Abstract
Habitual intake of black tea has been associated with relatively lower serum cholesterol concentrations in observational studies. However, clinical trial results evaluating the effects of black tea on serum cholesterol have been inconsistent. Several factors could explain these mixed results, in particular, uncontrolled confounding caused by lifestyle factors, e.g. diet. This diet-controlled clinical trial estimates the effect of black tea flavonoid consumption on cholesterol concentrations in 57 borderline hypercholesterolemic individuals (total cholesterol concentrations between 190 and 260 mg/dl (4.9 and 6.7 mmol/L)). A double blind, randomized crossover trial was conducted in Minneapolis, MN from April 2002 through April 2004, wherein key conditions were tightly controlled to minimize possible confounding. Participants consumed a controlled low-flavonoid diet plus 5 cups per day of black tea or tea-like placebo over two 4-week treatment periods. The flavonoid-free caffeinated placebo matched the tea in color and taste. Differences in cholesterol concentrations at the end of each treatment period were evaluated via linear mixed models. Differences (95% CI) in mg/dl among those treated with tea versus placebo were 3.43 (−7.08, 13.94) for total cholesterol, −1.02 (−11.34, 9.30) for low-density lipoprotein cholesterol (LDL-C), 0.58 (−2.98, 4.14) for high-density lipoprotein cholesterol (HDL-C), 15.22 (−40.91, 71.35) for triglycerides, and −0.39 (−11.16, 10.38) for LDL plus HDL cholesterol fraction. The LCL-C/HDL-C ratio decreased by −0.1 units (95% CI −0.41, 0.21). No results were statistically or clinically significant. Thus, the intake of 5 cups of black tea per day did not significantly alter the lipid profile of borderline hypercholesterolemic subjects.
Keywords: serum lipids, hypercholesterolemia, black tea, flavonoids, randomized crossover control trial
INTRODUCTION
Tea brewed from Camellia sinensis is the most commonly consumed beverage in the world after water.1,2 It is rich in polyphenolic flavonoids that possess antioxidant, anti-mutagenic, anti-inflammatory, and antiallergenic properties.3 These flavonoids may also be hypocholesterolemic,3–5 as shown in cell culture,6–8 animal,4,6,9–21 and observational22–32 studies of black tea, the most commonly consumed tea in the United States. Clinical trial results, however, have been inconsistent.33–39 Some randomized trials have found significant hypocholesterolemic associations between black tea consumption and lipid profiles,33,36,39 while others have reported no effect on lipid profiles.34,35,37,38 A review on the subject found limited evidence that tea has favorable effects on cardiovascular disease risk factors, including hypercholesterolemia.40 It urged cautious interpretation of the results due to the small number of potentially biased trials, and emphasized the need for further high quality trials with longer-term follow-up.
Several factors may contribute to the mixed results of previous black tea and cholesterol trials, such as varying study duration, strength and brewing method of tea, average habitual tea consumption, and differences in participants’ dietary habits. Prior studies, with the exception of one clinical trial, have not rigorously controlled all of these factors.36 The trial presented herein addresses prior deficits in the black tea and serum lipid literature by examining the effect of black tea beverage intake (5 cups/day, 700 mg tea solids/cup) on serum lipid concentrations using a standardized tea treatment and appropriate and consistent placebo in the presence of a completely controlled diet.
METHODS
Participants
Between April 2002 and April 2004, 1500 individuals were recruited via TV, radio, and print advertisements in Minneapolis and St. Paul, MN. They were screened via telephone for eligibility prior to secondary screening at the University of Minnesota General Clinical Research Center (GCRC, currently the Clinical and Translational Science Institute), where a screening blood draw occurred. Basic eligibility criteria included age 45–65 years, borderline hypercholesterolemia (total cholesterol concentrations 190–260 mg/dl or 4.9–6.7 mmol/L), 35–65 mg/dl (0.9–1.6 mmol/L) of high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) concentrations <600 mg/dl (6.8 mmol/L). A slightly wider range than standard borderline hypercholesterolemia was utilized.41 See on-line supplemental materials for additional inclusion/exclusion criteria.
Of the 1500 people initially screened, 400 (26.7%) were eligible and provided consent for secondary screening at the GCRC. Of these 400, 57 (14.3%) volunteers met all entry criteria, consented to participate, and were enrolled. Informed consent was obtained via a structured interview with the study coordinator prior to both screening and enrollment. The University of Minnesota, Twin Cities Institutional Review Board approved this study. The trial is registered at ClinicalTrials.gov (NCT01882283).
Study Design
At the end of a one-week run-in period, 57 participants were block-randomized by sex using a computer-generated list to initial consumption of either 5 cups per day of black tea or placebo (tea-like) beverage. Block randomization was used to equally distribute potential differences within sexes, e.g. metabolism, across randomization arms. Participants switched treatment assignments at the beginning of the second treatment period. Serum lipid values were measured at baseline, and the beginning and end of each treatment period. Research team members, participants and analysts remained blinded until the final analysis was completed. Only the study statistician had access to randomization codes.
