Abstract
Background
Cerebral cortical GABAergic interneuron dysfunction is hypothesized to lead to cognitive deficits co-morbid with human neuropsychiatric disorders, including schizophrenia, autism and epilepsy. We have previously shown that mice that harbor mutations in the Plaur gene, which is associated with schizophrenia, have deficits in frontal cortical parvalbumin expressing interneurons. Plaur mice have impaired reversal learning, similar to deficits observed in patients with schizophrenia.
Methods
We examined the role of parvalbumin interneurons in orbitofrontal cortex (OFC) during reversal learning by recording single unit activity from 180 control and 224 Plaur mouse neurons during a serial reversal task. Neural activity was analysed during correct and incorrect decision choices and reward receipt.
Results
Neurons in control mice exhibited strong phasic responses both during discrimination and reversal learning to decisions and rewards, and the strength of the response was correlated with behavioral performance. Although baseline firing was significantly enhanced in Plaur mice, neural selectivity for correct or erroneous decisions was diminished and not correlated with behaviour, and reward encoding was downscaled. In addition, Plaur mice showed a significant reduction in the number of neurons that encoded expected outcomes across tasks phases during the decision period.
Conclusions
These data indicate that parvalbumin interneurons are necessary for the representation of outcomes in OFC. Deficits in inhibition blunt selective neural firing during key decisions, contributing to behavioral inflexibility. These data provide a potential explanation for disorders of cognitive control that accompanies the loss of these GABAergic interneurons in human neuropsychiatric disorders, such as autism, epilepsy, and schizophrenia.
Keywords: schizophrenia, parvalbumin, Plaur, autism, interneuron, reversal learning, OFC
Introduction
GABAergic cortical interneurons are critical for network function, and loss of interneurons may figure prominently in the etiology of neuropsychiatric diseases, such as autism, epilepsy and schizophrenia (1–6). Altered development of forebrain GABAergic interneurons may compromise the assembly of local cortical networks (7–9). This developmental progression was modeled using mice with mutations in the urokinase plasminogen activator receptor (Plaur) (10–13). During embryonic development, the Plaur null mouse has decreased numbers of inhibitory cortical interneurons, due to abnormal neuronal migration (14). As a result, the adult Plaur null mouse has decreased fast-spiking parvalbumin-expressing (PV+) interneurons in prefrontal regions,(15–17) similar to observations in postmortem studies of humans with cognitive disorders. Plaur mice exhibit reversal and other behavioral impairments, much like impairments in adaptive behavioral control observed in patients with schizophrenia (18–20). However, it is important with animal models to show how gene mutations associated with human disorders may lead to the phenotypes observed in human patients (21).
Here we addressed this question directly by examining single unit activity in control and Plaur mouse OFC during performance of a cue-guided reversal task. The mouse must first learn to associate one stimulus with a rewarding outcome, and another stimulus with no reward. Subsequently the cue-outcome associations are reversed, and the mouse must switch its responding. We have previously shown that reversal performance in this setting depends upon mouse OFC, consistent with numerous prior reports of reversal deficits after OFC lesions in various species (3,22–25). Furthermore reversal performance is also selectively impaired in Plaur mice. Here we examine how an interneuron deficit affects associative encoding in the OFC, to address the hypothesis that fast-spiking interneurons are critical for proper OFC function during a task requiring flexible behavior.
Methods and Materials
Subjects
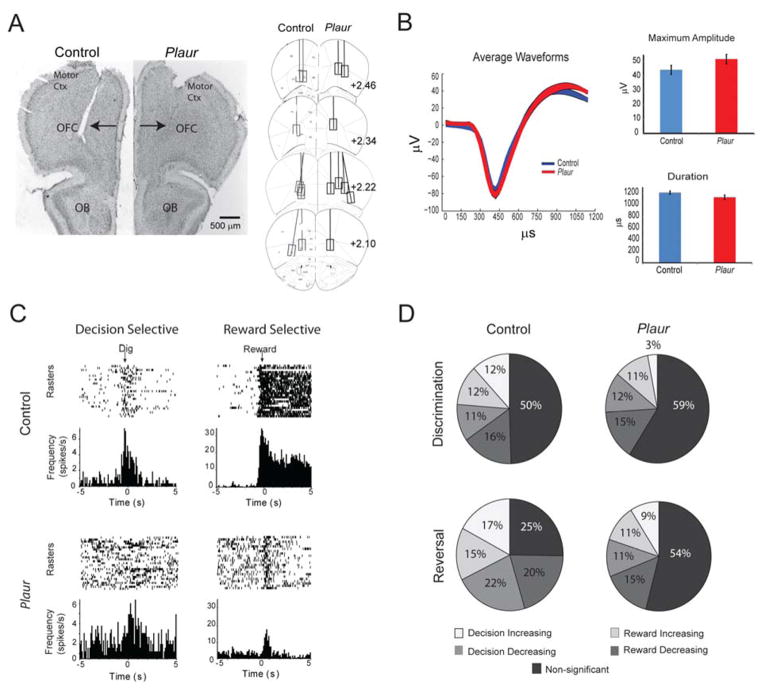
B6.129-Plaurtm1/Mlg/Plaurtm1/Mlg mice which have a null mutation in the gene that encodes the uPAR protein were genotyped as described previously (26). Behavioral and anatomical analyses were performed on adult (3–6 months old) male littermates, from at least 6 separate pedigrees bred on the C57BL/6J background for >20 generations. B6.129 male wild-type littermate mice were used as controls. Experiments were conducted in accordance with University of Maryland School of Medicine IACUC approved protocols and the Policies on the Use of Animals and Humans in Neuroscience Research. Under sterile conditions, an electrode with a drivable microarray of 9, 25-μm diameter FeNiCr wires (A-M Systems, Sequim, WA) in 27 gauge thin wall cannula (Small Parts, Miami Lakes, FL) consisting of 8 recording wires and 1 reference wire, was implanted in OFC (AP: 2.6; ML: −1.2; V: 2.1 mm, Figure 2A) (27). Prior to implantation, the wires were freshly cut and electroplated with platinum (H2PtCl6, Sigma-Aldrich, St. Louis, MO) to an impedance of approximately 300 kΩ. After testing, mice were transcardially perfused, and tissue was processed using routine laboratory protocols (15,28).
