Abstract
Background
Whether higher B vitamin (B6, B12, and folate) intake is protective against cognitive decline in later life remains uncertain. Several prospective, observational studies find higher B vitamin intake to be associated with lower risk of dementia; other studies, including most trials of B vitamin supplementation, observe no effect on cognition. We examine this question in a large population of older women carefully monitored for development of mild cognitive impairment (MCI) and probable dementia.
Objective
To determine whether baseline folate, vitamin B6 and/or B12 intake, alone or in combination, are associated with incident MCI/probable dementia among older women.
Design
Prospective, longitudinal cohort study. B vitamin intake was self-reported using a food frequency questionnaire administered at baseline between May 1996 and December 1999.
Participants/Setting
Postmenopausal women (n=7,030) free of MCI/probable dementia at baseline in the Women’s Health Initiative Memory Study.
Main outcome measures
Over a mean follow-up of 5.0 years, 238 cases of incident MCI and 69 cases of probable dementia were identified through rigorous screening and expert adjudication.
Statistical analyses
Cox proportional hazard models adjusting for sociodemographic and lifestyle factors examined the association of B vitamin intake above and below the recommended daily allowance and incident MCI/probable dementia.
Results
Folate intake below the RDA at study baseline was associated with increased risk of incident MCI/probable dementia (HR=2.0, 95% CI: 1.3, 2.9), after controlling for multiple confounders. There were no significant associations between vitamins B6 or B12 and MCI/probable dementia, nor any evidence of an interaction between these vitamins and folate intake.
Conclusions
Folate intake below the RDA may increase risk for MCI/probable dementia in later life. Future research should include long-term trials of folic acid supplementation to examine whether folate may impart a protective effect on cognition in later life.
Keywords: Folic acid, vitamin B12, vitamin B6, dementia, cognitive impairment, women’s health, aging
INTRODUCTION
Dementia and cognitive impairment are common in older populations, with an estimated prevalence of dementia ranging from 14% in those older than age 70, to 37% among those 90 years of age and older.1 It has also been estimated that the number of persons living with dementia worldwide will double every twenty years, resulting in a projected increase from 42.3 million individuals in 2020, to 81.1 million in 2040.2 Given the high prevalence and the loss of functioning associated with dementia and cognitive impairment, it is important to identify potential modifiable risk factors. Diet has been suggested as one area of potential intervention,3,4 and low intake of B vitamins may be a potential risk factor for cognitive impairment.5
Vitamin B12 deficiency has been associated with negative neurological outcomes; in particular, pernicious anemia, resulting from an inability to absorb B12 due to destruction of gastric cells, is associated with neurologic and psychiatric symptoms including cognitive impairment.6,7 Additionally, vitamin B12, along with folate and vitamin B6, are key actors in the homocysteine cycle. Disruption of the homocysteine cycle can lead to elevated homocysteine, which has been found among individuals with dementia8 and been shown to predict dementia prospectively.9
Despite potential biological mechanisms through which B vitamins could play a role in the onset of dementia, results from observational and intervention studies have been inconsistent. Several cross-sectional and longitudinal studies have suggested an association between lower levels of folate, B6 and B12 and cognitive impairment,5,10–12 while others have found no effect on cognition,13 or even an increased risk of cognitive decline associated with higher levels of folate intake.14 Several15–17 but not all18,19 intervention studies examining supplementation of these B vitamins and cognition have found null effects. With few exceptions,20 studies that seek to slow the progress of cognitive decline with supplemental B6, B12 or folic acid have not shown promising results.21,22 Several comprehensive reviews and meta-analyses discussing the impact of B vitamins on cognition have been published and generally point to null or inconclusive results.4,22–28
In the current study, the association of folate, vitamin B6 and vitamin B12 intake with incident MCI/probable dementia is examined among 7,030 post-menopausal women. The Women’s Health Initiative Memory Study (WHIMS) offers several advantages in examining the relation of B vitamin intake and cognitive impairment. The sample size in this study is larger than many of the previous studies examining this association. Additionally, mild cognitive impairment (MCI) and probable dementia were identified using rigorous, expert adjudicated diagnoses. The effect of B vitamins from dietary and supplemental sources was examined separately, and potential interactions between folate and vitamins B6 and B12, as well as with alcohol intake, were assessed.
METHODS
Study population
Postmenopausal women (n=27,347) ages 50 through 79 years were enrolled in the Women’s Health Initiative Hormone Trial (WHI-HT), a randomized, placebo-controlled clinical trial of hormone therapy (conjugated equine estrogen with and without medroxyprogesterone acetate).29,30 A subset of 7,479 women also participated in the Women’s Health Initiative Memory Study (WHIMS), an ancillary study designed to assess the effect of hormone therapy on dementia risk. WHIMS participants were ages 65–79 and free from probable dementia at baseline.31 Recruitment was initiated between May 1996 and December 1999 (henceforth referred to as “baseline”) and participants were followed through July 8, 2002 (for the estrogen plus progestin trial) and through Feb 29 2004 (for the estrogen only trial).32,33 Of the WHIMS participants, 7,201 provided dietary intake data, and of those 7,030 had complete information on important covariates and constitute the cohort for the current study. In addition to participating in the hormone therapy trials, some women in WHIMS were also enrolled in additional WHI trials examining health outcomes related to dietary modification, and calcium and vitamin D supplementation. All analyses were adjusted for randomization assignment in these trials. The National Institutes of Health and the institutional review boards for the WHI Clinical Coordinating Center and each WHI clinical center approved the study protocols, and all participants provided written informed consent.