Intervention
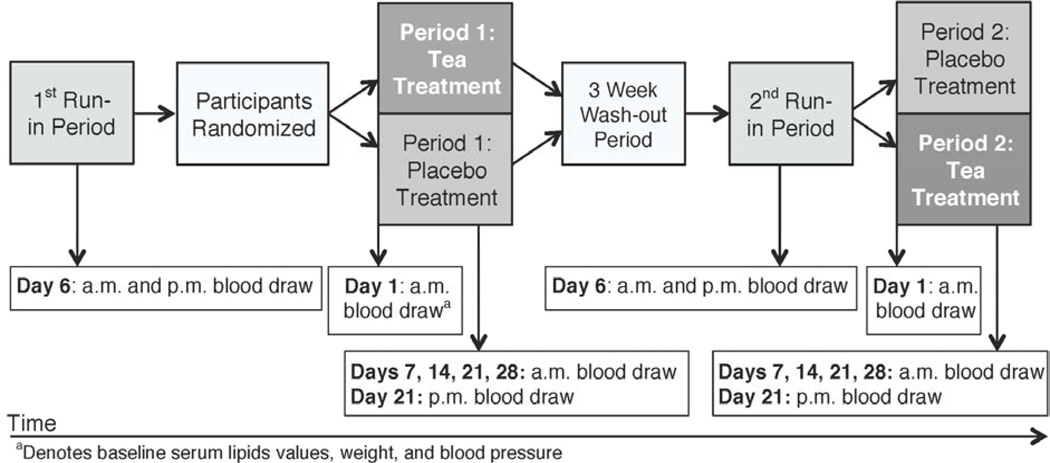
The intervention consisted of 5 cups per day of black tea or a tea-like placebo for 4 weeks, plus a provided, low-flavonoid diet. The trial included two 41-day treatment periods and a 3-week washout period, which occurred between the treatment periods. Each treatment period was comprised of 13 days of run-in time and 28 days of tea or placebo treatment. All participants consumed tea-like placebo during run-in periods. The isocaloric study diet was consumed throughout run-in and treatment periods, which allowed for a flavonoid washout from the self-selected diet consumed during the washout period. An overview of the entire study period and biological sample collection are described in Figure 1.
Figure 1.
Crossover study design overview, including timing of biological sample collection points, in a study of the effect of 5 cups per day of black tea on serum cholesterol concentrations (n=57)
Black tea was selected for this intervention because it is the most commonly consumed form of tea in U.S. The selection of five cups of tea per day was based on 1) its therapeutic potential, and 2), that if therapeutic, this volume could be reasonably incorporated into a patient’s daily routine. Significant effects on plasma cholesterol concentration, platelet aggregation, brachial artery reactivity, and oxidative damage, given response times, could occur within a one-month period of tea intake, hence the 28-day treatment period.3,42–45 The washout period duration allowed for re-equilibration of parent catechin and catechin metabolite concentrations to self-selected diet concentrations following tea intake.46–50
Tea Treatment and Placebo
Black tea and tea-like preparations arrived in identical individual serving packets from the Lipton Tea Company (currently Unilever-Best Foods NA), and were coded with randomly generated numbers that linked their content to the treatment assignment in a blinded fashion. They were pre-brewed, and matched in terms of color and taste, caffeine, aspartame, malic acid and fruit flavor content (On-line Supplemental Table 1). The flavonoid composition of the black tea treatment is described in the On-line Supplemental Table 2.
Participants were given specific treatment preparation instructions, and the tea/placebo was prepared for drinking and consumed off-site. If desired, the addition of sweetener to the beverage was allowed, but not milk. Participants were encouraged to consume the treatment at equal intervals throughout the day, but no mandatory times were specified. No specific limit was set on the amount of water that could be added to the preparation or the cup size.
Controlled Low-Flavonoid Diet
Participants were to consume the entire controlled low-flavonoid diet and daily water-soluble vitamin supplements (100% RDA), which were provided throughout each treatment period. Due to the low naturally occurring vitamin content of the controlled diet, vitamin supplementation was given for ethical reasons. With the exception of calorie-free and decaffeinated soft drinks, consumption of non-study provided foods was not permitted. All pain relievers (besides acetaminophen) were restricted.
The Nutrition Data System for Research Software for commercial entrees (Version 4.04_32, 2001, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) was used to determine the food composition of the study diet entrees, and to identify entree components that may contain flavonoids. Entrees with a possible significant flavonoid content were not used. Prior to the study, the nutritional composition of the entire study diet was calculated using Nutritionist V nutrient analysis software (Version 2.3, 2000, First Data Bank, San Bruno, CA). The macronutrient composition of the study diet was 15% protein, 51% carbohydrate, and 34% fat. The ratio of saturated, monounsaturated, and polyunsaturated fatty acids was 1:1:0.6 to meet usual intake values as reported in the National Health and Nutrition Examination Survey III.53
Nutritional composition was later re-analyzed using the Grand Forks Research Analysis of Nutrient Data software (Release 24, 2011, Grand Forks Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Grand Forks, ND), to determine the additional macronutrient values reported here.51,52 The diet contained 12.8 g saturated fat, 14.1 g monounsaturated fat, 7.8 g polyunsaturated fat and 6.3 g fiber per 1000 kcals. The diet, which contained no fruits and very small amounts of vegetables, was analyzed for multiple flavonoids. No flavonoids were detected. A detailed description of this analysis is found in the on-line supplemental materials.
Participants were assigned energy intake estimates congruent with weight maintenance. The study diet was comprised of a 5-day menu rotation, and calculated at five different energy levels: 2000, 2500, 3000, 3500, and 4000 kcal. Energy intake estimates were calculated using Harris Benedict Equation, multiplied by an estimated activity factor.54–56 The activity factors, ranging from 1.4-sedentary to 2.0-very active, were estimated using subjects’ self-reported physical activity levels.56 A unit-muffin of 100 kcal, which matched the diet’s exact macronutrient composition, was used to ensure weight maintenance. The results of daily weighs informed the need for energy intake modification.
