Abstract
Puberty is governed by the secretion of gonadotropin releasing hormone (GnRH), but the roles and identities of upstream neuropeptides that control and time puberty remain poorly understood. Indeed, how various reproductive neural gene systems change before and during puberty, and in relation to one another, is not well-characterized. We detailed the daily pubertal profile (from postnatal day [PND] 15 to PND 30) of neural Kiss1 (encoding kisspeptin), Kiss1r (the kisspeptin receptor), Tac2 (neurokinin B), and Rfrp (RFRP-3, mammalian GnIH) gene expression and day-to-day c-fos induction in each of these cell types in developing female mice. Kiss1 expression in the AVPV/PeN increased steadily over the pubertal transition, reaching adult levels around vaginal opening (PND 27.5), a pubertal marker. However, AVPV/PeN Kiss1 neurons were not highly activated, as measured by c-fos co-expression, at any pubertal age. In the ARC, Kiss1 and Tac2 cell numbers showed moderate increases across the pubertal period, and neuronal activation of Tac2/Kiss1 cells did not vary. Additionally, Kiss1r expression specifically in GnRH neurons was already maximal by PND 15 and did not change with puberty. Conversely, both Rfrp expression and Rfrp/c-fos co-expression in the DMN decreased markedly in the early pre-pubertal stage. This robust decrease of the inhibitory RFRP-3 population may result in diminishing inhibition of GnRH neurons during early puberty. Collectively, our data identify the precise timing of important developmental changes – and in some cases, lack thereof – in gene expression and neuronal activation of key reproductive neuropeptides during puberty, with several changes occurring well before vaginal opening.
Keywords: Puberty, Development, Sexual maturation, Reproduction, Kisspeptin, Kiss1, Kiss1r, RFRP-3, Rfrp, GnIH, Tac2, NKB, GnRH, GPR54
1. Introduction
The onset of puberty is generally defined as the activation of the previously-dormant neuroendocrine reproductive axis (Grumbach, 2002; Ojeda and Skinner, 2006; Plant and Witchel, 2006), reflected by increased secretion of gonadotropin-releasing hormone (GnRH). Several upstream hypothalamic circuits have been implicated in the control and modulation of GnRH secretion, but how and when these various reproductive circuits change during development to potentially time and trigger pubertal GnRH secretion is poorly understood (Kauffman, 2010; Ojeda et al., 2006, 2010; Richter, 2006; Tena-Sempere, 2012; Terasawa et al., 2013). Recognized upstream regulators of GnRH neurons range from stimulatory systems, like kisspeptin and neurokinin B (NKB), to inhibitory systems, such as GABA and RFRP-3 (the mammalian homolog of GnIH), not to mention epigenetic factors (Lomniczi et al., 2013; Semaan and Kauffman, 2013). Mutations in several of these systems have resulted in disrupted puberty in humans and animal models. For example, puberty is impaired in humans or mice lacking kisspeptin (encoded by Kiss1) or its receptor (Kiss1r) (de Roux et al., 2003; Lapatto et al., 2007; Seminara et al., 2003). Similarly, NKB can stimulate the reproductive axis (Billings et al., 2010; Wakabayashi et al., 2010), and humans with mutations in the gene for NKB, Tac2, fail to progress through puberty (Topaloglu et al., 2009; Young et al., 2010).
Developmental alterations in gene expression, protein synthesis, neuronal activation, and secretion of reproductive modulators are likely to be critical aspects driving pubertal progression. However, to date, pubertal changes in most reproductive neural systems have not been examined in sufficient temporal detail. Most studies have only compared gene or protein expression differences before and after puberty, or in some cases at just one or two single points during the pubertal period (which can last several weeks in rodents and years in primates). Thus, little is known about detailed, sequential changes during and throughout puberty. For example, whereas Kiss1 expression and kisspeptin-immunoreactivity are higher in the AVPV/PeN nucleus of adults compared to prepubertal rodents (Clarkson and Herbison, 2006; Clarkson et al., 2009; Han et al., 2005; Semaan et al., 2010; Takase et al., 2009; Takumi et al., 2011; Walker et al., 2012), the specific developmental pattern during multiple sequential days of the pubertal period itself remains underexplored. Data regarding pubertal changes in the kisspeptin population in the ARC similarly lack detailed temporal resolution, with most studies simply comparing prepubertal versus adult animals without focusing in detail on multiple pubertal ages in between. Moreover, the reported pubertal patterns of Kiss1 changes (or lack thereof) in the ARC are highly conflicting, often owing to inconsistencies and differences in experimental design, species, sexes, specific age(s) examined, methodology (e.g., qPCR versus in situ hybridization versus immunohistochemistry), and type of measure reported (e.g., cell number versus total expression levels) (Bentsen et al., 2010; Gill et al., 2010, 2012; Han et al., 2005; Lomniczi et al., 2013; Navarro et al., 2012; Takase et al., 2009; Takumi et al., 2011;Walker et al., 2012). The same caveats and limitations, in terms of inconsistencies in ages, sexes, and measures examined, also apply to newer identified reproductive players. For example, Tac2, which is coexpressed in virtually all ARC Kiss1 cells, has been compared between prepubertal and pubertal rodents and found to be higher in the latter (Gill et al., 2012; Navarro et al., 2012), but the temporal resolution of the observed changes were not studied in detail (only every 4–8 days), nor were temporal changes in Tac2 levels compared to changes in other reproductive genes or pubertal markers (e.g., vaginal opening). Likewise, RFRP-3, an inhibitor of the mammalian reproductive axis (Anderson et al., 2009; Ducret et al., 2009; Kriegsfeld et al., 2006; Wu et al., 2009), has been examined thus far at only sparse stages of development, and not yet during puberty. Interestingly, Rfrp expression in the mouse brain is higher in juveniles than adults (Poling et al., 2012), but exactly when or in what manner Rfrp expression levels change in peri-pubertal animals is unknown.
Previous examinations of reproductive gene differences primarily before and after puberty have left a critical gap regarding information on successive daily changes during and throughout the pubertal period. Furthermore, most previous reports have only studied one protein/gene, and it is therefore unknown how different reproductive factors (kisspeptin, NKB, RFRP-3, etc.) change during puberty in relation to one another. Here, we studied key developmental changes in gene expression and neuronal activation of multiple reproductive neural systems (Kiss1, Tac2, Rfrp) on a refined temporal level – day-by-day – throughout the pubertal period, and compared these day-by-day changes in one gene system to changes in another. We asked (1) which reproductive genes, or neuronal activation of those neurons, change first during the pubertal process and when? (2) Are specific changes in gene expression or neuronal activation gradual over the pubertal period, or is there an acute, rapid increase (or decrease) on a specific day(s)? (3) What is the temporal relationship between pubertal changes in different gene systems (e.g., between kisspeptin and RFRP-3) or to status of vaginal opening (VO), an oft-used external marker of female “puberty” in rodents?
2. Materials and methods
2.1. Animals
C57Bl6 mice were housed at the University of California, San Diego on a 12–12 light–dark cycle (lights off at 1800 h) with food and water available ad libitum. Female mice, generated from 7 breeder pairs, were weighed at postnatal day (PND) 15 (day of birth = PND 1) and again daily starting at weaning (PND 20). Weaned females were housed in groups of 2–3. Vaginal opening (VO), a commonly-used external marker of puberty, was monitored daily from the time of weaning until sacrifice. Mice were sacrificed at PND 15, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, or, for comparison, in adulthood (8–9 weeks; in diestrus stage) (n = 6–8 mice/group). To avoid litter effects, each age group contained mice from at least 3 different litters. All animals were sacrificed between 1100 h and 1300 h, and blood and brains collected. Blood was collected using Microtainer separator tubes and the serum was isolated for hormone measurements. Individual serum blood samples were assayed for LH by the University of Virginia’s Ligand Assay Core (Charlottesville, VA), using a sensitive mouse sandwich radioimmunoassay with a limit of detectability of 0.04 ng/ml and intra-assay variation of 7%. Brains were immediately frozen on dry ice and stored at −80 °C. All experiments were conducted with approval of the local Animal Care and Use Committee.
2.2. Single-label in situ hybridization (ISH)
Frozen brains were cut on a cryostat into five sets of 20 µm sections encompassing the entire forebrain and hypothalamus, thaw-mounted onto Superfrost plus slides, and stored at −80 °C. Single-label ISH was performed as previously described (Gottsch et al., 2004; Kauffman et al., 2007; Semaan et al., 2012). Riboprobes utilized were Kiss1 (Gottsch et al., 2004), Kiss1r (Poling et al., 2012), Tac2 (Kauffman et al., 2009), and Rfrp (Poling et al., 2012). Briefly, 1 complete set of slide-mounted sections spanning the entire AVPV/PeN (Plates 26–32 in the Franklin and Paxinos mouse stereotaxic atlas), ARC (Plates 41–52), or DMN (Plates 41–51) was fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air-dried. Radiolabeled (P33) antisense riboprobe (0.05 pmol/ml) was combined with yeast tRNA, heat-denatured, added to hybridization buffer, and applied to each slide (100 µl/slide). Slides were put in a humidity chamber at 55 °C for 17 h. The slides were then washed in 4× SSC, placed into RNAse treatment for 30 min at 37 °C, and washed in RNAse buffer without RNase at 37 °C for 30 min. After a wash in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62 °C, dehydrated in ethanols, and air-dried. Slides were then dipped in Kodak NTB emulsion, air-dried, and stored at 4 °C for 6–8 days (depending on the assay) before being developed and cover-slipped.