Figure 2.
Neural recording locations, waveforms and neuron types. (A) Example histology showing electrode tracts, with atlas plates demonstrating electrode locations. Boxes represent distance the electrode was advanced during the task. Recording locations were approximately equal, 5 electrodes in medial OFC and 3 in lateral OFC. (B) Average waveforms of putative pyramidal neurons for control (blue) and Plaur (red) plotted with SEM. Average waveforms were not different in maximum amplitude or duration. (C) Examples of single units presented in raster plots and peri-stimulus time histograms from neurons during the time of decision and reward receipt. Control mouse units show greater selectivity for choice and more robust activity for reward, compared to the Plaur mouse. (D) Pie chart breakdown of significant units. Approximately 24% of control single units increase on discrimination, split evenly between decisions and reward, while 14% of Plaur units increased for discriminations. On reversals, control discrimination and reward-neurons modestly increased in number, while Plaur decision-neurons increased. Additionally, the number of decreasing type neurons (decreasing during reward or decision) increased in controls (from 26% to 42%) between discrimination and reversal, and remained mostly unchanged in Plaur (27% to 26%).
Serial reversal learning task
Control (n = 8) and Plaur (n = 8) mice were tested on a modified naturalistic foraging reversal task (17,29,30). A reversal discrimination task was performed using 5 sets of discriminations and reversals (see Supplemental Tables S1 and S2 for experimental details, Figure 1A). Food deprived mice were trained for one day to dig in bowls of scented media to retrieve cereal rewards until they completed 8 consecutive correct trials. Placement of the baited bowl and assignment of relevant and irrelevant exemplars were randomized. At each trial start, the mice explored two identical bowls that contained combinations of odors and digging media. The bowls remained in the testing arena until the mouse dug in one bowl, signifying a choice. The bait was a piece of Honey Nut Cheerio cereal (~5 mg), and the cues, either olfactory (odor) or somatosensory and visual (texture of the digging medium hiding the bait) were relevant and irrelevant stimuli. Digging media were mixed with the odor (0.01% by volume) and Honey Nut Cheerio powder (0.1% by volume). No differences in approach or latency to dig were observed (Supplemental Data). Each discrimination/reversal pairing occurred on a new day.
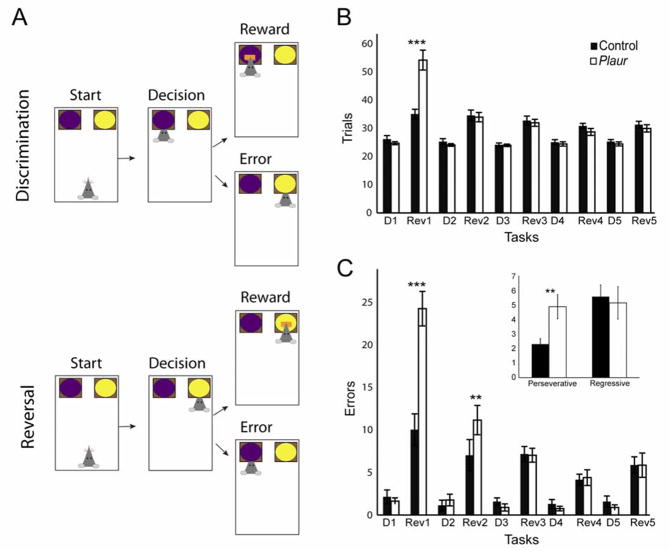
Figure 1.
Behavioral task schematic and data. (A) Schematic demonstrates the general layout, discrimination or reversal problem and reward or error outcomes. Initially, the mouse is presented with two bowls filled with different exemplars. After a period of exploration, the mouse makes a decision by digging in one of the two bowls. Either the decision is correct, and yields a food reward, or it is incorrect, and ends with no reward. After the outcome, the trial is ended and bowls are removed. (B,C) Behavioral responses: (B) Bar graphs of trials to reach criterion for control and Plaur mice. D1–5 show trials for discrimination problems, where mice learn the appropriate responses for each individual set of trials. Rev1–Rev5 groups show trials for the reversal of each prior discrimination problem. Both control and Plaur mice show increased trials compared to the previous discrimination learning, but only on the first reversal do Plaur mice show a behavioral deficit, compared to controls. (C) Bar graph of errors made prior to reaching the trials to criterion. The average number of errors shows that Plaur mice commit more errors on the first and second reversals, compared to control mice, and that all mice commit more errors on reversal problems than on discrimination problems. (Inset) demonstrates perseverative and regressive errors, whereby Plaur mice commit more perseverative, but not regressive errors. Asterisks (*) denote significance between genotypes.* p < 0.05, ** p < 0.01, *** p < 0.001.