Folate, vitamin B6 and B12 intake
Folate, vitamin B6 and vitamin B12 intake was assessed at baseline using a 122-item, self-administered food frequency questionnaire (FFQ). A validation study within a subpopulation of the cohort found that the Pearson correlation coefficient comparing dietary intake as assessed by 24-hour recall and 4-day food records with that measured by the FFQ was 0.59 for folate, 0.28 for vitamin B12 and 0.43 for vitamin B6.34 At baseline, 39.6% of women (N=2,783) were taking a multivitamin, and it was the most important source of total folate intake. Several common dietary sources of folate that account for the most variability in dietary folate intake found in other US study populations (NHANES and the Nurses’ Health Study) include cereal, orange juice, cooked beans, and green salad.35,36
Most of the women in WHIMS (69.9%) had baseline folate intake assessed prior to mandatory folic acid fortification of grain products in the US;37 however, for women whose baseline FFQs were returned post-fortification, folate intake was adjusted to account for the changes in folic acid content in foods and the different bioavailability of natural folate versus synthetic folic acid from fortification. Supplemental folic acid, vitamin B6 and vitamin B12 intake was assessed from pills brought into the WHI study sites by participants and was computed from combining intake from supplements, supplement mixtures (for example B-complex mixtures) and multivitamins.34 Total folate intake included intake from diet and supplemental sources. In statistical analyses, total and dietary folate, vitamin B6 and B12 intake were adjusted for overall caloric intake using the residuals method.38
Diagnosis of probable dementia and mild cognitive impairment
The WHIMS protocol for identifying cases of MCI and probable dementia has been described in-depth previously.32,33,39 In Phase 1 participants who scored below the Modified Mini-Mental State Examination (3MSE)27 cut-point received an in-depth multiphased evaluation including a battery of neuropsychological tests, history and physical, neuropsychiatric evaluation, and rigorous adjudication of MCI and dementia.39 Participants were first administered the 3MSE at baseline and then at yearly intervals. The 3MSE ranges from 0–100, and initial cut-points for further testing were 72 or lower for participants with less than 9 years of education, and 76 or lower for participants with 9 or more years; this cut-point was later raised to increase sensitivity to a score of 80 or lower for participants with less than 9 years of education, and 88 or lower for the participants with 9 or more years.32 In Phase 2, participants who scored below the 3MSE cut-point received the Modified Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery,40 the Primary Care Evaluation of Mental Disorders (PRIME-MD),41 and the Geriatric Depression Scale.42 The study participant, as well as a designated informant, answered questions related to the participant’s acquired cognitive and behavioral changes and functional abilities.32 In Phase 3, participants were assessed by a WHIMS clinic–based physician, experienced in diagnosis of dementia, who reviewed all materials from Phases 1 and 2 and conducted a face-to-face semi-structured neurologic evaluation of the participants neuropsychiatric status and history of vascular disease. The physician then made a determination regarding presence of no dementia, MCI, or probable dementia based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. The classification of MCI was based on accepted criteria at WHIMS baseline and defined as: poor performance (10th or lower percentile based on CERAD norms) on at least one CERAD test, evidence of functional impairment (but not severe enough to interfere with activities of daily living), and the absence of a diagnosis of another psychiatric or medical disorder (including probable dementia) that could explain the cognitive impairment.32,43 Participants considered for a diagnosis of MCI or probable dementia received blood tests and a noncontrast computed tomography brain scan to exclude reversible causes of cognitive decline. Final adjudication and diagnosis of dementia and MCI were conducted by expert raters at the WHIMS Clinical Coordinating Center.25
Participants classified as having MCI were considered cases of the combined endpoint of MCI/probable dementia at the first occurrence of MCI, but continued to be tested annually to determine whether they progressed to probable dementia. The main outcome in this study was a combined outcome of MCI or probable dementia (MCI/probable dementia), therefore participants with MCI who went on to develop probable dementia were considered “cases” (of MCI/probable dementia) at the time of their MCI diagnosis. Potential confounders were identified based on a priori subject knowledge as well as by examining bivariate relationships between possible confounding variables and B vitamin intake levels at baseline. Covariates that were significantly related to B vitamin intake and MCI/probable dementia risk (e.g., education), and covariates of particular importance for these outcomes in this population (e.g. hormone therapy32,33) were included in multivariable models. All models were adjusted for randomization assignment in the WHI study trials (hormone therapy, calcium and vitamin D supplementation and dietary modification). Age was included as a confounder by one-year intervals, education as less than high school, some college, and college graduate or above; income as <$19,000, $20,000–$34,999, $35,000–$49,999, $50,000–$74,999 and $75,000 and above; and race was dichotomized as white vs non-white. Smoking status was categorized as never, former, current, or missing; alcohol intake as less than 1 drink per week, 1–6 drinks per week, and 7 or more drinks per week; and frequency of recreational physical activity as no activity; some activity of limited duration, frequency or intensity; moderate or strenuous activity of at least 20 minutes duration and 2–4 times per week; moderate or strenuous activity of at least 20 minutes duration, 4 or more times per week). Self-rated general health status at baseline was rated from “excellent” to “poor” and past use of hormone therapy as ever vs never use. Body mass index (BMI) was estimated using the participant’s weight (kg) divided by height squared (m2).
Statistical analysis
Folate intake, vitamin B6 and vitamin B12 intake at baseline were categorized as falling below vs. meeting or exceeding the recommend dietary allowance (RDA) as defined by the Institute of Medicine. For folate, the RDA is 400 ug/day, for vitamin B6 it is 1.5 mg/day (among women older than 50 years), and for vitamin B12 it is 2.4 ug/day.44 Nutrients were also divided into quartiles based on the distribution of intake levels in the study population.
Cox proportional hazard models were used to estimate hazard ratios (HRs) and associated 95% confidence intervals (CIs) in relation to MCI/probable dementia, and non-cases were censored at the time of the last administration of the 3MSE. The endpoint of MCI/probable dementia is presented as a combined endpoint in primary analyses, but these endpoints were also examined separately. All models were stratified by randomization assignment in WHI trials and age, as well as, in multivariable models, race (as race was not found to meet the proportional hazards assumption). Tests of linear trend for quartiles of intake for B vitamins were conducted in full multivariable models.