2.3. Double-label ISH
Double label ISH was performed as described previously (Di Giorgio et al., 2014; Kim et al., 2013; Poling et al., 2012; Robertson et al., 2009). Briefly, slide-mounted brain sections were treated similarly to single-label ISH with the following modifications. Digoxigenin (DIG)-labeled antisense mouse Gnrh, Kiss1, Tac2, or Rfrp cRNA were synthesized with DIG labeling mix (Roche). Radiolabeled (33P) antisense c-fos or Kiss1r (0.05 pmol/ml) and DIG-labeled (1:500) riboprobes were combined with tRNA, heat denatured, and dissolved together in hybridization buffer. The probe mix was applied to slides (100 µl/slide) and hybridized at 55 °C overnight. After the 62 °C washes on day 2, slides were incubated in blocking buffer for 1 h at room temperature and then incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase [(Roche) diluted 1:500]. Slides were then washed with Buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Labs, CA) for 1 h at room temperature. The slides were then air-dried, dipped in emulsion, stored at 4 °C, and developed 7–10 days later, depending on the assay.
2.4. Quantification and statistical analysis
ISH slides were analyzed with an automated image processing system (Dr. Don Clifton, University of Washington) by a person blind to the treatment group. For single-label experiments, the software counted the number of silver grain clusters representing cells, as well as the number of silver grains in each specific cell cluster (a semi-quantitative index of mRNA content per cell) (Chowen et al., 1990). A relative measure of total mRNA for a specific gene in a brain area was determined by multiplying the total number of cells in that region by the relative amount of mRNA content per cell (Kim et al., 2013; Navarro et al., 2011). Cells were considered positive when the number of silver grains in a cluster exceeded that of background by threefold. For double-label assays, red fluorescent DIG-containing cells were identified under microscopy and the grain-counting software quantified the number of silver grains overlying each cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was >3 (Di Giorgio et al., 2014; Kauffman et al., 2014; Navarro et al., 2011).
For each gene, we quantified total cell number, relative mRNA level per cell, and total relative levels of mRNA in the entire brain region, as any one or more of these different measures might change during puberty. All data are expressed as the mean ± SEM for each group. Differences in group means were assessed via overall ANOVA or 2-way ANOVA with post-hoc analysis determined by Fisher’s LSD test. Statistical significance was set at p < 0.05. Correlation analysis between genes was performed via determination of Pearson’s correlation coefficient and analyzed for significance with Fisher’s r to z test (p < 0.05).
3. Results
3.1. Somatic and hormonal measures before and during puberty
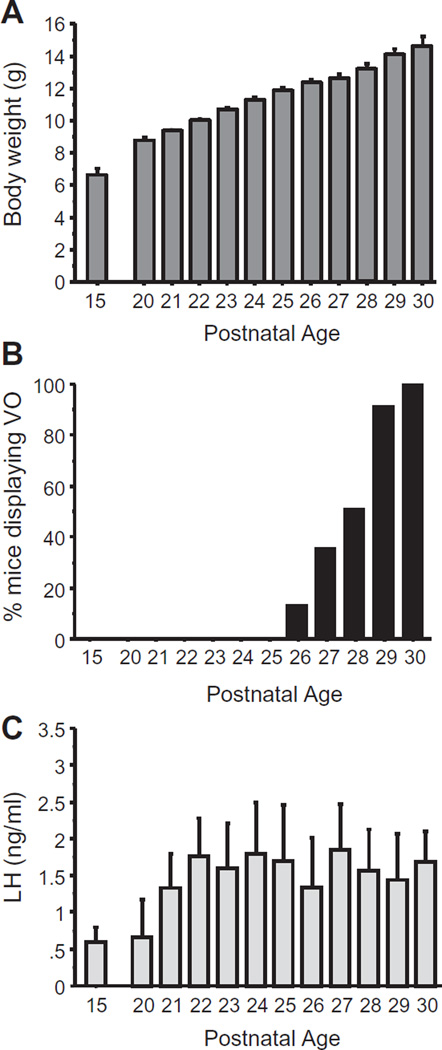
Body weight and the occurrence of VO (an external morphological indicator of female puberty) were measured before and during the pubertal transition (at PND 15 and every day from PND 20 to PND 30). Body weight steadily increased from PND 15 through PND 30, and the average age of VO was ~PND 28 (range of VO occurrence: PND 26–30; Fig. 1). LH levels in serum increased from PND 15 to PND 20 and remained elevated thereafter throughout the pubertal period (p < 0.05; Fig. 1).
Fig. 1.
Somatic and endocrine measures in peri-pubertal female mice. (A) Body weight of female mice over peri-pubertal development. (B) % of female mice displaying vaginal opening (VO), an external marker of puberty in rodents, at various developmental ages. (C) Mean serum LH levels in peri-pubertal females at different ages.
3.2. AVPV/Pen Kiss1 expression in female mice before and during puberty
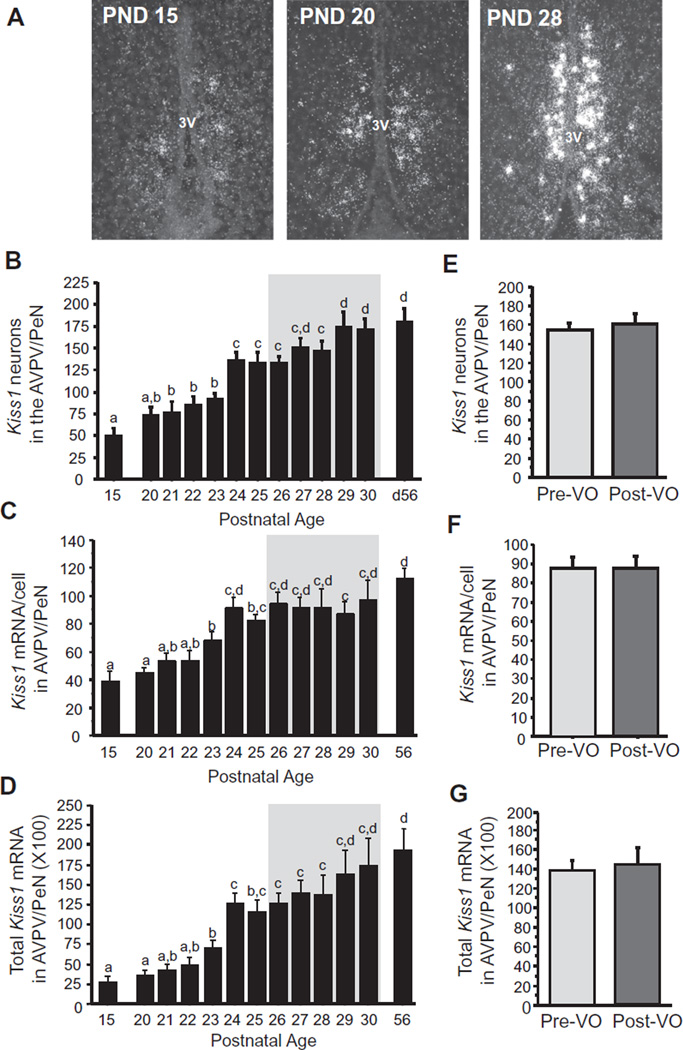
Previous developmental studies measured Kiss1/kisspeptin expression across largely spaced intervals of time, either before and after puberty or on just 1 or 2 days of the entire pubertal period. Here we examined a much more detailed, day-by-day pubertal profile of Kiss1 mRNA expression, looking separately at the AVPV/PeN and ARC populations. In the AVPV/PeN, Kiss1 neuron cell number increased steadily and consistently throughout the peripubertal ages examined, peaking to adult levels around the time of mean VO (~PND 27; Fig. 2). The relative level of Kiss1 mRNA/cell and total Kiss1 mRNA levels in AVPV/PeN also increased steadily across the pubertal period with a similar pattern (Fig. 2). Restricting analysis to just the age range when VO was observed, we determined that, independent of age, the levels of AVPV/PeN Kiss1 expression did not differ between females that had or had not already displayed VO at the time of sacrifice (termed pre-VO and post-VO, respectively; Fig. 2).
Fig. 2.
Kiss1 expression in the AVPV/PeN of female prepubertal and pubertal mice. (A) Representative images of Kiss1 expression, determined by ISH, in the AVPV/PeN of female mice. 3V, third ventricle. (B) Mean numbers of Kiss1 neurons in the AVPV/PeN, (C) mean relative Kiss1 mRNA content per neuron in the AVPV/PeN, and (D) mean relative total Kiss1 mRNA in the AVPV/PeN of female mice between PND 15 and PND 30, with adult diestrus female (PND 56) shown for comparison. The gray shading denotes the period when VO, an external marker of puberty, was observed (PND 26–30). (E–G) Kiss1 cell numbers, mRNA per cell, and total mRNA in the AVPV/PeN in female mice sacrificed just during the VO period (denoted by the gray shaded area in the other graphs) and analyzed based on status of VO, independent of age. Different letters denote significantly different from each other.