Measures
Participants visited the GCRC Monday through Friday throughout the study for daily weighs, and to collect meals. Once per week, GCRC nurses measured each subject’s weight, blood pressure, and pulse, and completed blood draws and urine collections. During these nurse evaluations, participants were interviewed to evaluate pharmaceutical usage, dietary intake, and motivation to maintain study requirements. The number of tea packets used was also counted to evaluate treatment compliance.
All compliance to treatment, provided diet, non-study beverage restriction, non-study food consumption, multivitamin intake, and pharmaceutical use was self-reported on daily compliance records, either at GCRC (weekdays) or at home (weekend days). Participants were asked to describe the type of deviation and its magnitude, e.g. mg of restricted medication. No biologic analyses were conducted to verify compliance.
Biological Samples
Morning measurements from Day 1 of the first tea/placebo treatment period established baseline data for weight, blood pressure, and serum lipids. Thereafter, fasting serum samples were collected on days 7, 14, 21 and 28 of each treatment period. Blood was collected in 2.5 ml Serum Separation Tubes (Becton Dickinson, Franklin Lanes, NJ), and the resulting serum sample was analyzed for TC, HDL-C, and TG at the University of Minnesota Medical Center, Fairview Collaborative Studies Clinical Laboratory (Minneapolis, MN), using the COBAS FARA instrument (Roche Analytical Instruments, Inc. Nutley, NJ). Serum total cholesterol, HDL-C and TG were measured enzymatically.57 HDL-C was determined after precipitation of LDL-containing lipoproteins with dextran sulfate/magnesium.58 LDL-C was calculated in participants with TG concentrations <400 mg/dl (<4.5 mmol/L) by the Friedewald equation.59
Analyses
At an α level of 0.05, the trial had 95% power to detect differences of 6.69 mg/dl of TC (0.2 mmol/L), 4.57 mg/dl of LDL-C (0.1 mmol/L), 1.37 mg/dl of HDL-C (0.04 mmol/L), and 22.79 mg/dl of TG (0.3 mmol/L) between treatment groups. These values are similar to, but more conservative than, what was found in Davies et al trial.36 Equivalence of mean baseline characteristic values was analyzed using one-way ANOVA to ensure that important covariates were equally distributed across randomization groups. A paired t-test was used to evaluate treatment carry-over effects between baseline and Day 1 lipid concentrations.
Differences in lipid concentrations across treatment groups were calculated using the Day 28 serum measurement taken at the end of each treatment period. The effect of tea consumption on each lipid concentration was analyzed as a repeated measures regression using the PROC MIXED procedure in SAS (Version 8.2, released 2001, SAS Institute, Inc., Cary, NC). Analyses were adjusted for treatment period via the inclusion of a period covariate. Treatment-period interactions were also tested. All data analyses followed intention-to-treat (ITT) principles. Results were considered statistically significant at an alpha level of 0.05.
RESULTS AND DISCUSSION
Participants and Compliance
The study sample consisted of 32 men and 25 women, who had a mean age of 52.4 years (range 45–65). Baseline characteristics of participants by treatment-order assignment are summarized in Table 1. Thirty participants were randomized to consume black tea treatment and 27 were randomized to placebo in the first treatment period. No statistically significant difference was observed in the baseline characteristics across treatment groups, except for HDL-C concentration, which was slightly lower among participants starting the tea treatment first (p=0.04).
Table 1.
Baseline characteristics by primary treatment assignment of participants in a study to examine the effect of 5 cups per day of black tea on serum cholesterol concentrations (n=57)
| Characteristic | Randomization Assignment | |
|---|---|---|
| Black Tea First (n=30) Mean (SD) |
Placebo First (n=27) Mean (SD) |
|
| % Female | 46.7 | 40.7 |
| Age (years) | 51.7 (5.1) | 53.2 (4.9) |
| BMI | 31.4 (5.8) | 30.0 (6.1) |
| Systolic Blood Pressure (mm Hg) |
130.1 (15.2) | 129.2 (12.0) |
| Total Cholesterol (mg/dl) | 207.2a (27.9) | 206.7 (22.8) |
| HDL-C (mg/dl)* | 38.6a (8.3) | 43.3 (9.2) |
| LDL-C (mg/dl) | 128.0b (20.0) | 133.6c (25.9) |
| VLDL-C (mg/dl) | 44.5a (34.9) | 34.8 (35.7) |
| TG (mg/dl) | 222.7a (174.4) | 174.1 (178.5) |
| AST (U/L)d | 22.6a (7.4) | 24.9 (7.7) |
| Glucose (mg/dl)d | 100.9a (15.1) | 99.1 (8.0) |
| Creatinine (mg/dl)d | 0.9a (0.2) | 1.0 (0.1) |
| Tea (cups per day)d | 1.3 (1.5) | 1.0 (1.4) |
p-value<0.05
Serum TC, HDL-C, VLDL-C, TG, AST, glucose, and creatinine value not available for 1 participant
LDL-C value calculated using Friedewald equation. Cannot be estimated for 5 participants with >400 mg/dl TG (>4.5 mmol/L)
LDL-C calculated using Friedewald equation. Cannot be estimated for 2 participants with >400 mg/dl TG (>4.5 mmol/L)
Values from eligibility visit.
