Abstract
Background
Several neural interface technologies that stimulate and/or record from groups of axons have been developed. The longitudinal intrafascicular electrode (LIFE) is a fine wire that can provide access to a discrete population of axons within a peripheral nerve fascicle. Some applications require, or would benefit greatly from, technology that could provide access to multiple discrete sites in several fascicles.
New Method
The distributed intrafascicular multi-electrode (DIME) lead was developed to deploy multiple LIFEs to several fascicles. It consists of several (e.g. six) LIFEs that are coiled and placed in a sheath for strength and durability, with a portion left uncoiled to allow insertion at distinct sites. We have also developed a multi-lead multi-electrode (MLME) management system that includes a set of sheaths and procedures for fabrication and deployment.
Results
A prototype with 3 DIME leads was fabricated and tested in a procedure in a cadaver arm. The leads were successfully routed through skin and connective tissue and the deployment procedures were utilized to insert the LIFEs into fascicles of two nerves.
Comparison with Existing Method(s)
Most multi-electrode systems use a single-lead, multi-electrode design. For some applications, this design may be limited by the bulk of the multi-contact array and/or by the spatial distribution of the electrodes.
Conclusion
We have designed a system that can be used to access multiple sets of discrete groups of fibers that are spatially distributed in one or more fascicles of peripheral nerves. This system may be useful for neural-enabled prostheses or other applications.
Keywords: peripheral nerve interface, multi-electrode, intrafascicular electrode, neuroprosthesis
1 Introduction
The past few decades have seen significant advances in the development of neurotechnology to stimulate neural tissue to replace function lost due to neurological disability or neurotrauma. Although the most widely clinically deployed systems stimulate the cochlea (Clark, 2006; von Ilberg et al., 2011), deep brain structures (Farris and Giroux, 2011) or the spinal cord (Cruccu et al., 2007), the peripheral nervous system has also been targeted extensively. For example, systems have been developed that utilize stimulation of the peroneal nerve for foot drop (Kottink et al., 2004), the vagal nerve to treat epilepsy or depression (Groves and Brown, 2005), the sacral nerve to treat urinary urge (Brazzelli et al., 2006) or fecal incontinence (Jarrett et al., 2004), gastric nerves to treat gastroparesis (Zhang and Chen, 2006), and motoneurons of ventilatory and abdominal muscles to provide sufficient ventilation (Creasey et al., 1996; Walter et al., 2011).
These systems typically use an arrangement in which a single lead is used to target one or more stimulation sites. In some instances, a single lead contains multiple electrode contacts in concentric circles along its longitudinal axis to stimulate sites at pre-determined distances along the lead (Cameron, 2004; Clark, 2006; Farris and Giroux, 2011; von Ilberg et al., 2011), while other designs use multi-contact cuffs (Tyler and Durand, 2002), 2-dimensional grids of electrode shanks (Branner and Normann, 2000), or a set of contacts on a single flexible substrate (tfLIFE: (Lago et al., 2007; Micera et al., 2010), TIME: (Badia et al., 2011a; Badia et al., 2011b)). In all of these designs, the contacts are embedded in a substrate such that the electrodes are spaced at fixed, predetermined distances from each other. This arrangement provides the opportunity for post-surgical selection of a single active channel or the selection and use of multiple channels while enabling a relatively simple implantation procedure and providing mechanical durability of the contact and its connection to the lead.
This single-lead, multi-electrode design has proven to be highly useful, but for some applications, the design may be limited by the bulk of the multi-contact array and/or by the spatial distribution of the electrodes. For example, there is great interest in interfacing with peripheral nerves of amputees in order to stimulate afferent fibers to provide sensation (Boretius et al., 2010; Dhillon et al., 2005; Dhillon et al., 2004; Micera and Navarro, 2009) and/or to record from efferent fibers to derive motor commands to control a prosthesis (Dhillon et al., 2005; Dhillon et al., 2004; Micera et al., 2010; Micera and Navarro, 2009). These systems require (or at least would benefit greatly from) interfaces at distinct sites at different locations in order to provide multiple channels of communication with a variety of fibers.
Previous work has demonstrated the feasibility of using longitudinal intrafascicular electrodes (LIFE) to provide access to small groups of axons within a peripheral nerve fascicle (Dhillon et al., 2005; Dhillon et al., 2004). LIFEs were made from Teflon-coated Pt/Ir wire (27.5μm diameter) by de-insulating approximately 1 mm of the wire (Lefurge et al., 1991; Malagodi et al., 1989) to form one active site. In human amputees, these electrodes were used to stimulate nerve fibers to elicit discrete sensations (Dhillon et al., 2005; Dhillon et al., 2004) and to record motor activity to drive the movement of a prosthetic arm (Dhillon et al., 2005; Dhillon et al., 2004). The small size of these fine-wire electrodes and their longitudinal orientation provides a safe and stable interface to the nerve subpopulations within a nerve fascicle (Lefurge et al., 1991; Yoshida and Stein, 1999). Since this system utilizes individual wires, it is possible to place multiple LIFEs in one fascicle, in different fascicles within one nerve or in multiple nerves to provide access to multiple sites that are distributed in a region of the body. The small diameter of the fine wire that comprise the electrode/lead provides a high degree of mechanical compatibility with the nerve fibers, but also presents risks of entanglement during surgical deployment and breakage if the fine wire lead is routed in a manner that subjects it to high mechanical stress.
