthermore, because retinal degeneration is associated with decreased expression of photoreceptor-specific proteins, Western blot analysis of the photoreceptor marker proteins cone transducin and rhodopsin was used to confirm photoreceptor survival. Wnt3a injection significantly increased levels of the cone photoreceptor marker protein cone transducin and the rod photoreceptor protein rhodopsin (Fig. 3A). Therefore, these data demonstrate that activation of the canonical Wnt pathway using exogenous application of Wnt3a leads to functional rescue of photoreceptors in the inherited retinal degeneration rd10 mouse.
Fig. 3.
Wnt3a protects photoreceptors in the rd10 mouse model of retinal degeneration. (A) Western blot analysis on subretinally injected retinas demonstrated that Wnt3a induced Wnt signaling in rd10 mice, as measured by increased β-catenin levels compared with the PBS control injection. Wnt3a also increased levels of the photoreceptor marker proteins cone transducin (Gαt2) and rhodopsin (n=4, *p<0.05). (B) The rd10 mice were subretinally injected with recombinant Wnt3a (0.01 μg) or PBS and analyzed after 7 days using flash ERGs that were recorded in dark-adapted (rod photoreceptor) or light-adapted (cone photoreceptor) conditions. Representative ERG waves (an average of 10 flash responses) are shown for injected and uninjected mice at a stimulus of 10 cd-s/m2. Wnt3a increased both scotopic (rod) and photopic (cone) responses in rd10 mice. (C) Maximum a- and b-wave amplitudes of the Wnt3a and PBS injected mice invoked by light flashes at various intensities measured at 7 days post-injection. (Wnt n=9, PBS n=10, * p<0.05). (D) Maximum a- and b-wave amplitudes of the Wnt3a and PBS injected mice invoked by light flashes at various intensities measured at 14 days post-injection. (Wnt n=4, PBS n=3, * p<0.05). (E) Wnt3a injection increased the ratio of pStat3 to total Stat3, compared with PBS, measured at 7 days post-injection. (n=4 *p<0.05). Mean ± SE are shown.
3.4 Muller glia are cellular mediators of protective Wnt signaling
Subretinal delivery of Wnt3a is expected to activate Wnt signaling in all cells that show endogenous Wnt signaling, including Muller glia, which upregulate Wnt signaling during retinal degeneration (Yi et al., 2007), and retinal ganglion cells, microglia, and amacrine cells, which have Wnt signaling in normal and degenerating retinas (Liu et al., 2006; Yi et al., 2007). We are particularly interested in Muller glia because our previous studies showed that they respond to Wnt3a by increasing growth factor expression such as BDNF (Yi et al., 2012). Therefore, we tested whether Wnt signaling activation specifically in Muller glia plays a protective role during inherited retinal degeneration.
In order to activate Wnt signaling specifically in Muller glia, we used a glia-specific promoter to drive expression of a non-secreted Wnt activator. Adenovirus was used for gene delivery because of its ability to infect Muller glia. The non-secreted Wnt regulator allowed modulation of Wnt signaling exclusively in the cells that express the constructs. Wnt signaling was activated with a mutagenized constitutively active version of β-catenin (β-cateninS33A), which is an established activator of Wnt signaling in the retina during development (Ouchi et al., 2005; Cho and Cepko, 2006; Koso et al., 2006; Miller et al., 2006; Liu et al., 2007). The negative control gene was the Wnt inhibitor dominant-negative β-eng fusion gene, in which the C-terminal Wnt transactivation domain is replaced by the transcriptional repressor domain from Drosophila Engrailed. β-eng specifically inhibits canonical β-catenin-induced Wnt signaling but retains the adhesion role of β-catenin (Montross et al., 2000; Tepera et al., 2003) The β-cateninS33A and β-eng genes were expressed specifically in Muller glia using the 2.2 kb GFAP promoter (Su et al., 2004) (see Methods) and were then packaged into adenoviral vectors, which has the advantage of rapid onset of expression and tropism to Muller glia.
Regulation of Wnt signaling by the GFAP-driven Wnt activator β-cateninS33A and inhibitor β-eng in the adenoviral vectors was confirmed in Muller glia and photoreceptors co-cultures (Yi et al., 2007). The β-cateninS33A construct induced 3-fold higher Wnt signaling (Fig 4A) compared with the GFAP-GFP control. The expression constructs were next subretinally injected into rd10 mice prior to degeneration onset and analyzed after 1 week. This short time period is frequently used with adenovirus because it is prior to an inflammatory reaction to the viral proteins. Heidelberg confocal scanning laser ophthalmoscopy of living injected mice, using GFP within the viral constructs for visualization, demonstrated that approximately a third of the retina expressed the delivered genes (Fig. 4C). GFP was also used to localize transduced cells in retina sections, and demonstrated that the GFP-positive cells had the elongated morphology characteristic of Muller glia with their nuclei in the inner nuclear layer, indicating efficient targeting to the Muller glia (Fig. 4B). Additionally, GFP exclusively co-localized with the Muller glia marker glutamine synthetase (GS), demonstrating cell type specific expression (Fig. 4D). Western blotting from the virally injected retinas showed that β-cateninS33A delivery increased endogenous β-catenin by 2.5-fold compared with saline injection, indicating activation of the Wnt pathway in vivo (Fig. 4E). Also, β-cateninS33A injected into adult C57Bl/6 mice induced Wnt signaling in Muller glia, which do not normally have endogenous Wnt signaling (Yi et al., 2007) (data not shown). Therefore, the GFAP-β-cateninS33A construct allowed short-term delivery of a Muller glia-specific Wnt activator.