Because alcohol can interfere with the absorption of folate,45 we tested for an interaction between the higher alcohol intake (>6 drinks per week) and folate intake. Additionally, as there is evidence that high folate may accelerate problems with cognition in the presence of B12 deficiency,6 we compared risk among those in the lowest B12 quartile and the highest folate quartile to individuals in the highest quartile for both vitamins. We also examined the interaction between randomized assignment to hormone therapy and vitamin B intake. Finally, in sensitivity analyses we examined whether results differed by whether folate intake was assessed prior to or after mandatory folate fortification by including a covariate in the multivariable model for the interaction of folate below the RDA and with FFQ administration before or after folate fortification. All statistical analyses were performed with SAS software.46
Subjects with missing information on several confounders, including education, alcohol intake, exercise, BMI, and general health status were excluded from analyses (N=171). Individuals excluded from analyses due to missing responses on these covariates did not differ from included individuals on age (70.0 years vs 70.1 years p=0.72) or smoking status (8.0% vs 6.8% current smokers, p=0.76), but were slightly more likely to be nonwhite (18.7% vs 12.8%, p=0.02). For individuals with missing information on smoking status (N=103), a missing smoking status indicator was created. Individuals with missing information on income (N=433) were assigned the mean income category for their education level. Analyses were also performed excluding individuals missing responses on income and smoking and results did not differ.
RESULTS
Nearly half (46.7%) of the participants (N=3,281) had baseline folate intake below the RDA, while only 2.5% (N=174) fell below the RDA for B12 intake, and 26.9% (N=1,890) fell below the RDA for B6 intake. The demographic and health characteristics of study participants, by whether they met the RDA for these B vitamins, are presented in Table 1. Individuals who fell below the RDA for these B vitamins were significantly less likely to be taking a multivitamin, more likely to be non-white, have lower educational attainment and income, and were less likely to be physically active.
Table 1.
Baseline characteristics of study population comparing participants in the Women’s Health Initiative Memory Study (WHIMS) above and below the recommended daily allowance (RDA) of folate, B12 and B6 intake (N=7,030)
| Characteristics | Folate <400ug |
Folate = > 400ug |
B12 <2.4ug | B12 => 2.4ug | B6 <1.5mg | B6 =>1.5mg |
|---|---|---|---|---|---|---|
| N=3281 | N=3749 | N=174 | N=6856 | N=1890 | N=5140 | |
| Age, N (%)*/ns/***δ | ||||||
| 63–69 | 1680 (51.2) | 1801 (48.0) | 87 (50.0) | 3394 (49.5) | 1020 (54.0) | 2461 (47.9) |
| 70–74 | 1096 (33.4) | 1336 (35.6) | 60 (34.5) | 2372 (34.6) | 608 (32.2) | 1824 (35.5) |
| >=75 | 505 (15.4) | 612 (16.3) | 27 (15.5) | 1090 (15.9) | 262 (13.9) | 855 (16.6) |
| Body mass index, mean (SD)*/*/*** | 28.7 (5.7) | 28.4 (5.7) | 27.6 (5.2) | 28.5 (5.7) | 28.9 (5.8) | 28.4 (5.6) |
| General health, N (%)**/ns/*** | ||||||
| Excellent | 464 (14.2) | 541 (14.4) | 22 (12.6) | 983 (14.3) | 249 (13.2) | 756 (14.7) |
| Very good | 1372 (41.8) | 1672 (44.6) | 72 (41.4) | 2972 (43.4) | 778 (41.2) | 2266 (44.1) |
| Good | 1143 (34.8) | 1277 (34.1) | 68 (39.1) | 2352 (34.3) | 670 (35.5) | 1750 (34.1) |
| Fair | 284 (8.7) | 252 (6.7) | 10 (5.8) | 526 (7.7) | 178 (9.4) | 358 (7.0) |
| Poor | 18 (0.6) | 7 (0.2) | 2 (1.2) | 23 (0.3) | 15 (0.8) | 10 (0.2) |
| Past hormone therapy, N (%) | ||||||
| Estrogen only ns/*/ns | ||||||
| Never | 2416 (73.6) | 2796 (74.6) | 117 (67.2) | 5095 (74.3) | 1414 (74.8) | 3798 (73.9) |
| Past | 865 (26.4) | 953 (25.4) | 57 (32.8) | 1761 (25.7) | 476 (25.2) | 1342 (26.1) |
| Estrogen + progestin**/*/ns | ||||||
| Never | 3074 (93.7) | 3449 (92.0) | 169 (97.1) | 6354 (92.7) | 1771 (93.7) | 4752 (92.5) |
| Past | 207 (6.3) | 300 (8.0) | 5 (2.9) | 502 (7.3) | 119 (6.3) | 388 (7.6) |
