SUMMARY
The μ opioid receptor (MOR) and κ opioid receptor (KOR) have been implicated in pair-bond formation and maintenance in socially monogamous species. Utilizing monogamous titi monkeys (Callicebus cupreus), the present study examined the potential role opioids play in modulating the response to separation, a potent challenge to the pair-bond. In Experiment 1, paired male titi monkeys were separated from their pair-mate for 30-minutes and then received saline, naloxone (1.0 mg/kg), morphine (0.25 mg/kg), or the KOR agonist, U50,488 (0.01, 0.03, or 0.1 mg/kg) in a counter-balanced fashion, immediately prior to a 30-minute reunion with their mate. Blood samples were collected immediately prior to and after the reunion. Males receiving morphine approached females less, initiated contact less, and females broke contact with the males less. The increase in cortisol in response to naloxone was greater compared to vehicle, and the increase in cortisol in response to the high dose of U50,488 compared to vehicle approached significance. In Experiment 2, paired males were treated with the KOR antagonist, GNTI (0.1, 0.3, or 1.0 mg/kg), or saline 24 h prior to a 60-min separation from their mate. Blood samples were collected at the time of injection and immediately before and after separation. Administration of the low dose of GNTI decreased the locomotor component of the separation response compared to vehicle. The present study found that the opioid system is involved in both the affiliative and separation distress components of a pair-bond, and these components are regulated by different opioid receptors.
Keywords: titi monkey, mu opioid, pair-bonding, cortisol, monogamy, kappa opioid
1.1
Socially monogamous species form long-term associations between two adults. In some species, these relationships have been shown to be classic attachment bonds (Hazan and Shaver, 1987) and result in pair-mates spending considerable time in physical contact with one another, providing social buffering, and exhibiting substantial behavioral and physiological agitation upon involuntary separation (Mason and Mendoza, 1998). Due to the rarity of monogamy in mammals (Kleiman, 1977) there is a paucity of data on the neurobiological underpinnings of adult attachment. Research on infant-mother attachments and monogamous prairie voles (Microtus ochrogaster) suggest that the opioid system may play a role. The monogamous titi monkey (Callicebus cupreus) is an animal model that we can use to further our understanding of the relationship between opioids and adult attachment. The overarching premise of this paper is that different components of the opioid system play distinct and, potentially, opposing roles in regulating the behavioral and physiological determinants of the emotional bond that characterize adult attachment relationships.
The opioid system regulates infant affiliation towards an adult attachment figure and the response to involuntary separation. μ opioid receptor (MOR) agonists, such as morphine, decrease physical contact between social partners; whereas, opioid antagonists, such as naloxone, increase physical contact (Keverne et al., 1989, Schino and Troisi, 1992, Kalin et al., 1995, Martel et al., 1995). Furthermore, MOR agonists reduce infant separation vocalizations in monkeys, dogs, guinea pigs, and rat pups (Herman and Panksepp, 1978, Panksepp et al., 1980, Kalin et al., 1988, Nelson and Panksepp, 1998). More generally, activation of the MOR system produces euphoria in humans and conditioned place preferences in rodents (Bardo et al., 1995, Boecker et al., 2008).
In contrast, the κ opioid system promotes attachment-like responses and seems to do so by regulating negative affect. κ opioid receptor (KOR) agonists increase ultrasonic vocalizations in rat pups during maternal separation, and they can induce ultrasonic vocalizations in situations where they do not usually occur (Carden et al., 1991, Carden et al., 1994). The KOR system is involved in producing unpleasant affective responses to stressors (McLaughlin et al., 2006, Land et al., 2008). KOR agonists produce conditioned place aversions (Land et al., 2008) and dysphoria in humans (Pfeiffer et al., 1986). Mice deficient in dynorphin (the endogenous ligand of this system) or animals administered a KOR antagonist prior to a forced swim stress test do not develop the expected conditioned aversions (Land et al., 2008).
The function of opioids in adult attachment is not well studied. Shapiro and colleagues (1989) found that morphine reduced side-by-side contact in monogamous prairie voles, however the antagonist naloxone had no effect on social behavior. MOR blockade prevents pair-bond formation in prairie voles possibly by blocking the rewarding components of initial social interactions such as sexual behavior. Peripheral administration of the opioid antagonist, naltrexone, or central administration of the MOR antagonist, CTAP, in the dorsal striatum or dorsomedial shell of the nucleus accumbens blocks partner preference formation in prairie voles without affecting physical contact (Burkett et al., 2011, Resendez et al., 2013). A recent study discovered that prairie voles have greater MOR binding in the brain in general compared to polygamous meadow voles (Inoue et al., 2013). There is also evidence that the KOR plays a role in pair-bond maintenance. A facet of an established pair-bond in prairie voles is mate-guarding, when individuals are aggressive towards a stranger conspecific (Aragona and Wang, 2004). When pair-bonded male prairie voles are administered the KOR antagonist, nor-BNI, peripherally or locally in the nucleus accumbens shell, there is a decrease in attacks towards stranger males (Resendez et al., 2012). This finding suggests that KOR are needed to maintain a pair-bond. It is possible that the KOR is driving the negative affective reaction to a stranger intruder (Resendez and Aragona, 2013).
The MOR and KOR also affect the physiological components of stress, particularly the hypothalamic-pituitary-adrenal (HPA) axis. In primates, including humans, and sheep MOR activation results in a decrease in cortisol concentrations (Zis et al., 1984, Parrott and Thornton, 1989, Broadbear et al., 2004), and opioid blockade with either naloxone or naltrexone increases cortisol concentrations (Williams et al., 2003, Wand et al., 2012, Ragen et al., 2013). KOR activation, in contrast, activates the HPA axis in humans, non-human primates, and rodents leading to increased corticotropin releasing hormone, adrenocorticotropic hormone, and glucocorticoids (Calogero et al., 1996, Pascoe et al., 2008, Ranganathan et al., 2012).
Currently, there is only one study examining the effects of opioid manipulation in a monogamous non-human primate. Administration of morphine or naloxone to monogamous titi monkey males in established pair-bonds has no effect on affiliative behavior or separation distress behavior (Ragen et al., 2013). However, the temporary absence of the pair-mate resulted in an increase in naloxone’s stress related effects, indicated by exaggerated increases in locomotion, cortisol, and vasopressin (AVP) suggesting that the presence of a pair-mate aids in homeostatic regulation of the opioid system in titi monkeys (Ragen et al., 2013).
The finding that MOR manipulation had no effect on affiliative behavior was surprising due to the several studies in nonhuman primates and prairie voles that found that MOR activation decreases physical contact (Keverne et al., 1989, Shapiro et al., 1989, Kalin et al., 1995) and opioid blockade increases physical (Keverne et al., 1989, Schino and Troisi, 1992, Martel et al., 1995). One possible reason for the null findings was due to methodology. A study by Kalin et al. (1995) found that morphine decreased physical contact and naltrexone increased physical contact between mother and infant macaques when they were administered the drug immediately prior to a reunion after a 30-minute separation. In the study by Ragen et al. (2013) titi monkeys were removed from their home cage, administered morphine or naloxone, and put immediately back. It is possible that a disruption of MOR via a separation was needed for MOR opioid manipulation to have an effect on social behavior in titi monkeys. In addition to affiliative behaviors, MOR manipulation had no effect on separation distress behaviors, particularly vocalizations. This is surprising due to the wealth of literature finding that MOR agonism decreases separation distress vocalizations in other attachment relations, specifically that between offspring and mother (Herman and Panksepp, 1978, Panksepp et al., 1978, Kalin et al., 1988, Nelson and Panksepp, 1998).