Fig. 4.
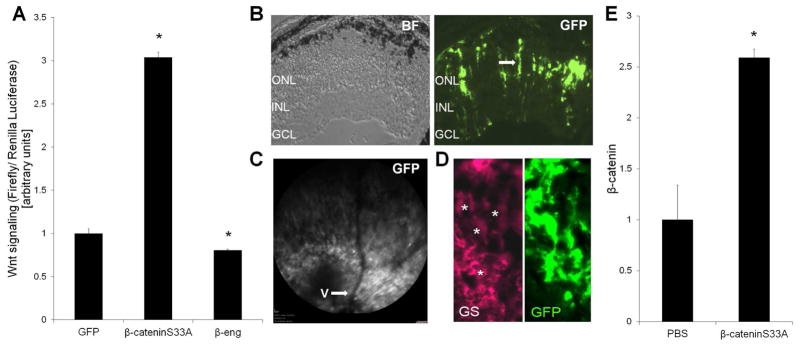
The Muller glia-specific β-cateninS33A construct activates Wnt signaling. The β-cateninS33A, β-eng and GFP genes, driven by the GFAP promoter to target Muller glia and packaged into adenovirus, were tested in retina primary cultures and in the retina. (A) Retina cultures were used to validate regulation of Wnt signaling, using a Wnt luciferase reporter. The GFAP-β-cateninS33A construct significantly induced Wnt signaling compared with control, whereas the β-eng construct reduced Wnt signaling (n=5, *p<0.05). (B) Distribution of the delivered genes in the retina, using fluorescence from the GFP reporter included in the virus. Detection of adenoviral β-cateninS33A in the rd10 retina, with corresponding phase image, shows that the GFAP promoter drove expression in Muller glia (arrow), based on morphology and position, with transduction across the retinal section. No GFP-positive astrocytes were observed and there was no indication of significant retinal inflammation or damage. (C) Efficient transduction by viral injections and promoter specificity. Fluorescence distribution across a retina 1 week after subretinal injection of β-cateninS33 was shown by imaging with Heidelberg confocal laser scanning ophthalmoscope using fluorescence from the GFP, which is included in the virus, to localize transduced regions. V, blood vessel. Approximately a third of the retina was transduced. (D) GFP expression is restricted to Muller glia by the GFAP promoter (*, codetection of GFP and glutamine synthetase (GS)). (E) Retinal lysates from rd10 injected with β-cateninS33A demonstrate that total β-catenin is elevated in the injected retinas compared with control injections, demonstrating expression and stability of the constitutively active β-cateninS33A construct. (n=4 *p<0.05). Mean ± SE are shown.
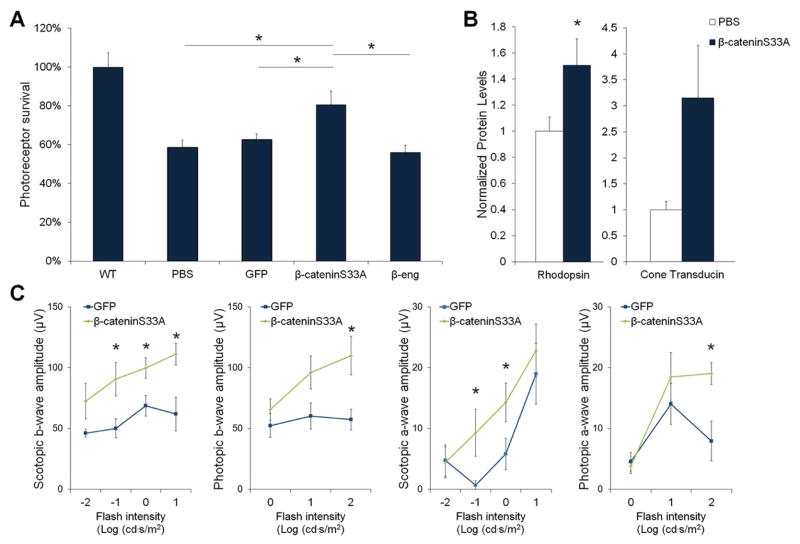
The rd10 mice were injected with β-cateninS33A, β-eng, the expression control GFP or the PBS control. As shown in Fig. 5, photoreceptor survival was increased by activating Wnt signaling specifically in Muller glia. Retinas injected with β-cateninS33A led to a 44% increase in the number of photoreceptor rows compared with control injections, demonstrating increased photoreceptor survival from Wnt activation (Fig. 5A). Furthermore, rod and cone photoreceptor ERG responses were also increased, which demonstrates functional rescue of the retina (Fig. 5B–C). Therefore, Muller glia specific activation of the Wnt pathway protected the rd10 retinas to a similar extent as pan-retinal Wnt signaling.
Fig. 5.