| B12 intake, mean (SD) (ug)***/***/*** | 5.95 (2.7) | 6.02 (4.0) | 1.9 (0.4) | 23.5 (90.4) | 14.5 (85.7) | 26.1 (90.5) |
| B6 intake, mean (SD) (mg)***/***/*** | 1.55 (0.5) | 1.66 (0.5) | 2.1 (0.6) | 7.6 (6.8) | 1.2 (1.2) | 9.8 (8.8) |
| Folate intake, mean (SD) (ug)***/***/*** | 242.8 (69.5) | 724.5 (259.5) | 257.5 (113.4) | 505.8 (310.6) | 261.4 (159.7) | 587.3 (305.3) |
| Multivitamin use, N (%)***/***/*** | 75 (2.3) | 2708 (72.2) | 0 (0.0) | 2783 (40.6) | 9 (0.5) | 2774 (54.0) |
| Alcohol intake, N (%) */*/ns | ||||||
| 0 (never, past, <1 drink/month, <1/wk) | 2180 (66.4) | 2393 (63.8) | 130 (74.7) | 4443 (64.8) | 1270 (67.2) | 3303 (64.3) |
| 1–6 drinks/wk | 711 (21.7) | 898 (24.0) | 28 (16.1) | 1581 (23.1) | 397 (21.0) | 1212 (23.6) |
| 7+ drinks/wk | 390 (11.9) | 458 (12.2) | 16 (9.2) | 832 (12.1) | 223 (11.8) | 625 (12.2) |
| Race/ethnicity, N (%)***/***/*** | ||||||
| White (non-Hispanic) | 2806 (85.5) | 3323 (88.6) | 137 (78.7) | 5992 (87.4) | 1521 (80.5) | 4608 (89.7) |
| Non-white§ | 475 (14.5) | 426 (11.4) | 37 (21.3) | 864 (12.6) | 369 (19.5) | 532 (10.4) |
| Education, N (%)***/***/*** | ||||||
| 0–8 years/some high school | 1492 (45.5) | 1483 (39.6) | 98 (56.3) | 2877 (42.0) | 917 (48.5) | 2058 (40.0) |
| High school/some college | 874 (26.6) | 1055 (28.1) | 37 (21.3) | 1892 (27.6) | 508 (26.9) | 1421 (27.7) |
| College grad/post-grad | 915 (27.9) | 1211 (32.3) | 39 (22.4) | 2087 (30.4) | 465 (24.6) | 1661 (32.3) |
| Income in the last year, $, N (%)***/**/*** | ||||||
| <19,999 | 896 (27.3) | 855 (22.8) | 61 (35.1) | 1690 (24.7) | 557 (29.5) | 1194 (23.2) |
| 20,000 to 34,999 | 1134 (34.6) | 1285 (34.3) | 59 (33.9) | 2360 (34.4) | 639 (33.8) | 1780 (34.6) |
| 35,000 to 49,999 | 643 (19.6) | 757 (20.2) | 30 (17.2) | 1370 (20.0) | 352 (18.6) | 1048 (20.4) |
| 50,000 to 74,999 | 410 (12.5) | 525 (14.0) | 19 (10.9) | 916 (13.4) | 225 (11.9) | 710 (13.8) |
| 75,000 or above | 198 (6.0) | 327 (8.7) | 5 (2.9) | 520 (7.6) | 117 (6.2) | 408 (7.9) |
| Smoking*/ns/*** | ||||||
| Never | 1751 (53.4) | 1971 (52.6) | 93 (53.5) | 3629 (52.9) | 983 (52.0) | 2739 (53.3) |
| Former | 1243 (37.9) | 1512 (40.3) | 64 (36.8) | 2691 (39.3) | 705 (37.3) | 2050 (39.9) |
| Current | 247 (7.5) | 223 (6.0) | 15 (8.6) | 455 (6.6) | 180 (9.5) | 290 (5.6) |
| Missing | 40 (1.2) | 43 (1.2) | 2 (1.2) | 81 (1.2) | 22 (1.2) | 61 (1.2) |
| Recreational physical activity***/*/*** | ||||||
| None | 636 (19.4) | 616 (16.4) | 40 (23.0) | 1212 (17.7) | 429 (22.7) | 823 (16.0) |
| Activity of limited duration, freq., intensity | 1522 (46.4) | 1634 (43.6) | 79 (45.4) | 3077 (44.9) | 889 (47.0) | 2267 (44.1) |
| Mod./strenuous activity >=20 min, 2–4 times/wk | 492 (15.0) | 635 (16.9) | 15 (8.6) | 1112 (16.2) | 264 (14.0) | 863 (16.8) |
| Mod./strenuous activity >=20 min, 4+ times/wk | 631 (19.2) | 864 (23.1) | 40 (23.0) | 1455 (21.2) | 308 (16.3) | 1187 (23.1) |
significance of comparison between folate below vs above RDA/ significance of comparison between B12 below vs above RDA/ significance of comparison between B6 below vs above RDA
ns = non-significant,
= p<0.05,
= p<0.01,
= p<0.001
Non-white race includes American Indian/Alaskan Native, Asian/Pacific Islander, African-American, Hispanic/Latino
Over a median length of follow-up of 5.0 years, there were 307 incident cases of MCI and probable dementia (classified as 238 of MCI and 69 of probable dementia at first occurrence of the combined endpoint). By the end of follow-up, 39 (16.4%) of the 238 cases of mild cognitive impairment progressed to probable dementia. The majority of cases of probable dementia in WHIMS were classified as Alzheimer’s disease (50%), vascular dementia (9.3%) or mixed dementia (15.7%); only 2 study participants experienced a stroke prior to onset of probable dementia.33
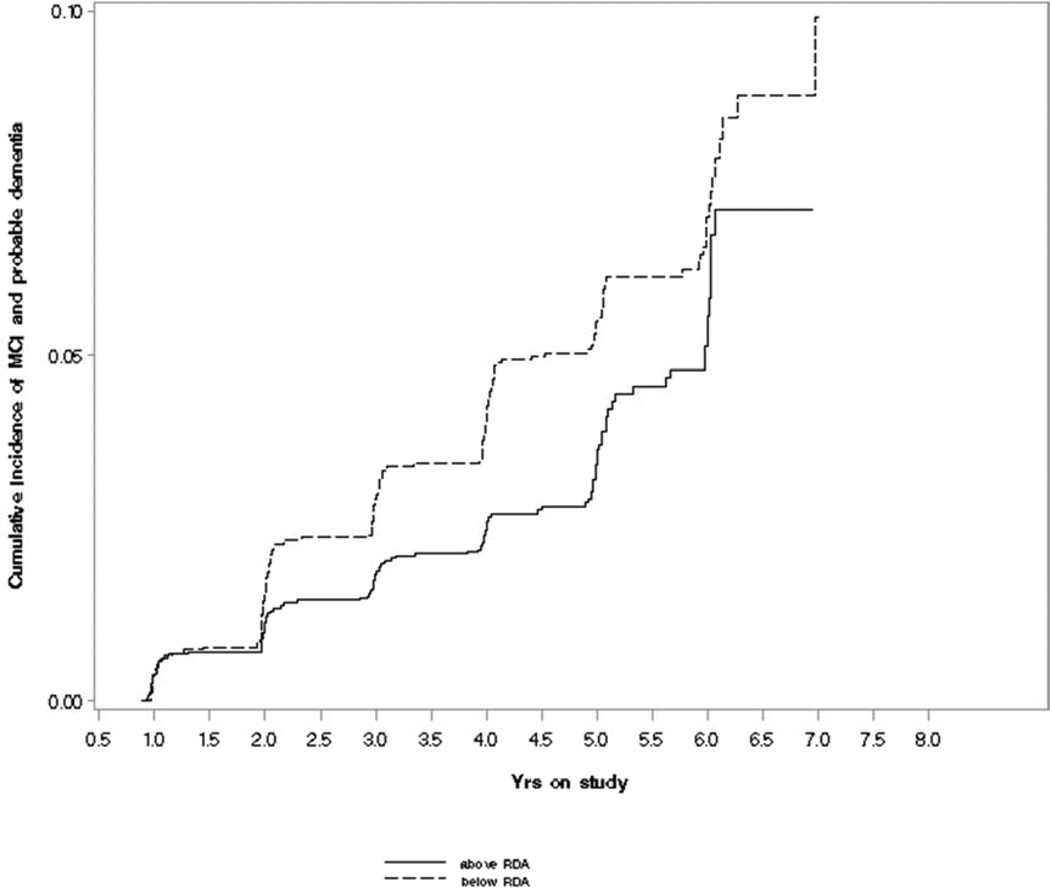