Post-randomization, two participants left the study for reasons unrelated to treatment during the first treatment period. One participant moved out of state, and another demonstrated willful non-participation in the study. No significant changes in body weight occurred during the study period. No compliance violations were found regarding drug intake, with the exception of one subject who took aspirin on two occasions. Compliance rates on all other indicators were above 96% (On-line Supplemental Table 3).
Blood Lipids
No period effects or treatment-period interactions were detected. At the end of the study period, mean differences in absolute values were +1.64% for total cholesterol (mean difference 3.43; 95%CI: −7.08, 13.94; p-value=0.2), −0.77% for low-density lipoprotein cholesterol (LDL-C) (mean difference −1.02; 95% CI: −11.34, 9.30; p-value=0.7), +1.40% for high-density lipoprotein cholesterol (HDL-C) (mean difference 0.58; 95%CI: −2.98, 4.14; p-value=0.3), +7.81% for triglycerides (mean difference 15.22; 95%CI: −40.91, 71.35; p-value=0.1), −0.22% for LDL plus HDL cholesterol fraction (mean difference −0.39 ; 95% CI: −11.16, 10.38; p-value=0.9) in those treated with tea versus placebo. There was a −3.08% difference in mean LDL-C/HDL-C ratio values across treatment periods (mean difference −0.1; 95%CI −0.41, 0.21; p-value=0.2). Mean absolute values (± standard error) across treatment groups, mean differences, and p-values for all lipid categories are shown in Table 2. The trial results indicated that consumption of 5 cups of tea per day for four weeks did not significantly change TC, LDL-C plus HDL-C, LDL-C, HDL-C, or TG concentrations.
Table 2.
Fasting cholesterol concentrations at the end of treatment period in a randomized cross-over study to examine the effect of 5 cups per day of black tea on serum cholesterol concentrations (n=57)
| Serum Lipid Type (mean±SE) |
Tea (n=57) | Placebo (n=57) |
Mean difference (95% CI) |
% Mean Difference |
p-value |
|---|---|---|---|---|---|
| Total cholesterol (mg/dl) |
212.30±3.76 | 208.87±3.74 | 3.43 (−7.08, 13.94) | 1.64 | 0.20 |
| LCL-C (mg/dl) | 130.80±3.43 | 131.82±3.40 | −1.02 (−11.34, 9.30) | −0.77 | 0.70 |
| HDL-C (mg/dl) | 41.90±1.27 | 41.32±1.27 | 0.58 (−2.98, 4.14) | 1.40 | 0.30 |
| Triglycerides (mg/dl) | 210.00±20.05 | 194.78±20.01 | 15.22 (−40.91, 71.35) | 7.81 | 0.10 |
| LDL-C + HDL-C (mg/dl) | 173.68±3.86 | 174.07±3.83 | −0.39 (−11.16, 10.38) | −0.22 | 0.90 |
| LDL-C/HDL-C ratio | 3.15±0.11 | 3.25±0.11 | −0.1 (−0.41, 0.21) | −3.08 | 0.20 |
The current study is the only clinical trial to date to isolate the effect of consuming the polyphenolic flavonoids found in black tea beverage on cholesterol concentrations, while strictly controlling dietary intake. This trial excluded current smokers, included stringent inclusion criteria, controlled caffeinated beverage and pharmaceutical use, and used participants as their own controls to maximize its internal validity. Critically, this study compared black tea beverage to an appropriate placebo (matched to tea treatment with the exception of flavonoids) according to a pre-specified protocol.
The results of this study contradict the majority of observational research and animal trials, and three clinical trials, one of which was also diet-controlled.9,10,17,19–21,23–27,33,36,39,60 The strong cholesterol lowering effects of black tea found in animal and cell culture studies may be due to the high doses (mg/kg) used in those studies versus most human research. Seven observational studies (six cross-sectional and one case-control) have reported that black tea consumption is correlated with lower total cholesterol in humans.23–27,31,32 However, other observational studies (three cross-sectional and one prospective) found no association between tea drinking and total cholesterol.22,28–30 Tea consumption may be a surrogate marker for lifestyle factors that could serve as confounders, e.g. exercise habits, smoking patterns, lower coffee consumption, or dietary differences. Incomplete adjustment for these factors, differences of consumed tea quantity, and/or differences in types (e.g. blended, decaffeinated, instant) and preparation (e.g. brewing time, amount used, brewing temperature) of tea consumed may explain the inconsistent results of observational studies.
Results of the current study are in agreement with four other clinical trials.34,35,37,38 While the direction of the results was similar, these trials differed in methodology from the current trial (treatment type, duration, target population). Some had methodological weaknesses that may have had an important effect on their results, e.g. allowing participants to consume variable amounts of tea and coffee, small sample size.35,37 Two other randomized clinical trials, which also did not control background diet, found that black tea had hypocholesterolemic effects.33,39 These two trials differed methodologically from the present study in multiple ways. Neither study compared within-participant serum lipid values. Bahorun et al. compared black tea intake to a hot water placebo in healthy adults. The study experienced differential loss to follow-up, totaling approximately 12% of their study population, and no ITT analysis was reported. Fujita et al. compared the effects of black tea extract capsules (333 mg x 3/day) to a placebo for a three-month period, and also measured serum lipids at 1 month post-intervention. These results indicated an 8.5% decrease in TC, a 8.4% decrease in TG and 11.8% decrease in LDL-C after 3 months, which were all statistically significant at p-value of 0.01. The difference in results from Fujita et al. and this study may be driven primarily by treatment type (extract capsules from fermented fenhai broad-leaf tea vs. powdered tea drink), due to the highly concentrated nature of the extract capsules. Also, analyses compared treatment groups to changes over time within each group, and did not present the results of comparisons between groups.
