Abstract
Objective
To compare the clinical presentation of ADHD between youth with autism spectrum disorder (ASD) and ADHD and a sample of youth with ADHD only.
Method
A psychiatrically referred sample of autism spectrum disorder (ASD) youth with ADHD attending a specialized ambulatory program for ASD (n = 107) and a sample of youth with ADHD attending a general child psychiatry ambulatory clinic (n = 74) were compared.
Results
Seventy-six percent of youth with ASD met Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) criteria for ADHD. The clinical presentation of ADHD in youth with ASD was predominantly similar to its typical presentation including age at onset (3.5 ± 1.7 vs. 4.0 ± 1.9; p = .12), distribution of diagnostic subtypes, the qualitative and quantitative symptom profile, and symptom severity. Combined subtype was the most frequent presentation of ADHD in ASD youth.
Conclusion
Despite the robust presentation of ADHD, a significant majority of ASD youth with ADHD failed to receive appropriate ADHD treatment (41% vs. 24%; p = .02). A high rate of comorbidity with ADHD was observed in psychiatrically referred youth with ASD, with a clinical presentation typical of the disorder.
Keywords: autism spectrum disorder, ADHD, youth
Background
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by variable presentation of difficulties with socialization and reciprocal communication in addition to restricted, repetitive behavior. An increasingly higher prevalence of ASD is documented in each successive epidemiological survey and the disorder is now estimated to affect 2% of school-aged children (Blumberg et al., 2013). In part, this rise in prevalence can be attributed to improved recognition of ASD in intellectually capable populations.
In addition to the disorder’s core features, the literature documents that a large number of individuals with ASD suffer from symptoms of ADHD (hyperactivity, impulsivity, and inattention) often regarded as associated features of ASD—symptoms that greatly add to their morbidity and dysfunction, coming at great costs to the family and society. ADHD is the most common psychiatric condition diagnosed in children with ASD, particularly in those with intact intellectual capacity (Tsai, 2000). Nearly two thirds (ranging from 59% to 83%) of referred youth with ASD suffer from ADHD (de Bruin, Ferdinand, Meester, de Nijs, & Verheij, 2007; Frazier et al., 2001; Gjevik, Eldevik, Fjaeran-Granum, & Sponheim, 2011; Joshi et al., 2010; Leyfer et al., 2006; Mattila et al., 2010; Sinzig, Walter, & Doepfner, 2009; Wozniak et al., 1997). Furthermore, up to three fourths of the clinically referred ASD population suffer from significant symptoms of ADHD (Gadow, DeVincent, & Pomeroy, 2006; Guerts et al., 2008; Lee & Ousley, 2006; Reiersen, Constantino, Volk, & Todd, 2007; Sinzig, Morsch, Bruning, Schmidt, & Lehmkuhl, 2008).
Appropriate recognition of ADHD in youth with ASD has important clinical implications, given that treatment interventions for these disorders differ. Considering that ADHD is known to respond to a variety of pharmacological and non-pharmacological interventions, identifying and treating ADHD in youth with ASD can greatly facilitate psychoeducational rehabilitation efforts unique to individuals with ASD. However, failure to recognize ADHD—especially in intellectually capable youth with ASD—can seriously undermine educational and social functioning, worsening already compromised social performance, and predispose these youth to increased risk for disruptive behaviors, mood dysregulation, and substance abuse (Biederman, Monuteaux, Spencer, Wilens, & Faraone, 2009; Biederman et al., 2014; Biederman et al., 2010; Fried et al., 2013). As diagnosis is paramount to the development of a rational treatment plan, improved diagnostic clarity of ADHD symptom presentation in ASD populations will aid clinical recognition of this highly treatment-responsive comorbidity.
This study focuses exclusively on examining the clinical correlates of ADHD in an ASD sample as compared with the typical presentation of ADHD with an aim to investigate the extent to which the presentation of ADHD in ASD is similar to the classic presentation of ADHD at the symptom level. In addition, we will explore the applicability of Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for discerning an ADHD diagnosis in intellectually capable populations with ASD.
To this end, we analyzed data from two large samples of psychiatrically referred populations of youth with ASD and with ADHD attending a specialty clinic for ASD and a general ambulatory child psychiatry clinic, respectively. On the basis of the extant literature, we hypothesized that the clinical presentation of ADHD in an ASD sample would be typical of the disorder and the DSM-based diagnostic criteria for ADHD would identify features of ADHD in ASD.
Method
Source of Study Populations
ASD participants were 107 children and adolescents 6 to 17 years old derived from consecutive referrals to a specialized ambulatory program for ASD at a major academic medical center. ADHD participants (n = 74) were children and adolescents 6 to 17 years old derived from consecutive referrals to a general child psychiatry ambulatory clinic at the same institution. Investigators received institutional review board approval to review, analyze, and report anonymously on these participants.
Assessment Procedures
All study participants were assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia-Epidemiologic Version (K-SADS-E; Orvaschel, 1994; Orvaschel & Puig-Antich, 1987), supplemented with a module to evaluate for ASD. Administered to the parent/guardian (usually the mother), the K-SADS-E provided Axis I psychiatric diagnoses based on DSM-III-R (3rd ed., rev.; American Psychiatric Association [APA], 1987) or DSM-IV (4th ed.; APA, 1994) criteria depending on the time of their interview. We have previously documented the concordance between DSM-III-R and DSM-IV definitions of ADHD, supporting diagnostic continuity between the two revisions of the DSM (Biederman et al., 1997).