The Muller glia-specific Wnt activator protects the retina of rd10 mice. (A) β-cateninS33A, β-eng, PBS and GFP were subretinally injected into rd10, and photoreceptor survival was measured in retina cross-sections. β-cateninS33A increased photoreceptor rows in rd10 mice (n=5, *p<0.05; 5 random sections in 5 mice were analyzed), compared with GFP, PBS and β-eng controls, and normalized to a wildtype mouse (WT). The Wnt inhibitor β-eng construct did not have a significant effect on the retina. (B) β-cateninS33A increased levels of the photoreceptor marker proteins rhodopsin (*p<0.05) and cone transducin (Gαt2) (p=0.061, not significant). Westerns were normalized to β-actin. (β-cateninS33A n=4, PBS n=4). (C) Scotopic (rod-driven) and photopic (cone) flash ERG analysis showed higher b-wave amplitudes in rd10 mice injected with the Wnt activator β-cateninS33A compared with GFP at multiple stimulus intensities. (β-cateninS33A n=11, GFP n =9, *p<0.05). Mean ± SE is shown.
3.5 Stat3 is a molecular mediator of Wnt-dependent neuroprotection
Several neuroprotective signaling pathways are induced by Wnt, including neurotrophins (Fragoso et al., 2011; Yi et al., 2012) and anti-apoptotic proteins (Toledo et al., 2008; Kim et al., 2011). We recently demonstrated that Wnt3a upregulates Stat3 in cultured cells, and that knocking-down Stat3 using siRNA abrogated the protective effect of Wnt3a in a retinal pigment epithelium (RPE) cell line (Fragoso et al., 2012). Stat3 has also been implicated as an essential protective molecule in the retina (Rhee et al.; Joly et al., 2008; Ueki et al., 2008). Therefore, we next tested whether Stat3 mediates Wnt-mediated protection of photoreceptors.
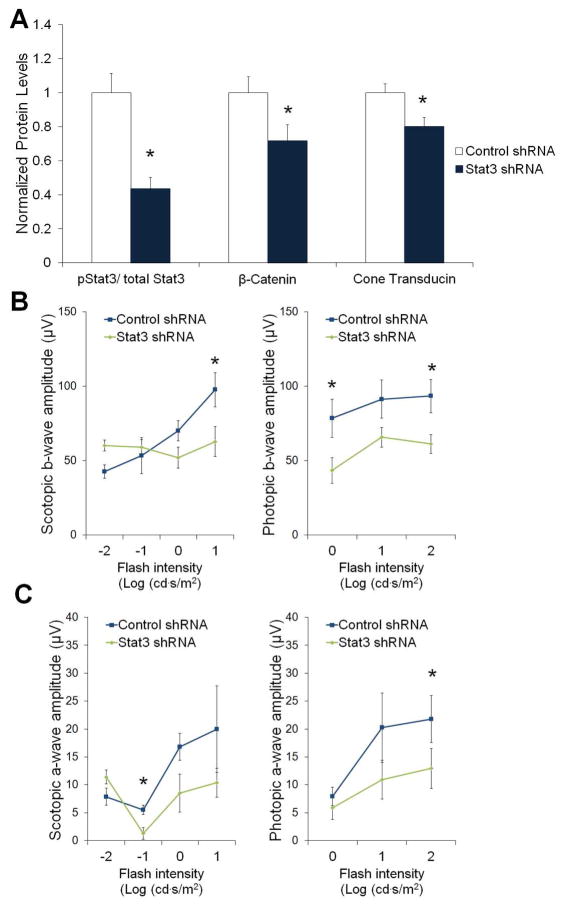
To test the involvement of Stat3, we knocked-down endogenous Stat3 in the retina of rd10 mice using Stat3-specific shRNA, delivered by lentiviral vector (Haghikia et al., 2011). The lenti-shRNA was co-injected with Wnt3a subretinally, and compared with scrambled control shRNA co-injected with Wnt3a. The Wnt3a molecule was used instead of GFAP-β-cateninS33A in adenovirus to avoid injecting two different viruses, and the lentivirus is used in order to target all the cell types that are responsive to Wnt3a. Control injections in wildtype mice indicated that there was no difference in photoreceptor layer thickness, visual acuity, and photoreceptor function between mice subretinally injected with the Stat3-specific shRNA compared with the control scrambled shRNA in lentivirus (Patel and Hackam, 2014), indicating that loss of Stat3 does not induce toxicity on its own. Efficiency of Stat3 knockdown was verified by Western blotting on whole retinas, which demonstrated that the ratio of activated (phosphorylated Stat3) to total Stat3 were reduced by 40% in eyes injected with lenti-Stat3 shRNA (Fig. 6A).
Fig. 6.
Wnt-induced photoreceptor protection is mediated by Stat3. (A) Lentivirus containing shRNA against Stat3, or a scrambled control shRNA, was used to knock-down Stat3 in the retina. Rd10 mice were subretinally injected with lenti-Stat3 shRNA+Wnt3a or lenti-control shRNA+Wnt3a. Western blotting indicated that Stat3 shRNA reduced levels of activated Stat3 (pStat3/tStat3) (n=6, *p<0.05). Furthermore, the levels of cone transducin (Gαt2) and β-catenin were lower in the retinas injected with lenti-Stat3 shRNA, indicating that Stat3 knockdown reduced the protection of photoreceptors by Wnt3a (*p<0.05). (B–C) ERG responses of the coinjected retinas were recorded. The Stat3 shRNA significantly reduced the protective effect of Wnt3a, compared with control shRNA, as indicated by lower a- and b-wave amplitudes at multiple light intensities (n=4, *p<0.05). Mean ± SE is shown.