3.3. Developmental profile of Kiss1 and Tac2 expression in the ARC before and during puberty
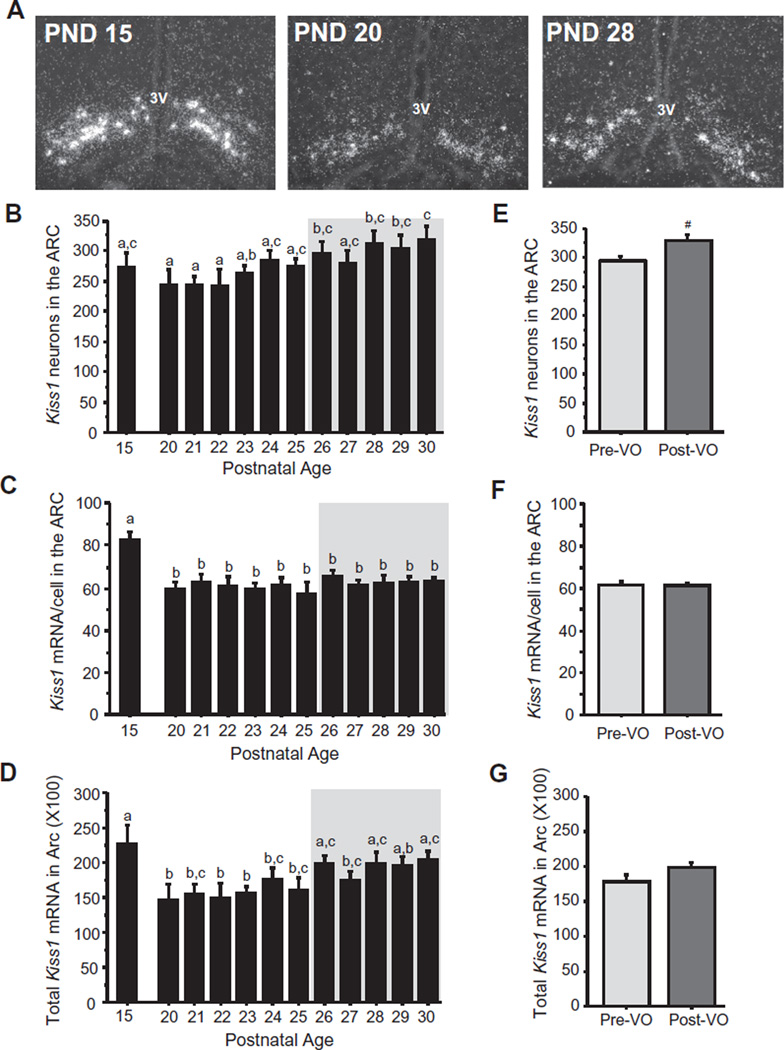
Prior data on rodent ARC Kiss1 expression during peri-pubertal stages are fairly inconsistent, confounded by differing or sporadic ages of analyses, with some reports of pubertal increases in Kiss1 expression and other reports of no changes (Gill et al., 2010; Lomniczi et al., 2013; Navarro et al., 2012; Takase et al., 2009; Takumi et al., 2011). Here, we found that the number of Kiss1 neurons in the ARC rose moderately and significantly over the pubertal transition, showing initial increases around PND 24 and reaching adult levels around the time of VO, with older pubertal ages (PND28–30) being significantly higher than earlier pubertal ages (PND 20–22) (p < 0.05; Fig. 3A and B). Intriguingly, unlike the pattern of cell number, the relative amount of Kiss1 mRNA per cell in the ARC was highest at PND 15, and dropped significantly by PND 20 (p < 0.01), remaining unchanged at all pubertal ages afterward (Fig. 3C). Levels of total relative Kiss1 mRNA in the ARC region were also high on PND 15 and dropped significantly by PND 20 (p < 0.05; Fig. 3D); total ARC Kiss1 levels then remained at this lower level from PND 20 to PND 25, after which they increased again and were significantly higher during most of the VO period (p < 0.05; Fig. 3D). When looking at females sacrificed just during the VO period (~PND26–29) and comparing pre-VO versus post-VO status, ARC Kiss1 cell number showed a strong trend for being higher after VO than before VO that just missed statistical significance (p = 0.054), Fig. 3E). Kiss1 mRNA/cell and total ARC Kiss1 levels did not differ between pre-VO and post-VO during the ages of the VO period (Fig. 3F and G).
Fig. 3.
Kiss1 expression in the ARC of female prepubertal and pubertal mice. (A) Representative images of Kiss1 expression, determined by ISH, in the ARC of female mice. 3V, third ventricle. (B) Mean numbers of ARC Kiss1 neurons, (C) mean relative Kiss1 mRNA content per neuron in the ARC, and (D) mean relative total Kiss1 mRNA in the ARC of female mice between PND 15 and PND 30, with adult diestrus female (PND 56) shown for comparison. The gray shading denotes the period when VO was observed (PND 26–30). Different letters denote significantly different from each other. (E–G) Kiss1 cell numbers, mRNA per cell, and total mRNA in the ARC in female mice sacrificed just during the VO period (denoted by the gray shaded area in the other graphs) and analyzed based on VO status, independent of age. #, non-significant trend (p = 0.054).
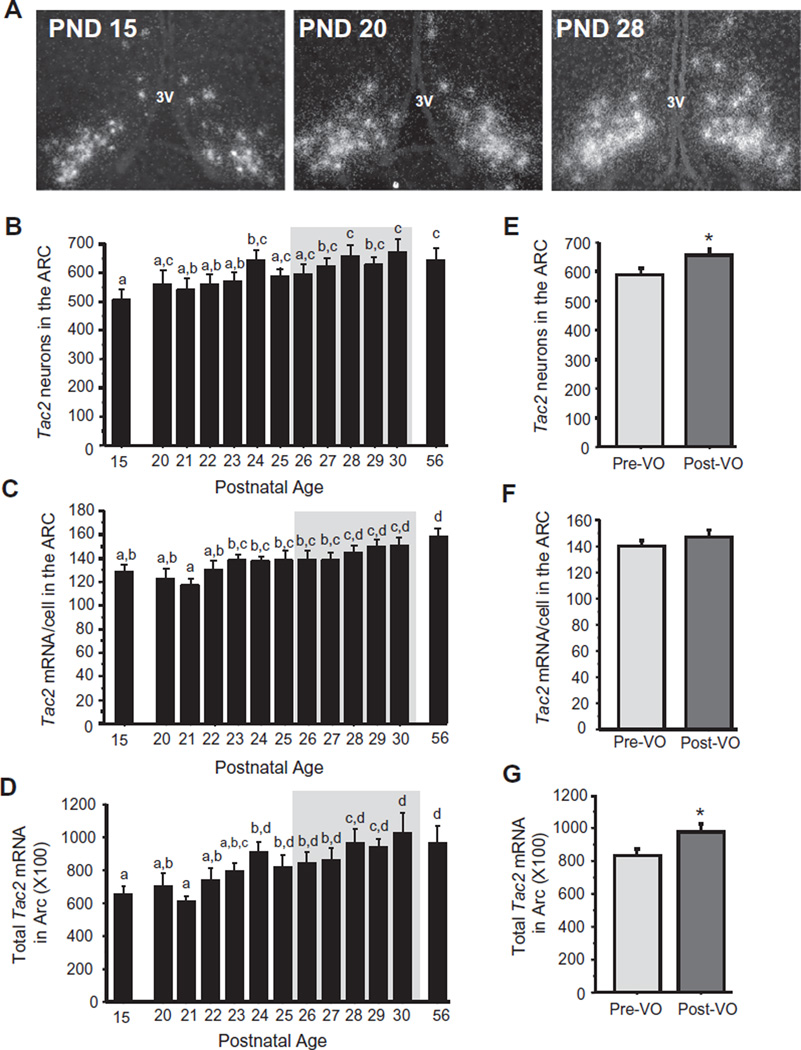
We next analyzed pubertal changes in Tac2 mRNA, which encodes the reproductive neuropeptide NKB and is coexpressed in virtually all ARC Kiss1 cells (often termed KNDy cells). As with ARC Kiss1 cell number, we found a modest gradual increase in Tac2 cell number in the ARC throughout the peri-pubertal ages examined (p < 0.05; Fig. 4B), with cell number reaching adulthood levels around PND 24. Similar gradual increases in the level of Tac2 mRNA per cell were also observed, reaching adult levels around PND 28 (p < 0.05; Fig. 4C). Likewise, total Tac2 mRNA levels in the ARC nucleus demonstrated a moderate increase at later pubertal ages compared to younger juvenile and peripubertal animals (PND 15, 20, 21, 22) (p < 0.05; Fig. 4D), culminating in adult levels around PND 24. During just the ages of the VO period, Tac2 cell number and total Tac2 expression were significantly higher in post-VO females versus pre-VO females, independent of age during this particular period (p < 0.05; Fig. 4E and G).
Fig. 4.