The present study sought to further explore the opioid system’s role in affiliative and separation distress behavior in titi monkey males with established pair-bonds, and attempt to discover if and how the opioid system is regulated social behavior and separation distress. Our first goal was to examine how MOR manipulation could affect affiliative behavior with a methodology different from Ragen et al. (2013) since no effect was observed. We predicted that MOR activation in paired titi monkeys with morphine would decrease affiliative behavior while opioid antagonism with naloxone would increase affiliative behavior after reunited with their pair-mate, which mirrors the paradigm used by Kalin et al. (1995). Our second goal was to explore whether the KOR was involved in separation distress in titi monkeys since it does not appear that the MOR is involved. We hypothesized that the KOR system may regulate the separation distress response because of its involvement in the induction of dysphoria (Land et al., 2008), its ability to induce ultrasonic vocalizations in rat pups (Carden et al., 1994), and its involvement in maintaining prairie vole pair-bonds (Resendez et al., 2012). Therefore we predicted that KOR activation via the KOR agonist, U50,488, would induce a separation distress response and that the KOR antagonist, GNTI, would attenuate the separation distress response.
Finally, we measured how these pharmacological manipulations affected HPA activity and vasopressin. We predicted that morphine would decrease cortisol concentrations, while naloxone and U50,488 would increase cortisol, and U50,488 would decrease vasopressin concentrations.
2.1 Experimental Procedures
2.2 Subjects
Eight titi monkey males living in established pairs were used as subjects for each experiment. See Table 1 for subject details. For experiment 1, one male was living with two offspring and four were living with one offspring. For experiment 2, two males were living with two offspring and three were living with one offspring. Seven of the eight subjects were the same in Experiment 2 as in Experiment 1. A subject from Experiment 1 had to be replaced due to the health of its offspring. At the beginning of Experiment 1 the mean (±SEM) age of the subjects was 116.3 ± 15.4 months, and the mean (±SEM) time after pairing was 48.8 ± 12.1 months. At the beginning of Experiment 2 the mean (±SEM) age of the subjects was 112.8 ± 15.3 months, and the mean (±SEM) time after pairing was 50.4 ± 12.2 months. Adult pairs and any offspring were housed in cages (1.2 m × 1.2 m × 1.8 m) at the California National Primate Research Center. Rooms were set on a 12:12 light:dark cycle with lights on at 0600 and lights off at 1800. The temperature was maintained at 21 °C. Housing conditions were similar to those found in Mendoza (1999). Subjects were fed daily at 0800 and 1300 h on a diet of New World monkey chow, apples, carrots, rice cereal, and bananas. Water was available ad libitum. All procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee.
Table 1.
Demographics of subjects in Experiment 1 and Experiment 2, and the treatments they received
| Experiment 1* | |||||
|---|---|---|---|---|---|
|
| |||||
| Subject ID | Pair-Mate ID | Subject Age (mo) | Pair-Mate Age (mo) | Time Paired (mo) | Offspring |
| 29775 | 37133 | 193 | 75 | 54 | |
| 35383 | 30557 | 100 | 172 | 54 | |
| 31716 | 29852 | 158 | 190 | 123 | 1M |
| 32878 | 34866 | 136 | 110 | 27 | 1F |
| 34531 | 36163 | 114 | 88 | 62 | 1M,1F |
| 36988 | 37929 | 79 | 63 | 17 | |
| 36187 | 35292 | 88 | 107 | 30 | 1F |
| 38064 | 30486 | 63 | 174 | 25 | 1F |
|
| |||||
| Experiment 2ˆ | |||||
|
| |||||
| Subject ID | Pair-Mate ID | Subject Age (mo) | Pair-Mate Age (mo) | Time Paired (mo) | Offspring |
|
| |||||
| 29775 | 37133 | 195 | 77 | 56 | |
| 35383 | 30557 | 102 | 174 | 56 | |
| 31716 | 29852 | 160 | 192 | 125 | 1M, 1M |
| 34531 | 36163 | 116 | 90 | 64 | 1M,1F |
| 36988 | 37929 | 81 | 65 | 19 | |
| 36187& | 35292 | 90 | 109 | 32 | 1F |
| 38064 | 30486 | 65 | 176 | 27 | 1F |
| 36150 | 34631 | 94 | 114 | 26 | 1M |
All animals received vehicle, 1.0 mg/kg naloxone, 0.25 mg/kg morphine, 0.01 mg/kg U50,488, 0.03 mg/kg U50,488 and 0.1 mg/kg U50,488
All animals received vehicle, 0.1 mg/kg GNTI, 0.5 mg/kg GNTI, and 1.0 mg/kg GNTI
Did not receive 0.1 mg/kg GNTI because of birth of an infant
2.3 Drugs
Dissolved morphine sulfate (Cardinal Health; Dublin, OH) was obtained in 10 ml vials at a concentration of 10 mg/ml, and any dilutions were made using physiological saline. Naloxone hydrochloride (Fisher Scientific; Pittsburg, PA) was dissolved in physiological saline and mixed fresh before test sessions. (±)-trans-U50,488 (Sigma-Aldrich; St. Louis, MO) was dissolved in saline and aliquots of each dose were stored at −20 °C and defrosted the day of testing. U50,488 has been shown to cross the blood-brain-barrier (BBB) via indirect evidence from experiments that block its analgesic effects (Aldrich et al., 2009). GNTI (5′-Guanidinyl-17-(cyclopropylmethyl)-6,7-dehydro-4,5α -epoxy-3,14-dihydroxy-6,7-2′,3′-indolomorphinan dihydrochloride) (Tocris Bioscience; Minneapolis, MN) was dissolved in saline, stored in aliquots at −20 °C and defrosted the day of testing. Since GNTI has not been used frequently to alter behavior, at least in rodents it is currently unclear whether GNTI crosses the BBB (Munro et al., 2012). However, it is important to note that peripheral administration of GNTI to macaques blocks the behavioral effects of peripheral administration of U50,488, which does cross the BBB (Negus et al., 2002). Naloxone, U50,488, and GNTI were all filtered through a 20 μm filter. Drugs and vehicle were administered by intramuscular (IM) injection in a volume of 0.1 ml/kg.
2.4 Blood Sample Collection
All animals were previously trained to enter a transport box (0.3 m × 0.3 m × 0.3 m). Subjects were hand captured from the transport box, the subject was manually restrained, and 1 ml of blood was collected from the femoral vein with a 1 cc syringe pretreated with heparin. The mean (±SEM) time from initial disturbance of the cage to completion of sample collection was 177.2 ± 5.0 seconds. Once collected, blood samples were stored on ice until all procedures for the day were completed; samples were then centrifuged for 15 minutes at 4° C, the plasma fraction extracted and stored frozen (−70° C) until assay. For Experiment 1, plasma was later assayed for cortisol and vasopressin (AVP), and plasma from Experiment 2 was assayed for cortisol. Plasma AVP concentrations were estimated in duplicate using commercial enzyme immunoassay kits (Enzo Life Sciences, Farmingdale, NY) that were validated for titi monkeys (Bales et al., 2005). Intra- and inter-assay coefficients of variation (CV) for AVP were 6.46 and 6.80, respectively. CVs for all samples did not exceed 10%. Plasma cortisol concentrations were estimated in duplicate using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA). Prior to assay, samples were diluted 1:4 in PBS gel buffer. Assay procedures were modified with the addition of 0.5 and 2.5 μg/dl concentrations of standards along with the provided range of 1.0–49 μg/dl. Assay sensitivity has been determined to be 0.26069 μg/dl. This assay procedure has been validated and used with titi monkeys (Bales et al., 2007, Jarcho et al., 2011, Ragen et al., 2013). Intra- and inter-assay CVs for cortisol were 5.01 and 3.67, respectively. All data points had CVs lower than 10%.