We collected the number of correct and error trials for all mice. Values are reported as the mean ± standard error of the mean (SEM). For trials to criteria and errors (any dig in a non-baited bowl), a two-way ANOVA was used to determine statistical significance between treatment groups and discriminations, with Student-Newman-Keuls (SNK) post hoc testing. We categorized errors into perseverative (errors from the start of reversal until the first correct response) or regressive (responding to the previously rewarded cue after committing at least one correct response). Behavioral analysis was performed with MATLAB (Natick, MA).
Data acquisition and analysis
Experiments were performed in a behavioral chamber (31). Active wires were screened daily, and the electrode assembly was advanced by ~60 μm per day at the end of the recording session. Neural activity was recorded using Plexon Multichannel Acquisition Processor systems (Dallas, TX). Signals from the electrode wires were amplified 20 times by an op-amp headstage (Plexon, HST/8o50-G20-GR). Immediately outside the training chamber, the signals were passed through a differential pre-amplifier (Plexon, PBX2/16sp-r-G50/16fp-G50), where the single unit (SU) signals were amplified 50 times and filtered at 150–9000 Hz. The SU signals were then sent to the Multichannel Acquisition Processor box, where they were filtered at 250–8000 Hz, digitized at 40 kHz and amplified at 1–32 times. Waveforms >2.5:1 signal-to-noise were extracted from active channels and recorded to disk. For unit quantification, units were separated into putative populations by action potential half-width and peak:trough ratio. All units were graphed and determined to belong to the putative fast-spiking (FS) or regular spiking populations, based upon peak:trough ratio and spike half-width. Units with a P:T ratio <0.5, a half-width less than 100 μs and a baseline firing rate greater than 5 Hz were deemed likely to be FS interneurons. The few FS cells observed were removed from analysis. Behavior specific timestamps were recorded by an observer simultaneous to performance. Units were identified and sorted using Off-line sorter (Plexon). Data were exported and analyzed using statistical and graphing routines in MATLAB to examine firing activity to decision and reward-epochs.
SU analysis epochs were computed as the total number of spikes divided by time. The particular epoch (decision, defined as the moment a dig was initiated, or reward, defined as the moment the mouse received the reward) was taken with a 500 ms window around the desired epoch and compared to baseline firing (average activity from trial start to 800 ms before a decision epoch. A t-test was used to determine neurons which significantly increased firing to an epoch (p < 0.05), and a multifactor ANOVA was used to determine neurons which fired preferentially for reward or decision-epochs (p < 0.05). Neural activity was normalized by Z-transform, and ANOVA and post hoc comparisons were used to measure differences in transformed firing rates within and across genotypes (p < 0.05) related to activity during behavioral epochs. Pearson chi-square tests (p < 0.05) were used to compare proportions of neurons. Line graphs centered on behavioral epochs (correct/error decisions, reward) show average activity around behavioral epochs with shaded standard error of the mean (SEM). Line graphs represent average trial activity by genotypes, and differences reported through ANOVA and post hoc comparisons (p < 0.05). Pearson linear correlation was used to determine correlation of neural activity (decision epoch) with behavioral performance (p < 0.05) and linear regression r value is calculated to provide direction of correlation. ANCOVA was performed by computing the covariance for both genotypes between the number of trials to criteria on reversals or discriminations, with z-transformed firing rates during decision epoch for all neurons.
Results
Behavioral impairment on first reversal
Research in rats (32) and primates (33) has consistently shown behavioral impairment of OFC lesions on reversal learning. However the effects of OFC lesions and other manipulations on reversal learning are typically transient (30,34,35), affecting only the first or early reversal problems in a set. Here we show a similar deficit in mice performing a serial reversal task (Figure 1A–C). As in our previous reports (17,28,36), control and transgenic mice learned discriminations without difficulty (Figure 1B), learning each discrimination (D1–D5) rapidly and with minimal errors (Figure 1C), but Plaur mice required more trials to meet criterion on the first reversal (Rev1). Two-way ANOVA revealed a main effect of genotype (F(1,154) = 5.0, p < 0.05), of discrimination vs reversal problem (F(9,154) = 48.7, p < 0.001), and a genotype × problem interaction (F(9,154) = 13.7, p < 0.001).
Similar to previous reports, the Plaur mouse behavioral deficit was limited to the first few reversal problems but not later reversals. Post hoc comparisons demonstrate that Plaur mice required significantly more trials to complete Rev1 as compared to control mice (t-test, p < 0.001). The difference in behavior between groups was not observed after the first reversal. A similar pattern was observed on number of errors (Figure 1C), except Plaur mouse deficits were still present during the second reversal. Two-way ANOVA revealed a main effect of genotype (F(1,154) = 13.3, p < 0.001), of problem (F(9,154) = 52.9, p < 0.001) and a genotype × problem interaction (F(9,154) = 11.0, p < 0.001). Post hoc comparisons showed increased errors during the reversal phase relative to the discrimination problem, consistent with animals having to take more time to override previously learned associations. Error-type was different between genotypes (inset, Figure 1C), with Plaur mice committing significantly more perseverative-type errors than controls (p < 0.01).