Tac2 expression in the ARC of female prepubertal and pubertal mice. (A) Representative images of Tac2 expression, determined by ISH, in the ARC of female mice. 3V, third ventricle. (B) Mean numbers of ARC Tac2 neurons, (C) mean relative Tac2 mRNA content per neuron in the ARC, and (D) mean relative total Tac2 mRNA in the ARC of female mice between PND 15 and PND 30, with adult diestrus female (PND 56) shown for comparison. The gray shading denotes the period when VO was observed (PND 26–30). (E–G) Tac2 cell numbers, mRNA per cell, and total mRNA in the ARC of female mice sacrificed during the VO period and analyzed based on the status of VO, independent of age. Different letters denote significantly different from each other. *, significantly different from pre-VO status.
3.4. Kiss1 neuronal activation in female mice before and during puberty
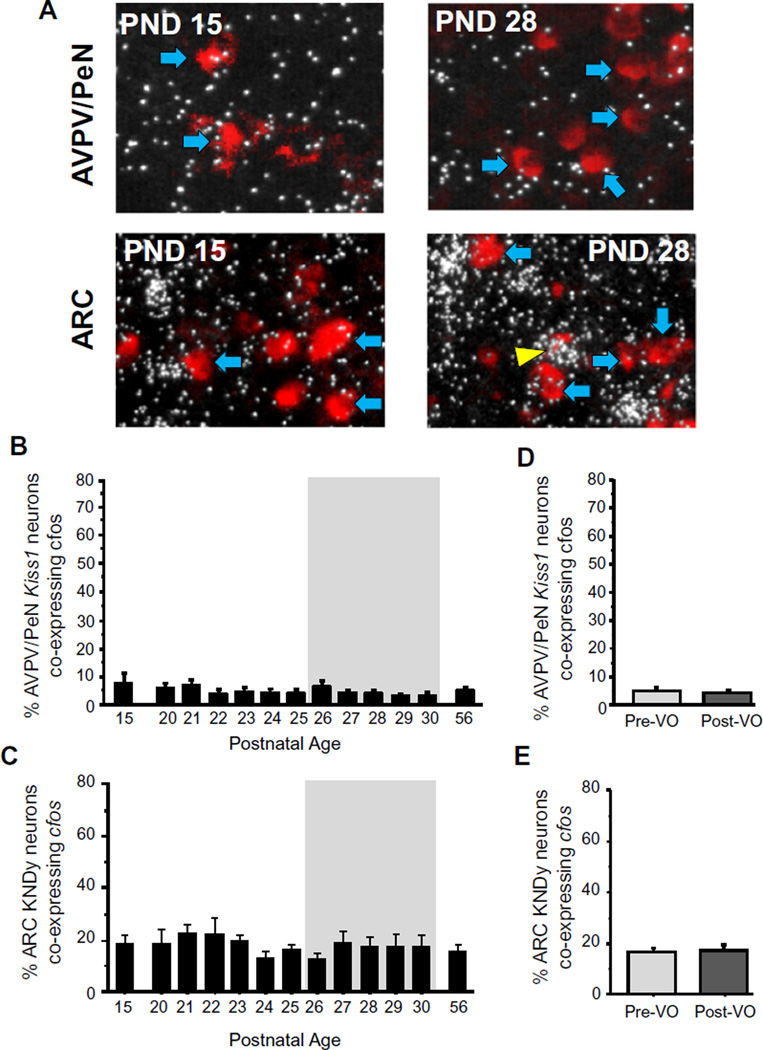
The pubertal profile of Kiss1 neuronal activation has not yet been determined for any species, and could change independent of pubertal changes in gene expression. Whereas Kiss1 levels in the AVPV/PeN increased markedly throughout the pubertal period (Fig. 2), cfos-Kiss1 coexpression in the AVPV/PeN was very minimal at all peripubertal ages, being typically ≤5% on most days examined (Fig. 5B). Moreover, no differences in cfos/Kiss1 co-expression in the AVPV/PeN were noted in females of differing VO status during the VO period (Fig. 5D).
Fig. 5.
Kiss1 neuronal activation during female peri-pubertal development. (A) Representative images of cfos mRNA expression (silver grains) in Kiss1 neurons (red fluorescence) in the AVPV/PeN and ARC of peri-pubertal female mice. Yellow arrowheads denote examples of Kiss1-cfos co-expression. Blue arrowheads denote example Kiss1 cells without cfos. (B and C) Mean percent of AVPV/PeN and ARC Kiss1 neurons expressing cfos in female mice between PND 15 and PND 30, and on PND 56 (diestrus adult female controls). The gray shading denotes the period when VO was observed. (D and E) Kiss1-cfos coexpression in female mice from the VO period analyzed based on each female’s status of VO. Different letters denote significantly different from each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Similar analyses were performed in the ARC for Kiss1 (“KNDy”) neuronal activation by measuring the numbers of these ARC neurons co-expressing cfos at each age. A moderate number of ARC Kiss1 cells co-expressed cfos at any given age, greater than that observed in the AVPV/PeN Kiss1 population. However, the degree of Kiss1 neuronal activation in the ARC did not fluctuate significantly throughout the peri-pubertal ages examined, remaining around 18–20%, nor was there any alteration in the level of ARC Kiss1-cfos co-expression based on VO status (Fig. 5C and E).
3.5. Kisspeptin receptor expression exclusively in GnRH neurons in mice before and during puberty
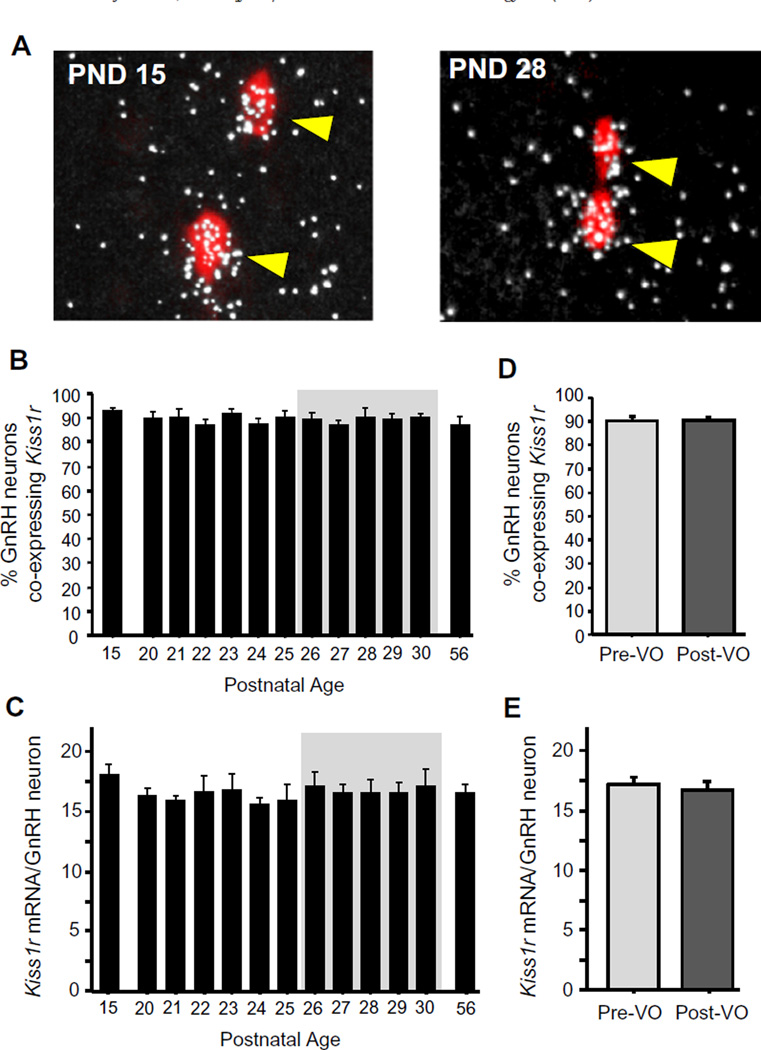
An important aspect of kisspeptin’s potential effects on puberty is its ability to activate GnRH neurons via signaling through Kiss1r. We therefore examined the successive day-by-day pattern of Kiss1r mRNA levels exclusively in GnRH neurons across the peripubertal transition. On PND 15, the percent of Gnrh neurons co-expressing Kiss1R was maximal and already at adult levels (Fig. 6); Kiss1r-Gnrh coexpression levels did not change at all across the pubertal period or in relation to VO (Fig. 6). In addition, the relative levels of Kiss1r mRNA specifically in GnRH neurons were not different at any age between PND 15 and PND 30 and were identical during all pubertal ages to adulthood levels (Fig. 6).
Fig. 6.