2.5 Experiment 1
The first goal of Experiment 1 was to determine if opioid manipulation via morphine or naloxone would affect affiliative behavior in males upon reunion with their pair-mate after a brief separation. The second goal was to observe whether administration of the KOR agonist, U50,488, would sustain the separation distress response in males after they have been reunited with their pair-mate after a brief separation. To achieve this, the female pair-mate and any offspring were removed from the home cage for 30 minutes and remained out of visual and auditory range. Separation of the mate is likely to result in an increase in isolation peeps, locomotion, and HPA activity; separation from the offspring however, has previously been found to neither elicit distress behavior nor activate the HPA system (Mendoza and Mason, 1986b). We removed the pair-mate and offspring from the home cage and not the male because we only wanted to observe the effects of separation from a pair-mate and not the effects of a combined stressor of separation from a pair-mate and exposure to a novel environment. The 30-minute session was filmed with the lone male being the focal animal, and an observer blind to the drug condition later scored separation behaviors such as isolation peeps and locomotion. After the 30-minute separation, the male was captured, blood was sampled, and an injection of a saline vehicle, morphine (0.25 mg/kg), naloxone (1.0 mg/kg), or U50,488 (0.01, 0.03, and 0.1 mg/kg) was administered IM. The male and his pair-mate were then returned to their home cage for 30 minutes. At the end of the 30-minute session, the male was recaptured and underwent a second blood draw. The second 30-minute session was filmed with the male being the focal animal. The timing of separation, drug administration, and reunion was based on Kalin et al. (1995) which found changes in physical contact between infant and mother macaques in response to opioid manipulation. Filmed sessions were later scored for both separation-related and affiliative behaviors (see Table 2 for ethogram). At the end of the entire 60-minute session any offspring were returned to their home cage. Subjects received all five drug treatments in a pseudo-counterbalanced fashion. Animals were tested once per week. It is unlikely that the drugs used had long lasting effects on behavior or HPA activity because the drugs used have a short duration of action, they were used acutely, and there was a seven day washout period between test sessions (Berkowitz, 1976, Pascoe et al., 2008, Wand et al., 2011).
Table 2.
Ethogram listing measured behaviors
| Behavior* | Description |
|---|---|
| Social Behaviors | |
| Contact (sec) | Passive physical contact between subjects that does not involve tail-twining |
| Female Approach | Female moves within <6 inches of the male |
| Female Breaks Contact | Female moves body and breaks contact with male |
| Female Initiates Contact | Female moves body and initiates contact with male |
| Female Leave | Female moves away from the male to a distance >6 inches |
| Grooming (sec) | Male combs through the fur of the female with his hands and/or mouth |
| Grooming Solicitations | Male presents body part in front of the female to be groomed |
| Male Approach | Male moves within <6 inches of the female |
| Male Breaks Contact | Male moves body and breaks contact with female |
| Male Initiates Contact | Male moves body and initiates contact with female |
| Male Leave | Male moves away from the female to a distance >6 inches |
| Proximity (sec) | Subject is within arm’s reach (~6 inches) of female |
| Receive Grooming (sec) | The focal animal is being groomed by the attachment figure |
| Tail-Twine (sec) | Sitting side-by-side with tails wrapped around each other for at least one rotation |
| Total Passive Contact (sec) | Combined time spent in contact and tail-twining |
| Separation Behaviors | |
| Isolation Peeps | Short, high pitched vocalizations which usually occur in rapid succession |
| Neutral Behaviors | |
| Locomotion (sec)§ | Male displaces his body by at least one body length |
| Scratch (sec) | Rapid and repeated raking of the fur or skin with own hand or foot |
Behaviors followed by (sec) are durations measured in seconds. All other behaviors are frequencies.
Also an indicator of separation distress and behavioral arousal
The dose of morphine was based on the finding that it had no effect on motor behavior in a previous study of titi monkeys (Ragen et al., 2013). The dose of naloxone was chosen due to its ability to increase affiliative behavior in juvenile macaques (Schino and Troisi, 1992) as well as elicit an increase in cortisol concentrations in titi monkeys (Ragen et al., 2013). U50,488 was chosen as the KOR agonist due to its high specificity for KOR (Emmerson et al., 1994) as well as its ability to increase ultrasonic vocalizations in rat pups and even induce ultrasonic vocalizations when they are with their littermates, a situation where ultrasonic vocalizations do not occur (Carden et al., 1991, Carden et al., 1994). Additionally, U50,488 has consistently been shown to produce conditioned place aversions (McLaughlin et al., 2006, Land et al., 2008, Land et al., 2009). The doses of U50,488 were chosen to avoid severe sedation, emesis and vomiting, which were based on observations in squirrel monkeys and rhesus macaques (Dykstra et al., 1987, Cox et al., 2007).
2.6 Experiment 2
The goal of Experiment 2 was to attenuate the separation distress response in male titi monkeys via administration of the KOR antagonist, GNTI. Behaviors of interest were locomotion and isolation peeps since both of these behaviors increase upon separation from a pair-mate (Mendoza and Mason, 1986a, Ragen et al., 2012). Male subjects were caught, underwent a blood draw, and administered GNTI (0.1, 0.5, or 1.0 mg/kg) or saline vehicle. Twenty-four hours after treatment administration, the male, female pair-mate, and offspring were caught and removed from the home cage. The male underwent a second blood draw and returned to his home cage for a 60-minute separation while the female and offspring remained outside of visual and auditory range from the male. After separation, the male was caught and an additional blood sample collected. The 60-minute separation was filmed and later scored for separation behaviors. After testing, the pair-mate and any offspring were returned to the home cage. A 60-minute separation was chosen since it has been effective in producing a strong separation response (Mendoza and Mason, 1986a, Ragen et al., 2013)
Most of the subjects in Experiment 1 were used in Experiment 2 (See Table 1). To prevent any possible long lasting effects of drug or behavioral manipulation we began Experiment 2 three weeks after the end of Experiment 1. This time between experiments likely eliminated issues related to reuse of certain subjects. Doses of GNTI were chosen based on their ability to block the KOR agonist, U50,488, in rhesus macaques (Negus et al., 2002). The separation test was conducted 24 hours after drug administration since that is when GNTI reaches its peak activity in the rhesus macaques (Negus et al., 2002). Testing occurred every other week since it takes two weeks for GNTI to be ineffective at blocking U50,488 (Negus et al., 2002). Doses were administered in a pseudo-counterbalanced order.