Characterization of neural activity
We recorded neuronal activity from regular spiking OFC neurons in control mice (180 discrimination, 180 reversal) and from Plaur mice (226 discrimination and 224 reversal, Figure 2A). Putative pyramidal neuron waveforms were virtually identical between Plaur and control mice (Figure 2B), with no differences in max amplitude (p = 0.11) or waveform duration (p = 0.38). As anatomical data predicted (15,17), few fast-spiking cells were encountered in the control mice (n = 8 cells) and Plaur mice (n = 2 cells), thus analysis of neural activity was performed on regular spiking putative pyramidal cells only. We observed increased firing in control mice before decisions and reward receipt (Figure 2C). Plaur mice showed similar responses, although the magnitudes of the firing rates were not as robust as control neurons. Since diminished PV+ interneuron numbers may lead to a constitutively overactive OFC, we first asked if baseline firing differed between the two groups. Consistent with this hypothesis, baseline firing was significantly different between the two genotypes for task related neurons (control: 4.0 spikes/s and Plaur: 12.4 spikes/s, p < 0.05, two-sample t-test). Due to baseline differences in firing, data are presented in both raw spikes/second and in z-transformed form.
Counts of task-related neurons are reduced in Plaur OFC
To determine the effect of interneuron loss, neural responses were divided into four categories: increasing-decision, decreasing-decision, increasing-reward and decreasing-reward. Interestingly, no overlap of activity change and behavioral epoch was observed in these OFC neurons (i.e., no neuron increased activity both for decision and reward). During discrimination learning, ~50% of recorded neurons were significantly active during behavioral performance in both genotypes (Figure 2). During reversal learning, we found two primary effects. First, significantly more control OFC neurons developed behavioral correlates during reversal learning (75%), compared to discrimination (χ2 = 9.2, p < 0.01), whereas Plaur mice had roughly equal percentages of neurons modulated during discriminations and reversals (χ2 =3.2, p = 0.08). This effect in the Plaur mice was mainly limited to increasing-type neurons, not decreasing-type (Figure 2D). Secondly, Plaur mice have diminished increasing-decision type neurons during discrimination (χ2 = 4.5, p < 0.05) and reversal learning (χ2 = 3.9 p < 0.05), compared to control mice. Thus, during reversal learning, neurons in control OFC exhibited more task-related activity than Plaur mice.
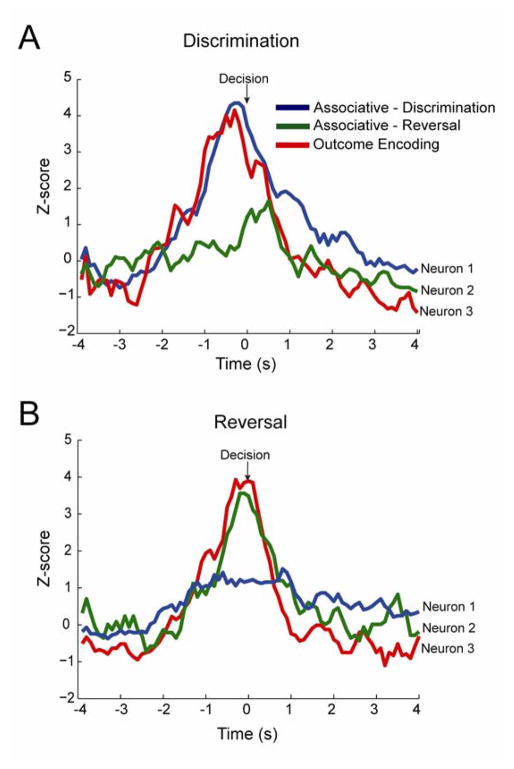
Previous research has identified OFC neurons that are highly associative, selectively firing based on either the initial or reversed associations. Rat and primate OFC neurons appear to encode expected outcomes, firing during decision-making to cues that predict reward both before and after reversal (31,32,37). To determine if similar neural representations exist in mouse OFC, we characterized neurons as: 1. associative-discrimination neurons which encoded correct decisions during discrimination learning, but not reversal (Figure 3A,B, blue lines); 2. associative-reversal neurons which encoded correct decisions during reversal, but not during initial discrimination learning (Figure 3A,B green lines); and 3. outcome-encoding neurons which encoded the correct decision both during initial discrimination learning and during reversal (Figure 3A,B red lines).
Figure 3.
Neuronal examples of functional associations. (A,B) Single neuron examples from control mouse showcasing 3 different types of neural associations. Blue lines show activity for an associative-discrimination type neuron, which fires more for discrimination than reversal trials. Green lines show activity for an associative-reversal neuron, which fires more for reversal than discrimination. Red lines show activity for an outcome expectant type neuron, which does not modulate its activity between task type.
As interneurons are critical for organizing local neuronal activity, we hypothesized that Plaur OFC neurons would show diminished encoding, compared to control neurons. During the decision epoch, Plaur mice had fewer outcome-encoding neurons than controls, (χ2 = 8.8, p < 0.01), and equal numbers of associative-type neurons (χ2 = 0.9, NS, Table 1). Interestingly, the nature of associative encoding was different for reward-neurons, where Plaur mice had increased numbers of outcome-encoding (χ2 = 4.4, p < 0.05) and diminished numbers of associative-type neurons (χ2 = 16.9, p < 0.01). Thus, Plaur mice exhibit significant reduction outcome-encoding neurons during the decision epoch, but an enhancement during reward epochs, as compared to control mice.
Table 1.