Figure 1 illustrates that the cumulative incidence of MCI/probable dementia among individuals whose daily intake of folate fell below the RDA was higher over follow-up than for those whose intake was above the RDA. Table 2 presents results from Cox proportional hazards models: folate intake below the RDA was significantly associated with risk for MCI/probable dementia in all models (multivariable, B6 and B12-adjusted HR=1.97, 95% CI: 1.32, 2.94). We also examined the association with folate intake in quartiles (Table 2), and the corresponding test for trend was borderline significant (pvalue for trend=0.09), although a linear dose-response relationship was not apparent. When examining MCI and probable dementia outcomes separately, we found that HRs were similar to those found for the combined endpoint (MCI multivariable-adjusted HR=1.52, 95% CI: 0.96, 2.4; probable dementia multivariable-adjusted HR=2.59, 95% CI: 1.31, 5.1).
Figure 1.
Cumulative incidence of mild cognitive impairment (MCI)/probable dementia by folate intake above or below the recommended daily allowance (RDA) in the Women’s Health Initiative Memory Study (WHIMS)
Table 2.
Hazard ratios for MCI/probable dementia over follow-up by folate intake above or below the RDA, and by quartile (N=7,030) in the Women’s Health Initiative Memory Study (WHIMS)
| Folate | MCI/ probable dementia cases |
Rate | Unadjusted | Age- and education- adjusted |
Multivariable adjusted |
Multivariable adj. and by B6, B12 quartile |
|---|---|---|---|---|---|---|
| Number of cases |
Cases per 100,000 person-yrs |
Hazard Ratio (95% CI) | ||||
| Less than RDA (<400 ug) |
179 | 3.2 per 100,000 |
1.50*** (1.19, 1.89) |
1.62*** (1.27, 2.07) |
1.50** (1.14, 1.97) |
1.97*** (1.32, 2.94) |
| 1st Quartile (<241.25 ug) |
85 | 2.7 per 100,000 |
1.15 (0.83, 1.60) |
1.28 (0.90, 1.84) |
1.14 (0.77, 1.69) |
1.61 (0.91, 2.87) |
| 2nd Quartile (241.25<449.6 6 ug) |
104 | 3.6 per 100,000 |
1.59** (1.16, 2.18) |
1.80*** (1.28, 2.52) |
1.70** (1.18, 2.46) |
2.33** (1.39, 3.89) |
| 3rd Quartile (449.66 <695.67 ug) |
55 | 1.8 per 100,000 |
0.80 (0.55, 1.15) |
0.87 (0.59, 1.28) |
0.88 (0.57, 1.33) |
0.99 (0.62, 1.56) |
| 4th Quartile (=>695.67 ug) |
63 | 2.2 per 100,000 |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
| P value of test for trend= 0.09 | ||||||
p<0.05,
p<0.01,
p<0.001
Multivariate model adjusted for: age, education, BMI, race, alcohol consumption, income, exercise, estrogen plus progestin use, smoking, general health status at baseline and arm of study enrollment (dietary modification trial, hormone therapy trial, calcium and vitamin D supplementation trial). Tests for linear trend conducted in full multivariate model adjusted for B6 and B12 quartile.
We also examined whether this association differed by source of folate. We hypothesized that folic acid from supplementation alone could be particularly protective among individuals with low levels of folate from diet (whereas supplemental folate for those whose dietary intake was already adequate would have less of an effect on the outcome). We found that among individuals with less than the RDA of folate from diet, there was a suggestion that folic acid supplementation above the RDA was inversely associated with MCI/probable dementia (multivariable-adjusted HR=0.75, 95% CI: 0.54, 1.05), although this association did not reach statistical significance (p=0.09). Given the high correlation between multivitamin use and total folate intake, multivitamin use was not included in the final multivariable model. However, in a sensitivity analysis we found that the effect of total folate intake below the RDA remained significantly associated with MCI/probable dementia after adjusting for multivitamin use (HR=1.69, 95% CI: 1.09, 2.62, p=0.02).
We also examined the effect of vitamin B6 below the RDA on risk of MCI/probable dementia (Table 3) and found no significant association (multivariable, folate and B12 adjusted HR=0.84, 95% CI: 0.61, 1.14). Vitamin B12 below the RDA also showed no significant association with probable dementia/MCI in the multivariable-adjusted model (multivariable, folate and B6 adjusted HR=1.19, 95% CI: 0.63, 2.24). Both vitamins B6 and B12 were examined as quartiles, and neither showed a significant linear trend with risk of MCI/probable dementia.
Table 3.