2.7 Statistics
Data were analyzed using generalized linear mixed models (GLMM) (Littell et al., 1996) in SAS 9.2 (SAS Institute, Cary, NC). The goal of Experiment 1 was to examine how MOR manipulation influences affiliative (i.e. contact, proximity, etc.) and locomotor behavior and how KOR activation affects separation behavior and locomotor behaviors (i.e. isolation peeps). Therefore for Experiment 1 we performed two different comparisons. The first comparison was the comparison between morphine, naloxone, and vehicle. The second comparison was between the three doses of U50,588 and vehicle. The model for behavior for both of the comparisons included Treatment with animal ID (identification) as a random variable to account for repeated measures. The model for testing opioid manipulation on hormone concentrations included Treatment, Time, and a Treatment × Time interaction with the animal’s ID as a random variable. For Experiment 1, we also made comparisons between treatments on the change in hormone concentration from the first blood sample to second blood sample. This model only included Treatment with animal ID as a random variable. If data were not normally distributed, a square root or quad root transformation was performed to normalize the data. The behaviors Grooming and Receive Grooming were unable to be normalized, and the GLMM was still performed because an F test is still considered to be robust for non-normally distributed data (Feir-Walsh and Toothaker, 1974). Post-hoc comparisons were performed with Least Squares Means. Post-hoc tests only compared drug treatment to vehicle. This allows for clarity of interpretation of results as well as reducing the number of comparisons. To correct for multiple comparisons a Hochberg-Benjamini false discovery rate was used (Benjamini and Hochberg, 1995). Significant p-values were set at 0.05, and all post-hoc tests were two-tailed.
3.1 Results
3.2 Experiment 1
3.2.1 Behavior
Values and statistics for all MOR related behaviors can be viewed in Table 3. There was a significant effect of Treatment on Locomotion (F(2,14)=5.98, P=0.01). In spite of a significant p-value for the omnibus test, post-hoc tests revealed no significant effect of morphine compared to vehicle (F(1,14)=4.28; P=0.114) or naloxone compared to vehicle (F(1,14)=1.36; P=0.195). Since there was a significant effect of Treatment on Locomotion and previous research has found opioid manipulation affects locomotion (Ragen et al., 2013) we included Locomotion as a covariate when analyzing the effects of drugs on variables such as Male Approach, Male Leave, Male Initiates Contact and Male Breaks Contact because these variables could be influenced by alterations in locomotor behavior. Opioid manipulation affected Male Approach (F(2,13)=4.00, P=0.04), Male Initiates Contact (F(2,13)=4.98, P=0.025, Figure 1A), and Female Breaks Contact (F(2,14)=17.38, P=0.0002, Figure 1B). There was a trend for morphine to reduce the number of Male Approaches compared to vehicle (F(1,13)=5.76; P=0.064). Morphine resulted in significantly fewer Male Initiates Contact compared to vehicle (F(1,13)=6.40; P=0.05). When males were administered morphine there was a significant decrease in Female Breaks Contact compared to vehicle (F(1,14)=12.96; P=0.006). Furthermore, when males were administered naloxone there was a significant increase in Female Breaks Contact compared to vehicle (F(1,14)=5.02; P<0.042). Since Male Initiates Contact followed a similar pattern to that of Female Breaks Contact we wanted to explore whether the effects of drug administration on the males were correlated to those found in females. Male Initiates Contact was positively correlated with Female Breaks Contact (r=0.73; P<0.0001) (Pearson’s product-moment correlation) (Figure 2). This correlation included data points from the vehicle, morphine, and naloxone conditions in all eight subjects. MOR manipulation had no effect on Contact, Female Approach, Female Initiates Contact, Female Leave, Grooming, Grooming Solicitations, Male Breaks Contact, Male Leave, Proximity, Receive Grooming, Tail-Twine or Total Passive Contact (P>0.05).
Table 3.
Values (Mean ± SEM) for behaviors measured when subjects were given vehicle, 1.0 mg/kg of naloxone, and 0.25 mg/kg of morphine. Post-hoc test only compare drug treatment to vehicle
| Condition
| |||||
|---|---|---|---|---|---|
| Behavior | Vehicle | 1.0 mg/kg Naloxone | 0.25 mg/kg Morphine | F-Statistic | P-value |
| Contact | 621.4±88.8 | 543.4±152.7 | 541.9±171.7 | 0.18 | 0.83 |
| Female Approach | 4.5±1.8 | 3.6±1.3 | 1.6±0.6 | 1.93 | 0.18 |
| Female Breaks Contact | 6.9±1.3 | 9.8±1.6* | 2.3±0.7** | 17.38 | <0.001 |
| Female Initiates Contact | 4.0±1.6 | 5.3±1.3 | 1.9±0.5 | 2.97 | 0.08 |
| Female Leave | 6.5±1.5 | 8.4±2.3 | 8.1±5.6 | 0.96 | 0.41 |
| Grooming | 1.4±1.4 | 1.4±1.4 | 1.9±1.9 | 0.03 | 0.97 |
| Grooming Solicitations | 0.1±0.1 | 0±0 | 0.4±0.2 | 2.88 | 0.09 |
| Locomotion | 41.9±11.8 | 84.9±43.6 | 17.3±7.7 | 5.98 | 0.01ˆ |
| Male Approach | 6.4±1.7 | 11.3±4.3 | 3.1±1.2 | 5.32 | 0.04ˆ |
| Male Breaks Contact | 4.6±2.5 | 6.5±2.0 | 2.2±1.2 | 2.86 | 0.09 |
| Male Initiates Contact | 8.0±2.1 | 11.5±3.1 | 3.4±1.6* | 5.62 | 0.03 |
| Male Leave | 3.8±1.8 | 5.9±3.1 | 1.5±0.8 | 2.00 | 0.17 |
| Proximity | 131.4±53.5 | 167.0±40.4 | 100.4±49.4 | 1.25 | 0.32 |
| Receive Grooming | 10.9±10.3 | 11.6±10.7 | 56.0±53.2 | 0.16 | 0.86 |
| Scratch | 40.8±14.8 | 17.8±6.6 | 64.4±35.4 | 2.00 | 0.17 |
| Tail-Twine | 458.1±138.5 | 450.4±136.3 | 635.0±230.0 | 0.47 | 0.63 |
| Total Passive Contact | 1079±190.6 | 993.8±171.5 | 1176.88±220.1 | 0.55 | 0.59 |
Bold significant omnibus comparison
≤0.05 compared to Vehicle
<0.01 compared to Vehicle
No significant difference between drug and vehicle
Fig. 1.