Kisspeptin receptor levels in GnRH neurons during female peri-pubertal development. (A) Representative images of Kiss1r mRNA expression (silver grains) in Gnrh neurons (red fluorescence) in peri-pubertal female mice. (B) Mean percent of Gnrh neurons expressing Kiss1r in female mice between PND 15 and PND 30, and on PND 56 (diestrus adult female controls). (C) Mean relative levels of Kiss1r mRNA per GnRH neuron across the pubertal transition. (D and E) Gnrh-Kiss1r coexpression in female mice from the VO period analyzed based on each female’s status of VO. Different letters denote significantly different from each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Peripubertal decreases in Rfrp expression and neuronal activation in the DMN of female mice
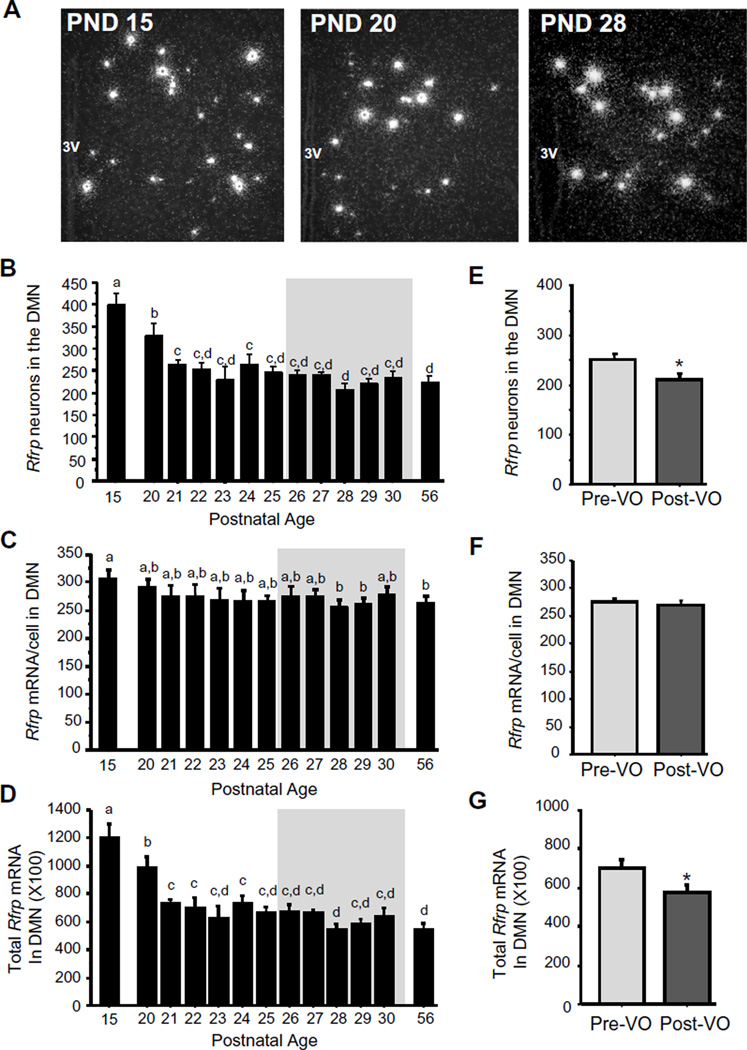
The neuropeptide RFRP-3 can inhibit the reproductive axis (Anderson et al., 2009; Ducret et al., 2009), and Rfrp cell number is lower in adulthood than in juvenile mice (Poling et al., 2012). We therefore determined exactly when this developmental reduction occurs and whether Rfrp neuronal activity was similarly changed during puberty. Interestingly, both the number of Rfrp neurons and total Rfrp mRNA in the DMN region were highly elevated on PND 15 and dropped significantly around PND 20–21 to adult levels (p < 0.05; Fig. 7B and D). The relative level of Rfrp mRNA in each cell remained generally constant (Fig. 7C). During the VO period (~PND26–29), females who had already undergone VO demonstrated significantly lower Rfrp cell number and total Rfrp mRNA levels versus pre-VO females, independent of age (p < 0.01; Fig. 7E and G).
Fig. 7.
Rfrp expression in the DMN of female peri-pubertal mice. (A) Representative images of Rfrp expression, determined by ISH, in the DMN of female mice. 3V, third ventricle. (B) Mean numbers of Rfrp neurons, (C) mean relative Rfrp mRNA content per neuron, and (D) mean relative total Rfrp mRNA in of female mice between PND 15 and PND 30, with adult diestrus female (PND 56) shown for comparison. The gray shading denotes the period when VO was observed (PND26–30). (E–G) Rfrp cell numbers, mRNA per cell, and total mRNA in the DMN in female mice from the VO period (shaded gray area in other graphs) and analyzed based on each female’s VO status, independent of age. Different letters denote significantly different from each other. *, significantly different from pre-VO status.
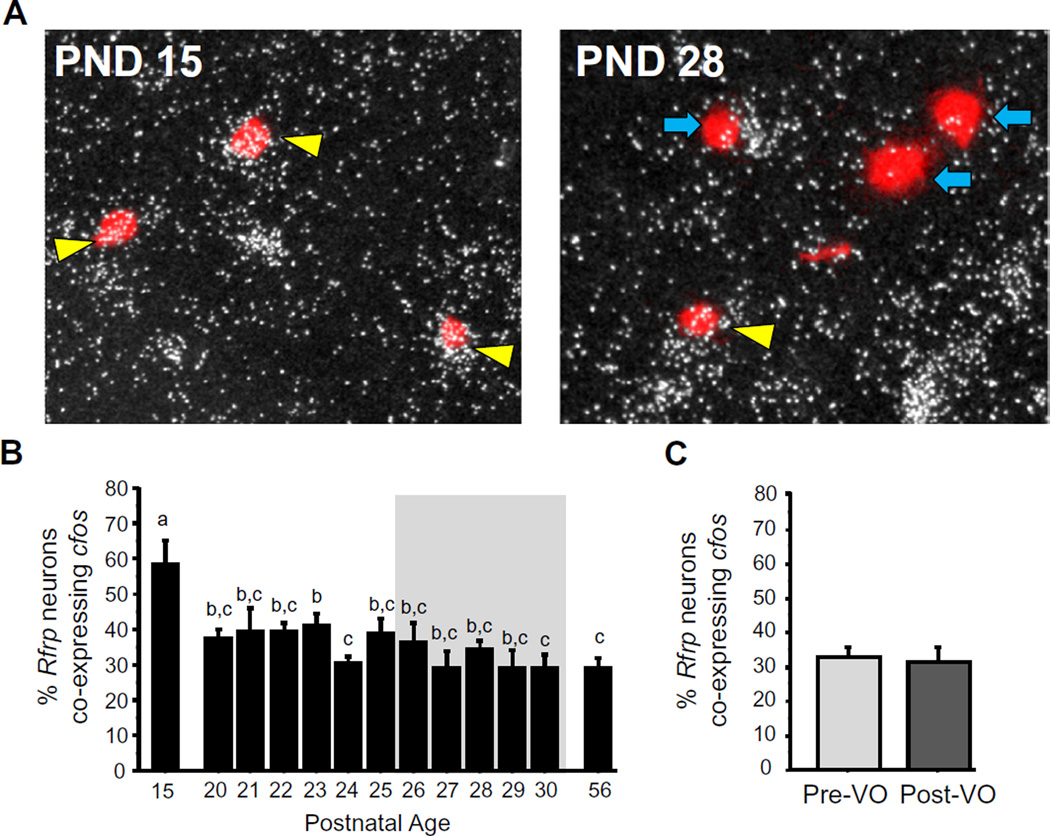
In addition to the marked peripubertal decreases in Rfrp expression levels, the activational status of Rfrp neurons, as measured by c-fos co-expression, also decreased substantially between PND 15 and PND 20 (p < 0.05; Fig. 8A and B), remaining relatively constant thereafter with a slight non-significant trend for further decreases in the later pubertal period.
Fig. 8.
RFRP-3 neuronal activation during female peri-pubertal development. (A) Representative images of cfos mRNA expression (silver grains) in Rfrp neurons (red fluorescence) in the DMN of peri-pubertal female mice. Yellow arrowheads denote examples of Rfrp-cfos co-expression. Blue arrowheads denote example Rfrp cells without cfos. (B) Mean percent of Rfrp neurons expressing cfos in female mice between PND 15 and PND 30, and on PND 56 (diestrus adult female controls). The gray shading denotes the period when VO was observed (PND26–30). (C) Rfrp-cfos coexpression in female mice from the VO period analyzed based on each female’s status of VO, independent of age. Different letters denote significantly different from each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Correlation analysis of peri-pubertal reproductive gene expression
To further analyze the relationship between the various brain measures studied across the pubertal transition in our female mice, we performed a correlation analysis of neural reproductive gene expression and neuronal activation during the pubertal period. Table 1 depicts the Pearson correlation coefficients, demonstrating several significant correlations between the various gene systems during the pubertal period. Some pubertal gene measures were strongly negatively correlated (e.g., Rfrp and AVPV/PeN Kiss1 cells; Rfrp neuronal activation levels and Tac2 cell number, etc), whereas others were strongly positively correlated (e.g., ARC Kiss1 cells and ARC Tac2 mRNA; Rfrp mRNA and Rfrp neuronal activation, etc.) (Table 1).
Table 1.