Classification of encoding neurons
| Genotype | Total neurons | Decision | Reward | ||
|---|---|---|---|---|---|
| Outcome | Associative | Outcome | Associative | ||
| Control | 180 | 20 | 11 | 10 | 27 |
| Plaur | 224 | 5* | 16* | 22* | 4* |
About 65% of control decision-increasing neurons were outcome-expectant, while 24% of Plaur neurons were. Control mice had 35% associative-type neurons, while Plaur had significantly more (76%). Among reward-neurons, control mice had 27% outcome-expectant neurons, while Plaur had significantly more, 85%. Plaur mice had fewer associative-type neurons, 15% compared to 73% among controls. Significantly different groups (p < 0.05) are designated by an asterisk (*).
Reward and Decision-related activity is attenuated in Plaur mice
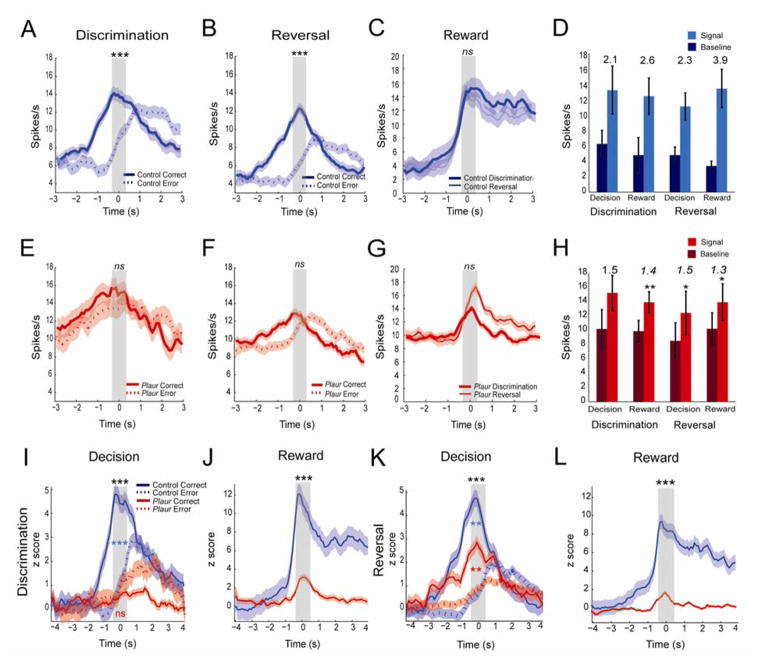
PV+ interneurons are hypothesized to coordinate firing patterns of neuronal ensembles. We hypothesized that fewer PV+ interneurons would lead to diminished encoding strength of associations and outcomes due to lowered coordinated activity of OFC neurons. In Figure 4, we address this issue by plotting average firing on correct and incorrect trials for all increasing-type neurons for control (blue) and Plaur mice (red) in both raw spikes/s (Figure 4A–H) and in the z-transformed scale (Figure 4I–L). Control increasing-type neurons display different firing patterns on correct vs error trials during discrimination and reversals (p < 0.001, Figure 4A,B). In contrast, Plaur OFC increasing-type decision neurons showed no differential firing between correct or error trials on discrimination (p = 0.23) or reversal (p = 0.14, Figure 4E,F). Control reward neurons are not significantly different on discrimination or reversal reward receipt epochs (p = 0.88, Figure 4C), nor are Plaur reward neurons (p = 0.39, Figure 4G). In addition, the signal:baseline ratio (firing during epoch divided by baseline firing) was significantly different between genotypes for decision neurons during reversal (control 2.3, Plaur 1.5, p < 0.05) and for reward neurons in discrimination (control 2.6, Plaur 1.4, p < 0.01) and reversal (control 3.9, Plaur 1.3, p < 0.05, Figure 4D,H).
Figure 4.
Averaged neural activity is shown during decision and reward-epochs for increasing type neurons. (A–C) Control increasing-type neurons show differential encoding of correct vs error decisions. Firing rates increased during reward receipts, but the rates were not different comparing discrimination and reversal problems. Significant differences during the decision or reward epochs, as denoted by grey rectangles, are marked by asterisks. (D) By definition of increasing neurons, the value of the signals were greater than baseline. The signal:baseline ratios were calculated for each epoch for control mice. (E–G) Plaur increasing-type neurons show no differential encoding of correct or error decisions. Similar to control mice, reward encoding in the Plaur mice did not exhibit differences between discrimination and reversal problems. (H) Plaur units have a higher baseline firing rate than controls, which alters signal:baseline ratio (epoch firing divided by baseline firing), with control increasing-type neurons (D) having significantly higher ratio than Plaur neurons. Asterisks denote differences between control and Plaur mouse signal firing rates (p < 0.05). (I,K) Average z-scored neural activity of decision related neurons from control and Plaur mice on discrimination (I,J) and reversal trials (K,L). Control mice (blue lines) demonstrate significantly increased neural activity at time of decision for correct (solid line) and error (dashed line) compared to Plaur mice (red lines). Plaur mice show little activation for correct choice on the discrimination task (I), and less increased activity during the reversal task (K). (J,L) Control mice (blue lines) show significantly increased activity for reward receipt compared to Plaur (red) mice on both discriminations and reversals. Control mouse neurons increase activity during reward receipt, and maintain higher activity rates during consumption, while Plaur mice show a modest increase to reward receipt only. ** denote p < 0.01, *** p < 0.001. Black asterisks denote comparison between genotypes. Blue asterisks comparison between control correct and error epochs. Red asterisks comparison between Plaur correct and error epochs.