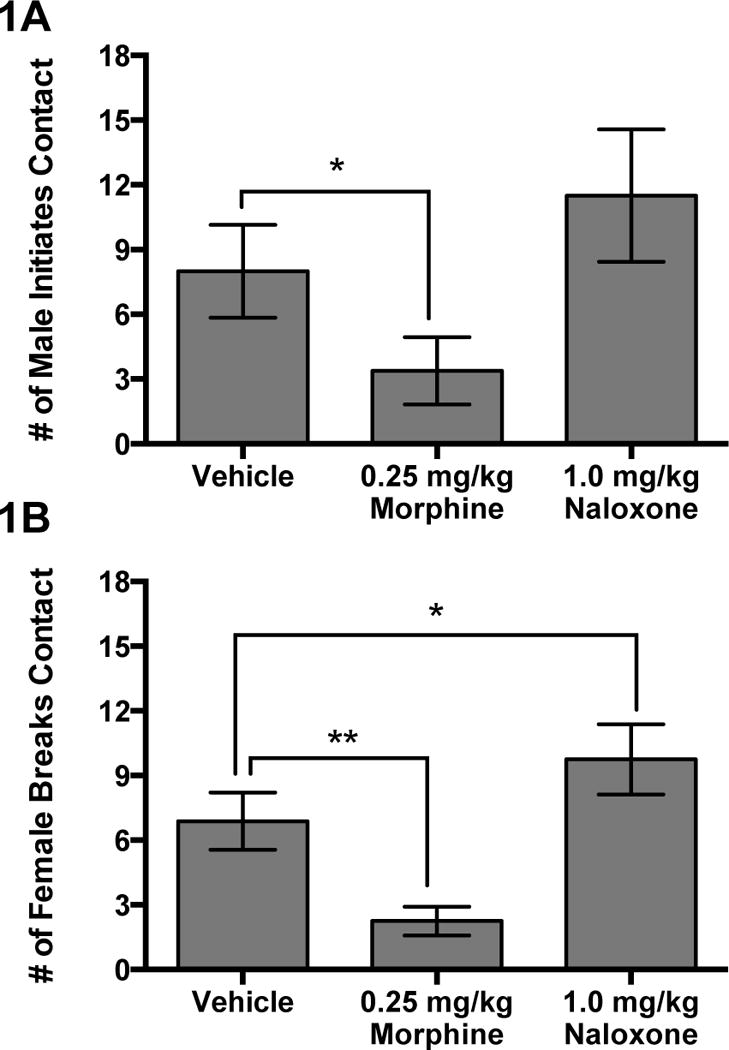
Mean (±SEM) (A) number of Male Initiates Contact and (B) number of Female Breaks Contact comparing morphine and naloxone to vehicle. *p≤0.05, **p<0.01,
Fig. 2.
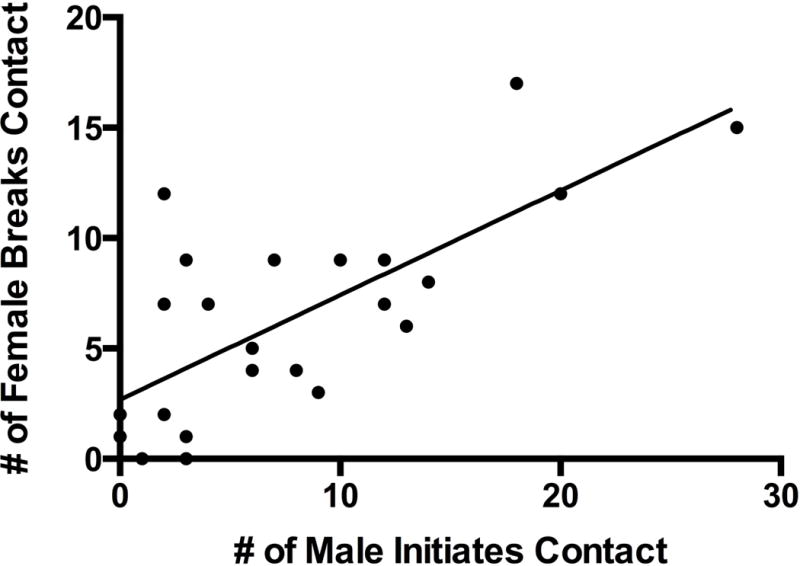
Correlation between Male Initiates Contact and Female Breaks Contact.
For KOR behavioral analyses, there were no significant effects of Treatment on separation related behaviors (P>0.05) (Table 4). Since KOR treatment did not affect separation behavior, we wanted to confirm that the subjects were actually exhibiting a separation response at all. Therefore we performed an analysis on locomotion and isolation peeps collapsed over all treatments comparing when subjects were separated from their pair-mate (first 30 minutes) and when they were reunited with their pair-mate (second 30 minutes). We used a GLMM including Time and ID to analyze the data. As predicted, when males were separated from their pair-mate there was a significant increase in Locomotion (With: 49.13±8.29 seconds, Without: 136.94±8.29 seconds) (F(1,55)=14.26; P=0.0004) and Isolation Peeps (With: 0.32±0.28, Without: 292.13±50.84) (F(1,55)=47.41; P<0.0001). This indicates that the animals showed the predicted distress response upon separation, but that the chosen doses of U50,488 had no effect.
Table 4.
Values (Mean ± SEM) for behaviors measured when subjects were given vehicle, 0.01 mg/kg U50,488, 0.03 mg/kg U50,488, and 0.1 mg/kg U50,488 mg/kg.
| Condition
| ||||||
|---|---|---|---|---|---|---|
| Behavior | Vehicle | 0.01 mg/kg U50,488 | 0.03 mg/kg U50,488 | 0.1 mg/kg U50,488 | F-Statistic | P-value |
| Locomotion | 41.9±11.8 | 50.1±14.9 | 33.9±11.3 | 70.6±25.0 | 1.96 | 0.15 |
| Isolation Peeps | 0.13±0.13 | 0±0 | 1.13±1.13 | 0±0 | 1.00 | 0.41 |
3.2.2 Hormones
A Pre morphine sample underwent a freeze-thaw cycle therefore it was removed from AVP analysis due to AVP breaking down during a freeze thaw cycle. Additionally a Pre Vehicle sample was unable to be collected from one subject so cortisol and AVP data could not be obtained. In the MOR manipulation model on AVP, there was a significant effect of Time (F(1,31)=5.51; P=0.03) but no effect of Treatment (Vehicle: 226.74±16.9 pg/mL; Morphine: 205.62±13.46 pg/mL; Naloxone:257± 22.3 pg/mL) (F(2,31)=2.85; P=0.07) or Treatment × Time interaction (F(2,31)=2.23; P=0.12). Plasma AVP was significantly greater in the Post sample (249.45±17.17 pg/mL) compared to the Pre sample (209.59±11.12 pg/mL) (F(1,31)=5.51; P=0.03). In the MOR manipulation model on cortisol, there was a significant effect of Time (F(1,32)=34.72; P<0.0001 μg/dL), and Treatment (F(2,32)=20.43; P<0.0001), but not a Time × Treatment interaction (F(2,32)=2.61; P=0.09). Levels of plasma cortisol were significantly greater in the Post sample (49.46±2.32 μg/dL) compared to the Pre sample (38.32± 2.29 μg/dL) (F(1,32)=34.72; P<0.0001). Naloxone treatment resulted in significantly higher plasma cortisol levels (50.31±3.67 μg/dL) compared to vehicle (43.94±2.15 μg/dL) (F(1,32)=7.13; P=0.01), and morphine (37.65±3.67 μg/dL) treatment resulted in significantly lower plasma cortisol levels compared to vehicle (F(1,32)=13.72; P=0.0008).
We also analyzed the effects of Treatment on the change in hormone concentrations from the Pre to Post sample. There was a significant effect of Treatment on the change in cortisol levels (F(2,12)=7.66; P=0.007) (Figure 3A). The change in cortisol in response to naloxone was significantly greater compared to vehicle (F(1,12); P=0.016). There was a statistical trend for an effect of Treatment on the change of AVP levels (F(2,11)=3.66; P=0.06) from Pre to Post (Figure 3B).
Fig. 3.