Diagnostic interviews were administered by highly trained and closely supervised psychometricians with bachelor’s or master’s degrees in psychology or a related field. To establish rater reliability, board-certified child and adult psychiatrists and licensed clinical psychologists independently diagnosed participants based on audiotaped interviews completed by raters. Kappa coefficients of agreement were computed between rater and senior clinician diagnoses. The median kappa coefficient was .98, calculated using 500 assessments from interviews of adults and children.
Diagnoses were considered positive by interviewers if full DSM-III-R/DSM-IV criteria (including clinical impairment) were met unequivocally. To resolve diagnostic uncertainties, all interviews were reviewed by a committee of board-certified child and adult psychiatrists and experienced clinical psychologists who were blind to the participant’s referral source, diagnostic status, and all other non-diagnostic data (e.g., socio-economic status [SES], family and social functioning). Diagnoses presented for review were considered positive only if diagnostic criteria were met to a degree that would be considered clinically meaningful based on the nature of the symptoms, the associated impairment, and the coherence of the clinical picture as collected during the interview. The reliability of the diagnostic review process was estimated by computing kappa coefficients of agreement for clinician reviewers. The median reliability between individual clinicians and review committee diagnoses was .87.
A diagnosis of ADHD-Inattentive or ADHD-Hyperactive was defined as six or more symptoms of either inattention or hyperactivity, respectively, with an onset at or before age 7 and cross-sectionality, defined as impairment in two or more settings. A diagnosis of ADHD-Combined type required meeting a full diagnosis of both ADHD-Inattentive and ADHD-Hyperactive.
For every diagnosis, information regarding the age of symptom onset and offset was obtained. Treatment histories included rates of disorder-specific counseling, pharmacotherapy, and hospitalization.
As the K-SADS-E lacks a module to evaluate ASD, we adopted DSM-III-R pervasive developmental disorder (PDD) diagnostic criteria into interview format to evaluate for this disorder. ASD was defined as meeting DSM-III-R diagnostic criteria for autistic disorder or pervasive developmental disorder–not otherwise specified (PDD-NOS). A diagnosis of autistic disorder required the full presence of 8 out of 16 symptoms with at least 2 symptoms from each of the three domains of ASD; in the absence of autistic disorder, a diagnosis of PDD-NOS required the presence of more than 2 of the required symptoms with symptom(s) present from each of the three ASD domains. To establish rater reliability for the PDD module, an independent clinician with expertise in the diagnosis of ASD (first author) independently diagnosed participants based on audiotaped interviews completed by raters. Based on 20 interviews, the median kappa coefficient of agreement between raters and clinician was .90. The kappa for reliability between independent raters and the final diagnostic decision made by a clinician-reviewer was .88. In addition, concurrent and discriminant validity of the PDD module for diagnosing ASD has been established (Joshi et al., 2011). Excellent sensitivity for the PDD module is observed with clinical diagnosis of ASD (94%) and with the Social Responsiveness Scale (T-score ≥ 60; 96%; Constantino et al., 2003).
Although ADHD could not be diagnosed in the presence of PDD per DSM-III-R and DSM-IV criteria, the present study employed a non-hierarchical approach to diagnostic endorsements, which required meeting full DSM symptom and impairment criteria for diagnosis. This approach allowed for empirical examination of all present disorders in an effort to fully characterize the clinical picture of each participant.
ASD participants also completed a clinical diagnostic interview with a board-certified psychiatrist experienced in evaluating ASD and comorbid psychiatric conditions. The psychiatric diagnostic interview was conducted with the participant and parent/guardian(s), and information was incorporated from multiple sources when available (e.g., psychiatric records, schools, social services).
All diagnoses of ADHD were determined by the K-SADS-E, regardless of referral source. Diagnoses of ASD in participants referred to the general psychiatry clinic were made based on the K-SADS-E PDD module; in participants referred to the specialized ambulatory clinic, ASD diagnoses were made based on clinical diagnostic interview.
The DSM Global Assessment of Functioning (GAF) Scale (Endicott, Spitzer, Fleiss, & Cohen, 1976) was used to assess overall adaptive functioning. To evaluate school functioning, three indices of difficulties were used: placement in special classes, extra tutoring, and repeated grades. SES was established by using categories delineated by Hollingshead (1975). Full-scale IQ of ASD participants was assessed with the Vocabulary and Matrix subsets of Wechsler Abbreviated Scale of Intelligence; that of ADHD participants was assessed with the Wechsler Intelligence Scale for Children (Wechsler, 1991, 1999). Participants with IQ < 70 were excluded.
Data Analysis
Data for this study are expressed as mean ± standard deviation unless otherwise specified. Continuous data were analyzed using t tests and categorical data were analyzed via Pearson chi-square analyses. Logistic regression was used to adjust for potential confounders. All analyses were two-tailed; statistical significance was set at the 5% level (p < .05).