This manuscript reports on the development of a system that can be used to access multiple sets of discrete populations of fibers that are spatially distributed in one or more peripheral nerves. Our approach utilizes LIFE technology because it has been demonstrated to provide a stable interface to a small group of fibers. This work addresses the challenge of scaling this technology for use in distributed multi-channel systems by facilitating the surgical implantation of several LIFEs at various sites in the periphery and enhancing the strength and durability of the leads. We have developed a novel distributed intrafascicular multi-electrode (DIME) lead consisting of multiple-LIFEs packaged in a single lead and an approach to deploy a plurality of these leads.
2 Materials and methods
2.1.1 DIME Design objectives
In the peripheral nervous system, groups of nerve fibers are surrounded by perineurium to form a fascicle; groups of fascicles are surrounded by epineurium to form a nerve bundle. In humans, the diameter of the fascicles range from 0.1- 1mm and the diameter of peripheral nerves range from 2-12mm (Gustafson et al., 2009; Gustafson et al., 2005). In the human arm the diameters of the median and ulnar nerves are approximately 3mm (Heinemeyer and Reimers, 1999) and the number of fascicles and distribution of motor and sensory fascicles vary along the length of the nerve (Stewart, 2003; Sun et al., 2009).
The primary objective in the design of the DIME was to provide access to small groups of axons from different fascicles within a single nerve or from different nerves within a region of the body. The system should be suitable for chronic use and therefore should not induce trauma or physiological reactions that would impair nerve function or degrade health. The system should be sterilizable and implantation should utilize procedures that can be executed readily and reliably by a trained surgeon.
2.1.2 DIME design overview
Each LIFE consists of a highly flexible insulated wire (typically platinum-iridium approximately 25 μm in diameter) with an exposed area that forms the single electrode contact and a rigid needle that is used to insert the wire into an exposed fascicle; the needle is removed after the wire is inserted. The DIME lead (Figure 1) consists of multiple LIFEs to enable several channels of communication with small groups of nerve fibers within neighboring fascicles. The proximal ends of the LIFEs within a DIME lead are coiled and housed in a sheath to provide robustness and ease of handling during the implantation procedure and for durability when implanted for extended periods of time. For systems that require access to nerves at different sites, multiple DIME leads can be deployed individually or clustered to form a single lead to further facilitate routing and connections.
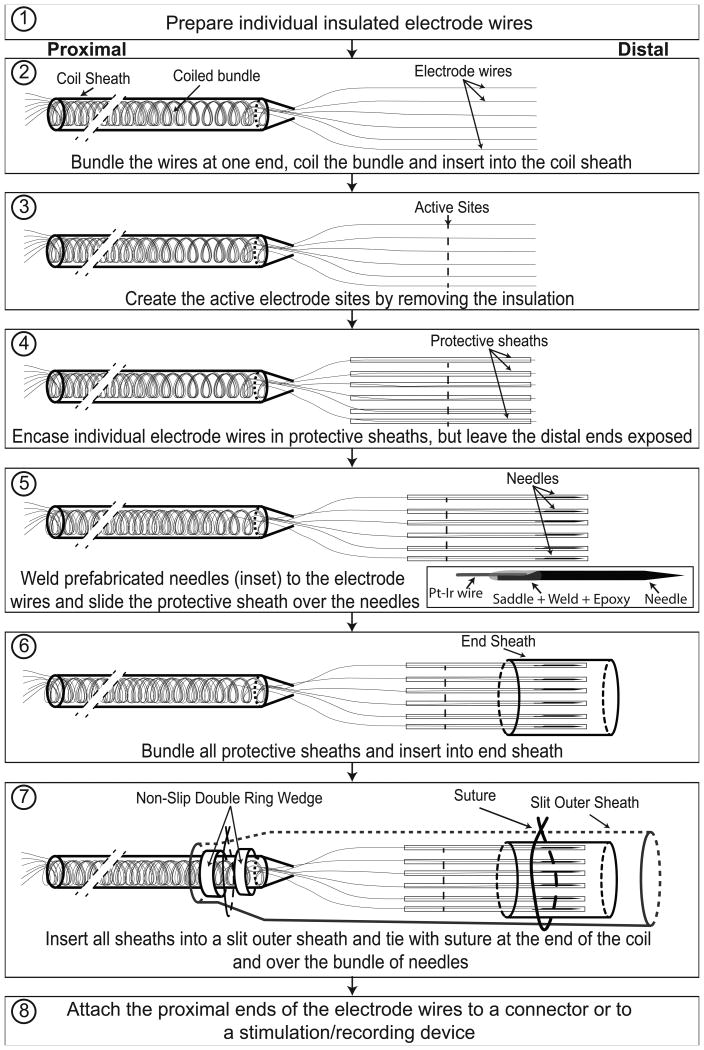
Figure 1.