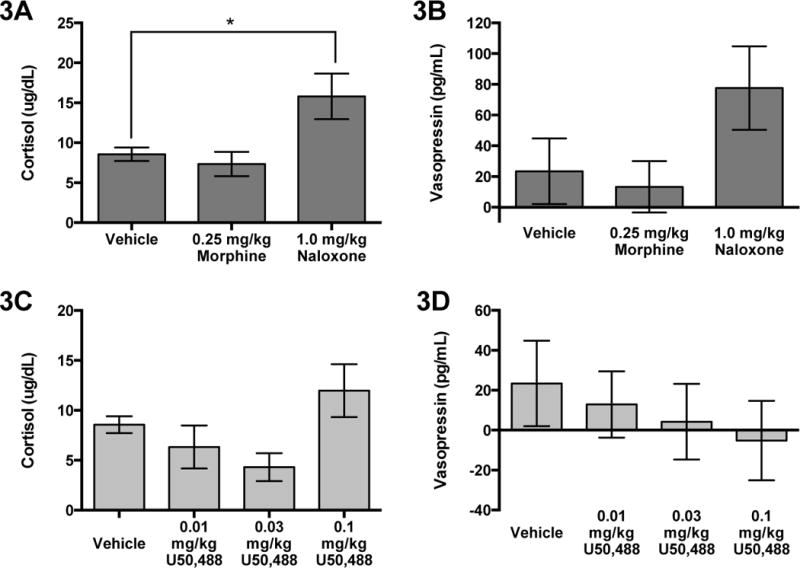
Mean (±SEM) change in (A) cortisol and (B) vasopressin from pre to post samples comparing naloxone and morphine to vehicle. Mean (±SEM) change in (C) cortisol and (D) vasopressin from pre to post samples comparing all three doses of U50,488 to vehicle. *p<0.05, **p<0.01
For KOR manipulation on AVP, there was no significant effect of Time (F(1,14)=0.53; P=0.47) (Pre: 230.71±14.06 pg/mL; Post: 231.86±14.34 pg/mL), Treatment (F(3,14)=0.08; P=0.97) (Vehicle: 226.74±16.9 pg/mL; Low: 225.19±13.00 pg/mL; Medium: 230.19±20.4 pg/mL; High: 234.52±19.70 pg/mL), or Time × Treatment interaction (F(3,14)=0.20; P=0.89). For KOR manipulation on cortisol, there was a significant effect of Time (F(1,14)=12.25; P=0.001), but no effect of Treatment (Vehicle: 43.94±2.15 μg/dL; Low: 41.64±3.4 μg/dL; Medium: 42.61±2.26 μg/dL; High: 45.76±2.69 μg/dL) (F(3,14)=0.85; P=0.47) or Time × Treatment interaction (F(3,14)=0.57, P=0.64). Plasma cortisol levels were significantly greater in the Post (47.63±1.63 μg/dL) sample compared to the Pre (39.21±1.84 μg/dL) sample (F(1,14)=0. 12.25; P=0.001). There was a significant effect of KOR manipulation on the change in cortisol from the Pre to Post sample (F(3,19)=4.19; P=0.02) (Figure 3C). Despite this significant omnibus test there were no significant effects when comparing the low (F(1,19)=0.07; P=0.798), medium (F(1,19)=2.27; P=0.149), or high (F(1,19)=3.60; P=0.073) dose to vehicle. There was no significant effect of KOR manipulation on the change in AVP from the Pre to Post sample (F(3,19)=0.29; P=0.83) (Figure 3D).
3.3 Experiment 2
3.3.1 Behavior
KOR blockade had a significant effect on Locomotion (F(3,20)=3.25; P=0.04) (Figure 4B). Administration of the 0.1 mg/kg dose of GNTI resulted in significantly less Locomotion compared to vehicle (F(1,20)=8.01; P=0.03). KOR blockade with GNTI had no effect on Isolation Peeps (F(3,20)=2.06; P=0.14) (Figure 4A).
Fig. 4.
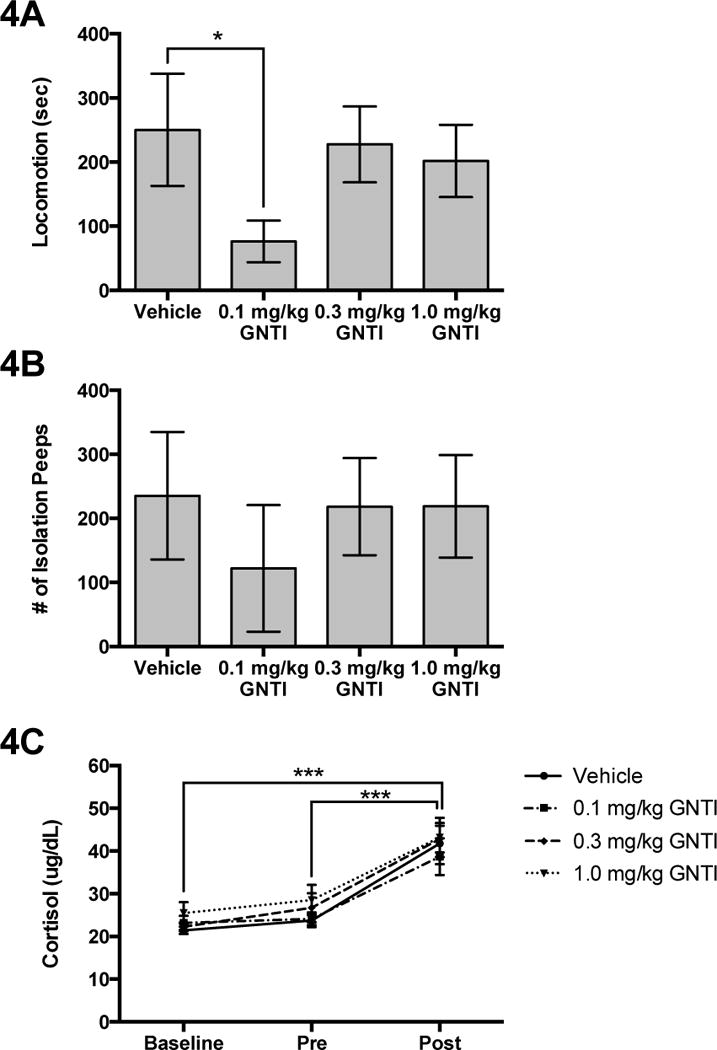
The effects of GNTI on (A) Locomotion, (B) Isolation Peeps, and (C) cortisol when a male is separated from his pair-mate. *p<0.05, ***p<0.0001
3.3.2 Hormones
For KOR blockade on cortisol, there was a significant effect of Time (F(1,71)=71.98; P<0.0001; Figure 4C), but no effect of Treatment (Vehicle: 29.20±2.52 μg/dL; Low: 28.67±2.20 μg/dL; Medium: 31.35±2.5 μg/dL; High: 32.40±2.58 μg/dL) (F(3,71)=1.48; P=0.23) or Treatment × Time interaction (F(6,71)=0.29; P=0.94). Plasma levels of cortisol were significantly greater in the Post sample compared to baseline (F(1,71)=118.37; P<0.0001) and the Pre sample (F(1,71)=93.90; P<0.0001). There was no difference between the baseline and the Pre sample (F(3,71)=1.68; P=0.20).