Results
Socio-demographics
Out of 283 children aged 6 to 17 years old referred to the ASD program and with a clinical diagnosis of ASD, all had structured interviews and 150 (53%) had available IQ data. Participants with IQ scores < 70 (7%) were excluded. Of the remaining 140 participants, 76% had a lifetime diagnosis of ADHD based on the K-SADS-E, producing a final sample size of n = 107 (Figure 1). Of these, 87 (81%) had ADHD symptom-level data.
Figure 1.
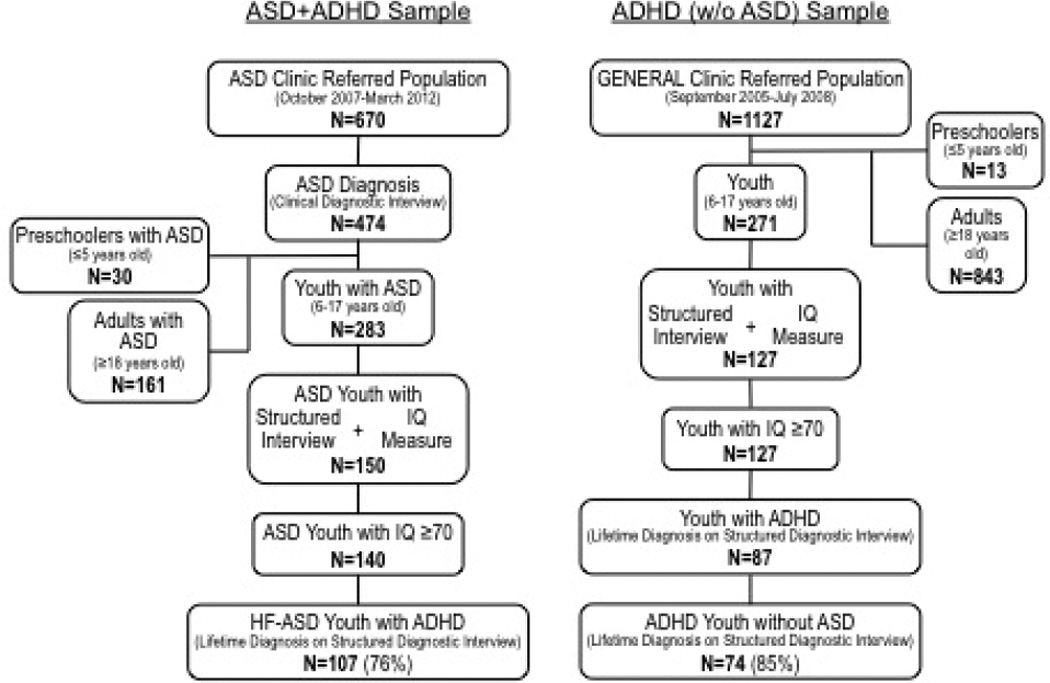
Flow diagram of sample ascertainment process.
Note. ASD = autism spectrum disorder; IQ = Intelligence quotient; HF-ASD = high-functioning ASD.
Out of 271 children between the ages of 6 and 17 years old referred to the general child psychiatric clinic, 265 completed a structured interview (K-SADS-E). IQ data were available for 127 of these participants, all of whom had an IQ ≥ 70. Of this sample, 87 (69%) had a lifetime diagnosis of ADHD; 74 (85%) of the ADHD-positive participants did not have ASD based on the K-SADS-E PDD module, giving a total sample size of n = 74 (Figure 1). Of these, 56 had ADHD symptom-level data.
Thus, comparisons were made between ASD children with ADHD (ASD + ADHD; n = 107) and ADHD children without ASD (ADHD; n = 74). Symptom-level comparisons were made between ASD children with ADHD (ASD + ADHD; n = 87) and ADHD children without ASD (ADHD; n = 56). Age and race (majority Caucasian) were similar between groups. Analysis was controlled for SES and IQ, which differed significantly between ASD and ADHD samples (Table 1).
Table 1.
Demographics and Clinical Features.
| ASD + ADHD (n = 107) |
ADHD (n = 74) |
Test statistic | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 11.0 ± 3.4 | 11.4 ± 3.2 | t = 0.76 | .45 |
| Gender (male) | 94 (88) | 57 (77) | χ2(1) = 3.71 | .05 |
| Race (Caucasian) | 88 (83) | 67 (91) | χ2(1) = 2.06 | .15 |
| SES | 2.04 ± 0.97 | 1.38 ± 0.62 | t = −5.06 | <.0001 |
| IQ | ||||
| M | 97 ± 14 | 102 ± 16 | t = 2.20 | .03 |
| Range | 70–133 | 71–132 | ||
| >85 | 85 (79) | 62 (84) | χ2(1) = 0.54 | .46 |
| ASD diagnosis (DSM-IV) | ||||
| Autistic disorder | 67 (63) | NA | ||
| Asperger’s disorder | 25 (23) | NA | ||
| PDD-NOS | 15 (14) | NA | ||
| Psychosocial functioning | ||||
| Global assessment of functioning | ||||
| Lifetime | 45.9 ± 5.0 | 48.1 ± 7.5 | t = 2.35 | .02 |
| Current | 51.1 ± 4.7 | 53.2 ± 6.4 | t = 2.51 | .01 |
| School functioning | ||||
| Repeated grade | 10 (19) | 8 (11) | χ2(1) = 2.15 | .14 |
| Extra tutoring | 82 (77) | 59 (80) | χ2(1) = 0.24 | .62 |
| Special class | 55 (51) | 17 (23) | χ2(1) = 14.76 | <.001 |
Note. Values expressed as n (%) or M±SD. Bolded values indicate statistical significant at p ≤ 0.05. ASD = autism spectrum disorder; SES = socio-economic status; IQ= intelligence quotient; NA = not applicable; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.); PDD-NOS = pervasive developmental disorders–not otherwise specified.