As shown in Fig. 6B, coinjection of Wnt3a+Stat3 shRNA resulted in a decrease in photoreceptor b-wave amplitudes compared with Wnt3a+control shRNA injections, indicating that downregulation of Stat3 reduced the level of Wnt3a-induced protection. Similarly, the a-wave amplitudes were also lower in the Wnt3a+Stat3 shRNA injected mice compared with the Wnt3a+control shRNA. The ERG response in the Wnt3a+control shRNA was slightly lower than the Wnt3a alone, possibly due to a negative effect of the lentivirus, or from needing to inject a larger volume into the eye for these experiments. Furthermore, the Wnt3a-induced rescue of the photoreceptor markers cone transducin and rhodopsin were also reduced by coinjection of Stat3 shRNA (Fig. 6B). Therefore, Stat3 shRNA reversed the prosurvival effects of Wnt3a, suggesting that Stat3 is an important mediator of Wnt3a-dependent photoreceptor protection.
3.6 Wnt signaling activation reduced markers of retinal remodeling
We next investigated whether activation of Wnt signaling reduced markers of tissue remodeling, which can be used as an additional indicator of retinal protection in rd10 mice (Pang et al., 2011). Pathologic tissue remodeling occurs during photoreceptor death and includes aberrant neurite growth and gliosis and is believed to be a major obstacle for treating retinal degenerations. Retinal remodeling is typically assessed using markers of glial activation, such as elevated GFAP, and markers of bipolar cell neurite growth, such as PKCα (Lin et al., 2012). The GFP reporter in the β-cateninS33A and β-eng constructs allowed us to co-localize the Wnt regulators with GFAP. To determine the cellular phenotype of the virally transduced Muller glia, all GFP-positive cells were assessed for codetection of GFAP. Retinas injected with the Wnt activator β-cateninS33A had a significant reduction in GFAP-positive activated Muller glia, compared with the Wnt inhibitor β-eng (Fig. 7A). Also, Western blotting indicated that Wnt activation reduced PKCα levels by 2-fold, suggesting reduced neurite growth in the rescued retinas (Fig. 7B) (reduced PKCα may also be due to loss of bipolar cells, although bipolar cell death is not reported in rd10 or any retinal degeneration models). Therefore, activating Wnt signaling led to functional rescue of photoreceptors and reduced pathologic tissue changes.
Fig. 7.
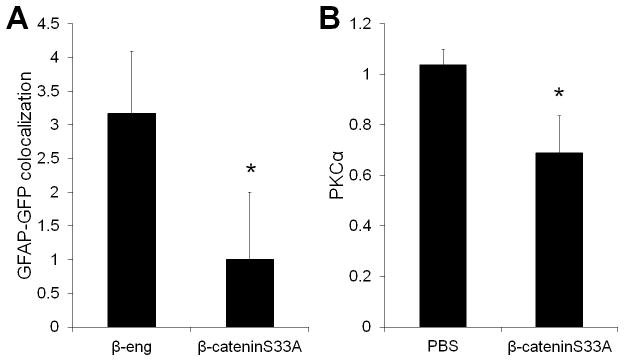
Wnt signaling reduced markers of tissue reorganization. (A) Cells injected with the Wnt regulators were detected by co-expressed GFP in retina sections. Gliotic changes in Muller glia was quantified by co-localization of GFP with the glial proliferation marker GFAP. The average number of cells per section that showed codetection of GFAP and GFP was calculated. β-cateninS33A injected retinas had fewer GFP-positive cells that co-localized with GFAP, indicating reduction of glial remodeling with Wnt activation (n=3, p<0.01). (B) Neuritic changes were quantified by Western blot detection of the bipolar neuron marker PKCα. The levels of PKCα were lower in retinas with Wnt activation (n=4, p<0.05).