Correlation matrix showing correlation analysis of neural reproductive gene expression and neuronal activation during the pubertal period of female mice. Values shown are Pearson correlation coefficients, which can range from −1.0 to 1.0, with positive and negative values reflecting a positive correlation or negative correlation, respectively. Values in bold (positive) or italic (negative) are statistically significant (p < 0.05); non-bold, non-italic values are not statistically significant.
| AVPV Kiss1 cells |
AVPV Kiss1 total mRNA |
ARC Kiss1 cells |
ARC Kiss1 total mRNA |
ARC Tac2 cells |
ARC Tac2 total mRNA |
Rfrp cells |
Rfrp total mRNA |
AVPV neural activat. |
ARC neural activat. |
Rfrp neural activat. |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| AVPV Kiss1 cells | 0.884 | 0.422 | 0.206 | 0.399 | 0.469 | −0.521 | −0.560 | −0.200 | 0.228 | −0.383 | |
| AVPV Kiss1 total mRNA | 0.884 | 0.296 | 0.105 | 0.329 | 0.410 | −0.455 | −0.495 | −0.204 | −0.270 | −0.301 | |
| ARC Kiss1 cells | 0.422 | 0.296 | 0.782 | 0.537 | 0.546 | −0.058 | −0.067 | −0.090 | −0.120 | −0.364 | |
| ARC Kiss1 total mRNA | 0.206 | 0.105 | 0.782 | 0.301 | 0.328 | 0.263 | 0.246 | −0.030 | −0.051 | −0.191 | |
| ARC Tac2 cells | 0.399 | 0.329 | 0.537 | 0.301 | 0.898 | −0.211 | −0.243 | −0.132 | −0.107 | −0.354 | |
| ARC Tac2 total mRNA | 0.469 | 0.410 | 0.546 | 0.328 | 0.898 | −0.241 | −0.277 | −0.224 | −0.019 | −0.322 | |
| Rfrp cells | −0.521 | −0.455 | −0.058 | 0.263 | −0.211 | −0.241 | 0.883 | 0.114 | 0.123 | 0.379 | |
| Rfrp total mRNA | −0.560 | −0.495 | −0.067 | 0.246 | −0.243 | −0.277 | 0.883 | 0.050 | 0.176 | 0.471 | |
| AVPV Kiss1 neural activat. | −0.200 | −0.204 | −0.090 | −0.030 | −0.132 | −0.224 | 0.114 | 0.050 | 0.041 | 0.006 | |
| ARC Kiss1 neural activat. | 0.228 | −0.270 | −0.120 | −0.051 | −0.107 | −0.019 | 0.123 | 0.176 | 0.041 | 0.091 | |
| Rfrp neural activat. | −0.383 | −0.364 | −0.364 | −0.191 | −0.354 | −0.322 | 0.379 | 0.471 | 0.006 | 0.091 |
4. Discussion
Several hypothalamic circuits have been implicated in governing the timing and progression of maturation of the reproductive axis, converging on GnRH neurons to trigger puberty onset. The kisspeptin system, along with NKB, is strongly implicated in pubertal development in mammals, including humans (de Roux et al., 2003; Lapatto et al., 2007; Seminara et al., 2003; Topaloglu et al., 2009, 2012; Young et al., 2010). Yet, despite their proposed involvement in the developmental maturation of reproductive capabilities, how these various reproductive neural systems change developmentally has primarily been compared before and after puberty (Clarkson et al., 2009; Gill et al., 2012; Navarro et al., 2004; Poling et al., 2012; Takumi et al., 2011), with far less analysis on their changes during and throughout puberty, either on their own or in relation to one another. Here, we provide the first detailed, day-by-day peripubertal gene expression profiles in developing female mice, as well as neuronal activation patterns via c-fos co-expression, in several key neural populations influencing GnRH.
4.1. Kisspeptin in the AVPV/PeN
Sexual maturation is impaired in humans and mice with mutations in the Kiss1r or Kiss1 genes (de Roux et al., 2003; Seminara et al., 2003; Topaloglu et al., 2012), and exogenous kisspeptin administered to prepubertal animals induces various aspects of puberty (such as increased LH secretion or VO) (Navarro et al., 2004; Shahab et al., 2005). Although in vivo secretion of neuropeptides in mice, especially at younger ages, is nearly impossible to study, work performed in non-human primates demonstrated increased kisspeptin secretion at later stages of puberty versus before puberty (Guerriero et al., 2012). Moreover, ex vivo kisspeptin secretion from hypothalamic explants is higher in female pubertal monkeys compared to juveniles (Keen et al., 2008). However, the neuroanatomical source of this pubertal kisspeptin secretion (AVPV/PeN versus ARC) remains unclear, as does the timing of endogenous kisspeptin’s developmental onset. Previous studies in mice indicated that AVPV/PeN Kiss1 mRNA and kisspeptin protein are undetectable prior to PND 10 and 15, respectively (Clarkson et al., 2009; Semaan et al., 2010), and then increase from PND 15 to adulthood, as assessed every 5 days of age (Clarkson et al., 2009). As in mice, AVPV/PeN kisspeptin cells in rats are not detectable on or before PND 8, but are present by the next age examined (PND 22), and are even higher in adulthood (Takumi et al., 2011). Yet in these previous studies, it remained unclear if AVPV/PeN kisspeptin/Kiss1 gradually increases across puberty or if this increase happens quickly at a potentially important age or pubertal stage. Likewise, whether the increase in AVPV/PeN kisspeptin/Kiss1 occurs before, at, or after changes in other genes was not previously determined. Using a detailed, day-by-day pubertal analysis of Kiss1 mRNA expression, our present findings demonstrate that Kiss1 cell number, Kiss1 mRNA per cell, and total Kiss1 mRNA levels in the AVPV/PeN markedly, but consistently and gradually, increase from PND 15 through PND 30. The daily increases in AVPV/PeN Kiss1 expression occur well before VO and finally resembled adulthood levels around the period of VO (by PND 26–29, depending on the specific measure). The AVPV/PeN kisspeptin system has been implicated in the preovulatory LH surge in adult females, but at present has not yet been linked functionally to puberty. Thus, whether these robust pubertal increases in AVPV/PeN Kiss1 levels are involved in puberty onset or progression currently remains unknown. Interestingly, while AVPV/PeN Kiss1 levels increased consistently with age, AVPV/PeN Kiss1 did not vary with VO status at ages specifically during the VO period, suggesting that Kiss1 increases at that particular period are age-dependent and may not relate to puberty or VO status.
To provide additional insight, we also examined whether the neuronal activation of the AVPV/PeN Kiss1 population also changed with pubertal status. At all developmental ages examined, c-fos co-expression in Kiss1 AVPV/PeN cells was very minimal and did not change over the pubertal period. Thus, despite dramatic increases in Kiss1 levels throughout the pubertal transition, AVPV/PeN Kiss1 neurons do not appear to be activated, as reflected by cfos induction, during this developmental period, at least at the specific times of day examined. This lack of AVPV/PeN Kiss1 neuronal activation at early and mid-pubertal ages is in alignment with the proposed role of these particular Kiss1 neurons not as drivers of pulsatile GnRH but as generators of the preovulatory GnRH/LH surge, an event which does not first occur until the very end of puberty, typically signifying attainment of reproductive capability. Of note, we only studied c-fos and therefore cannot rule out that other less commonly-used markers of neuronal activation might reveal a different result with respect to AVPV/PeN Kiss1 neuronal activation.
4.2. Kisspeptin and NKB in the ARC
Unlike in the AVPV/PeN, ARC Kiss1 expression is readily detectable prenatally and at birth in rodents, and continues to be expressed throughout postnatal development (Cao and Patisaul, 2011; Kumar et al., 2014; Poling and Kauffman, 2012). However, previous data regarding peripubertal changes in ARC Kiss1 gene expression are either lacking, incomplete, or conflicting. Some studies report small increases in ARC Kiss1 expression around early puberty (Bentsen et al., 2010; Takase et al., 2009) and a more dramatic pubertal increase has also recently been reported (Lomniczi et al., 2013). Conversely, other studies have reported no major differences in ARC Kiss1 levels between juvenile and adult rodents (Gill et al., 2010; Han et al., 2005; Navarro et al., 2012), leaving the issue unresolved. Here, our detailed day-by-day assessment determined that between PND 20 and PND 30, the number of ARC Kiss1 neurons and total levels of Kiss1 mRNA in the ARC increased moderately, with significant increases first evident around PND 24–26 and the overall increase from PND 20 to PND 30 being ~25%. However, interestingly, the amount of Kiss1 mRNA per cell in the ARC was highest at PND 15; between PND 15 and PND 20, the level of Kiss1 mRNA per cell dropped ~27% and, correspondingly, the total Kiss1 mRNA in the ARC also decreased during this pre-pubertal stage. This decrease in Kiss1 levels per cell may be due in part to increasing estradiol levels during puberty, since estradiol is known to repress Kiss1 expression in the ARC (i.e., negative feedback) (Smith et al., 2005). It is not clear if or how the pre-pubertal decrease in ARC Kiss1 levels relates to the triggering pubertal onset, but suggests that enhanced kisspeptin synthesis per cell occurs well before the pubertal period. Intriguingly, this matches the fact that female mice lacking estradiol negative feedback in kisspeptin cells initiate very early VO, evident around PND 15 (Mayer et al., 2010).