For direct comparison of the relative change in activity between genotypes, the z-scored data were compared (Figure 4I–L). Control mouse OFC neurons robustly encoded correct and erroneous decisions during the decision-epoch during discrimination and reversal trials (Figure 4I,J). Furthermore, activity was significantly stronger on correct versus incorrect trials (p < 0.001). By contrast, firing in Plaur OFC was not elevated during correct trials during the decision-epoch in the discrimination phase (p < 0.6). During reversals, Plaur mice did show significant selectivity, firing more strongly during correct trials relative to baseline and error, but the change in firing rate from baseline during decision epoch was diminished for correct vs error trials, compared to control mice (p < 0.01). The smaller change in firing in Plaur OFC may reflect that increased baseline (noise) due to interneuron loss.
During reward delivery, neurons significantly increased firing (Figure 4J,L). Similar to activity during decision epoch, the response to reward was dramatically reduced in Plaur mice. Consistent with the selection of increasing-reward-type neurons, firing in response to reward was significantly stronger for both groups (p < 0.01, Figure 4J,L). However, the magnitude of change from baseline during reward epoch was reduced for Plaur mouse reward neurons, relative to control mouse neurons (p < 0.05). These data suggest that a reduction in fast-spiking PV+ interneurons in the Plaur mouse leads to diminished encoding of correct and erroneous decisions and rewards. It may be argued that these findings reflect differences in behavior (i.e., Plaur mice performed worse that control mice) and not PV+ interneuron deficit. To address this concern, we excluded the first reversal phase and redid the above analysis and found the same pattern of results (Supplemental Figure S1). The same analyses were performed on decreasing-type cells (Supplemental Figure S2), and decreasing-type neurons had no significant activity between genotypes. Finally, the OFC has been shown to represent reward, we observed reward expectancy in control, but not Plaur neurons (Supplemental Figure S3).
Activity in mouse OFC is correlated with behavior
We know OFC is critical for reversal learning (19,24,30,38–40), and Plaur mice have reversal deficits that we have hypothesized are due to disrupted encoding of decisions in OFC (17). Thus, we predict that activity in OFC should be correlated with decision-making, specifically during reversal decisions, with activity corresponding to task difficulty. We plotted the z-scored neural activity during decision epoch with respect to trials to criterion for control and Plaur mice (Figure 5A,B). Neither control nor Plaur mouse neurons were correlated with performance during acquisition of the discrimination problems (r = −0.06, p = 0.8 control, r = 0.74 and p = 0.09 for Plaur). However, for reversal leaning, high control neuron firing rates were observed when performance was impaired (r = 0.43, p < 0.05). Plaur neurons did not show this correlation between firing rate and reversal learning (r = 0.21, p < 0.4). In addition, ANCOVA demonstrated a significant difference between control and Plaur neural firing rates as a function of task performance (F(1,42) = 4.35, p < 0.05) in reversal, but not discrimination problems (F(1,25) = 0.16, p = 0.69) demonstrating that the two genotypes are not only differently correlated, but are significantly different from each other only during reversals. These data demonstrate that increased activity in control OFC is correlated with behavioral performance during reversal learning, as observed previously in rats (41), suggesting that an optimal change in firing rate is required for OFC function. Analyses of decreasing-type and non-significant type neurons showed no significant correlations (Supplemental Figure S4).
Figure 5.
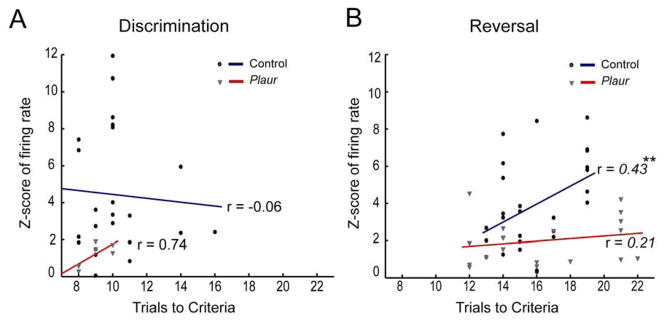
Correlation of neural activity with behavioral performance. Correlation of neural activity during discrimination (A) or reversal problems (B) with performance of each mouse as the number of trials to criteria (early correct and errors, + 8 consecutive correct). Neither control nor Plaur neural activity are significantly correlated with number of trials required to complete criteria for discrimination, but control mice show a significant positive correlation on reversal problems, such that the more trials required to reach criteria, the more OFC neurons fired. Plaur mouse OFC neurons do not show this correlation. Groups were significantly different from each other, as illuminated by ANCOVA, ** denote p < 0.01.
Discussion
We show that neurons in mouse OFC exhibit the same correlates as observed in rats and primates during reversal learning, encoding outcome expectations and associations formed to reward predictive cues (31,37,42–44). Mouse OFC neurons were responsive during decision and reward delivery epochs in discrimination and reversal phases. However, neural activity was only significantly correlated to behavioral performance during reversal. Our data also show that a neurodevelopmental example of an interneuron deficit leads to difficulty navigating a reversal task (15,17,26) in agreement with reports from human patients with schizophrenia and impaired cognition (45–48). In the Plaur mice, these behavioral deficits likely reflect decreased numbers of fast-spiking PV+ interneurons, which led to altered encoding of decisions (correct vs error), elevated baseline OFC activity, diminished differences between baseline firing and trial epochs, a reduction in predicted outcome encoding, and a lack of correlation between OFC firing and behavior.