As expected, both ASD and ADHD study samples were predominantly male (88% and 77%, respectively). The ADHD sample demonstrated a mean full-scale IQ score significantly higher than that of the ASD sample (102 ± 16 vs. 97 ± 14; p = .03). A majority of participants in both study samples had intact intellectual capacity (IQ > 85; ASD + ADHD = 79% and ADHD = 84%). Autistic disorder was the most common form of ASD diagnosed in the ASD sample (63%), followed by Asperger’s disorder (23%) and PDD-NOS (14%; Table 1).
Participants with ASD and ADHD demonstrated significantly poorer global functioning based on GAF scores compared with ADHD participants, both currently (51.1 ± 4.7 vs. 53.2 ± 6.4; p = .01) and over their lifetime (45.9 ± 5.0 vs. 48.1 ± 7.5; p = .02). ASD + ADHD participants were more likely to be enrolled in a special education class at school (51% vs. 23%; p < .001), while rates of extra tutoring and repeated grades did not differ significantly between ASD + ADHD and ADHD groups (Table 1).
Characteristics of ADHD
The age at onset and severity of ADHD were similar between groups (Table 2). The percentage of participants with moderate/severe ADHD was also similar between samples (ASD + ADHD = 93% vs. ADHD = 92%; p = .88). Significantly more ADHD participants met criteria for the Hyperactive-Impulsive subtype of ADHD compared with ASD + ADHD participants (57% vs. 33%; p = .02).
Table 2.
Clinical Characteristics of ADHD.
| Clinical correlates of ADHD | ASD + ADHD (n = 107) |
ADHD (n = 74) |
Test statistic |
p value |
|---|---|---|---|---|
| Age at onset | 3.5 ± 1.7 | 4.0 ± 1.9 | t = 1.57 | .12 |
| Current diagnosis | 92 (86) | 66 (89) | χ2(1) = 0.41 | .52 |
| Lifetime diagnosisa | DSM-IV | DSM-III-R | ||
| Hyperactive/impulsive only | 29 (33) | 32 (57) | z = −2.24 | .03 |
| Inattentive only | 7 (8) | 1 (2) | z = 1.55 | .12 |
| Combined (Inattentive + Hyperactive) | 51 (59) | 23 (41) | z = 1.50 | .14 |
| Number of symptomsa | ||||
| Inattentive | 6.0 ± 2.2 | 5.0 ± 2.9 | z = 1.89 | .06 |
| Hyperactive/impulsive | 7.7 ± 1.5 | 8.1 ± 1.1 | z = −1.49 | .14 |
| Inattentive + Hyperactive | 13.7 ± 2.6 | 13.1 ± 3.4 | z = 0.93 | .35 |
| Severity (moderate to severe) | 99 (93) | 68 (92) | χ2(1) = 0.024 | .88 |
| Treatment history | ||||
| No treatment | 44 (41) | 18 (24) | χ2(1) = 5.48 | .02 |
| Only counseling | 16 (15) | 4 (5) | χ2(1) = 4.06 | .04 |
| Pharmacotherapy | 47 (44) | 52 (70) | χ2(1) = 12.25 | <.001 |
| Lifetime comorbidity with ASD subtypes (n = 140) | ||||
| Autistic disorder (n = 86) | 67 (78) | NA | χ2(2) = 2.02 | .37 |
| Asperger’s disorder (n = 31) | 25 (81) | NA | ||
| PDD-NOS (n = 23) | 15 (65) | NA | ||
Note. Values expressed as n (%) or M±SD. Bolded values indicate statistical significant at p ≤ 0.05. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.); DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.); ASD = autism spectrum disorder; NA = not applicable; PDD-NOS = pervasive developmental disorders–not otherwise specified.
Total N ASD + ADHD = 87 and ADHD = 56.
The symptom profiles for hyperactivity and impulsivity were similar between ADHD cases with and without ASD, with a few exceptions (Figure 2). Specifically, those with ASD were less likely to be careless (ADHD + ASD = 74% vs. ADHD = 91%; p = .02), and less likely to have problems listening (ADHD + ASD = 86% vs. ADHD = 100%; p = .004). Participants with ASD were more likely to have difficulty waiting their turn (ADHD + ASD = 87% vs. ADHD = 61%; p = .001), and more likely to interrupt or intrude (ADHD + ASD = 91% vs. ADHD = 61%; p < .001).
Figure 2.