Kiss1 neurons in the ARC highly co-express Tac2, encoding NKB (Goodman et al., 2007; Navarro et al., 2009; Rance and Bruce, 1994) (the so-called KNDy neurons). NKB can stimulate LH secretion, likely by triggering kisspeptin secretion (Billings et al., 2010; Navarro et al., 2009;Wakabayashi et al., 2010), and mutations in the NKB system impair puberty (Topaloglu et al., 2009; Young et al., 2010). Recently, it was shown that Tac2 mRNA in the MBH of mice (measured with qPCR of MBH dissections) is higher in pubertal than prepubertal mice (Gill et al., 2012), and another study in female rats reported increased MBH Tac2 levels before puberty and in the later pubertal period, but no changes in between (measured every 4–6 days) (Navarro et al., 2012). In both cases, the temporal resolutions of these potentially important developmental increases were not studied in detail, nor were Tac2 levels compared to changes in other genes or pubertal markers. Besides the lack of temporal resolution, a further limitation of these previous findings was that analyses were done on MBH brain dissections, which encompass portions of neighboring non-ARC brain regions which also express Tac2, thereby lacking anatomical resolution. In our present study, we used ISH to focus solely on the ARC Tac2 population (i.e., KNDy neurons) and documented a steady, moderate increase in Tac2 cell number and total Tac2 mRNA levels in the ARC across the peripubertal period, being ~30–35% higher at later pubertal ages (PND 28–30) than at PND 15. Between PND 20 and PND 30, total ARC Tac2 mRNA expression increased by ~30%, which is similar in magnitude to a previous report studying just those two ages using qPCR (Gill et al., 2012). In the present study, an additional comparison of mice before and after VO status, during just the age period when VO is observed, demonstrated notable age-independent increases in both ARC Tac2 and Kiss1 cell numbers, as well as total Tac2 expression, which correlated with VO status. This suggests that elevations in Tac2 and Kiss1 in the ARC may be a good indicator of VO status and, hence, pubertal progress. Unlike Tac2 and Kiss1 gene expression, neuronal activation of ARC KNDy cells did not change significantly over the pubertal period. However, overall neuronal activation levels were notably higher in the ARC Kiss1 versus AVPV Kiss1 cells at all pubertal ages analyzed, suggesting that ARC Kiss1 cells exhibit more neural activity in general at this peripubertal period than AVPV Kiss1 cells, regardless of gene expression levels. The functional significance of this regional Kiss1 difference in neuronal activation is not yet known, but may relate to the proposed involvement of the ARC, but not AVPV/PeN, in driving GnRH pulses (which increase at puberty).
4.3. Kisspeptin receptor in GnRH neurons
In addition to Kiss1, changes in the kisspeptin receptor, Kiss1r, may also be involved in pubertal maturation, though this has received little attention. Although kisspeptin administered to prepubertal rodents and monkeys induces various aspects of precocious puberty (Navarro et al., 2004; Shahab et al., 2005), low kisspeptin doses are less effective at stimulating gonadotropin secretion and GnRH neuronal firing activity in juvenile than adult rodents (Castellano et al., 2006; Han et al., 2005), suggesting that kisspeptin has a reduced ability to activate the GnRH system before puberty. Moreover, in rats of both sexes and female monkeys, though not male mice, hypothalamic Kiss1r expression is higher in adulthood than in juveniles (Han et al., 2005; Navarro et al., 2004; Shahab et al., 2005). However, in almost all cases, Kiss1r was measured in whole hypothalamus, rather than in specific cell-types, preventing identification of which specific neuronal populations the changes occur in. Here, we determined that the level of Kiss1r expression specifically in Gnrh neurons in female mice is already at maximal adult levels by PND 15 and does not vary during any stage of the pubertal transition. A recent mouse study utilizing lacZ expression as a proxy for Kiss1r reported that at some undetermined point between PND 5 and PND 20, there was a significant increase in the number of GnRH cells expressing Kiss1r (Herbison et al., 2010). Our data suggest that this previously-reported developmental increase in Kiss1r-GnRH coexpression occurs before PND 15, because no change in the percent of Kiss1r-Gnrh colocalization was observed in our mice after this age.
We also found that, like the prevalence of Kiss1-Gnrh colocalization, the relative amount of Kiss1r mRNA in Gnrh neurons showed minimal variation during the pubertal period and was virtually equivalent to adult levels at all pubertal ages examined. This suggests that the ability for kisspeptin to signal to its receptor is already present and maximal well before external markers of puberty (such as VO) occur. Previous studies that reported increases in hypothalamic Kiss1r levels between juvenile life and adulthood measured Kiss1r in whole hypothalamus and therefore combined multiple hypothalamic regions and cell-types that may express this receptor. Our present ISH data allow for cell-type specificity and clearly demonstrate no significant pubertal increases in Kiss1r specifically in GnRH neurons. This indicates that the previously-reported developmental increase occurs in non-GnRH cells, the identity and function (reproductive or otherwise) of which remain to be determined.
4.4. RFRP-3 in the DMN
In contrast to kisspeptin and NKB, which stimulate the reproductive axis, RFRP-3 is characterized as an inhibitor of reproductive hormone secretion (Anderson et al., 2009; Ducret et al., 2009). Because puberty onset could include modulation of an upstream “brake” on GnRH secretion, we assessed whether Rfrp neurons (which are located exclusively in the DMN) show notable changes before or during puberty. Neural Rfrp expression in mice was previously examined at a few developmental ages, with total Rfrp cell number being higher in juveniles than adults (Poling et al., 2012), but it was unknown exactly when and how Rfrp levels change in post-juvenile (i.e., pubertal) mice. Strikingly, we found that both the number of Rfrp neurons and total Rfrp mRNA dropped significantly, by 30% and 40%, respectively, between PND 15 and PND 21, after which they stabilized at adult levels. This large decrease in Rfrp expression over the course of a few days may point to this particular peripubertal stage as a critical period for a reduction of RFRP-3-mediated inhibition of GnRH or kisspeptin neurons (Poling et al., 2013). Notably, this drop in Rfrp expression occurs almost a week prior to VO, suggesting that hypothalamic changes in reproductive circuits occur well before external physical signs of pubertal onset. However, interestingly, during the VO period itself, mice that had already undergone VO also had significantly lower Rfrp expression, both in cell number and total mRNA levels (~20% reduction for each), than mice of the same ages that had not yet shown VO. Moreover, unlike the AVPV/PeN and ARC Kiss1 cells, the Rfrp population exhibited a large peripubertal change in neuronal activation. In juvenile animals, the number of Rfrp cells co-expressing c-fos was dramatically higher – by nearly twofold – than on any subsequent pubertal age examined. What is causing the Rfrp neuron activation at PND 15 but not at older pubertal ages is currently unknown, but may potentially comprise an integral part of the pubertal process. However, at present, very little – if anything – is known regarding the identity of upstream or internal factors that regulate Rfrp neurons.
The observed peri-pubertal decreases in Rfrp levels and Rfrp neuronal activation accord with the proposed role of RFRP-3 as an inhibitor of the reproductive axis (Anderson et al., 2009; Ducret et al., 2009), and may indicate that RFRP-3 signaling is reduced prior to, or at, the onset of puberty in order to disinhibit the reproductive axis. Indeed, it has been proposed that upstream networks may control puberty through the relief of inhibition on stimulatory factors, like kisspeptin and NKB, to allow GnRH secretion to be enhanced at puberty (Kauffman, 2010; Lomniczi et al., 2013; Tena-Sempere, 2012; Terasawa et al., 2013). Based on our current findings, RFRP-3 signaling may be one possible aspect of inhibitory control during puberty onset. Despite this possibility, a recent report suggested that RFRP-3 signaling via GPR147 is not crucial for puberty onset, as GPR147 KO mice exhibited normal puberty onset (Leon et al., 2014). However, RFRP-3 can also bind the receptor GPR74, which was not only intact in those KO mice but actually upregulated in some tissues, perhaps providing compensatory pathways and maintaining functional RFRP-3 signaling. Thus, whether RFRP-3 signaling plays a role in pubertal timing still remains to be determined.
4.5. General considerations and conclusions
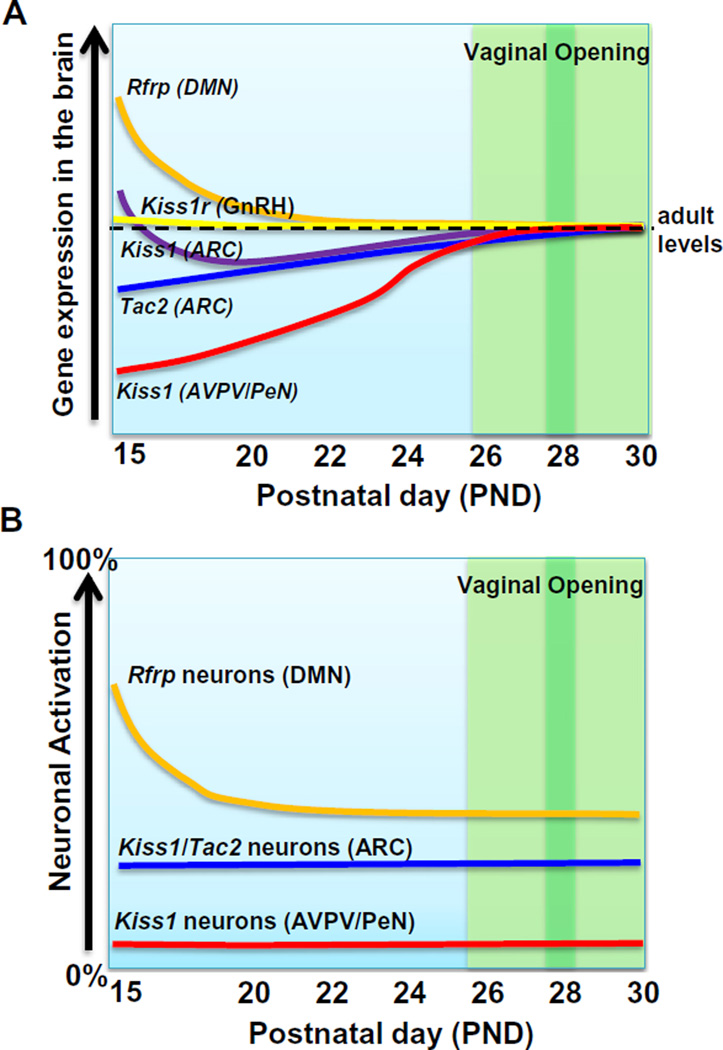
The present study identifies several notable changes in gene expression and neuronal activation at various stages of the pubertal period (summarized in Fig. 9). The timing of the observed changes is interesting for several reasons. First, not all the changes occurred at the same time or over the same number of days, indicating differential regulation of these various reproductive systems during the peripubertal period. For example, RFRP-3 changed dramatically early in the peripubertal period but not later on, whereas ARC Kiss1 and Tac2 changed very gradually and constantly throughout the pubertal period. Kiss1 in the AVPV/PeN, like the ARC, changed throughout the entire period but on a much larger scale day to day. Second, many of the observed changes, including Rfrp and AVPV/PeN Kiss1, occurred well before VO (Fig. 9), an external morphological marker of puberty. Indeed, the Rfrp changes occurred over a week before mean VO, and likewise, AVPV/PeN Kiss1 was already showing notable increases a week before VO. These observations support the idea that pubertal changes in the brain may actually occur much earlier than VO, which is merely a morphological marker of “sufficient” estradiol exposure and, as such, is not a good indicator of puberty onset, but rather of pubertal occurrence or progression. Though many studies of puberty in rodent models use VO as an indicator of the “onset of puberty”, it appears that neuroendocrine puberty in the brain has likely begun well before the VO event.