The current trial most resembles the randomized double-blind crossover trial conducted by Davies et al., which provided a standardized placebo and a controlled diet to participants. The study included similar treatment duration, tea dosages, and population as the current trial. The work by Davies et al., however, differed from the current trial in that they provided the National Cholesterol Education Program Step I-type diet and, initially, a placebo without caffeine. Furthermore, the study added a post hoc treatment arm with a caffeinated placebo. Their results showed that 15 mildly hypercholesterolemic adults experienced declines in TC (3.8%; p=0.06, trend) and LDL-C (7.5%; p=0.01) concentrations after consumption of black tea compared to placebo without caffeine. The comparison of tea consumption to a caffeinated placebo during a post hoc treatment period resulted in differences of 6.5% TC (p=<0.001) and 11.1% LDL-C (p=0.002) across treatment groups, larger differences than found when comparing tea to the non-caffeinated placebo. These results contradict the results of multiple clinical trials published on this topic to date, including the current trial. The incorporation of a post hoc third treatment period with 12 of 15 original trial participants, during which all participants consumed a caffeinated placebo drink, may have contributed to the large effects observed in this trial.36
Participants in the current study served as their own controls, which reduced error variance and the potential for observation bias. Participants were highly compliant with study procedures, and there was minimal loss-to-follow-up. We estimated that the respective lengths of the treatment and washout periods were appropriate to observe the effect of interest and eliminate carry over effects of treatment due to the short time required for re-equilibration of the parent catechin and catechin metabolite concentrations after a high intake of tea.42,46–48,50 Conversely, lipophilic metabolites of catechins may need a longer period of time for re-equilibration, but no carry over effect was seen as lipid concentrations returned to baseline during the washout period. The use of absolute cholesterol concentrations in the primary outcome analyses, versus measures of difference, was appropriate, as cholesterol concentration equilibration occurs within two weeks.42
Limitations of the present trial include an inability to test for dose response relationships between tea and lipid concentrations, and to evaluate the effects of tea consumption against different background diets. Also, while study staff carefully monitored participant compliance via multiple mechanisms, no biological analyses were conducted to validate self-reported compliance, a potential weakness shared with most other trials on this subject.
CONCLUSIONS
In summary, the intake of 5 cups of tea in combination with a low-flavonoid typical American diet did not significantly alter the lipid profile of borderline hypercholesterolemic participants. While tea may have very small beneficial effects on lipid profiles, this trial indicates that there is little therapeutic benefit to using black tea beverage to prevent hypercholesterolemia progression. Further research is needed to explore other tea-consumption-related mechanisms of potential importance in cardiovascular disease etiology, e.g. improvement of endothelial function, inflammation reduction, platelet hyperactivity, and oxidative damage.
Supplementary Material
ACKNOWLEDGMENTS
We would like to recognize and thank Linda Lewis, Anhtung Chau, Poonguzhali Kailash, Peter Wilker, Chris Lessard, Kevin Viken, Nicki Teig, Danielle Babl, Archana Bargaje, GCRC kitchen and nurses for their contributions to this research.
This research was supported by the Lipton Tea Company/Unilever-Best Foods NA, the Minnesota Medical Foundation, and by NIH grant number M01-RR-00400 of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Rasa Troup, MS, RD, CSSD, LD
- Jennifer H. Hayes, MEd, MPH
- Susan K. Raatz, PhD, MPH, RD
- Bharat Thyagarajan, MD, PhD, MPH, MBBS
- Waseem Khaliq, MD
- David R. Jacobs, Jr., PhD
- Nigel S. Key, MB, ChB
- Bozena M. Morawski, MPH
- Daniel Kaiser, PhD
- Alan J. Bank, MD
- Myron Gross, PhD
Contributor Information
Rasa Troup, Current: Sports Dietitian, Department of Intercollegiate Athletics, University of Minnesota, 516 15th Ave SE, Minneapolis, MN 55455, USA, Tel: 612-708-3314, Fax: 612-379-4871, mich0232@umn.edu; At time of research: Nutrition Department, University of Minnesota, 1334 Eckles Ave, St. Paul, MN 55108, USA; Department of Laboratory Medicine and Pathology, University of Minnesota, MMC 609, 420 Delaware Street SE, Minneapolis, MN, 55455. USA.
Jennifer H. Hayes, Current: Senior Epidemiologist, Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene, 201 W Preston Street #400, Baltimore, MD 21201, USA, Tel: 410-767-5459, Fax: 410-333-5218, jennifer.hayes@maryland.gov; At time of research: Department of Laboratory Medicine and Pathology, University of Minnesota, MMC 609, 420 Delaware Street SE, Minneapolis, MN, 55455. USA.