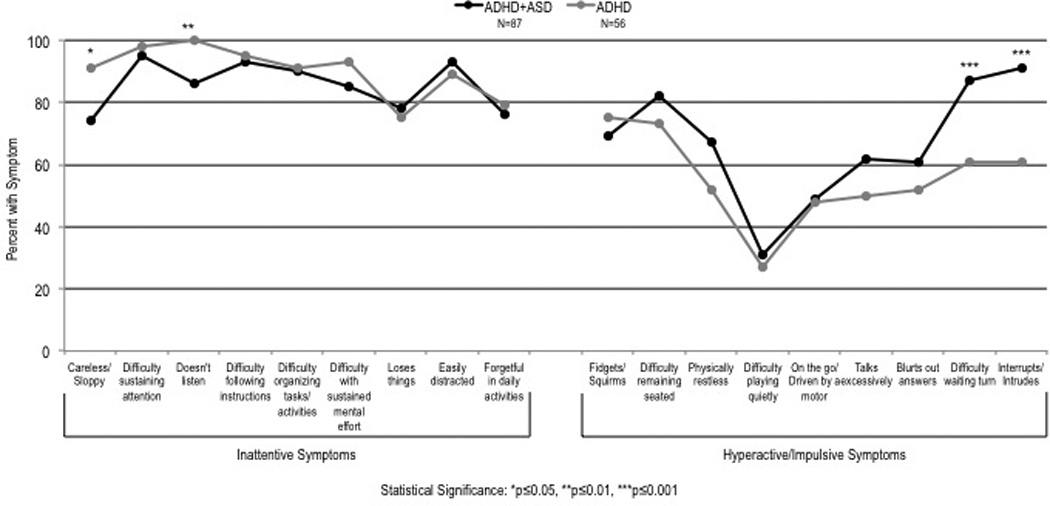
ADHD symptom profile in the context of ASD.
Note. ASD = autism spectrum disorder.
*p ≤ .05. **p ≤ .01. ***p ≤ .001.
Discussion
We conducted a systematic assessment of the phenotypic correlates of ADHD in youth with and without ASD to investigate the extent to which the clinical presentation of ADHD in youth with ASD is consistent with the disorder’s typical presentation. The comorbidity of ADHD with ASD was substantial and clinically significant within psychiatrically referred samples of youth with either disorder. The phenotypic features of ADHD were largely similar in youth with ASD compared with youth with ADHD alone. The clinical presentation of ADHD in youth with ASD was remarkably analogous to its typical presentation by variables such as age at onset, distribution of diagnostic subtypes, the qualitative and quantitative symptom profile, and severity of symptoms (Table 2). The frequency of ADHD comorbidity was equally high in all subtypes of ASD. Among the diagnostic subtypes of ADHD, the combined subtype—symptomatically the most robust form of ADHD—was the most frequent in youth with ASD (although its rate in ASD + ADHD youth did not significantly differ from its rate of frequency in ADHD youth). Moreover, a significantly greater proportion of ADHD youth with ASD failed to receive pharmacotherapy or counseling for their ADHD. Taken together, these findings confirm that the clinical presentation of ADHD in ASD populations is substantially similar to the typical presentation of the disorder, though ADHD in the comorbid state with ASD remains largely untreated. In addition, DSM-based diagnostic features of ADHD were successfully discerned in youth with ASD, suggesting that DSM criteria are equally applicable for diagnosing ADHD in ASD populations as in typically developing populations.
Prevalence of ASD and ADHD Comorbidity
In our study sample, the overlap between ASD and ADHD was bidirectional though asymmetrical. Reciprocal ASD + ADHD comorbidity was present in a majority of the youth with ASD and in a minority of youth diagnosed with ADHD, suggesting that the observed comorbidity is substantially higher in psychiatrically referred populations of youth with ASD than in other psychiatrically referred populations. The ability of a DSM-based structured diagnostic interview to diagnose ADHD in an ASD sample endorses the applicability of DSM-based diagnostic criteria for evaluating ADHD in individuals with ASD. The high rate of comorbidity with ADHD observed in youth with ASD is consistent with the literature documenting ADHD as the most common psychiatric condition in referred populations of youth with ASD, with a prevalence documented as high as 83% (de Bruin et al., 2007; Frazier et al., 2001; Joshi et al., 2010; Mukaddes, Herguner, & Tanidir, 2010; Sinzig et al., 2009; Wozniak et al., 1997). In our sample of ASD youth, the rates of ADHD were equally high and evenly distributed among subtypes of ASD.
Clinical Presentation of ADHD
The clinical presentation of ADHD in the presence of ASD was substantially similar to the archetypal DSM presentation of ADHD in youth without ASD (Table 2). Initial onset of ADHD as reported by the caretaker occurred in early preschool years, irrespective of comorbidity with ASD. At time of assessment, a majority of the study participants with and without ASD were experiencing moderate to severe ADHD.
With few exceptions, a striking similarity was observed in the symptom profile of ADHD in youth with and without ASD. Noted disparities included that in the presence of ASD, youth with ADHD experienced hyperactive/impulsive symptoms of “difficulty waiting turn” and “interrupts or intrudes” at a higher frequency and inattention symptoms of “careless and sloppy” and “doesn’t listen” at a lower frequency than typically developing ADHD youth.
The expression of ADHD symptoms was robust in youth with ASD who met diagnostic criteria for the combined subtype of ADHD more often than either other subtype (in our non-ASD sample, the hyperactive/impulsive subtype was most frequently diagnosed). Despite this robust presentation of ADHD, a significant proportion of the ASD + ADHD youth failed to receive ADHD-specific treatment, whereas a majority of the sampled ADHD youth without ASD received treatment for ADHD.