DIME fabrication process: Illustrations of steps involved in fabrication of DIME lead with multiple sheaths. See text for descriptions of each step. Diagrams are not to scale to allow illustration of thin, long components; for reference, the length of the needle is 2.5 cm and the diameter of the silicone-encased coiled bundle is 640 μm.
Because the individual wires are very fine and flexible and there are rigid needles attached at the end, the DIME has been designed with a system of sheaths to protect the electrodes and prevent entanglement of the wires while handling during surgery. The multi-lead multi-electrode (MLME) management system consists of a set of sheaths for each LIFE and an outer sheath that covers the bundle of individual sheathed LIFEs. The outer sheath and then the individual sheaths are sequentially removed during the implantation procedure. Implant-grade materials are used in the construction of all components and the system is suitable for sterilization using standard ethelyne-oxide processes.
2.1.3 DIME fabrication process
Steps 1 to 3 in Figure 1 show a schematic of the process for fabrication of a DIME with six electrodes. In step 1, the proximal portions of six insulated electrode wires (25μm diameter Pt/Ir coated with 6 μm thick PTFE (polytetrafluoroethylene) to result in 37μm diameter insulated wire, A-M Systems, Carlsborg, WA) are coiled to provide strain relief and flexibility. In step 2, the coiled portion of the bundle is ensheathed within an implant-grade silicone tube (MED4515, Nusil, Carpinteria, CA) of 300μm inner diameter (ID) and 640μm outer diameter (OD) to protect the coiled bundle and provide strain relief, while maintaining flexibility. In step 3, the active site is created by de-insulating an approximately 1mm portion of each of the free hanging wires at a distance of approximately 2cm from the distal end. De-insulation is achieved by a using a heated tungsten rod (or, alternatively, by using a narrow laser beam). The distance between active site and the distal end of the wire can be varied depending upon anchoring requirements. This set of free hanging portions of individual wires can be used as 6 LIFEs for stimulation or as 5 LIFEs and 1 extrafascicular reference electrode for recording.
2.1.4 DIME packaging process
The DIME packaging process is illustrated in steps 4 to 8 of Figure 1. In step 4, each of the free hanging LIFEs is inserted into a protective sheath. The protective sheath is a polyimide tube (160 μm ID, 179 μm OD, A-M Systems, Carlsborg, WA) that ensheathes each LIFE and is shorter in length, such that a portion of the LIFE emerges from the protective sheath. Polyimide was selected as the material for these sheaths because it provides suitable protection and because the sheaths can be custom fabricated with multiple micro-apertures to accelerate the diffusion of gas during sterilization (although microapertures were not incorporated into the samples used in the prototypes). In step 5, using a process that has been modified from that described by Malagodi and colleagues (Malagodi et al., 1989), tungsten needles are prepared from tungsten rods (we have used rods of 1.5cm length, 75μm diameter; and rods of 2.5cm length, 100μm diameter in prototypes) by etching one end to create a sharp tip and grinding the other end to create a saddle, the saddled portion is then welded to the distal end of each LIFE, and a thin coat of conforming medical epoxy (Epotek-301, Billerica, MA) is applied to smoothen the wire and needle junction in order to limit the potential for nerve damage (see inset in Figure 1, step 5). After attaching the needles, the protective sheaths are moved to cover the needles entirely. In step 6, the six protective sheaths (with LIFEs and needles encased) are bundled and inserted into the end sheath, a silicone tube (508 μm ID, 939 μm OD). In step 7, the end sheath, protective sheaths, and part of the coil sheath are inserted into the outer sheath, which is a silicone tube (1.5mm ID, 2mm OD), that is slit along its length (shown as dotted lines in Figure 1, step 7). The purpose of the outer sheath is to protect the junction where wires come out of the coil sheet and to provide mechanical stability while routing during surgery; the slit is included to facilitate insertion and removal of its contents without damage. The outer sheath is closed using suture at the distal end and at the proximal end, where it overlaps the coil sheath. Note that the coil sheath has silicone rings (0.65mm ID and 1.2mm OD) to help stabilize the suture that holds the outer sheath on the coil sheath.
Although our long-term objective is to use the DIME with implanted electronics, to allow for short term (weeks to months) studies with implanted electrodes we have fabricated a system that uses percutaneous leads and an external connector. The external connector sytem was designed to enable a set of 3 DIMEs and a ground electrode to be quickly and reliably connected to external electronics during sessions in the laboratory and to have a low-profile and conformable structure to protect the exposed portion of the percutaneous leads when not in use (Figure 3B, C). To fabricate the external connector (step 8), individual wires are soldered (using SMD291SNL10, Chip-quick, Mashpee, MA) to the pads of a custom-fabricated PCB that houses a multi-channel connector (Hirose LX, Hirose Electric Ltd, Simi Valley, CA) and the pads were insulated with medical grade epoxy (Epotek-301, Billerica, MA). Susequently, custom fabricated stainless steel molds were used to encapsulate the external connector assembly in silicone. The assembly has a low profile (approximately 6 mm) and the taper allows strain relief for the leads. The silicone tabs allow the external connector to be firmly and comfortably attached to the skin using medical-grade adhesive tape.
