Abstract
Intraperitoneal infection with Taenia crassiceps cysticerci in mice alters several behaviors, including sexual, aggressive, and cognitive function. Cytokines and their receptors are produced in the central nervous system (CNS) by specific neural cell lineages under physiological and pathological conditions, regulating such processes as neurotransmission. This study is aimed to determine the expression patterns of cytokines in various areas of the brain in normal and T. crassiceps-infected mice in both genders and correlate them with the pathology of the CNS and parasite counts. IL-4, IFN-γ, and TNF-α levels in the hippocampus and olfactory bulb increased significantly in infected male mice, but IL-6 was downregulated in these regions in female mice. IL-1β expression in the hippocampus was unaffected by infection in either gender. Our novel findings demonstrate a clear gender-associated pattern of cytokine expression in specific areas of the brain in mammals that parasitic infection can alter. Thus, we hypothesize that intraperitoneal infection is sensed by the CNS of the host, wherein cytokines are important messengers in the host–parasite neuroimmunoendocrine network.
Introduction
The immune and neuroendocrine systems communicate through a network in which hormones, antigens, receptors, cytokines, antibodies, and neuropeptides modulate the immune response, in connection with neuroendocrine changes, while maintaining homeostasis (Besedovsky and del Rey 1996, 2000; Buckingham and others 1996).
Cytokines are highly inducible secretory proteins that mediate intercellular communication in the immune, endocrine, and nervous systems. They are grouped into several families: tumor necrosis factors, interleukins, interferons, and colony-stimulating factors (Dinarello 2007). Certain cytokines and their receptors have recently been demonstrated to be produced in the central nervous system (CNS) by specific neural cell lineages under physiological and pathological conditions (Deverman and Patterson 2009). Cytokines regulate various processes in the CNS, including neurotransmission (Camacho-Arroyo and others 2009).
Thus, this interneuronal communication through synaptic, presynaptic, and parasynaptic interactions is complicated by the potential involvement of cytokines in these processes, requiring this network to be delicately balanced throughout the ontogeny of an organism (Deverman and Patterson 2009). The mutual regulatory influences between neurotransmitters, hormones, and cytokines demonstrate that under normal conditions, they function in concert. The increase in the discovery of the number of factors that determine the result of these neuronal interactions expands our understanding of brain function (Deverman and Patterson 2009).
Murine intraperitoneal cysticercosis is caused by the taeniid, Taenia crassiceps. This experimental system has been used to explore the physiological host factors that are associated with porcine cysticercosis and human neurocysticercosis (Larralde and others 1990). Male and female mice that are infected with T. crassiceps experience significant changes in sex steroid levels (Morales-Montor and Larralde 2005; Arteaga-Silva and others 2009), affecting their sexual behavior (Morales and others 1996), aggressiveness (Gourbal and others 2001), social status (Gourbal and others 2002), and prey avoidance of predator (Gourbal and others 2002).
Recently, it has been demonstrated that chronic cysticercotic infection impairs short-term memory in male and female mice—more so in the latter (Morales-Montor and others 2014). Correlating with these changes, serotonin levels decline in the hippocampus of infected females, whereas noradrenaline levels rise significantly in infected males (Morales-Montor and others 2014). Notably, infected females showed a significant increase in the number of counts in the forced swimming tests and had decreased counts in immobility, whereas male mice showed an increment in the number of counts in total activity and ambulation tests. Serotonin levels fell by 30% in the hippocampus of infected females, and noradrenaline levels increased significantly in infected males (Morales-Montor and others 2014). Concomitantly, IL-6, IFN-γ, and TNF-α in the hippocampus were upregulated in infected male and female mice, and IL-4 expression increased in infected females, but decreased in infected males (Morales-Montor and others 2014). Moreover, c-fos and progesterone receptor expression in the hypothalamus (HYP), brain cortex, and preoptic area (POA) of infected mice oscillate during the infection, which does not occur in other areas of the brain or in other organs (Morales-Montor and others 2004a; Rodriguez-Dorantes and others 2007). This finding indicates that the brain senses intraperitoneal infections and responds to it.
Thus, during chronic murine cysticercosis, a host–parasite NIE network is established, involving the brain. Yet, we do not know whether classical messengers of the immune system, such as cytokines, mediate communication in this network with the host brain.