Implications of Undertreatment of ADHD in the Presence of ASD
Considering the high prevalence of ADHD noted in intellectually capable populations of youth with ASD, untreated ADHD could have significant social consequences, not limited to impaired academic performance and increased risk for comorbid mood and substance use disorders (Biederman et al., 2009; Biederman et al., 2014; Biederman et al., 2010; Fried et al., 2013). Forty-one percent of our ASD sample (a majority of whom had intact intellectual capacity) never received prior treatment for ADHD. Given the current lack of pharmacological treatment regimens for the improvement of core ASD features and the availability of effective ADHD treatment options, properly identifying and treating ADHD in ASD populations represents an important opportunity for improving patient quality of life. Limited research on pharmacological treatments for ADHD symptoms in ASD has demonstrated the efficacy of methylphenidate, atomoxetine, and guanfacine, among others. Importantly, in ASD patients with ADHD, rates of response to traditional anti-ADHD medications tend to be lower and rates of adverse events are higher than what is seen in patients with ADHD without ASD, although the magnitude of difference varies by medication (Harfterkamp et al., 2012; Mahajan et al., 2012; Murray, 2010). In spite of such differences, anti-ADHD medications remain a viable treatment option for ADHD symptoms in ASD and, as our findings suggest, may be underutilized.
Given the differential treatment response documented between ADHD patients with and without ASD, it remains unclear whether the simultaneous presence of ADHD and ASD constitutes an independent comorbid disorder or represents a unique subtype of ADHD. Some researchers have suggested that ADHD is in fact part of the autism spectrum, supported by evidence of symptom overlap, comorbidity rates, common risk factors, and neuropsychological findings which demonstrate subgroups that differ mainly in symptom severity rather than distinct categorical entities (Taurines et al., 2012). When comorbid symptoms and cognitive profiles of a large sample of children were compared, latent class analysis revealed an absence of an ASD class without ADHD symptoms and the presence of an ADHD class without ASD, lending support to this hypothesis of a “gradient overarching disorder” (van der Meer et al., 2012). However, to date, no population-based study on ASD diagnoses in children with a primary diagnosis of ADHD has been conducted (Taurines et al., 2012). More research is needed to further explore the overlap of ASD and ADHD to determine whether there is a truly shared etiology. Although the clinical expression of ADHD in individuals with ASD is strikingly similar to the presentation observed in individuals without ASD, it remains to be explored whether the neural correlates of ADHD in ASD are equally typical. Future studies examining the neural correlates of ADHD in ASD populations are warranted.
In summary, in the presence of comorbid ASD, the clinical presentation of ADHD—including variables such as age at onset, type and number of ADHD symptoms, symptom severity, and the pattern of diagnostic subtypes of ADHD—was remarkably similar to the classic DSM presentation of ADHD, suggesting that the DSM criteria used for diagnosing ADHD in typical populations can be successfully applied to identify ADHD in ASD populations.
Our findings should be evaluated in the light of certain limitations. As we examined psychiatrically referred samples, these findings may not generalize to community samples. In addition, this analysis assessed clinically referred samples that were largely Caucasian; thus, these findings may not generalize to community samples or other ethnic groups. Diagnoses of ASD for each study group were made based on different versions of the DSM. The Autism Diagnostic Interview–Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) were not performed to confirm ASD diagnoses, although the feasibility of conducting the ADI-R/ADOS in clinical settings and large population-based studies is limited given the specialized training and significant time required for administration.
Conclusion
Despite these considerations, the results of this study demonstrate that psychiatrically referred populations of youth with ASD can have high levels of comorbid ADHD that may go untreated. This emphasizes the importance of recognizing and appropriately treating this pharmaco-responsive psychiatric comorbidity in ASD populations. More importantly, the clinical presentation of ADHD in youth with ASD is substantially similar to typical presentation of ADHD, suggesting that the available diagnostic criteria for ADHD can be applied to identify ADHD in ASD populations.
Key Points.
It is known that referred populations of youth with ASD can have high levels of comorbid ADHD. • We found the presentation of ADHD in youth with ASD to be remarkably similar to the disorder’s classic presentation in typically developing populations. • Classic DSM definition of ADHD is applicable to ASD populations. • ADHD may go untreated in ASD populations.
Acknowledgments
Dr. Gagan Joshi is supported by the National Institute of Mental Health (NIMH) of the National Insitutes of Health (NIH) under Award Number K23MH100450. He receives research support from Duke University and Sunovion Pharmaceuticals as a site principal investigator for multi-site clinical trials and from Pfizer as a principal investigator for an investigator-initiated clinical trial. He is a co-investigator for clinical trials sponsored by Merck/Schering-Plough Corporation, the U.S. Department of Defense, and Pamlab, LLC. In 2012, Dr. Joshi received research support from Forest Research Laboratories, Duke University, Shire, Merck/Schering-Plough Corporation, ElMindA, and Pamlab, LLC.
In the past year, Dr. Stephen V. Faraone received consulting income and/or research support from Akili Interactive Labs, VAYA Pharma, and SynapDx and research support from the National Institutes of Health (NIH). His institution is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts.