4. Discussion
The results of this study demonstrate that activating the canonical Wnt signaling pathway, by using the pan-retinal Wnt3a ligand or the Muller glia-specific constitutively active β-catenin, induced neuroprotection in the retina and reduced tissue remodeling. This study is the first to examine the role of a natural Wnt activator, the Wnt ligand Wnt3a, on photoreceptor survival during inherited retinal degeneration. These data also add to the growing body of evidence on the role of Wnt signaling in neuroprotection in the CNS (Marchetti et al., 2013). For example, Wnt signaling protected retinal neurons in vivo and in vitro from acute injuries such as oxidative stress and laser damage (Braunger et al., 2013; Liu et al., 2013; Sanges et al., 2013), and induced functional improvement in a spinal cord injury model (Suh et al., 2011). Additionally, β-catenin overexpression, which activates Wnt signaling, reduced apoptosis in a chicken ethanol neurodegeneration model (Flentke et al., 2014), and blocking Wnt inhibitors in several different in vitro and in vivo models of neuronal injury significantly reduced apoptosis (Caricasole et al., 2004; Toledo et al., 2008). Downregulation of Wnt signaling, by pharmacologic inhibition or overexpression of the antagonists Axin2 or Dkk1, promoted neurodegeneration in cellular and animal models of Alzheimer’s disease (Toledo et al., 2008) and hippocampal neurons in vivo (Kim et al., 2011). Although exogenous activation of Wnt signaling in Muller glia led to increased photoreceptor survival, we observed that inhibiting Wnt signaling in Muller glia did not worsen degeneration, which suggests different relative importance of exogenous and endogenous Wnt signaling to neuroprotection in the retina. A potential explanation that could reconcile these findings is that the baseline increase in Wnt signaling that is observed in Muller glia during retinal degeneration is not sufficiently protective in the presence of the continuing damage caused by the retinal mutation. Therefore, reducing baseline Wnt signaling by β-eng would not worsen degeneration whereas increasing Wnt signaling further, by overexpression of β-cateninS33A, leads to protection. Wnt was shown in our previous studies to induce injury-specific protection in cultured cells (Fragoso et al., 2011), and thus follow-up experiments in vivo would test the relative importance of endogenous and exogenous Wnt signaling in protection from different types of retinal injuries, such as acute toxicity.
4.1 Cellular mechanisms of action
We demonstrated the novel finding that Wnt-dependent photoreceptor protection was mediated through Muller glia, and did not depend on Wnt signaling activation directly in the photoreceptors themselves. Although GFAP is also expressed in astrocytes, the use of the long 2.2 kb form of the GFAP promoter (Su et al., 2004; de Leeuw et al., 2006) restricted expression to Muller glia, and GFP-positive cells were not observed within the nerve fiber layer where astrocytes reside. The current study is consistent with our previous work that demonstrated that endogenous Wnt signaling is activated in Muller glia during retinal degeneration, and that Muller glia mediated the protective effect of Wnt3a on cocultured photoreceptors exposed to oxidative stress (Yi et al., 2007). Muller glia were also proposed to mediate neuroprotection of RGCs by the atypical Wnt pathway activator Noggin in vivo (Seitz et al., 2010). These findings are also similar to Muller glia being the cellular mediators of growth factor protection of photoreceptors by BDNF and other neurotrophins (Harada et al., 2000).
Although it is not possible to directly compare the level of neuroprotection from the virally delivered β-cateninS33A in Muller glia with that of the recombinant Wnt3a ligand, it would appear their level of rescue of ERG responses was approximately equivalent, suggesting that Muller glia are primary contributors to Wnt-induced neuroprotection. Future studies would directly compare the effects of pan-retinal and Muller glia-specific Wnt signaling using lentiviral vectors, or by blocking Wnt signaling in Muller glia at the same time as delivering the Wnt ligand.
A central role for Muller glia in Wnt-induced neuroprotection is supported by accumulating evidence that demonstrates the importance of Wnt signaling in glia elsewhere in the CNS. For example, glial-derived Wnt ligand activators are a major component of the microenvironment that regulates stem cell biology and neuronal development (Moon et al., 2004; Schmidt and Patel, 2005), and astrocyte-derived Wnt3 controls proliferation and neuronal differentiation during hippocampal neurogenesis (Lie et al., 2005). Additionally, astrocyte-derived Wnt1 contributes to neuroprotection in the MPTP-induced dopaminergic neurotoxicity model of Parkinson’s disease (L’Episcopo et al., 2011). Analogous to our observations in retinal Muller glia (Yi et al., 2007), elevated endogenous Wnt3a expression and nuclear β-catenin were detected in glia in the spinal cord of SOD1G93A ALS transgenic mice (Chen et al., 2012), which may serve to protect neurons from further damage. Therefore, a protective role for glial Wnt may be common throughout the CNS.
The β-cateninS33A expression data above, as well as previous findings indicating that Wnt3a induces Wnt signaling in Muller glia (Yi et al., 2007), suggests that Wnt3a-STAT3 signaling is present in Muller glia. In the Wnt + Stat3 shRNA experiments, multiple cell types are likely infected by the lenti-shRNA constructs that are not involved in photoreceptor survival, such as RGCs, but because they would also be infected in the control shRNA experiment they are less likely to contribute to the reversal of the pro-survival effects of Wnt3a. We did not directly test the involvement of the other Wnt-responsive cell types, and it remains possible that other cells may be involved in Wnt3a-mediated neuroprotection. Other cell types that may mediate protective Wnt signaling in the retina include RPE cells and the photoreceptors themselves. The RPE have low levels of Wnt activity in post-developed retinas (Westenskow et al., 2009) but can respond to Wnt ligands in culture (Fragoso et al., 2012; Hu et al., 2013). Although previous studies using transgenic Wnt reporter mouse lines indicated Wnt signaling activation was not evident in photoreceptors (Liu et al., 2003; Yi et al., 2007), photoreceptors do express Wnt3a (Fig 1) and other Wnt ligands (Hunter et al., 2004), suggesting that Wnt ligands may act directly on photoreceptors in an autocrine manner. Wnt3a was also detected throughout the adult retina, including the photoreceptor layer, using in situ hybridization (Blackshaw et al., 2001).