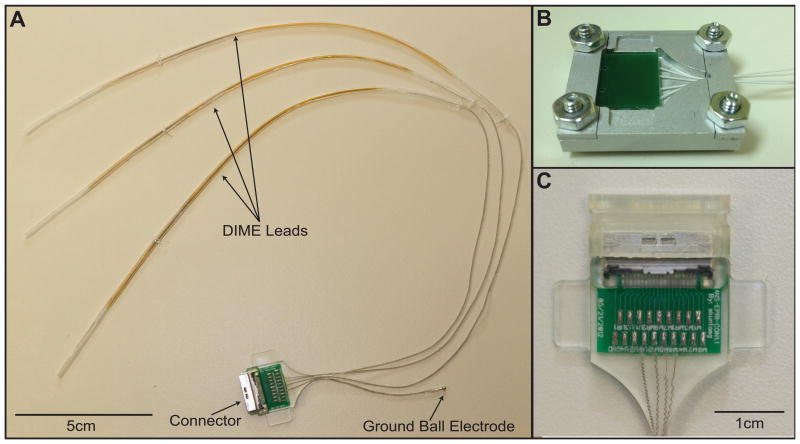
Figure 3.
A: Electrode and connector assembly: Fabricated system with 3 percutaneous DIME leads, a ball ground electrode with a percutaneous lead and an external connector assembly. B: The mold that was used to encapsulate the external connector in silicone. C: The external connector assembly that was designed for use with percutaneous leads.
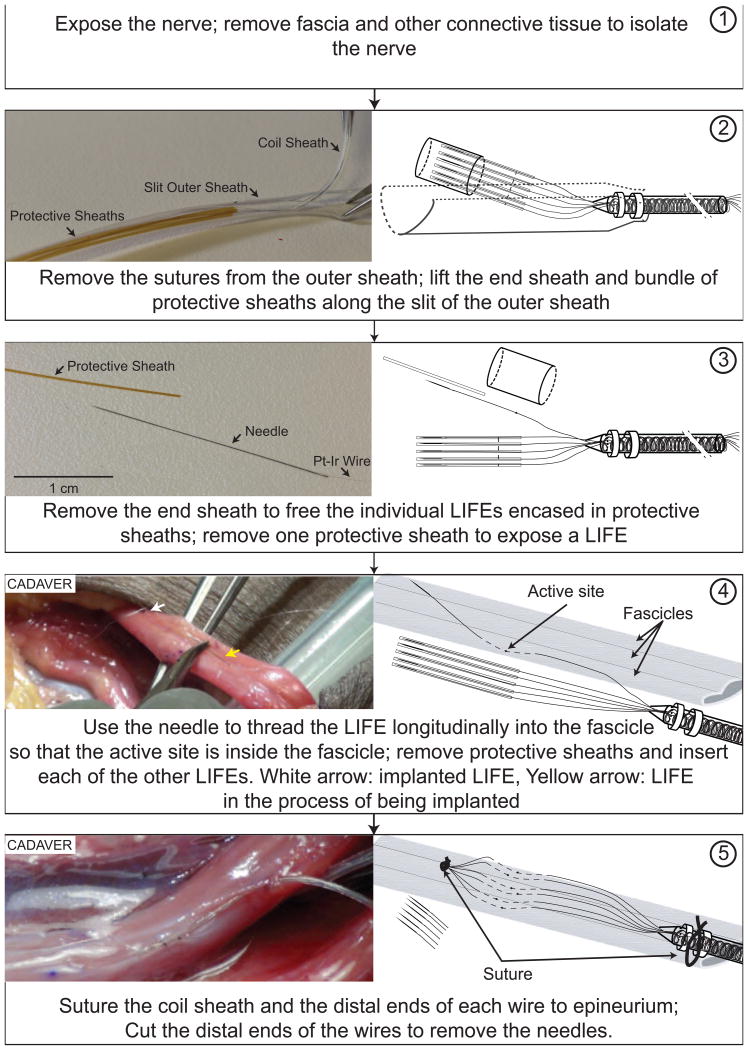
3 DIME deployment process
The DIME deployment process is illustrated in Figure 2. In step 1, the nerve is exposed and isolated from the surrounding tissue. In step 2, the sutures securing the outer sheath are removed and discarded and the bundle of protective sheaths is removed from the outer sheath through the longitudinal slit. In step 3, the end sheath is removed and discarded and then one of the protective sheaths is removed to free an individual LIFE. In step 4, the attached needle is used to thread the LIFE longitudinally into a nerve fascicle until the active region is inside the fascicle. The arrows in the picture show one implanted LIFE and another which is in the process of being implanted in a different fascicle of the same nerve. For each of the remaining electrodes, the end sheath is removed and the LIFE is inserted into a fascicle. In step 5, the coil sheath and the bundle of distal ends of the electrode wires are sutured to the epineurium. The needles and excess wires are clipped and discarded.