4.1 Discussion
The present study had two aims. The first was to determine whether manipulation of the opioid system via morphine and naloxone would affect physical affiliative behavior in paired male titi monkeys; an effect observed in primate infant-mother relationships (Kalin et al., 1995) as well as adult, nonmonogamous primate relationships (Keverne et al., 1989, Martel et al., 1995). Unlike previous studies administering morphine and naloxone to prairie voles (Shapiro et al., 1989) and nonmonogamous monkeys (Keverne et al., 1989, Schino and Troisi, 1992, Kalin et al., 1995) we did not find a change in overall physical contact, which includes grooming and total passive contact. These findings do replicate our previous study in titi monkeys (Ragen et al., 2013). Morphine did, however, result in males initiating contact with their pair-mate less frequently. This effect is not due to morphine’s effect on locomotion since it was included as a covariate in the model. It is unlikely that alterations in initiating physical contact in titi monkeys are related to changes in sexual motivation. Even though naltrexone is able to decrease mating bouts in prairie voles during pair-bond formation (Burkett et al., 2011), sexual behavior in established titi monkey pairs is infrequent (Ragen et al., 2012) and rarely observed in the present study (data not presented). The decrease in initiating contacting is also likely not an artifact of nausea since there were no indicators of emesis such as salivation, vomiting, or food aversion. It is important to note that high doses of morphine can bind to KOR, which is observed in macaque brain membranes (Emmerson et al., 1994). It is unknown whether KOR agonists can alter affiliative behavior, but there is evidence that KOR blockade in prairie voles has no effect on affiliative behavior (Resendez et al., 2012).
It is interesting that males administered morphine decreased the number of times they initiated contact with females but that there was no overall change in physical contact. This finding could be explained by the female pair-mate’s behavior, since she would break physical contact less frequently when the male was administered morphine. Furthermore, there was a positive correlation between the number of times the male initiated contact with the female and the number of times she broke contact with him suggesting some coordination of behavior. It has been hypothesized that individuals maintain an optimal level of opioidergic tone via physical contact and disruption of the opioid system (ex. morphine administration) will alter physical contact (Nelson and Panksepp, 1998). The results of the present study support this hypothesis since the over activation of MOR by morphine may have been the reason for a reduction of physical contact being sought out by males and why the females broke contact less frequently. It is important to note that naloxone is relatively non-specific and that the use of more specific MOR antagonist could have different effects. It is possible that selective MOR blockade would be able to increase motivation to engage in physical contact, but the blockade of other opioid receptors prevents this effect. However, the choice of naloxone in the present study was due to its precedent of activating HPA activity and increasing social behavior in other species.
In addition to altering behavior, we found that the separation/reunion paradigm and opioid manipulation affected plasma AVP and cortisol. Levels of AVP and cortisol increased from the pre to post sample. These increases are likely due to the stressor of being captured, undergoing a blood draw, and receiving an injection. Similar effects have previously been found in titi monkeys (Ragen et al., 2013). Additionally, naloxone administration resulted in a greater increase in cortisol compared to vehicle and morphine. This is an effect previously shown in titi monkeys (Ragen et al., 2013), and the HPA activating effects of naloxone are well established (Parrott and Thornton, 1989, Wand et al., 2012). The increase in AVP in response to opioid manipulation only approached significance (p=0.06). A previous study done in titi monkeys found that naloxone increased plasma AVP (Ragen et al., 2013). It is possible that the effect of naloxone on plasma AVP is less robust, and the presence of the pair-mate during reunion buffered the effects of naloxone on AVP release.
Our second goal for Experiment 1 was to activate the KOR and induce a separation distress response in males after they had been reunited with their female pair-mate following a brief separation. We also wanted to examine whether KOR manipulation resulted in changes in plasma AVP and cortisol. The KOR agonist, U50,488, did not sustain separation behaviors (i.e. isolation peeps) when males were reunited with their pair-mate after separation which is contrary to our prediction. This is in contrast to its effects in rat pups (Carden et al., 1996), in which KOR activation produces isolation calls in the presence of litter mates. There could be multiple explanations for why U50,488 had no effect in the current study. First, the effects of KOR agonists on separation distress behaviors could differ depending on species as well as the type of attachment. Another possibility is that administration of the drug during the reunion (and thus the female’s presence) was able to fully buffer any aversive effect of a KOR agonist. If we had administered U50,488 when a male was separated from his pair-mate, there may have been a potentiation of separation distress behaviors as seen by Carden et al. (1994). Another, but not mutually exclusive explanation, is that the dose of U50,488 was not high enough to induce separation distress behaviors. The doses of U50,488 used in the present study were relatively low and were chosen to prevent emesis and extreme sedation, which could have confounded the separation distress response (Cox et al., 2007). It is also possible that if we had removed the male from the home cage in addition to the separation the stress level would have been heightened enough for U50,488 to have a greater effect and therefore sustain a separation distress response when reunited with his pair-mate in the home cage.
The effects of KOR activation increasing cortisol were not robust. The increase in cortisol in response to the high dose of U50,488 only approached significance compared to vehicle (p=0.07). This is not similar to the findings in previous studies (Calogero et al., 1996, Pascoe et al., 2008, Ranganathan et al., 2012). It is likely that the cause of the null effect is due to low doses of U50,488 that were chosen.
The reason for Experiment 2 was examine further examine if the KOR is involved in the separation distress response and we predicted that KOR blockade with GNTI would attenuate the separation distress response in male titi monkeys. KOR blockade has been found to attenuate aggressive behavior in paired prairie vole males when exposed to a stranger male, an indicator of an established pair-bond (Resendez et al., 2012). We found that GNTI was able to attenuate the locomotor component of the separation distress response, but it did not have a significant effect on isolation vocalizations. This effect is likely not due to GNTI impacting gross locomotion since only very high doses GNTI can have a sedative effect (Negus et al., 2002), and the present study found that only the lowest dose of GNTI had a significant effect. Higher doses of GNTI may not be affecting locomotion in titi monkeys because they may be blocking MOR (Metzger et al., 2001). Despite GNTI’s great specificity for KOR, mutations in the MOR can result in GNTI having a greater affinity towards the MOR (Metzger et al., 2001). Therefore, at higher doses GNTI may be blocking MORs, which results in an increase in locomotion in titi monkeys separated from their pair-mate (Ragen et al., 2013). Even though GNTI was able to attenuate the behavioral component of the separation distress response, it did not alter the effect of social separation on HPA activation. This finding is similar to previous research by McLaughlin and colleagues (2006) who found that KOR blockade was able to reduce the negative affective component of a stressor but not the endocrine response. The lack of effects could also be due to the repeated use of GNTI, since to our knowledge repeated use of GNTI and its effects on HPA activity has not been studied. The data from Experiment 2 suggest that KOR plays a role in the behavioral but not the physiological separation distress response potentially by reducing the negative affective component of separation. GNTI could have had a greater effect on brain areas responsible for the affective components of KOR activation, such as the nucleus accumbens, dorsal raphe, ventral tegmental area (Land et al., 2009, Chefer et al., 2013), compared to areas that more directly activate the HPA axis (i.e. hypothalamus).