Role of OFC during reversal learning
Lesion and behavioral studies have shown that rat and some primates display initial reversal learning impairments but soon solve the problem at the same rate as the control group (49,50). Yet, there is also evidence of prolonged reversal deficits due to OFC lesions in Rhesus monkeys (51), and data from macaque OFC lesions suggest that reversal deficits may come from damage to fibers of passage (52), potentially highlighting species anatomical differences, behavioral task differences, or an interaction of both. Our mouse reversal data fits with the rat and New World monkey reports (but see (53)). During Rev1, Plaur mice make >2-fold the errors as control mice and require nearly 1/3 more trials to complete the initial reversal problem. While the number of errors was higher in Rev2 for Plaur mice, behavioral performance in terms of trials to criteria was the same as the control group. After multiple reversals, both genotypes show trends towards decreasing their number of trials to criteria (and errors) on reversals. The magnitude of reversal deficit observed in the Plaur mice is nearly identical to the mouse OFC lesion study (30). Our data are in accordance with rat and primate lesion and pharmacological data, and the most recent mouse studies (23,25,49,54–56), demonstrating the necessity of a functioning OFC for reversal learning across species.
OFC encoding of sensory associations and expected outcomes
Research has demonstrated that OFC is critical for the development of outcome expectancies and encoding of specific sensory associations (31,37,42,43). This is the first paper to describe how neurons in mouse OFC encode aspects of reversal learning. Many control OFC neurons reliably were selective during the decision period, but only during either discrimination or reversal. Other neurons responded strongly during decision during both discrimination and reversal problems, and these neurons are thought to be ‘outcome-encoding’, as they represent the expected outcome regardless of context. This type of encoding is consistent with previous reports of OFC neurons in other animal models (43,57) and was significantly reduced in Plaur mice.
Optogenetic control of PV+ interneurons demonstrated their role in coordinating cellular networks (58–60), and our data support a role for OFC PV+ cells in facilitating local networks to tune responses during changes in cue-outcome associations, as seen in rats (61). Unlike Plaur reward-neurons, control reward-neurons showed evidence of representing the expected outcome of the decision. With limited numbers of PV+ interneurons, the ability of OFC to effectively coordinate neural encoding of reward expectancy is diminished. Indeed, Plaur reward-neurons failed to encode associations with cues during either discrimination or reversal trials, suggesting a diminished capacity to associate a stimulus with a predicted rewarding outcome. A reward expectation deficit may lead to an incomplete association between cues and outcomes, and drive weak, if any, outcome expectancies. After the first reversal, other brain areas, such as basolateral amygdala (BLA) (62) and dorsal striatum (63–65) may become more critical.
Our data show that diminishing the number of FS PV+ interneurons in OFC leads to elevated baseline firing rate of pyramidal neurons, but no difference in the average maximum firing rate during a behavioral epoch. This suggests that it is not OFC neural firing rate, per se, that leads to behavioral modification, but rather the change from baseline at any given epoch that elicits behavioral change. Neurons in OFC are responsible for driving flexible encoding in BLA, and therefore, an inflexible OFC (from lesion) results in inflexible encoding in BLA during reversal learning (39,57). In Plaur mice, the BLA has normal PV+ interneuron numbers (36). PV+ interneurons inhibit the regular spiking putative pyramidal neurons (60), and loss of PV+ fast-spiking cells implies disinhibition and diminished encoding of information (66). In this context, Plaur mouse OFC putative pyramidal neurons show elevated baseline firing rates (three-fold greater than control mice) and altered associative encoding. These changes in neural activity lead to an altered signal to noise ratio in OFC, leaving OFC inflexible and projecting noisy or incomplete information to BLA, possibly underlying the initial reversal behavior deficit.
Research into flexible encoding in OFC of associations during reversal learning has shown an inverse correlation with reversal performance (41). As seen in the rat, control mouse OFC neurons demonstrate an inverse relationship with reversal performance and neural activity, where worse behavioral performance is associated with higher OFC neuronal firing. In the Plaur mouse, which has elevated baseline neural activity and diminished strength of associative encoding, there is no such correlation between activity and reversal performance. This may be due to Plaur OFC neurons already displaying elevated firing, leaving less room for signalling flexible encoding. Thus, while control mouse OFC appears to form and modify associations as observed in other animals, diminished numbers of FS PV+ interneurons in OFC of the Plaur mouse may alter signal to noise ratio, driving inflexible behavior.
Many neuropsychiatric disorders have developmental origins which have been linked to altered GABAergic inhibition (10,67,68). Patients with schizophrenia demonstrate impaired performance and increased errors when tested on reversals, implying compromised OFC function (18–20,69,70). The specific anatomical and cognitive deficits seen in our mouse model strongly recapitulate human disease (71,72). Our data demonstrate that changing the GABAergic tone in OFC interferes with the associative and outcome expectant functions, akin to observations in humans with psychiatric conditions.
Supplementary Material
Acknowledgments
Financial support for this study was provided by NARSAD Young Investigator Award (EMP); National Institute on Drug Abuse grant DA108826 (PI:EMP), DA015718 (PI:GS) and DA031695, (PI:MRR).
We thank D. Calu for neural recording assistance, S. Scahill for histology assistance, G. Martins, S. Mullins, A. Gruber, A. Keller, P O’Donnell, and J. Smith for insightful comments on the manuscript.