Figure 2.
DIME deployment process: Illustrations of steps involved in deployment of DIME lead during surgical implantation of LIFEs in peripheral nerves. See text for descriptions of each step. Diagrams are not to scale to allow illustration of thin, long components; for reference, the length of the needle is 2.5 cm and the diameter of the silicone-encased coiled bundle is 640 μm.
4 System tests
A prototype system consisting of three DIMEs was fabricated to assess the feasibility of using several DIMEs and the multi-lead multi-electrode (MLME) system to interface with peripheral nerves at multiple sites in the upper arm. In this application, the objective is to stimulate small groups of fibers within fascicles of peripheral nerves to elicit discrete sensations of grip force or hand opening (Dhillon et al., 2005) and/or to record from small groups of fibers to record motor signals to control movements of a prosthesis (Dhillon et al., 2005; Dhillon et al., 2004). To elicit a range of sensations or to control multiple degrees of freedom, it would be desirable to interface with groups of axons from several fascicles from two or more nerves. A prototype was fabricated with three DIMEs, a separate ball ground electrode and an external connector (Figure 3).
The prototype system was tested in a human cadaver arm. The objective of the surgery was to place the external connector on the lateral aspect of the upper arm and insert electrodes from 2 DIMEs into the median nerve (at two different sites) and electrodes from the third DIME into the ulnar nerve through a medial exposure. This preparation therefore presented a significant challenge because it required the leads to be routed through the skin and fascial planes and it required insertion of electrodes into multiple fascicles of two nerves.
The surgical procedure was initiated by making a 8-10 cm incision on the medial side of the arm approximately at the midpoint between the elbow and the shoulder. For each DIME lead the skin was punctured with a trocar on the lateral aspect of the arm near the insertion of the deltoid tendon. Each DIME lead was routed through the skin using a guide tube and then the set of three leads was routed across the arm using a tendon passer. The DIME leads were routed between the belly of the brachialis muscle and the humerus to the site of the exposed median and ulnar nerves. Once the leads were routed, the DIME deployment process (Figure 2) was followed to implant LIFEs from 2 DIMEs (12 electrodes) in the median nerve and LIFEs from 1 DIME (6 electrodes) in the ulnar nerve.
The procedure in the cadaver arm was successful in routing the leads, and the MLME system with the protective sheaths enabled efficient handling of the DIME electrode wires without becoming entangled. No mechanical damage was noted upon visual inspection of the leads and individual wires. Using other prototypes of the system in acute studies in rodents, LIFEs implanted in tibial and peroneal fascicles of sciatic nerve were demonstrated to be functional by stimulating to selectively produce ankle movements and/or record afferent activity produced by passively manipulating the limb. An example of a recording from a LIFE inserted in the tibial fascicle is shown in Figure 4. Furthermore, preliminary results from extended fatigue tests of the wires and wire bundles of the DIME have demonstrated high durability (Pena et al., 2014).
Figure 4.
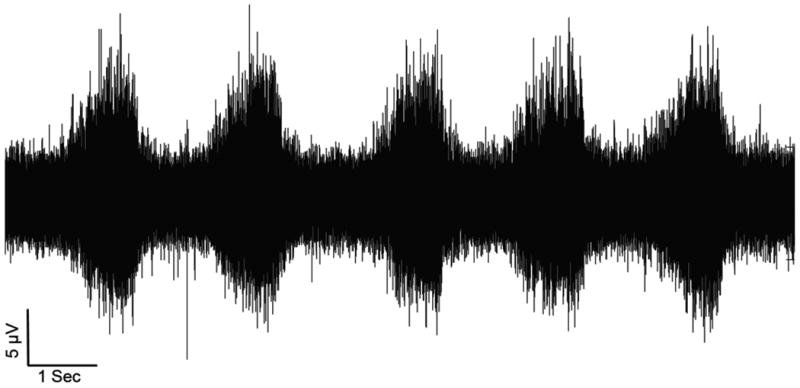
Differential recording of neural activity from a rodent model using a LIFE inserted into the tibial fascicle of the sciatic nerve and aLIFE used as a reference inserted outside the fascicle within the nerve. In this experiment, the length and diameter of the needle used for insertion were 1.5 cm and 75 μm, respectively; the diameter of the Pt/Ir wire was 25 μm. The location of the electrode into the tibial fascicle was first confirmed by observing a plantarflexion twitch in response to stimulation. During the recording of afferent activity shown in the plot, the foot was manipulated to repeatedly dorsiflex (periods of high activity) and plantarflex the ankle.