Hazard ratios for MCI/probable dementia over follow-up, by vitamin B6 and B12 intake above or below the RDA, and by quartile in the Women’s Health Initiative Memory Study (WHIMS) (N=7,030)
| B6 | MCI/ probable dementia |
Rate | Unadjusted | Age- and education- adjusted |
Multivariate adjusted |
Multivariable adj, and by B12 and folate |
| Number of cases |
Cases per 100,000 person-yrs |
Hazard Ratio (95% CI) | ||||
| Less than RDA (<l.5 mg) |
96 | 3.0 per 100,000 |
1.24 (0.97, 1.57) |
1.28* (1.00, 1.63) |
1.02 (0.79, 1.31) |
0.84 (0.61, 1.14) |
| 1st Quartile (<1.46 mg) |
91 | 3.1 per 100,000 |
1.33 (0.97, 1.82) |
1.48* (1.05, 2.08) |
1.24 (0.85, 1.81) |
1.00 (0.53, 1.88) |
| 2nd Quartile (1.47–<2.18 mg) |
86 | 2.9 per 100,000 |
1.24 (0.90, 1.70) |
1.39 (0.98, 1.97) |
1.11 (0.76, 1.62) |
0.99 (0.55, 1.77) |
| 3rd Quartile (2.19<3.78 mg) |
62 | 2.1 per 100,000 |
0.91 (0.65, 1.29) |
1.03 (0.71, 1.50) |
1.03 (0.69, 1.53) |
1.23 (0.77, 1.99) |
| 4th Quartile (= >3.79 mg) |
68 | 2.3 per 100,000 |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
| P value of test for trend=0.99 | ||||||
| B12 | MCI/ probable dementia cases |
Rate | Unadjusted | Age- and education- adjusted |
Multivariate adjusted |
Multivariable adjusted and adj by B6 and folate quartile |
| Number of cases |
Cases per 100,000 person-yrs |
Hazard Ratio (95% CI) | ||||
| Less than RDA (<2.4 ug) |
15 | 5.3 per 100,000 |
1.94* (1.15, 3.27) |
1.57 (0.90, 2.76) |
1.37 (0.75, 2.48) |
1.19 (0.63, 2.24) |
| 1st Quartile (<5.23 ug) |
94 | 3.2 per 100,000 |
1.19 (0.88, 1.61) |
1.32 (0.71, 1.34) |
1.14 (0.79, 1.65) |
0.71 (0.41, 1.24) |
| 2nd Quartile (5.24– <8.62 ug) |
69 | 2.3 per 100,000 |
0.87 (0.62, 1.20) |
0.89 (0.62, 1.27) |
0.79 (0.53, 1.17) |
0.51* (0.29, 0.88) |
| 3rd Quartile (8.63– <12.70 ug) |
66 | 2.2 per 100,000 |
0.83 (0.60, 1.16) |
0.89 (0.62,1.26) |
0.81 (0.55, 1.20) |
0.66 (0.42, 1.04) |
| 4th Quartile (= >12.70 ug) |
78 | 2.7 per 100,000 |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
1.0 (Reference) |
| P value of test for trend =0.43 | ||||||
p<0.05,
p<0.01,
p<0.001
Multivariate model adjusted for: age, education, BMI, race, alcohol consumption, income, exercise, estrogen plus progestin use, smoking, general health status at baseline and arm of study enrollment (dietary modification trial, hormone therapy trial, calcium and vitamin D supplementation trial). Tests for linear trend conducted in full multivariate model.
We examined whether individuals with high levels of folate and low levels of B12 were at increased risk of MCI/probable dementia. We found no significant difference in risk between those in the lowest quartile of B12 and highest quartile of folate compared with those in the highest quartile of both nutrients (p=0.91). We also did not find a significant interaction between folate and high alcohol intake (p-value for interaction=0.61) or with folate intake and randomization to hormone therapy (p-value for interaction=0.22).
Finally, we examined whether associations with folate intake and risk of MCI/dementia differed by when the baseline dietary assessment was conducted: i.e., prior to or after mandatory folate fortification. While fewer people fell below the RDA for folate intake post-versus pre-fortification (21.2% post-fortification versus 57.7% pre-fortification), the interaction of folate below the RDA and timing of the FFQ was not significant (p=0.98), and folate intake at baseline below the RDA remained significantly associated with risk of MCI/probable dementia (HR=2.57, 95% CI: 1.4, 4.7, p=0.002).
DISCUSSION
We found that folate intake below the RDA was associated with an increased risk of MCI/probable dementia over a median follow-up of 5.0 years after controlling for many potential confounders including vitamin B6 and B12 intake. There was no clear linear trend between folate intake quartile and MCI/probable dementia risk; while risk was elevated in both the first and second quartiles of folate intake versus the highest quartile, somewhat unexpectedly, risk was slightly more elevated in the second versus the first quartile. This finding remains after controlling for several key potential confounders, but does not have a clear physiological basis and may be due to chance.
Our results are consistent with several other studies that found that lower levels of folate are associated with cognitive impairment or decline in older populations.5,10,47–51 However, other studies of folate find null,13 or even negative effects,14 of higher levels of folate on cognition. A recent meta-analysis by Wald and colleagues reviewed nine randomized trials of folic acid supplementation and did not find any significant protective effect of supplementation on cognitive decline. However, the length of follow-up in these studies was relatively short (median follow-up time less than 1 year), which may not provide the time needed for folic acid supplementation to exert a protective effect.22
There are several proposed mechanisms for how B vitamins may play a protective role in cognition during aging. Vitamins B6, B12 and folate play important roles in the homocysteine cycle, and without an adequate supply of these vitamins, homocysteine accumulates intracellularly and in the bloodstream.23,24,27 Higher levels of homocysteine are related to increased risk of cardiovascular disease,52 which is associated with risk for vascular dementia and Alzheimer’s disease.53 A recent MRI study found that elevated homocysteine may be related to cognitive impairment through increased white matter hyperintensity and cerebral infarcts.54 Additionally, both vitamin B6 and folate are involved with the synthesis of several key neurotransmitters, and folate deficiency has been related to oxidative stress and DNA damage in neurons,23,55 although these pathways have been less well explored.28
In our study, we did not find associations with intakes of vitamins B6 or B12 with cognition, either singularly or in combination with folate intake. Results from studies examining the effect of vitamins B6 and B12 on cognition have been inconsistent. Several intervention studies15,24,25,56 as well as an observational study, found no strong evidence that vitamin B12 was related to cognitive impairment.57 However, some studies have found vitamins B6 and B12 to be protective for cognitive decline.6,58,59 While a recent finding from the Framingham Heart Study identified cognitive decline to be particularly accelerated in the context of low plasma B12 and high plasma folate,6 we did not replicate this result when examining risk among individuals with low intake of B12 and high intake of folate.