Susan K. Raatz, Current: Research Nutritionist, Agricultural Research Service, U.S. Department of Agriculture, Grand Forks Human Nutrition Research Center, 2420 2nd Ave North Grand Forks, ND 58203, USA; Department of Food Science and Nutrition, University of Minnesota, 1334 Eckles Ave, St Paul, MN 55108, USA, Tel: 701-795-8294, Fax: 701-795-8240, susan.raatz@ars.usda.gov; At time of research: University of Minnesota, General Clinical Research Center, 251 Masonic, 424 Harvard Street SE, Minneapolis, MN 55455, USA; Department of Medicine, University of Minnesota, 14-142C PWB, 516 Delaware Street SE, MMC 480, Minneapolis, MN 55455, USA.
Bharat Thyagarajan, Current: Assistant Professor, Department of Laboratory Medicine and Pathology, School of Medicine, University of Minnesota, MMC 609 Mayo 8609, 420 Delaware Street SE, Minneapolis, MN 55455, USA, Phone: 612-624-1257, Fax: 612-624-8950, thya0003@umn.edu; At time of research: Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 1300 S. 2nd Street, Suite 300, Minneapolis, MN 55454, USA.
Waseem Khaliq, Current: Instructor of Medicine, Johns Hopkins Bayview Medical Center, Johns Hopkins, University School of Medicine, 5200 Eastern Avenue, MFL Building, West Tower 6th Floor, Baltimore, MD 21224 USA, Tel: 410-955-9434, Fax: N/A, khaliqmd@gmail.com; At time of research: School of Public Health, Division of Epidemiology, University of Minnesota, 1300 South Second Street, Suite 300, Minneapolis, MN 55454, USA.
David R. Jacobs, Jr, Current: Professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 1300 S. 2nd Street, Suite 300, Minneapolis, MN 55454, USA, Tel: 612-624-4196, Fax: 612-624-0315; At time of research: Professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 1300 S. 2nd Street, Suite 300, Minneapolis, MN 55454, USA.
Nigel S. Key, Current: Harold R Roberts Professor, Director, UNC Hemophilia and Thrombosis Center, Departments of Medicine and Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 303 Mary Ellen Jones Building, CB #7035, Chapel Hill, NC 27599, USA, Tel: 919-966-3311, Fax: 919-966-7639, nigel_key@med.unc.edu; At time of research: Department of Medicine, University of Minnesota, 14-142C PWB, 516 Delaware Street SE, MMC 480, Minneapolis, MN 55455, USA.
Bozena M. Morawski, Graduate Research Assistant, Department of Laboratory Medicine and Pathology, School of Medicine, University of Minnesota, 420 Delaware Street SE, Minneapolis, MN 55455, USA; Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 1300 S. 2nd Street, Suite 300, Minneapolis, MN 55454, USA, Tel: 612 625 4891, Fax: 612 624 0315, bozena@umn.edu.
Daniel Kaiser, Current: Greatbatch, Inc., 2595 Dallas Parkway, Suite 310, Frisco, TX 75034, USA, Tel: 214 618 5240, Fax: N/A, drkaiser@greatbatch.com; At time of research: St. Paul Heart Clinic, 255 North Smith Avenue, Suite 100, St. Paul, MN 55109.
Alan J. Bank, Current: United Heart and Vascular Clinic, 225 N. Smith Ave, Suite 400, St. Paul, MN 55102; Division of Cardiology, Department of Medicine, School of Medicine, University of Minnesota, 420 Delaware Street SE, Minneapolis, MN 55455, United Heart and Vascular Clinic, Tel: 651-241-2047, Fax: 651-241-2910, Alan.Bank@allina.com; At time of research: St. Paul Heart Clinic, 255 North Smith Avenue, Suite 100, St. Paul, MN 55109.
Myron Gross, Current: Department of Laboratory Medicine and Pathology, School of Medicine, University of Minnesota, MMC 609, 420 Delaware Street SE, Minneapolis, MN 55455, USA. Tel.: 612-624-5417 Fax: 612-273-6994 gross001@umn.edu; At time of research: Department of Laboratory Medicine and Pathology, School of Medicine, University of Minnesota, MMC 609, 420 Delaware Street SE, Minneapolis, MN 55455, USA.