4.2 Molecular mechanisms of action
Although much has been discovered about the molecular mechanisms by which Wnt signaling regulates development of the embryonic retina (Aldiri et al., 2013), little has been reported on how Wnt regulates neuronal survival. Our study indicates that the survival protein Stat3 is a potential mediator of the protective effect of Wnt signaling in the retina. Wnt signaling activated Stat3 and knock-down of Stat3 reduced photoreceptor protection. These findings are the first identification of Wnt-Stat3 regulation in the retina and implicate Wnt as a previously unknown upstream activator of Stat3 signaling during photoreceptor survival. Stat3 is activated in multiple cell types in the retina (Peterson et al., 2000), making it difficult to identify the cell type that upregulates Stat3 in response to Wnt, although most evidence supports a role for Muller glia in both signaling pathways. It is possible that reduced Stat3 blocked Wnt3a-induced effects in Muller glia, or may block photoreceptors from responding to Muller glia. Wnt-Stat3 cross-talk was also observed in cancer cell lines where both pathways have activating mutations (Kawada et al., 2006; Yan et al., 2008; Armanious et al., 2010), and in embryonic stem cells (Hao et al., 2006), further supporting a functional interaction between these prosurvival pathways. Other candidate molecules for mediating Wnt-dependent protection include BDNF, which is induced in Muller glia by Wnt activation (Seitz et al., 2010; Yi et al., 2012), CNTF (Yi et al., 2007; Seitz et al., 2010; Fragoso et al., 2011), acting upstream of Stat3, and IGF-1/Akt (Toledo et al., 2008; Kim et al., 2011).
4.3 Other effects of Wnt in the retina
Additional positive effects of Wnt in the retina were demonstrated in recent studies. For example, Wnt activation induces neural regeneration and stem cell proliferation (Osakada et al., 2007; Del Debbio et al., 2010), and regulates Muller glia dedifferentiation and proliferation after acute injury (Wan et al., 2012; Liu et al., 2013; Sanges et al., 2013). Because these additional activities of Wnt are relatively long term outcomes of Wnt pathway activation, they are unlikely to contribute to the neuroprotective effect that we demonstrated in our analysis using a relatively short duration of Wnt activation. The neuroprotection in our study raises an important translational question of whether long term activation of the Wnt pathway could be utilized as a therapy for retinal degeneration diseases. Activating the Wnt pathway has been proposed to as a therapy for neurodegenerations (Hackam, 2005; Osakada and Takahashi, 2009; Marchetti et al., 2013) and one would predict that continued neuroprotection is the outcome of sustained Wnt signaling. However, excessive Wnt activation in certain cell types has deleterious effects, including tumorigenesis and angiogenesis, the extent of which varies with the method of Wnt activation (Zhang et al., 2010; Cui et al., 2013). In contrast to the present study, previous studies that showed deleterious effects from Wnt activation used substantially different models, including mice that were genetically manipulated to be oversensitive to Wnt signaling by knocking-out inhibitor genes, or used acute injury models (laser light, oxidative stress), or a non-cell type-specific Wnt activator (Zhou et al., 2010; Wan et al., 2012; Liu et al., 2013; Sanges et al., 2013). Also, low numbers of cells were shown to have off-target effects, for example, proliferation and expression of neural stem/progenitor genes occurred in less than 1% of Muller glia (Das et al., 2006; Osakada et al., 2007; Del Debbio et al., 2010; Ramachandran et al., 2011), and Wnt does not regulate proliferation in late stages of retinal development (Sanchez-Sanchez et al., 2010). Therefore, further development of Wnt as a therapy would require careful characterization of the extent of off-target effects by addressing whether negative effects, such as tumorigenesis and angiogenesis, are lower in retinas with cell-specific Wnt activation compared with pan-retinal activation.
Any future treatment for retinal diseases based on Wnt signaling would be provided after retinal degeneration has started. For the purposes of testing efficacy and to determine whether Wnt signaling has therapeutic potential for retinal degeneration, we activated Wnt signaling prior to disease onset in the mouse, which is not as relevant in the clinical setting. It is possible that Wnt may have different or the same efficacy if injected after degeneration has started, and therefore follow-up studies using the doses that were protective here as a starting point would need to be performed at different stages of disease. The retina rescue observed here, combined with previous work, suggests a possible sentinel function for Wnt: injury to photoreceptors from mutation or oxidative stress stimulates Muller glia, and possibly other cell types, which leads to secretion of Wnt activators, such as Wnt ligands previously identified in degenerating retinas (Yi et al., 2007), and results in Wnt signaling activation. Wnt signaling may have direct effects on photoreceptor viability, and/or indirect effects by increasing levels of pro-survival molecules in Muller glia. In the presence of significant or continuing damage, the Wnt pathway is stimulated but may not be sufficient to prevent degeneration. However, over-expression of Wnt activators would increase Wnt signaling, and elevate the threshold for apoptosis and lead to reduced photoreceptor death. Therefore, manipulating Wnt signaling, by itself or in combination with other survival factors, could halt or delay neuronal degeneration in the retina. Delaying blindness by even a few years would have a meaningful impact on quality of life. Furthermore, Wnt-induced rescue would be applicable to all photoreceptor degenerations, regardless of the primary cause or genetic mutation.
