Abstract
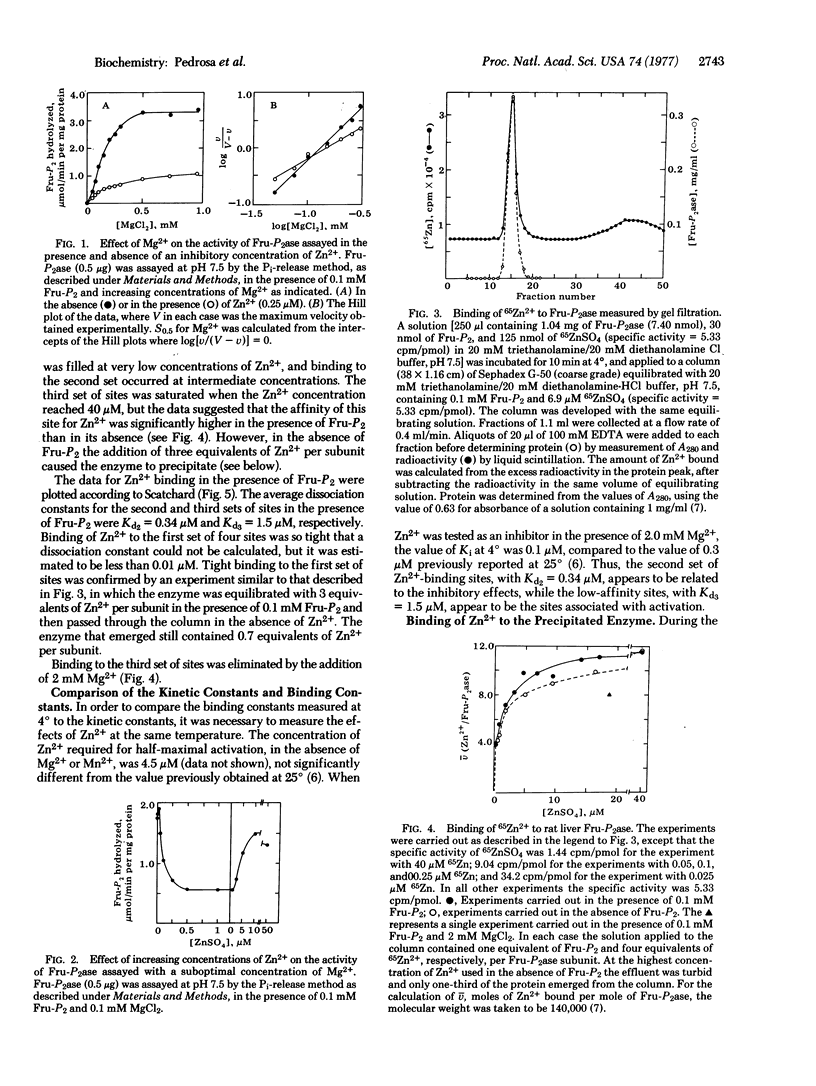
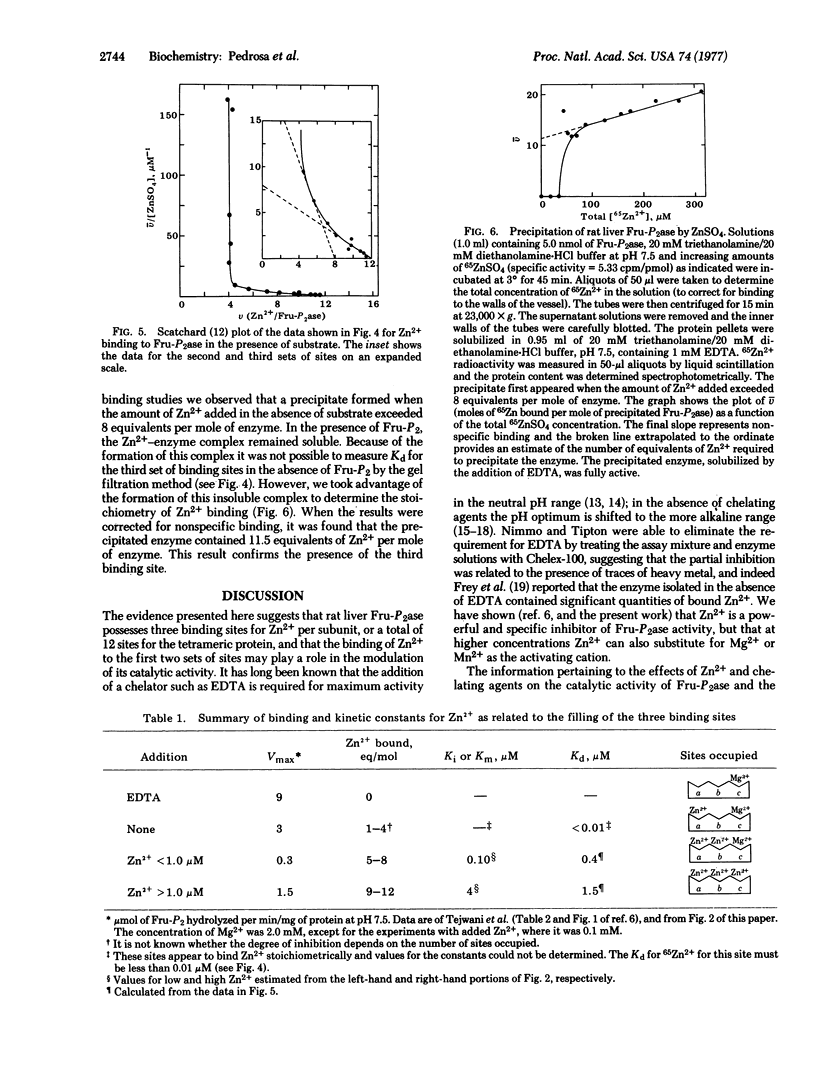
Rat liver fructose-1,6-bisphosphatase (D-fructose-1,6-bisphosphate 1-phosphohydrolase, EC 3.1.3.11) contains 12 binding sites for Zn2+ per molecule, or 3 per subunit, as determined by gel filtration and by precipitation of an insoluble Zn2+-enzyme complex. The first set of sites binds Zn2+ with very high affinity, and the binding constant for these sites could not be determined. The average values of the dissociation constants for the second and third sets of sites were approximately 0.4 and 1.5 muM, respectively. The third set of sites, having lowest affinity, appears to be identical to the binding sites for the activating cation, Mg2+, and the binding of Zn2+ to this set of sites is prevented by the addition of Mg2+. Binding of the first 4 equivalents of Zn2+ yields an enzyme of intermediate activity, while the binding of 8 equivalent results in almost complete inhibition of catalytic activity. Thus Zn2+ appears to function as both an activator and a negative allosteric regulator of fructose-1,6-bisphosphatase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Datta A. G., Abrams B., Sasaki T., van den Berg J. W., Pontremoli S., Horecker B. L. The activation of rabbit muscle, liver, and kidney fructose bisphosphatases by histidine and citrate. Arch Biochem Biophys. 1974 Dec;165(2):641–645. doi: 10.1016/0003-9861(74)90292-6. [DOI] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., WOODFORD M. FRUCTOSE 1, 6-DIPHOSPHATASE IN STRIATED MUSCLE. Biochem J. 1965 Feb;94:436–445. doi: 10.1042/bj0940436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The purification of fructose 1,6-diphosphatase from ox liver and its activation by ethylenediaminetetra-acetate. Biochem J. 1975 Feb;145(2):323–334. doi: 10.1042/bj1450323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Grazi E., Accorsi A. Fructose 1,6-diphosphatase from rabbit liver. 