5 Discussion
The design of the DIME enables deployment of multiple LIFEs to access discrete and spatially distributed subpopulations of axons in peripheral nerves while reducing the surgical complexity and risk of entanglement. This capability opens up new opportunities for scientific investigations of sensory, motor or autonomic systems. Perhaps more importantly, the technology may open new opportunities for clinical intervention. Neural sensation, computation and control are inherently distributed processes that involve interactions between nearby and distant neuronal populations. Interpreting the pattern of activity across a set of peripheral nerves may require simultaneous recording from multiple sites; specific and accurate control of organ function without side effects may require the production of precise patterns of activity across distributed populations of axons. The design of the DIME enables these types of interfaces because multiple electrodes can be implanted at a single site to enable access to several distinct groups of axons within one fascicle or at separate sites to enable access to distinct distributed axons across fascicles and/or nerves.. These capabilities may prove to be highly advantageous in systems designed to control or influence epileptic seizures (Penry and Dean, 1990), pain (Cameron, 2004), motility in various parts of the alimentary canal (Zhang and Chen, 2006), appetite (McNearney, 2007; Shikora et al., 2009), immunological responses (Borovikova et al., 2000; Famm et al., 2013), metabolism (Ruffin and Nicolaidis, 1999; Vijgen et al., 2013) or bladder and bowel function (Creasey et al., 2001; Johnston et al., 2005). In sensorimotor systems, the DIME may be particularly useful in systems that require coordinated activation of distributed sets of muscles, such as systems that activate the diaphragm, chest wall and airway musculature to provide ventilatory assistance to people with high level spinal cord injury (Creasey et al., 1996; DiMarco, 2005).
Recently, one of the primary drivers of the development of peripheral neural interface technology has been the need to provide amputees with sensation and multiple degree of freedom control of a prosthesis (Micera et al., 2010; Micera and Navarro, 2009). In considering the relative merits of the DIME and other electrode systems, there clearly are tradeoffs that may make one type more suitable than the others for a given application. For example, the multi-contact cuff (Tyler and Durand, 2002) has the advantages of relatively straightforward surgical deployment and it is less invasive to the nerve, but its selectivity may be a limiting factor for some applications. The Utah Slanted Electrode Array (Branner and Normann, 2000) is also relatively simple to implant and may provide high-resolution, multi-channel access to the peripheral nerve, but the mechanical impedance mismatch with the nerve may trigger tissue responses that hinder long-term functionality. The tfLIFE and TIME (Boretius et al., 2010; Yoshida et al., 2000) are also both relatively simple to deploy and are less invasive and much more compliant (Badia et al., 2011b; Lago et al., 2007) than the Utah Slanted Electrode Array, but transverse deployment of the TIME across fascicles may limit the ability of fascicles to glide with respect to eachother (Millesi et al., 1990). Both the tfLIFE and the TIME have multiple contacts and therefore may provide access to discrete populations within a fascicle (Badia et al., 2011a; Badia et al., 2011b; Kundu et al., 2014), but the spatial distribution of the sites on each device is small and therefore the range of sensations and/or control sites may be limited. For applications that require access to distributed sites, the key concepts of the system described here (bundling of leads and a management system) could be adopted to facilitate the deployment of several tfLIFE or TIME devices. Although the design of the DIME simplifies the implantation of multiple LIFEs, surgical deployment of this system clearly requires more surgical time and effort than the other approaches. The LIFE is more invasive than a cuff, but the size, flexibility and orientation of the electrode may make it more compatible with peripheral nerve than the other invasive approaches and it can provide higher selectivity than a multi-contact cuff. Given that the diameter of some of the targeted fascicles may be more than 30 times larger than the diameter of the electrode, it may be possible to place several LIFEs at a single point along the fascicle to access distinct subgroups of axons. If multiple insertions at a single site raises concerns for a particular deployment, the DIME could be fabricated as described here or with different wire lenths within a given bundle and the individual wires could be inserted at locations distributed along a fascicle.
6 Conclusion
We have developed a system that can be used for recording or stimulation of multiple discrete sites from one or more peripheral nerves. The system utilizes a set of LIFEs, each of which provides discrete access to a small group of axons within a fascicle. The DIME lead allows each of the electrodes to be inserted individually and allows for some distance between insertion sites. A MLME management system includes a set of sheaths and procedures for surgical deployment that were designed to avoid entanglement of the fine wires. A prototype of an electrode and connector assembly for recording from multiple peripheral nerves of the upper extremity was fabricated using DIME leads and the MLME management system. The DIME leads were routed successfully through the skin and between the muscles to the vicinity of the median and ulnar nerve in a cadaver arm. LIFEs were successfully implanted and secured at two sites in the median and in one site in the ulnar nerve. This system may be particularly useful in systems that require access to multiple discrete sets of axons in peripheral nerves, such as neural-prostheses for amputees.
Highlights.
Developed a distributed intrafascicular multi-electrode for neural interfacing.