There are limitations to the current study. First, we were only able to examine B vitamin intake, rather than serum or red blood cell levels. Due to several factors, the intake amounts of these vitamins might not reflect the amount available for cellular processes. For example, there are several factors that can affect folate bioavailability, such as MTHFR genotype,60 and drugs including methotrexate and metformin.23,61,62 Vitamin B6 absorption may be affected by the presence of chronic inflammation.63 Additionally, lowered production of gastric acid (necessary for the breakdown of vitamin B12 from food), due to gastric inflammation or drugs such as proton pump inhibitors (PPIs), can reduce levels of bioavailable vitamin B12.27 To address possible drug interaction with B-vitamins, we examined rates of use of medications that commonly interact with these vitamins. We found these commonly interacting drugs were used by relatively small percentages of the population (metformin 0.8% of women in the WHI clinical trials; PPIs by 2.1% of the WHI population). Therefore, we believe that drug interactions do not largely explain our null results for B6 and B12. However, we cannot rule out other sources of disparity between intake and bioavailability, especially for vitamin B12. If B12 or B6 intake does not very well reflect the amount of the vitamin available to the physiological processes hypothesized to affect cognitive functioning, this may explain our null effect. Additionally, only a small percentage of the population fell below the RDA for B12 intake (2.4% of the study population), so our statistical power to examine the effect of B12 below the RDA on MCI/probable dementia is limited.
Another potential limitation is that B vitamin intake is most likely related to levels of other healthful nutrients, which could themselves be protective for cognitive decline, and we are unable to disentangle specifically the effect of B vitamins from other potentially beneficial nutrients (although the association of low folate intake with MCI/probable dementia remained even after controlling for multivitamin use and B6 and B12 intake). In addition, we were only able to assess folate intake at one time point (baseline), so we are not able to track how change in folate intake over time with increasingly large numbers of individuals exposed to folic acid fortification may relate to risk of MCI/probable dementia. However, we did not find any evidence of a significantly different association between folate intake below the RDA and MCI/probable dementia risk by whether folate at baseline was measured pre or post-fortification. Finally, about 20% of probable dementia cases were identified without a prior presentation of MCI, suggesting a relatively rapid decline in cognitive status among some participants. These more rapid onset cases were not explained by incident stroke, as only two individuals with probable dementia experienced a stroke prior to probable dementia diagnosis.32,33 It is more likely that the identification of such cases was associated with the raising of the 3MSE screening cut-point that occurred mid-study to increase the sensitivity to detect probable dementia cases;32 thus, the presence of such cases would indicate that our study population was not singular and that our results are generalizable.
The current study also has several strengths. Study outcomes of MCI and probable dementia were carefully screened and diagnosed by expert adjudication. This study also includes a larger sample size than the majority of studies investigating the effect of B vitamins on MCI/dementia risk. Additionally, we are able to examine a wide range of confounders (including education, income, exercise, and alcohol intake) that could be associated with both B vitamin intake and MCI/dementia risk. Finally, we are able to jointly examine the association of levels of folate, vitamins B6 and B12 on incidence of MCI and probable dementia, which has not been as widely examined as the association with these vitamins individually.51
In conclusion, we found evidence that folate intake below the RDA was related to increased risk for MCI/probable dementia among older women. Levels of vitamin B6 and B12 intake were not found to be associated with increased risk. To date, the majority of intervention studies examining the effect of folic acid supplementation on cognitive performance in later life have not supported a protective role for folic acid, while several longitudinal observational studies have found this association. It is important to better understand this apparent inconsistency, as modifiable risk factors for dementia would be an important public health intervention for a rapidly aging population. Future intervention studies with longer follow-up times will be more directly comparable to longer-term prospective cohort studies like this one, and will allow for a longer time period over which folic acid supplementation may reveal a neuroprotective effect. In our current study including a large number of older women followed prospectively for 5 years, we found evidence that low levels of folate intake are indeed associated with a higher risk of MCI/probable dementia.
ACKNOWLEDGMENTS
Sponsor’s Role: None.
Short list of WHI investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. One author was also supported by a training grant from NIMH (NIH T32MH017119) while completing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Agnew-Blais, Wassertheil-Smoller, Kang, Hogan, Coker, Snetselaar, and Smoller: study concept and design. Agnew-Blais, Wassertheil-Smoller, Hogan, Kang: design and data analysis. All authors listed contributed to the interpretation of data and manuscript preparation. WHI Study Group and Clinical Site Investigators as listed below: acquisition of subjects and data.
Conflict of Interest Disclosures:
No authors have any financial or personal conflicts of interest.