From 2011–2013, Dr. Janet Wozniak has received research support from Merck/Schering-Plough, McNeil and Shire. In the past she has received research support, consultation fees or speaker’s fees from: Eli Lilly, Janssen, Johnson and Johnson, McNeil, Pfizer, Shire. She is the author of the book, “Is Your Child Bipolar” published May 2008, Bantam Books. In 2011–2013, her spouse John Winkelman MD, PhD received consultation fees from Pfizer, UCB, and Zeo for his role as consultant. He received research support from GlaxoSmithKline for his role as research study staff. In the past, he has received research support, consultation fees or speaker’s fees from: Axon Labs, Boehringer-Ingelheim, Covance, Cephalon, Eli Lilly, GlaxoSmithKline, Impax, Jazz Pharmaceuticals, King, Luitpold, Novartis, Neurogen, Novadel Pharma, Pfizer, Sanofi-Aventis, Sepracor, Takeda, UCB (Schwarz) Pharma, Wyeth, Zeo.
Dr. Joseph Biederman is currently receiving research support from the following sources: American Professional Society of ADHD and Related Disorders (APSARD), the Department of Defense, ElMindA, Janssen, McNeil, Shire, and Vaya Pharma/Enzymotec. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded continuing medical education (CME) courses. In 2011, Dr. Joseph Biederman gave a single unpaid talk for Juste Pharmaceutical Spain, received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course, and received honoraria for presenting at international scientific conference on ADHD. He also received an honorarium from Cambridge University Press for a chapter publication. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Eli Lilly, Shire, and AstraZeneca; these royalties are paid to the Department of Psychiatry at MGH. In 2010, Dr. Joseph Biederman received a speaker’s fee from a single talk given at Fundación Dr. Manuel Camelo A.C. in Monterrey, Mexico. Dr. Biederman provided single consultations for Shionogi Pharma, Inc. and Cipher Pharmaceuticals, Inc.; the honoraria for these consultations were paid to the Department of Psychiatry at the MGH. Dr. Biederman received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Glaxo, Gliatech, Hastings Center, Janssen, McNeil, Medice Pharmaceuticals (Germany), Merck, MMC Pediatric, National Alliance for Research on Schizophrenia and Depression (NARSAD), National Institute on Drug Abuse (NIDA), New River, National Institute of Child Health and Human Development (NICHD), NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma, Inc., Veritas, and Wyeth.
Biographies
Gagan Joshi, MD, is the medical director of the Alan and Lorraine Bressler Clinical and Research Program for Autism Spectrum Disorder and a psychiatrist in the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital in Boston, Massachusetts. He is an Assistant Professor of Psychiatry at Harvard Medical School, Boston and is board certified in general and child psychiatry. His clinical and research interests focus on the clinical and neural characterization and psychopharmacotheraputics of ASD and related psychopathology.
Stephen V. Faraone, PhD, is the director of the Medical Genetics Research Center and distinguished professor of psychiatry and neuroscience and physiology at SUNY Upstate Medical University, Syracuse. He is also senior scientific advisor to the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital and a lecturer at Harvard Medical School, Boston, Massachusetts. His research contributions include psychiatric genetics, psychopharmacology, diagnostic issues, and methodology.
Janet Wozniak, MD, is the Director of the Pediatric Bipolar Disorder Clinical and Research Program and Director of the Child and Adolescent Psychiatry Outpatient Service at Massachusetts General Hospital, Boston, Massachusetts. She is an Associate Professor of Psychiatry at Harvard Medical School and a clinical and research psychiatrist. Dr. Wozniak’s research focuses on the characteristics, longitudinal course, and treatment of pediatric bipolar disorder.
Laura Tarko, MPH, worked as a biostatistician in the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital in Boston, Massachusetts.
Ronna Fried, EdD, is the director of neuropsychology in the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital, Boston, Massachusetts. She oversees, trains, and supervises psychometricians that assess thousands of participants in the program every year for both clinical and research purposes. She is also an Associate Professor of Psychology at Harvard Medical School, Boston, Massachusetts
Maribel Galdo, MA, LCSW, is the director of business and operations of the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital in Boston, Massachusetts.
Stephannie L. Furtak, BA, is a clinical research coordinator in the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital in Boston, Massachusetts.
Joseph Biederman, MD, is the chief of the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD and director of the Alan and Lorraine Bressler Clinical and Research Program for Autism Spectrum Disorders at Massachusetts General Hospital, Boston, Massachusetts. He is a Professor of Psychiatry at Harvard Medical School, Boston and is board certified in general and child psychiatry. His clinical and research interests include ADHD in children and adults and pediatric mood and anxiety disorders.