Developed multi-electrode multi-lead packaging processes.
Developed surgical procedures to implant the distributed multi-electrode
Tested surgical procedures in a cadaver arm.
Acknowledgments
This work was supported by a grant from National Institutes of Health (R01EB008578) and a contract from the Defense Advanced Research Projects Agency (N6001-12-C-4195) to R.J.
Abbreviations
- DIME
distributed intrafascicular multielectrode
- LIFE
longitudinal intrafascicular electrode
- MLME
multilead multi-electrode
- ID
inner diameter
- OD
outer diameter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badia J, Boretius T, Andreu D, Azevedo-Coste C, Stieglitz T, Navarro X. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. Journal of neural engineering. 2011a;8:036023. doi: 10.1088/1741-2560/8/3/036023. [DOI] [PubMed] [Google Scholar]
- Badia J, Boretius T, Pascual-Font A, Udina E, Stieglitz T, Navarro X. Biocompatibility of chronically implanted transverse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE transactions on bio-medical engineering. 2011b:58. doi: 10.1109/TBME.2011.2153850. [DOI] [PubMed] [Google Scholar]
- Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T. A transverse intrafasci cular multichannel electrode (TIME) to interface with the peripheral nerve. Biosensors & bioelectronics. 2010;26:62–9. doi: 10.1016/j.bios.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain research bulletin. 2000;51:293–306. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol. 2006;175:835–41. doi: 10.1016/S0022-5347(05)00326-5. [DOI] [PubMed] [Google Scholar]
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. Journal of neurosurgery. 2004;100:254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- Clark GM. The multiple-channel cochlear implant: the interface between sound and the central nervous system for hearing, speech, and language in deaf people - a personal perspective. Philos T R Soc B. 2006;361:791–810. doi: 10.1098/rstb.2005.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasey G, Elefteriades J, DiMarco A, Talonen P, Bijak M, Girsch W, Kantor C. Electrical stimulation to restore respiration. Journal of rehabilitation research and development. 1996;33:123–32. [PubMed] [Google Scholar]
- Creasey GH, Grill JH, Korsten M, Betz R, Anderson R, Walter J. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch Phys Med Rehabil. 2001;82:1512–9. doi: 10.1053/apmr.2001.25911. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952–70. doi: 10.1111/j.1468-1331.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Dhillon GS, Kruger TB, Sandhu JS, Horch KW. Effects of short-term training on sensory and motor function in severed nerves of long-term human amputees. J Neurophysiol. 2005;93:2625–33. doi: 10.1152/jn.00937.2004. [DOI] [PubMed] [Google Scholar]
- Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs. J Hand Surg-Am. 2004;29A:605–15. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Restoration of respiratory muscle function following spinal cord injury - Review electrical and magnetic stimulation techniques. Resp Physiol Neurobi. 2005;147:273–87. doi: 10.1016/j.resp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: A jump-start for electroceuticals. Nature. 2013;496:159–61. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S, Giroux M. Deep brain stimulation: a review of the procedure and the complications. JAAPA. 2011;24:39–40. 2–5. doi: 10.1097/01720610-201102000-00007. [DOI] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav R. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gustafson KJ, Pinault GCJ, Neville JJ, Syed I, Davis JA, Jean-Claude J, Triolo RJ. Fascicular anatomy of human femoral nerve: Implications for neural prostheses using nerve cuff electrodes. Journal of rehabilitation research and development. 2009;46:973–84. doi: 10.1682/jrrd.2008.08.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson KJ, Zelkovic PF, Feng AH, Draper CE, Bodner DR, Grill WM. Fascicular anatomy and surgical access of the human pudendal nerve. World J Urol. 2005;23:411–8. doi: 10.1007/s00345-005-0032-4. [DOI] [PubMed] [Google Scholar]
- Heinemeyer O, Reimers CD. Ultrasound of radial, ulnar, median, and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound in medicine & biology. 1999;25:481–5. doi: 10.1016/s0301-5629(98)00187-2. [DOI] [PubMed] [Google Scholar]
- Jarrett ME, Mowatt G, Glazener CM, Fraser C, Nicholls RJ, Grant AM, Kamm MA. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. The British journal of surgery. 2004;91:1559–69. doi: 10.1002/bjs.4796. [DOI] [PubMed] [Google Scholar]
- Johnston T, Betz R, Smith B, Benda B, Mulcahey M, Davis R, Houdayer T, Pontari M, Barriskill A, Creasey G. Implantable FES system for upright mobility and bladder and bowel function for individuals with spinal cord injury. Spinal Cord. 2005;43:713–23. doi: 10.1038/sj.sc.3101797. [DOI] [PubMed] [Google Scholar]
- Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJ IJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artificial organs. 2004;28:577–86. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- Kundu A, Harreby KR, Yoshida K, Boretius T, Stieglitz T, Jensen W. Stimulation selectivity of the ′thin-film longitudinal intrafascicular electrode′ (tfLIFE) and the ′transverse intrafascicular multi-channel electrode′ (TIME) in the large nerve animal model. IEEE Trans Neural Syst Rehabil Eng. 2014;22:400–10. doi: 10.1109/TNSRE.2013.2267936. [DOI] [PubMed] [Google Scholar]
- Lago N, Yoshida K, Koch KP, Navarro X. Assessment of biocompatibility of chronically implanted polyimide and platinum intrafascicular electrodes. IEEE transactions on bio-medical engineering. 2007;54:281–90. doi: 10.1109/TBME.2006.886617. [DOI] [PubMed] [Google Scholar]
- Lefurge T, Goodall E, Horch K, Stensaas L, Schoenberg A. Chronically implanted intrafascicular recording electrodes. Ann Biomed Eng. 1991;19:197–207. doi: 10.1007/BF02368469. [DOI] [PubMed] [Google Scholar]
- Malagodi MS, Horch KW, Schoenberg AA. An Intrafascicular Electrode for Recording of Action-Potentials in Peripheral-Nerves. Ann Biomed Eng. 1989;17:397–410. doi: 10.1007/BF02368058. [DOI] [PubMed] [Google Scholar]
- McNearney TA. Gastric/intestinal electrical stimulation modulates appetite regulatory peptide hormones in the stomach and duodenum in rats. Obesity surgery. 2007;17:406–13. doi: 10.1007/s11695-007-9049-7. [DOI] [PubMed] [Google Scholar]
- Micera S, Citi L, Rigosa J, Carpaneto J, Raspopovic S, Di Pino G, Rossini L, Yoshida K, Denaro L, Dario P, Rossini PM. Decoding Information From Neural Signals Recorded Using Intraneural Electrodes: Toward the Development of a Neurocontrolled Hand Prosthesis. P Ieee. 2010;98:407–17. [Google Scholar]
- Micera S, Navarro X. Bidirectional Interfaces with the Peripheral Nervous System. Int Rev Neurobiol. 2009;86:23–38. doi: 10.1016/S0074-7742(09)86002-9. [DOI] [PubMed] [Google Scholar]
- Millesi H, Zoch G, Rath T. The gliding apparatus of peripheral nerve and its clinical significance. Annales de chirurgie de la main et du membre superieur : organe officiel des societes de chirurgie de la main = Annals of hand and upper limb surgery. 1990;9:87–97. doi: 10.1016/s0753-9053(05)80485-5. [DOI] [PubMed] [Google Scholar]
- Pena A, Kunteagowdanahalli S, Abbas J, Jung R. Fatigue testing of longitudinal intrafascicular electrodes as a peripheral nerve interface. Neural Interfaces Conterence; Dallas, TX. 2014. [Google Scholar]
- Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31:S40–S3. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- Ruffin MP, Nicolaidis S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Res. 1999;846:23–9. doi: 10.1016/s0006-8993(99)01922-8. [DOI] [PubMed] [Google Scholar]
- Shikora SA, Bergenstal R, Bessler M, Brody F, Foster G, Frank A, Gold M, Klein S, Kushner R, Sarwer DB. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009;5:31–7. doi: 10.1016/j.soard.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Stewart JD. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle & nerve. 2003;28:525–41. doi: 10.1002/mus.10454. [DOI] [PubMed] [Google Scholar]
- Sun K, Zhang J, Chen TY, Chen ZW, Chen ZG, Li Z, Li H, Hu P. Three-Dimensional Reconstruction and Visualization of the Median Nerve from Serial Tissue Sections. Microsurg. 2009;29:573–7. doi: 10.1002/micr.20646. [DOI] [PubMed] [Google Scholar]
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- Vijgen GHEJ, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, Brans B, Lichtenbelt WDV. Vagus Nerve Stimulation Increases Energy Expenditure: Relation to Brown Adipose Tissue Activity. Plos One. 2013;8:e77221. doi: 10.1371/journal.pone.0077221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ilberg CA, Baumann U, Kiefer J, Tillein J, Adunka OF. Electric-Acoustic Stimulation of the Auditory System: A Review of the First Decade. Audiol Neuro-Otol. 2011;16:1–30. doi: 10.1159/000327765. [DOI] [PubMed] [Google Scholar]
- Walter JS, Wurster RD, Zhu Q, Laghi F. Respiratory muscle pacing with chronically implanted intramuscular Permaloc electrodes: A feasibility study. Journal of rehabilitation research and development. 2011;48:103–14. doi: 10.1682/jrrd.2010.05.0086. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Pellinen D, Pivin D, Rousche P, Kipke D. Development of the thin-film longitudinal intra-fascicular electrode. Proceedings of the fifth Annual Conf of the IFESS. 2000:279–84. [Google Scholar]
- Yoshida K, Stein RB. Characterization of signals and noise rejection with bipolar longitudinal intrafascicular electrodes. IEEE transactions on bio-medical engineering. 1999;46:226–34. doi: 10.1109/10.740885. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Alimentary pharmacology & therapeutics. 2006;24:991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]