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet and risk of Alzeimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris M. Vitamins and cognitive development and performance: Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71(1):1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer Disease in the elderly. Arch Neurol. 2007;64(1):86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Selhub J, Jacques P. Vitamin B-12 and folate status in relation to decline in scores on the Mini-Mental State Examination in the Framingham Cohort Study. J Am Geriatr Soc. 2012;60(8):1457–1464. doi: 10.1111/j.1532-5415.2012.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toh B, van Driel I, Gleeson P. Pernicious anemia. NEJM. 1997;337(20):1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55(11):1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. NEJM. 2002;289(20):2651–2662. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 10.Ramos MI, Allen LH, Mungas DM, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005;82(6):1346–1352. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 11.Riggs KM, Spiro A, Tucker K, Rush D. Relations of vitamin B12, vitamin B6, folate and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63(3):306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in healthy elderly population. JAMA. 1983;249(21):2917–2921. [PubMed] [Google Scholar]
- 13.Morris M, Evans D, Schneider J, Tangney C, Bienias J, Aggarwal N. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer's disease. J Alzheimer Dis. 2006;9(4):435–443. doi: 10.3233/jad-2006-9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris M, Evans D, Bienias J, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62(4):641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 15.Kang JH, Cook N, Manson J, Burimng JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hvas A, Juul S, Lauritzen L, Nexo E, Ellegaard J. No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. J Affective Dis. 2004;81(3):269–273. doi: 10.1016/S0165-0327(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 17.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. NEJM. 2006;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 18.Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B-12 or B-6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002;132(6):1345–1356. doi: 10.1093/jn/132.6.1345. [DOI] [PubMed] [Google Scholar]
- 19.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic-acid supplementation on cognitive function in older adults in the FACIT trial a randomized double blind controlled trial. Lancet. 2007;369(208–216):208. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 20.Connelly P, Prentice N, Cousland G, Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's Disease. Int J Geriatr Psychiatr. 2008;23(2):155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 21.Clarke R, Harrison G, Richards S Vital Trial Collaborative Group. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in peeople at high risk of dementia. J Int Med. 2003;254(1):67–75. doi: 10.1046/j.1365-2796.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- 22.Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am J Med. 2010;123(6):522–527. doi: 10.1016/j.amjmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Malouf R, Evans JG. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD004514.pub2. Art. No: CD004514. [DOI] [PubMed] [Google Scholar]
- 24.Malouf R, Grimley Evans J. Vitamin B6 for cognition. Cochrane Database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD004393. Art no CD004393. [DOI] [PubMed] [Google Scholar]
- 25.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch Int Med. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Schneider J, Tangney C, Morris M. Folic acid and cognition in older persons. Expert Opin Drug Saf. 2006;5(4):511–522. doi: 10.1517/14740338.5.4.511. [DOI] [PubMed] [Google Scholar]
- 27.Malouf R, Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD004326. CD004326. [DOI] [PubMed] [Google Scholar]
- 28.Morris M, Schneider J, Tangney C. Thoughts on B-vitamins and dementia. J Alzheimer Dis. 2006;9(4):429–433. doi: 10.3233/jad-2006-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 31.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women, the Women’s Health Initiative Memory Study: A Randomized Controlled Trial. JAMA. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 32.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin in the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 33.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 34.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 35.Subar A, Block G, James L. Folate intake and food sources in the US population. Am J Clin Nutr. 1989;50(3):508–516. doi: 10.1093/ajcn/50.3.508. [DOI] [PubMed] [Google Scholar]
- 36.Kang J. Personal communication: Sources of folate intake in the Nurses' Health Study. Agnew-Blais. 2014 [Google Scholar]
- 37.US Department of Health and Human Services FDA. Food standards: amendment of the standards of identity for enriched grain products to require addition of folic acid. Fed Register. 1996;61(151):40513. [Google Scholar]
- 38.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 39.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): A trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19(6):604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 40.Morris J, Heyman A, Mohs R, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 42.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatr Neurol. 1991;4(3):173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 43.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 44.Institute of Medicine, editor. Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, D.C.: National Academy Press; 1998. [PubMed] [Google Scholar]
- 45.Halsted CH, Villanueva JA, Devlin AM, Chandler CJ. Metabolic interactions of alcohol and folate. J Nutr. 2002;132(8 Suppl):2367S–2237sS. doi: 10.1093/jn/132.8.2367S. [DOI] [PubMed] [Google Scholar]
- 46.SAS [computer program] Version 9.3. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 47.Kado DM, Karlamangla AS, Huang MH, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118(2):161–169. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Wang HX. Vitamin B12 and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56(9):1188–1194. doi: 10.1212/wnl.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 49.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer’s disease. Am J Clin Nutr. 2005;82(3):636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 50.Quadri P, Fragiacomo C, Pezzati R, et al. Homocysteine, folate and vitamin B-12 in mild cognitive impairment, Alzheimer disease and vascular dementia. Am J Clin Nutr. 2004;80(1):114–122. doi: 10.1093/ajcn/80.1.114. [DOI] [PubMed] [Google Scholar]
- 51.Hassing L, Wahlin A, Winblad B, Backman L. Further evidence on the effects of vitamin B12 and folate levels on episodic memory functioning: A population-based study of healthy very old adults. Biol Psychiatr. 1999;45(11):1472–1480. doi: 10.1016/s0006-3223(98)00234-0. [DOI] [PubMed] [Google Scholar]
- 52.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202–1208. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman AB, Fitzpatrick AK, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study Cohort. J Am Geriatr Soc. 2005;53(7):1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 54.Tangney C, Aggarwal N, Li H, et al. Vitamin B12, cognition, and brain MRI: A cross-sectional examination. Neurology. 2011;77(13):1276–1282. doi: 10.1212/WNL.0b013e3182315a33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. PNAS. 1997;94(7):3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ford AH, Flicker L, Alfonso H, et al. Vitamins B12, B6 and folic acid for cognition in older men. Neurology. 2010;75(17):1540–1547. doi: 10.1212/WNL.0b013e3181f962c4. [DOI] [PubMed] [Google Scholar]
- 57.Wahlin A, Hill RD, Winblad B, Backman L. Effects of serum vitamin B12 and folate status on episodic memory performance in very old age: A population-based study. Psychol Aging. 1996;11(3):487–496. doi: 10.1037//0882-7974.11.3.487. [DOI] [PubMed] [Google Scholar]
- 58.Moorthy D, Peter I, Scott TM, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142(8):1554–1556. doi: 10.3945/jn.112.161828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Jager CA, Oulhaj O, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int J Geriatr Psychiatr. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 60.Ma J, Stampfer MJ, Hennekens CH, et al. Methylenetetrahyrdofolate reductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation. 1996;94(10):2410–2416. doi: 10.1161/01.cir.94.10.2410. [DOI] [PubMed] [Google Scholar]
- 61.Kamen BA, Nylen PA, Camitta BM, JR B. Methotrexate accumulation and folate depletion in cells as possible mechanisms of chronic toxicity of the drug. British J Haematol. 1981;49(3):355–360. doi: 10.1111/j.1365-2141.1981.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 62.Wulffele MG, Kooy A, Lehert P, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: A randomized, placebo-controlled trial. J Int Med. 2003;254(5):455–463. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 63.Morris M, Sakakeeny L, Jacques P, Picciano M, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140(1):103–110. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]