REFERENCES
- 1.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21(3):334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson JM, Croft KD. Tea flavonoids and cardiovascular health. Mol Asp Med. 2010;31(6):495–502. doi: 10.1016/j.mam.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 3.McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21(1):1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 4.Yang TT, Koo MW. Hypocholesterolemic effects of Chinese tea. Pharmacol Res. 1997;35(6):505–512. doi: 10.1006/phrs.1997.0176. [DOI] [PubMed] [Google Scholar]
- 5.Habauzit V, Morand C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Ther Adv Chronic Dis. 2012;3(2):87–106. doi: 10.1177/2040622311430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda I, Yamahira T, Kato M, Ishikawa A. Black-tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats. J Agric Food Chem. 2010;58(15):8591–8595. doi: 10.1021/jf1015285. [DOI] [PubMed] [Google Scholar]
- 7.Singh DK, Banerjee S, Porter TD. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J Nutr Biochem. 2009;20(10):816–822. doi: 10.1016/j.jnutbio.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAnlis GT, McEneny J, Pearce J, Young IS. Black tea consumption does not protect low density lipoprotein from oxidative modification. Eur J Clin Nutr. 1998;52(3):202–206. doi: 10.1038/sj.ejcn.1600540. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda I, Imasato Y, Sasaki E, et al. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992;1127(2):141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 10.Vinson JA, Dabbagh YA. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998;433(1–2):44–46. doi: 10.1016/s0014-5793(98)00880-1. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda H, Chisaka T, Kubomura Y, et al. Effects of crude drugs on experimental hypercholesterolemia. I. Tea and its active principles. J Ethnopharmacol. 1986;17(3):213–224. doi: 10.1016/0378-8741(86)90138-8. [DOI] [PubMed] [Google Scholar]
- 12.Chisaka T, Matsuda H, Kubomura Y, Mochizuki M, Yamahara J, Fujimura H. The effect of crude drugs on experimental hypercholesteremia: mode of action of (-)-epigallocatechin gallate in tea leaves. Chem Pharm Bull. 1988;36(1):227–233. doi: 10.1248/cpb.36.227. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Ishigaki A, Hara Y. Long-term effect of a trace amount of tea catechins with perilla oil on the plasma lipids in mice. Int J Vitam Nutr Res. 1998;68(4):272–274. [PubMed] [Google Scholar]
- 14.Hayek T, Fuhrman B, Vaya J, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arter Thromb Vasc Biol. 1997;17(11):2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 15.Miura Y, Chiba T, Tomita I, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131(1):27–32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Yokoyama WH, Irving D, Rein D, Walzem RL, German JB. Effect of dietary catechin and vitamin E on aortic fatty streak accumulation in hypercholesterolemic hamsters. Atherosclerosis. 1998;137(1):29–36. doi: 10.1016/s0021-9150(97)00248-7. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto N, Okushio K, Hara Y. Effect of black tea polyphenols on plasma lipids in cholesterol-fed rats. J Nutr Sci Vitaminol. 1998;44(2):337–342. doi: 10.3177/jnsv.44.337. [DOI] [PubMed] [Google Scholar]
- 18.Yokozawa T, Dong E, Nakagawa T, Kim DW, Hattori M, Nakagawa H. Effects of Japanese black tea on atherosclerotic disorders. Biosci Biotechnol Biochem. 1998;62(1):44–48. doi: 10.1271/bbb.62.44. [DOI] [PubMed] [Google Scholar]
- 19.Alshatwi AA, Al Obaaid MA, Al Sedairy SA, Ramesh E, Lei KY. Black and green tea improves lipid profile and lipid peroxidation parameters in Wistar rats fed a high-cholesterol diet. J Physiol Biochem. 2011;67(1):95–104. doi: 10.1007/s13105-010-0053-3. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer MA, Mulder TPJ, Molhuizen HOF. Theaflavins from black tea, especially theaflavin-3-gallate, reduce the incorporation of cholesterol into mixed micelles. J Agric Food Chem. 2008;56(24):12031–12036. doi: 10.1021/jf8022035. [DOI] [PubMed] [Google Scholar]
- 21.Fujita H, Yamagami T. Extract of black tea (pu-ehr) inhibits postprandial rise in serum cholesterol in mice, and with long term use reduces serum cholesterol and low density lipoprotein levels and renal fat weight in rats. Phytother Res. 2008;22(10):1275–1281. doi: 10.1002/ptr.2477. [DOI] [PubMed] [Google Scholar]
- 22.Hakim Ia, Alsaif Ma, Alduwaihy M, Al-Rubeaan K, Al-Nuaim AR, Al-Attas OS. Tea consumption and the prevalence of coronary heart disease in Saudi adults: results from a Saudi national study. Prev Med. 2003;36(1):64–70. doi: 10.1006/pmed.2002.1130. [DOI] [PubMed] [Google Scholar]
- 23.Stensvold I, Tverdal A, Solvoll K, Foss OP. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21(4):546–553. doi: 10.1016/0091-7435(92)90062-m. [DOI] [PubMed] [Google Scholar]
- 24.Solvoll K, Selmer R, Løken EB, Foss OP, Trygg K. Coffee, dietary habits, and serum cholesterol among men and women 35–49 years of age. Am J Epidemiol. 1989;129(6):1277–1288. doi: 10.1093/oxfordjournals.aje.a115247. [DOI] [PubMed] [Google Scholar]
- 25.Tuomilehto J, Tanskanen A, Pietinen P, et al. Coffee consumption is correlated with serum cholesterol in middle-aged Finnish men and women. J Epidemiol Community Heal. 1987;41(3):237–242. doi: 10.1136/jech.41.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green M, Jucha E. Association of serum lipids with coffee, tea, and egg consumption in free-living subjects. J Epidemiol Community Heal. 1986;40(4):324–329. doi: 10.1136/jech.40.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little JA, Shanoff HM, Csima A, Toronto MA, Yano R. Coffee and serum-lipids in coronary heart-disease. Lancet. 1966;1(7440):732–734. doi: 10.1016/s0140-6736(66)90890-7. [DOI] [PubMed] [Google Scholar]