Footnotes
Financial Disclosures
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 2.Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 4.Aronica E, Redeker S, Boer K, Spliet WG, van Rijen PC, Gorter JA, et al. Inhibitory networks in epilepsy-associated gangliogliomas and in the perilesional epileptic cortex. Epilepsy Res. 2007;74:33–44. doi: 10.1016/j.eplepsyres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence YA, Kemper TL, Bauman ML, Blatt GJ. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand. 2010;121:99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roll P, Vernes SC, Bruneau N, Cillario J, Ponsole-Lenfant M, Massacrier A, et al. Molecular networks implicated in speech-related disorders: FOXP2 regulates the SRPX2/uPAR complex. Hum Mol Genet. 2010;19:4848–4860. doi: 10.1093/hmg/ddq415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Zhang B, Wang T, Liang QC, Jing XR, Zheng J, et al. Increased expression of urokinase-type plasminogen activator receptor in the frontal cortex of patients with intractable frontal lobe epilepsy. J Neurosci Res. 2010;88:2747–2754. doi: 10.1002/jnr.22419. [DOI] [PubMed] [Google Scholar]
- 13.Royer-Zemmour B, Ponsole-Lenfant M, Gara H, Roll P, Leveque C, Massacrier A, et al. Epileptic and developmental disorders of the speech cortex: ligand/receptor interaction of wild-type and mutant SRPX2 with the plasminogen activator receptor uPAR. Hum Mol Genet. 2008;17:3617–3630. doi: 10.1093/hmg/ddn256. [DOI] [PubMed] [Google Scholar]
- 14.Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 15.Powell EM, Campbell DB, Stanwood GD, CD, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eagleson KL, Bonnin A, Levitt P. Region- and age-specific deficits in gamma-aminobutyric acidergic neuron development in the telencephalon of the uPAR(−/−) mouse. J Comp Neurol. 2005;489:449–466. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- 17.Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Astrocyte-mediated hepatocyte growth factor/scatter factor supplementation restores GABAergic interneurons and corrects reversal learning deficits in mice. J Neurosci. 2010;30:2918–2923. doi: 10.1523/JNEUROSCI.5268-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 24.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Baxter MG, Browning PG. Two wrongs make a right: deficits in reversal learning after orbitofrontal damage are improved by amygdala ablation. Neuron. 2007;54:1–3. doi: 10.1016/j.neuron.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Bae MH, Bissonette GB, Mars WM, Michalopoulos GK, Achim CL, Depireux DA, et al. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Exp Neurol. 2010;221:129–135. doi: 10.1016/j.expneurol.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- 28.Martins GJ, Shahrokh M, Powell EM. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience. 2011;176:199–209. doi: 10.1016/j.neuroscience.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colacicco G, Welzl H, Lipp HP, Wurbel H. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res. 2002;132:95–102. doi: 10.1016/s0166-4328(01)00391-6. [DOI] [PubMed] [Google Scholar]
- 30.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:1–4. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 35.van der Plasse G, La Fors SS, Meerkerk DT, Joosten RN, Uylings HB, Feenstra MG. Medial prefrontal serotonin in the rat is involved in goal-directed behaviour when affect guides decision making. Psychopharmacology (Berl) 2007;195:435–449. doi: 10.1007/s00213-007-0917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Selective prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav Brain Res. 2014;259:143–151. doi: 10.1016/j.bbr.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 38.Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–1174. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke KA, Takahashi YK, Correll J, Brown PL, Schoenbaum G. Orbitofrontal inactivation impairs reversal of Pavlovian learning by interfering with ‘disinhibition’ of responding for previously unrewarded cues. Eur J Neurosci. 2009;30:1941–1946. doi: 10.1111/j.1460-9568.2009.06992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 45.Weiler JA, Bellebaum C, Brune M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- 46.McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med. 2009;39:1289–1293. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- 47.Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed P, Staytom L, Stott S, Truzoli R. Comparison of conditioning impairments in children with Down syndrome, autistic spectrum disorders and mental age-matched controls. J Intellect Disabil Res. 2011;55:988–997. doi: 10.1111/j.1365-2788.2011.01454.x. [DOI] [PubMed] [Google Scholar]
- 49.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 50.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–39. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- 52.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Racz A, Ponomarenko AA, Fuchs EC, Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J Neurosci. 2009;29:2563–2568. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruber AJ, Powell EM, O’Donnell P. Cortically activated interneurons shape spatial aspects of cortico-accumbens processing. J Neurophysiol. 2009;101:1876–1882. doi: 10.1152/jn.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delamater AR. The role of the orbitofrontal cortex in sensory-specific encoding of associations in pavlovian and instrumental conditioning. Ann N Y Acad Sci. 2007;1121:152–173. doi: 10.1196/annals.1401.030. [DOI] [PubMed] [Google Scholar]
- 62.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 63.Pisa M, Cyr J. Regionally selective roles of the rat’s striatum in modality-specific discrimination learning and forelimb reaching. Behav Brain Res. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 64.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biol Psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Duuren E, Lankelma J, Pennartz CM. Population coding of reward magnitude in the orbitofrontal cortex of the rat. J Neurosci. 2008;28:8590–8603. doi: 10.1523/JNEUROSCI.5549-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 68.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 69.Rubin P, Hemmingsen R, Holm S, Moller-Madsen S, Hertel C, Povlsen UJ, et al. Relationship between brain structure and function in disorders of the schizophrenic spectrum: single positron emission computerized tomography, computerized tomography and psychopathology of first episodes. Acta Psychiatr Scand. 1994;90:281–289. doi: 10.1111/j.1600-0447.1994.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 70.Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med. 1995;25:619–630. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- 71.O’Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- 72.Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.