Fig. 9.
Cartoon schematic summarizing the various changes in reproductive gene expression and neuronal activation during the pubertal transition in female mice. (A) Summary of the changes in neural gene expression in reproductive genes during the pubertal transition in female mice. Levels are plotted relative to typical adult levels, which are designated by the horizontal black dotted line. (B) Summary of the changes in neuronal activation in reproductive circuits (kisspeptin and RFRP-3 neuronal populations) during the pubertal transition. Relative levels are plotted as 0–100% of each neuronal population showing activation. In both (A) and (B), the green shaded area designates the period when vaginal opening (VO) is observed, with the darker green bar denoting the mean age of VO. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Whether any of the notable changes observed in the present study actually reflect an involvement in the pubertal mechanism versus other important physiological processes remains to be determined. Likewise, it is unknown if some of the alterations observed are secondary responses to pubertal changes in gonadal sex steroids, which presumably rise during the pubertal period, as demonstrated in other rodents. In particular, the large AVPV/PeN Kiss1 increases are likely due, at least in part, to rising estradiol levels during pubertal development, as AVPV/PeN Kiss1 expression can be strongly upregulated by activational effects of sex steroids (Smith et al., 2005). However, current mouse estradiol assays are not sensitive enough to detect very low levels of estradiol, especially at young ages (Kauffman, unpublished observations), and we were therefore unable to correlate serum estradiol in our pubertal mice to changing gene expression. Regardless, many of the observed pubertal changes in gene expression cannot be solely due to sex steroids, as the different genes changed with different patterns, magnitudes, and time-courses (or in some cases, did not change at all), despite being exposed to the same hormonal milieu within each animal. In fact, interestingly, the ARC Kiss1 and Tac2 systems increased over the pubertal period, despite presumably rising sex steroid levels (which normally inhibit these two genes, at least in adults). In addition, we note that all animals in the present study were sacrificed during a 2-hour time window during the day (from 11 am to 1 pm), and gene expression levels and neuronal activation of these various reproductive populations may change differently (or not at all) at other circadian times outside of this period. Given the large scale of the present study, it was not logistically possible to include analyses at multiple time points each day.
Future analyses of the same neural genes in pubertal males will be critical. Indeed, it will be informative to determine what commonalities and differences exist between male and female reproductive genes, especially given known sex differences in both normal puberty onset (earlier in females than males) and pubertal disorders in humans (precocious puberty is more common in females, delayed puberty more prevalent in males). Moreover, our study focused on gene expression and neuronal activation, but did not measure neuropeptide protein levels. Future studies can perform similar large-scale analysis of protein levels across puberty to complement and extend our mRNA findings.
In summary, we report that multiple reproductive genes are in flux during the peripubertal and pubertal period, with marked and continual increases in AVPV/PeN Kiss1 expression, smaller gradual in ARC Kiss1 and Tac2 expression, and a more rapid and sizable drop in Rfrp expression and neuronal activation in the early portion of the peripubertal period. The observed increases in Kiss1 and Tac2 may relate to increased stimulation of the maturing reproductive axis, whereas the reduction in Rfrp levels and neuronal activation may reflect disinhibition of reproductive circuits to facilitate puberty onset. Several of these changes occurred well before external morphological signs of puberty, though interestingly, many of the genes changed on quite different timescales and patterns than each other.
Acknowledgements
This work was funded by NICHD R01 HD065856. Additional support provided by NICHD SCCPIR grants U54 HD012303 (UCSD) and U54 HD28934 (University of Virginia). SJS supported by F32 HD066849.
References
- Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–1840. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides. 2010;31(2):275–283. doi: 10.1016/j.peptides.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J. Comp. Neurol. 2011;519(15):2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, et al. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol. Cell. Endocrinol. 2006:257–258. 75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52(6):581–588. doi: 10.1159/000125647. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150(7):3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U.S.A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio NP, Semaan SJ, Kim J, Lopez PV, Bettler B, Libertun C, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155(3):1033–1044. doi: 10.1210/en.2013-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS ONE. 2010;5(7):e11911. doi: 10.1371/journal.pone.0011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153(10):4883–4893. doi: 10.1210/en.2012-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Horm. Res. 2002;57(Suppl. 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta) Endocrinology. 2012;153(4):1887–1897. doi: 10.1210/en.2011-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Coming of age in the Kisspeptin Era: sex differences, development, and puberty. Mol. Cell. Endocrinol. 2010;324:51–63. doi: 10.1016/j.mce.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am. J. Physiol. Endocrinol. Metab. 2009;297(5):E1212–E1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Sun Y, Kim J, Khan AR, Shu J, Neal-Perry G. Vasoactive intestinal peptide modulation of the steroid-induced LH surge involves kisspeptin signaling in young but not in middle-aged female rats. Endocrinology. 2014;155(6):2222–2232. doi: 10.1210/en.2013-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Tolson KP, Dhamija S, Kauffman AS. Developmental GnRH signaling is not required for sexual differentiation of kisspeptin neurons but is needed for maximal Kiss1 gene expression in adult females. Endocrinology. 2013;154(9):3273–3283. doi: 10.1210/en.2013-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc. Natl. Acad. Sci. U.S.A. 2006;103(7):2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J. Neurosci. 2014;34(10):3756–3766. doi: 10.1523/JNEUROSCI.5123-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Leon S, Garcia-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the npff1 receptor null mouse. Endocrinology. 2014;155(8):2953–2965. doi: 10.1210/en.2014-1030. [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, et al. Epigenetic control of female puberty. Nat. Neurosci. 2013;16(3):281–289. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. U.S.A. 2010;107(52):22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J. Physiol. 2004;561(Pt 2):379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J. Neurosci. 2012;32(7):2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. Knobil and Niell’s Physiology of Reproduction. third ed. Elsevier; 2006. pp. 2061–2126. [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, et al. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147(3):1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Loche A, Matagne V, Kaidar G, Sandau US, et al. The transcriptional control of female puberty. Brain Res. 2010;1364:164–174. doi: 10.1016/j.brainres.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Witchel SM. Puberty in non-human primates and humans. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. third ed. Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153(2):782–793. doi: 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J. Neuroendocrinol. 2013;25(10):876–886. doi: 10.1111/jne.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- Richter LM. Studying adolescence. Science. 2006;312(5782):1902–1905. doi: 10.1126/science.1127489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Kauffman AS. Emerging concepts on the epigenetic and transcriptional regulation of the Kiss1 gene. Int. J. Dev. Neurosci. 2013;31(6):452–462. doi: 10.1016/j.ijdevneu.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Dhamija S, Kim J, Ku EC, Kauffman AS. Assessment of epigenetic contributions to sexually-dimorphic Kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology. 2012;153(4):1875–1886. doi: 10.1210/en.2011-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. U.S.A. 2005;102(6):2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J. Neuroendocrinol. 2009;21(6):527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J. Mol. Neurosci. 2011;43(2):138–145. doi: 10.1007/s12031-010-9430-1. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. Deciphering puberty: novel partners, novel mechanisms. Eur. J. Endocrinol. 2012;167(6):733–747. doi: 10.1530/EJE-12-0669. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Guerriero KA, Plant TM. Kisspeptin and puberty in mammals. Adv. Exp. Med. Biol. 2013;784:253–273. doi: 10.1007/978-1-4614-6199-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 2012;366(7):629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol. Reprod. 2012;87(6):129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J. Physiol. 2009;587(Pt 7):1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 2010;95(5):2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]