- 28.Klatsky AL, Petitti DB, Armstrong MA, Friedman GD. Coffee, tea and cholesterol. Am J Cardiol. 1985;55(5):577–578. doi: 10.1016/0002-9149(85)90250-4. [DOI] [PubMed] [Google Scholar]
- 29.Carson CA, Cauley JA, Caggiula AW. Relation of caffeine intake to blood lipids in elderly women. Am J Epidemiol. 1993;138(2):94–100. doi: 10.1093/oxfordjournals.aje.a116839. [DOI] [PubMed] [Google Scholar]
- 30.Nichols AB, Ravenscroft C, Lamphiear DE, Ostrander LD. Independence of serum lipid levels and dietary habits. The Tecumseh study. JAMA. 1976;236(17):1948–1953. [PubMed] [Google Scholar]
- 31.Green MS, Harari G. Association of serum lipoproteins and health-related habits with coffee and tea consumption in free-living subjects examined in the Israeli CORDIS Study. Prev Med. 1992;21(4):532–545. doi: 10.1016/0091-7435(92)90061-l. [DOI] [PubMed] [Google Scholar]
- 32.Kark JD, Friedlander Y, Kaufmann NA, Stein Y. Coffee, tea, and plasma cholesterol: the Jerusalem Lipid Research Clinic prevalence study. Br Med J (Clin Res Ed) 1985;291(6497):699–704. doi: 10.1136/bmj.291.6497.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahorun T, Luximon-Ramma A, Neergheen-Bhujun VS, et al. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev Med. 2012;54(Suppl):S98–S102. doi: 10.1016/j.ypmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Kubota K, Sumi S, Tojo H, et al. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract. Nutr Res. 2011;31(6):421–428. doi: 10.1016/j.nutres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Mukamal KJ, MacDermott K, Vinson Ja, Oyama N, Manning WJ, Mittleman Ma. A 6-month randomized pilot study of black tea and cardiovascular risk factors. Am Hear J. 2007;154(4):724. doi: 10.1016/j.ahj.2007.07.008. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies MJ, Judd JT, Baer DJ, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133(10):3298S–3302S. doi: 10.1093/jn/133.10.3298S. [DOI] [PubMed] [Google Scholar]
- 37.Bingham SA, Vorster H, Jerling JC, et al. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br J Nutr. 1997;78(1):41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 38.Trautwein EA, Du Y, Meynen E, et al. Purified black tea theaflavins and theaflavins/catechin supplements did not affect serum lipids in healthy individuals with mildly to moderately elevated cholesterol concentrations. Eur J Nutr. 2010;49(1):27–35. doi: 10.1007/s00394-009-0045-7. [DOI] [PubMed] [Google Scholar]
- 39.Fujita H, Yamagami T. Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr Res. 2008;28(7):450–456. doi: 10.1016/j.nutres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane database Syst Rev. 2013;6(6) doi: 10.1002/14651858.CD009934.pub2. CD009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269(23):3015–3023. [PubMed] [Google Scholar]
- 42.Hodson L, Skeaff CM, McKenzie JE. Maximal response to a plasma cholesterol-lowering diet is achieved within two weeks. Nutr Metab Cardiovasc Dis. 2002;12(5):291–295. [PubMed] [Google Scholar]
- 43.Tijburg LB, Wiseman SA, Meijer GW, Weststrate JA. Effects of green tea, black tea and dietary lipophilic antioxidants on LDL oxidizability and atherosclerosis in hypercholesterolaemic rabbits. Atherosclerosis. 1997;135(1):37–47. doi: 10.1016/s0021-9150(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 44.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 45.Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean ME. Tea flavonoids and cardiovascular health. QJM. 2001;94(5):277–282. doi: 10.1093/qjmed/94.5.277. [DOI] [PubMed] [Google Scholar]
- 46.Hollman PC, Tijburg LB, Yang CS. Bioavailability of flavonoids from tea. Crit Rev Food Sci Nutr. 1997;37(8):719–738. doi: 10.1080/10408399709527799. [DOI] [PubMed] [Google Scholar]
- 47.Yang CS, Lee MJ, Chen L. Human salivary tea catechin levels and catechin esterase activities: implication in human cancer prevention studies. Cancer Epidemiol Biomarkers Prev. 1999;8(1):83–89. [PubMed] [Google Scholar]
- 48.Ishikawa T, Suzukawa M, Ito T, et al. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997;66(2):261–266. doi: 10.1093/ajcn/66.2.261. [DOI] [PubMed] [Google Scholar]
- 49.Princen HMG, van Duyvenvoorde W, Buytenhek R, et al. No effect of consumption of green and black tea on plasma lipid and antioxidant levels and on LDL oxidation in smokers. Arter Thromb Vasc Biol. 1998;18(5):833–841. doi: 10.1161/01.atv.18.5.833. [DOI] [PubMed] [Google Scholar]
- 50.Van het Hof KH, de Boer HS, Wiseman SA, Lien N, Westrate JA, Tijburg LB. Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 1997;66(5):1125–1132. doi: 10.1093/ajcn/66.5.1125. [DOI] [PubMed] [Google Scholar]
- 51.USDA, ARS GFHNRC. Grand Forks Analysis of Nutrient Data. 2011 [Google Scholar]
- 52.Winham DM, Hutchins AM. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr J. 2011;10:128. doi: 10.1186/1475-2891-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ervin RB, Wright JD, Wang C-Y, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data. 2004;(348):1–6. [PubMed] [Google Scholar]
- 54.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40(1):168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 55.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Carnegie Institution of Washington; 1919. [Google Scholar]
- 56.World Health Organization. Energy and protein requirements (Report of Joint FAO/WHO/UNE Expert Consultation) Geneva: World Health Organization; 1985. p. 205. [PubMed] [Google Scholar]
- 57.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzym. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 58.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379–1388. [PubMed] [Google Scholar]
- 59.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 60.Huang H-C, Lin J-K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012;3(2):170–177. doi: 10.1039/c1fo10157a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.