This study provides additional support to the idea that the opioid system regulates different components of the pair-bond in titi monkeys. More specifically, the opioid system could be playing a role in maintaining the pair-bond. The MOR system may be acting as a positive reinforcer for pair-maintenance whereby MORs are activated upon reunion after a separation and produce a positive affective state. In support of the hypothesis that individuals aim to maintain a homeostatic level of MOR activity, over activation of MORs upon reunion with morphine would result in a decrease in motivation to initiate contact. In contract to MOR, KOR may act as a negative reinforcer to promote proximity maintenance. Upon involuntary separation there may be KOR activation and an induction of a negative affective state which would result in distress behaviors. Therefore, if you were to block KORs with an antagonist, there would be an attenuation of separation behavior. The idea that KORs act as a negative reinforcer to promote pair-bond maintenance is supported by studies done in prairie voles (Resendez et al., 2012, Resendez and Aragona, 2013). Future research will map MOR and KOR binding in the titi monkey brain. Mapping the location of MOR and KOR binding in the titi monkey brain would provide insight into whether the role of MORs and KORs are due to differences in their neuroanatomical locations or just differences in how these receptors function. It would also provide information on where opiate drugs are acting to produce behavioral and physiological changes.
Acknowledgments
Funding was provided by the Good Nature Institute, NICHD: HD053555, Office of Research Infrastructure programs: Grant P51OD01107. We would like to acknowledge the California Primate Research Center research services and husbandry for their daily care of the animals, as well as Dr. Angela Colagross-Schouten and the veterinary staff for their excellent veterinary care. We are grateful to Rebecca Larke and Dr. Michael Jarcho for their invaluable help in running test sessions. We would also like to thank Dr. Sara Freeman for help with constructing the figures.
ABBREVIATIONS
- AVP
vasopressin
- BBB
blood-brain-barrier
- GLMM
generalized linear mixed model
- GNTI
5′-Guanidinyl-17-(cyclopropylmethyl)-6,7-dehydro-4,5α -epoxy-3,14-dihydroxy-6,7-2′,3′-indolomorphinan dihydrochloride
- HPA
hypothalamic-pituitary-adrenal
- KOR
κ opioid receptor
- MOR
μ opioid receptor
- U50,488
(±)-trans-U50,488
References
- Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci U S A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang ZX. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. Ilar J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, Mendoza SP. Validation of oxytocin and vasopressin plasma assays for primate: What can blood tell us? [Abstract] Am J Primatol. 2005;66:73. [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B-Methodol. 1995;57:289–300. [Google Scholar]
- Berkowitz BA. The relationship of pharmacokinetics to pharmacological activity: Morphine, methadone and naloxone. Clin Pharmacokinet. 1976;1:219–230. doi: 10.2165/00003088-197601030-00004. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The Runner’s High: Opioidergic Mechanisms in the Human Brain. Cereb Cortex. 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero AE, Scaccianoce S, Burrello N, Nicolai R, Muscolo LAA, Kling MA, Angelucci L, Dagata R. The kappa-opioid receptor agonist MR-2034 stimulates the rat hypothalamic-pituitary-adrenal axis: Studies in vivo and in vitro. J Neuroendol. 1996;8:579–585. [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-day-old, 10-day-old and 18-day-old rat pups in isolation and the home cage. Dev Psychobiol. 1994;27:65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- Carden SE, Hernandez N, Hofer MA. The isolation and companion comfort responses of 7- and 3-day-old rat pups are modulated by drugs active at the opioid receptor. Behav Neurosci. 1996;110:324–330. doi: 10.1037//0735-7044.110.2.324. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Backman CM, Gigante ED, Shippenberg TS. Kappa Opioid Receptors on Dopaminergic Neurons Are Necessary for Kappa-Mediated Place Aversion. Neuropsychopharmacology. 2013;38:2623–2631. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H, Togasaki DM, Chen L, Langston W, Di Monte DA, Quik M. The selective kappa-opioid receptor agonist U50,488 reduces L-dopa-induced dyskinesias but worsens parkinsonism in MPTP-treated primates. Exp Neurol. 2007;205:101–107. doi: 10.1016/j.expneurol.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta, and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Feir-Walsh BJ, Toothaker LE. An empirical comparison of the ANOVA F-test, normal scores test, and Kruskal-Wallis test under violation of assumptions. Educ Psychol Meas. 1974;34:789–799. [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. J Pers Soc Psychol. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Burkett JP, Young LJ. Neuroanatomical distribution of mu-opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non-monogamous meadow voles (Microtus pennsylvanicus) Neuroscience. 2013;244:122–133. doi: 10.1016/j.neuroscience.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quartely Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R. SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996. p. 633. [Google Scholar]
- Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Dev Psychobiol. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SP. Squirrel monkeys. In: Poole T, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. Vol. 1. Oxford: Blackwell Science Ltd; 1999. pp. 591–600. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiol Behav. 1986a;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Animal Behaviour. 1986b;35:1336–1347. [Google Scholar]
- Metzger TG, Paterlini MG, Ferguson DM, Portoghese PS. Investigation of the selectivity of oxymorphone- and naltrexone-derived ligands via site-directed mutagenesis of opioid receptors: Exploring the ‘address’ recognition locus. J Med Chem. 2001;44:857–862. doi: 10.1021/jm000381r. [DOI] [PubMed] [Google Scholar]
- Munro TA, Huang XP, Inglese C, Perrone MG, Van’t Veer A, Carroll FI, Beguin C, Carlezon WA, Colabufo NA, Cohen BM, Roth BL. Selective kappa Opioid Antagonists nor-BNI, GNTI and JDTic Have Low Affinities for Non-Opioid Receptors and Transporters. PLoS One. 2012;8:9. doi: 10.1371/journal.pone.0070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. Kappa opioid antagonist effects of the novel kappa antagonist 5′-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology (Berl) 2002;163:412–419. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. Biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Parrott RF, Thornton SN. Opioid influences on pituitary function in sheep under basal conditions and during psychological stress. Psychoneuroendocrinology. 1989;14:451–459. doi: 10.1016/0306-4530(89)90044-9. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33:478–486. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38:2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Mendoza SP, Mason WA, Bales KL. Differences in titi monkey (Callicebus cupreus) social bonds affect arousal, affiliation, and response to reward. Am J Primatol. 2012;74:758–769. doi: 10.1002/ajp.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Rev Neurosci. 2013;24:51–60. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ. μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci. 2013;33:9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-opioids receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Troisi A. Opiate receptor blockade in juvenile macaques: effect on affiliative interactions with their mothers and group companions. Brain Res. 1992;576:125–130. doi: 10.1016/0006-8993(92)90617-i. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiol Behav. 1989;46:719–723. doi: 10.1016/0031-9384(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Frost JJ, Xu XQ, McCaul ME. Naloxone-induced cortisol predicts mu opioid receptor binding potential in specific brain regions of healthy subjects. Psychoneuroendocrinology. 2011;36:1453–1459. doi: 10.1016/j.psyneuen.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Wong DF, Xu XQ, McCaul ME. The relationship between naloxone-induced cortisol and mu opioid receptor availability in mesolimbic structures is disrupted in alcohol dependent subjects. Alcohol. 2012;46:511–517. doi: 10.1016/j.alcohol.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Ko MCH, Rice KC, Woods JH. Effect of opioid receptor antagonists on hypothalamic-pituitary-adrenal activity in rhesus monkeys. Psychoneuroendocrinology. 2003;28:513–528. doi: 10.1016/s0306-4530(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Zis AP, Haskett RF, Albala AA, Carroll BJ. Morphine inhibits cortisol and stimulates prolactin secretion in man. Psychoneuroendocrinology. 1984;9:423–427. doi: 10.1016/0306-4530(84)90050-7. [DOI] [PubMed] [Google Scholar]