Footnotes
Conflicts of Interest
All other authors have nothing to disclose.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Biederman J, Faraone SV, Weber W, Russell RL, Rater M, Park K. Correspondence between DSM-III-R and DSM-IV Attention Deficit Hyperactivity Disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 1997;36(12):1682–1687. doi: 10.1097/00004583-199712000-00016. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Faraone SV. Do stimulants protect against psychiatric disorders in youth with ADHD? A 10-year follow-up study. Pediatrics. 2009;124(1):71–78. doi: 10.1542/peds.2008-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty C, Spencer TJ, Woodworth KY, Bhide PG, Zhu J, et al. Is ADHD a risk for posttraumatic stress disorder (PTSD)? Results from a large longitudinal study of referred children with and without ADHD. World J Biol Psychiatry. 2013 doi: 10.3109/15622975.2012.756585. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010;167(4):409–417. doi: 10.1176/appi.ajp.2009.09050736. [DOI] [PubMed] [Google Scholar]
- Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in Prevalence of Parent-Reported Autism Spectrum Disorder in School-Aged U.S. Children: 2007 to 2011–2012. National Health Statistics Report. 2013;65 http://www.cdc.gov/nchs/data/nhsr/nhsr065.pdf?utm_source=Just+Released--New+Data+from+the+2011%2F12+National+Survey+of+Children%27s+Health&utm_campaign=New+Data+from+2011%2F12+NSCH&utm_medium=archive. [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, de Nijs PF, Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord. 2007;37(5):877–886. doi: 10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Biederman J, Bellordre CA, Garfield SB, Geller B, Coffey BJ, et al. Should the diagnosis of Attention-Deficit/Hyperactivity Disorder be considered in children with Pervasive Development Disorder? Journal of Attention Disorders. 2001;4(4):203–211. [Google Scholar]
- Fried R, Petty C, Faraone SV, Hyder LL, Day H, Biederman J. Is ADHD a Risk Factor for High School Dropout? A Controlled Study. J Atten Disord. 2013 doi: 10.1177/1087054712473180. [Epub ahead of print] (4 Feb 2013). [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord. 2006;36(2):271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- Gjevik E, Eldevik S, Fjaeran-Granum T, Sponheim E. Kiddie-SADS Reveals High Rates of DSM-IV Disorders in Children and Adolescents with Autism Spectrum Disorders. J Autism Dev Disord. 2011;41(6):761–769. doi: 10.1007/s10803-010-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerts H, Grasman R, Verte S, Oosertlaan J, Roeyers H, van Kammen S, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46(13):3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Harfterkamp M, van de Loo-Neus G, Minderaa RB, van der Gaag RJ, Escobar R, Schacht A, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733–741. doi: 10.1016/j.jaac.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale Press; 1975. [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, et al. The Heavy Burden of Psychiatric Comorbidity in Youth with Autism Spectrum Disorders: A Large Comparative Study of a Psychiatrically Referred Population. J Autism Dev Disord. 2010;40(11):1361–1370. doi: 10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty CR, Fried R, Wozniak J, Micco JA, Henin A, et al. Discriminant and concurrent validity of a simplified DSM-based structured diagnostic instrument for the assessment of autism spectrum disorders in youth and young adults. BMC Psychiatry. 2011;11(1):204. doi: 10.1186/1471-244X-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DO, Ousley OY. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(6):737–746. doi: 10.1089/cap.2006.16.737. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Mahajan R, Bernal MP, Panzer R, Whitaker A, Roberts W, Handen B, et al. Clinical practice pathways for evaluation and medication choice for attention-deficit/hyperactivity disorder symptoms in autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S125–S138. doi: 10.1542/peds.2012-0900J. [DOI] [PubMed] [Google Scholar]
- Mattila M, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with Asperger's syndrome/high-functioning autism: A community- and clinic-based study. J Autism Dev Disord. 2010;40(9):1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- Mukaddes NM, Herguner S, Tanidir C. Psychiatric disorders in individuals with high-functioning autism and Asperger's disorder: similarities and differences. World J Biol Psychiatry. 2010;11(8):964–971. doi: 10.3109/15622975.2010.507785. [DOI] [PubMed] [Google Scholar]
- Murray MJ. Attention-deficit/Hyperactivity Disorder in the context of Autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382–388. doi: 10.1007/s11920-010-0145-3. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children Epidemiologic Version. 5th Edition ed. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version. Fort Lauderdale, FL: Nova University; 1987. [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child Adolesc Psychiatry Ment Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Atten Disord. 2009;13(2):117–126. doi: 10.1177/1087054708326261. [DOI] [PubMed] [Google Scholar]
- Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord. 4(3):115–139. doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- Tsai L. Children with Autism Spectrum Disorder: Medicine Today and in the New Millennium. Focus Autism Other Dev Disabl. 2000;15(3):138–145. [Google Scholar]
- van der Meer JM, Oerlemans AM, van Steijn DJ, Lappenschaar MG, de Sonneville LM, Buitelaar JK, et al. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry. 51(11):1160–1172 e1163. doi: 10.1016/j.jaac.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children - Third Edition. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich, Inc.; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) 4th ed. San Antonio, Tx: The Psychological Corporation; 1999. [Google Scholar]
- Witwer AN, Lecavalier L. Validity of Comorbid Psychiatric Disorders in Youngsters with Autism Spectrum Disorders. J Dev Phys Disabil. 2010;22:367–380. [Google Scholar]
- Wozniak J, Biederman J, Faraone SV, Frazier J, Kim J, Millstein R, et al. Mania in children with pervasive developmental disorder revisited. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1552–1559. doi: 10.1016/S0890-8567(09)66564-3. discussion 1559–1560. [DOI] [PubMed] [Google Scholar]