5. Conclusion
The findings presented in this study indicate that Muller glial Wnt signaling triggers neuroprotective signaling through the Stat3 pathway in a genetic mouse model of neuronal degeneration, and identify Wnt as a novel therapeutic target for diseases of the central nervous system.
Highlights.
The study tested the role of Wnt/β-catenin signaling during retinal degeneration.
Wnt signaling protected photoreceptors in the rd10 mouse model.
Wnt-induced photoreceptor protection was mediated by Stat3.
Radial Muller glia are cellular mediators of protective Wnt signaling.
Acknowledgments
This study was supported by NEI RO1 EY017837, NEI 3R01EY017837-03S1, the Karl Kirchgessner Foundation, a NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant. We thank Hany Azcuy, Patricia Garcia and BaoXiang Li for technical support. We are grateful to Dr. Denise Hilfiker-Kleiner and Michaela Scherr from Hannover Medical School for the shRNA constructs, and Dr. M. Brenner (University of Alabama at Birmingham) for the GFAP promoter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, Leitao CN, Fodde R, Smits R. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–1560. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- Aldiri I, Moore KB, Hutcheson DA, Zhang J, Vetter ML. Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/beta-catenin signaling. Development (Cambridge, England) 2013;140:2867–2878. doi: 10.1242/dev.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanious H, Gelebart P, Mackey J, Ma Y, Lai R. STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. International journal of clinical and experimental pathology. 2010;3:654–664. [PMC free article] [PubMed] [Google Scholar]
- Barhoum R, Martinez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, de la Rosa EJ, de la Villa P, Cuenca N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008;155:698–713. doi: 10.1016/j.neuroscience.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Fraioli RE, Furukawa T, Cepko CL. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiology of disease. 2013;50:1–12. doi: 10.1016/j.nbd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Burgi S, Samardzija M, Grimm C. Endogenous leukemia inhibitory factor protects photoreceptor cells against light-induced degeneration. Mol Vis. 2009;15:1631–1637. [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H, Wang X. Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;420:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Cui L, Guan Y, Qu Z, Zhang J, Liao B, Ma B, Qian J, Li D, Li W, Xu GT, Jin Y. WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. The Journal of clinical investigation. 2013;123:1647–1661. doi: 10.1172/JCI65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Developmental biology. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- de Leeuw B, Su M, ter Horst M, Iwata S, Rodijk M, Hoeben RC, Messing A, Smitt PS, Brenner M. Increased glia-specific transgene expression with glial fibrillary acidic protein promoters containing multiple enhancer elements. J Neurosci Res. 2006;83:744–753. doi: 10.1002/jnr.20776. [DOI] [PubMed] [Google Scholar]
- Del Debbio CB, Balasubramanian S, Parameswaran S, Chaudhuri A, Qiu F, Ahmad I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. PloS one. 2010;5:e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Hernandez M, Smith SM. CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J Neurochem. 2014;128:523–535. doi: 10.1111/jnc.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso MA, Yi H, Nakamura RE, Hackam AS. The Wnt signaling pathway protects retinal ganglion cell 5 (RGC-5) cells from elevated pressure. Cellular and molecular neurobiology. 2011;31:163–173. doi: 10.1007/s10571-010-9603-z. [DOI] [PubMed] [Google Scholar]
- Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS. The Wnt/beta-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One. 2012;7:e46892. doi: 10.1371/journal.pone.0046892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt Signaling in Eye Organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. The Journal of comparative neurology. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam AS. The Wnt signaling pathway in retinal degenerations. IUBMB life. 2005;57:381–388. doi: 10.1080/15216540500137586. [DOI] [PubMed] [Google Scholar]
- Haghikia A, Missol-Kolka E, Tsikas D, Venturini L, Brundiers S, Castoldi M, Muckenthaler MU, Eder M, Stapel B, Thum T, Haghikia A, Petrasch-Parwez E, Drexler H, Hilfiker-Kleiner D, Scherr M. Signal transducer and activator of transcription 3-mediated regulation of miR-199a-5p links cardiomyocyte and endothelial cell function in the heart: a key role for ubiquitin-conjugating enzymes. European heart journal. 2011;32:1287–1297. doi: 10.1093/eurheartj/ehq369. [DOI] [PubMed] [Google Scholar]
- Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Developmental biology. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma JX. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Investigative ophthalmology & visual science. 2013;54:141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nature reviews Neuroscience. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Joly S, Lange C, Thiersch M, Samardzija M, Grimm C. Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J Neurosci. 2008;28:13765–13774. doi: 10.1523/JNEUROSCI.5114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, Shimahara Y, Chiba T. Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer research. 2006;66:2913–2917. doi: 10.1158/0008-5472.CAN-05-3460. [DOI] [PubMed] [Google Scholar]