13. The number of Mn++ binding sites measured with 54Mn++. Biochem Biophys Res Commun. 1969 Nov 6;37(4):597–602. doi: 10.1016/0006-291x(69)90851-1. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., De Flora A., Horecker B. L. Regulation of fructose, 1,6-bisphosphatase by histidine under gluconeogenic conditions. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2166–2168. doi: 10.1073/pnas.71.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E., SCHROEDER E. A. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Arch Biochem Biophys. 1958 Apr;74(2):326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., Tashima Y., Horecker B. L., Pontremoli S. Activation of rabbit kidney fructose diphosphatase by Mg-EDTA, Mn-EDTA and Co-EDTA complexes. Arch Biochem Biophys. 1973 Jan;154(1):283–291. doi: 10.1016/0003-9861(73)90059-3. [DOI] [PubMed] [Google Scholar]
- THIERS R. E., VALLEE B. L. Distribution of metals in subcellular fractions of rat liver. J Biol Chem. 1957 Jun;226(2):911–920. [PubMed] [Google Scholar]
- Tashima Y., Yoshimura N. Control of rabbit liver fructose-1, 6-diphosphatase activity by magnesium ions. J Biochem. 1975 Dec;78(6):1161–1169. doi: 10.1093/oxfordjournals.jbchem.a131012. [DOI] [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. Dual role of Zn2+ as inhibitor and activator of fructose 1,6-bisphosphatase of rat liver. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2692–2695. doi: 10.1073/pnas.73.8.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. The purification of properties of rat liver fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1976 Nov;177(1):253–264. doi: 10.1016/0003-9861(76)90435-5. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol A., Black W. J., Horecker B. L. Activation of rabbit muscle fructose diphosphatase by EDTA and the effect of divalent cations. Arch Biochem Biophys. 1972 Aug;151(2):591–596. doi: 10.1016/0003-9861(72)90536-x. [DOI] [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]