- Kim H, Won S, Hwang DY, Lee JS, Kim M, Kim R, Kim W, Cha B, Kim T, Kim D, Costantini F, Jho EH. Downregulation of Wnt/beta-catenin signaling causes degeneration of hippocampal neurons in vivo. Neurobiology of aging. 2011;32:2316 e2311–2315. doi: 10.1016/j.neurobiolaging.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Koso H, Ouchi Y, Tabata Y, Aoki Y, Satoh S, Arai K, Watanabe S. SSEA-1 marks regionally restricted immature subpopulations of embryonic retinal progenitor cells that are regulated by the Wnt signaling pathway. Developmental biology. 2006;292:265–276. doi: 10.1016/j.ydbio.2005.09.051. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D’Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiology of disease. 2011;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, Lavail MM, Laties AM, Wen R. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PloS one. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lin S, Cheng M, Dailey W, Drenser K, Chintala S. Norrin attenuates protease-mediated death of transformed retinal ganglion cells. Mol Vis. 2009;15:26–37. [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jones BW, Liu A, Tucker JF, Rapp K, Luo L, Baehr W, Bernstein PS, Watt CB, Yang JH, Shaw MV, Marc RE. Retinoid receptors trigger neuritogenesis in retinal degenerations. Faseb J. 2012;26:81–92. doi: 10.1096/fj.11-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA. Wnt signaling promotes muller cell proliferation and survival after injury. Investigative ophthalmology & visual science. 2013;54:444–453. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Marchetti B, L’Episcopo F, Morale MC, Tirolo C, Testa N, Caniglia S, Serapide MF, Pluchino S. Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson’s disease: the Wnt/beta-catenin signaling cascade as the common final pathway for neuroprotection and self-repair. The European journal of neuroscience. 2013;37:1550–1563. doi: 10.1111/ejn.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development. 2005;132:1539–1553. doi: 10.1242/dev.01714. [DOI] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami M, Souchelnytskyi N, Kiuchi Y, Kanamoto T. Wnt14 inhibits death of retinal precursor cells. Exp Eye Res. 2009;89:462–468. doi: 10.1016/j.exer.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Montross WT, Ji H, McCrea PD. A beta-catenin/engrailed chimera selectively suppresses Wnt signaling. J Cell Sci. 2000;113(Pt 10):1759–1770. doi: 10.1242/jcs.113.10.1759. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth factors (Chur, Switzerland) 2010;28:232–242. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Takahashi M. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: targeting the Wnt pathway and transplantation therapy as strategies for retinal repair. J Pharmacol Sci. 2009;109:168–173. doi: 10.1254/jphs.08r19fm. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Tabata Y, Arai K, Watanabe S. Negative regulation of retinal-neurite extension by beta-catenin signaling pathway. Journal of cell science. 2005;118:4473–4483. doi: 10.1242/jcs.02575. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S, Everhart D, Dinculescu A, Liu L, Umino Y, Lei B, Chang B, Barlow R, Strettoi E, Hauswirth WW. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Hackam AS. A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Molecular and cellular neurosciences. 2014;63C:38–48. doi: 10.1016/j.mcn.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D, Yang XJ. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Muller glial cells. Proceedings of the National Academy of Sciences of the United States of America. 110:E4520–4529. doi: 10.1073/pnas.1303604110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Ruiz A, Duncan JL, Hauswirth WW, Lavail MM, Bok D, Yang XJ. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007;48:1389–1400. doi: 10.1167/iovs.06-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature neuroscience. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Sanchez-Sanchez AV, Camp E, Leal-Tassias A, Mullor JL. Wnt signaling has different temporal roles during retinal development. Dev Dyn. 2010;239:297–310. doi: 10.1002/dvdy.22168. [DOI] [PubMed] [Google Scholar]
- Sanges D, Romo N, Simonte G, Di Vicino U, Tahoces AD, Fernandez E, Cosma MP. Wnt/beta-catenin signaling triggers neuron reprogramming and regeneration in the mouse retina. Cell reports. 2013;4:271–286. doi: 10.1016/j.celrep.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Patel K. Wnts and the neural crest. Anat Embryol (Berl) 2005;209:349–355. doi: 10.1007/s00429-005-0459-9. [DOI] [PubMed] [Google Scholar]
- Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Expression specificity of GFAP transgenes. Neurochem Res. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta neurochirurgica. 2011;153:1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- Tell S, Yi H, Jockovich ME, Murray TG, Hackam AS. The Wnt signaling pathway has tumor suppressor properties in retinoblastoma. Biochemical and biophysical research communications. 2006;349:261–269. doi: 10.1016/j.bbrc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–796. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Developmental cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenskow P, Piccolo S, Fuhrmann S. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development (Cambridge, England) 2009;136:2505–2510. doi: 10.1242/dev.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zhou C, Zhang W, Zhang G, Zhao X, Yang S, Wang Y, Lu N, Zhu H, Xu N. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer letters. 2008;271:85–97. doi: 10.1016/j.canlet.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Yi H, Hu J, Qian J, Hackam AS. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport. 2012;23:189–194. doi: 10.1097/WNR.0b013e32834fab06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Nakamura RE, Mohamed O, Dufort D, Hackam AS. Characterization of Wnt signaling during photoreceptor degeneration. Investigative ophthalmology & visual science. 2007;48:5733–5741. doi: 10.1167/iovs.07-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, He X, Ma JX. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6900–6905. doi: 10.1073/pnas.0906764107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Investigative ophthalmology & visual science. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]