Because intraperitoneal infection with T. crassiceps alters various behaviors in mice, in association with changes in the expression of soluble messengers in the NIE network in various areas of the brain, we hypothesized that this infection also affects the levels of molecules that mediate intercellular communication in the NIE network in these regions, such as cytokines. Thus, we examined the changes in IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, and IFN-γ expression in the POA, olfactory bulb (OB), hippocampus, HYP, lateral cortex (LC), and frontal cortex (FC) of mice during intraperitoneal T. crassiceps infection and correlated these data with brain inflammation and the parasite burdens in the peritoneum. Our results demonstrate that as the infection advances and the intraperitoneal parasite load increases, cytokine expression levels in various areas of the brain undergo persistent, sexually dimorphic changes, without any histological damage to the brain. Thus, we report novel expression patterns of cytokines in the brain of a host during a helminth infection.
Materials and Methods
Ethics statement
The Animal Care and Use Committee at the Instituto de Investigaciones Biomédicas evaluated the animal care and experimentation practices per Mexican regulations (NOM-062-ZOO-1999). These regulations are in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH and The Weatherall Report), ensuring compliance with international regulations and guidelines. The ethics committee of the Instituto de Investigaciones Biomédicas approved this protocol (Permission Number: 2009-16).
Animals and experimental infection
Male and female Balb/c AnN (H2-d) inbred mice, obtained from Harlan (Mexico City), were used for all experiments and housed in the animal care facilities at the Instituto de Investigaciones Biomédicas, UNAM, at controlled temperatures and under 12-h dark–12-h light cycles, with the lights on between 07:00 and 19:00. They were fed sterilized Purina Diet 5015 (Purina, St. Louis, MO) and given sterilized tap water ad libitum. Estrous was monitored in females.
The fast-growing ORF strain of T. crassiceps was used for infection in all experiments (Smith and others 1972). Larvae were obtained from 3- to 6-month-infected female donor mice. Ten nonbudding T. crassiceps larvae (∼2 mm in diameter) were suspended in 0.3 mL sterile phosphate buffered saline (0.15 M NaCl, 0.01 M sodium phosphate buffer, pH 7.2) and injected intraperitoneally into 42-day-old male and female mice using a 0.25-gauge needle. Eight uninfected mice of each sex were used as age-matched controls.
Mice were euthanized by cervical dislocation after anesthesia with ketamine (Pfizer, Mexico D.F., Mexico) at 4, 8, and 16 weeks of infection. All tissue sections were collected immediately after rinsing. To avoid variations due to circadian rhythms, all animals were sacrificed at the same time each day (08:00). Females were sacrificed in the same phase of estrous (proestrus).
Collection and processing of brain tissue
The POA, HYP, OB, hippocampus, and FC and LC from control and infected male and female mice were obtained at 4, 8, and 16 weeks postinfection as per the The Mouse Brain in Stereotaxic Coordinates. Briefly, POA, and HYP were dissected by making a razor cut just rostral to the optic chiasm to the anterior commissure, the anteroventral limit of which was the nucleus of the diagonal band of the lateral Broca. The hippocampus was obtained by making a razor cut underneath the FC, and the FC was excised directly from the frontal lobes; the LC was excised directly from the lateral lobes.
The brain tissue from 5 animals was pooled by region to increase the sample quantity. All experiments were performed in triplicate.
RNA extraction
Total RNA was isolated from the POA, HYP, hippocampus, OB, LC, FC, and spleen (positive control tissue for cytokine expression) of control and infected mice by using TRIzol (Gibco-BRL, Grand Island, NY). Briefly, each tissue sample was removed and disrupted immediately in TRIzol (1 mL/0.1 g tissue); 0.2 mL chloroform was then added per 1 mL TRIzol. The aqueous phase was recovered after a 10-min centrifugation at 14,000 g. RNA was precipitated with isopropyl alcohol, washed with 75% ethanol, and dissolved in RNase-free water. RNA concentration was measured, based on its absorbance at 260 nm, and its purity was verified after electrophoresis in a 1.0% denaturing agarose gel in the presence of 2.2 M formaldehyde. Total RNA from all extracted tissues was reverse-transcribed, from which IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α, and 18S ribosomal RNA (control) were PCR-amplified.
IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IFN-γ, and TNF-α expression in brain tissues
The sequences of the primers that were used for amplification have been published (Rodriguez-Dorantes and others 2007). Briefly, 1 μg of total RNA from each tissue was incubated at 37°C for 1 h with 400 units of M-MLV reverse-transcriptase (Applied Biosystems, Boston, MA) in a 50-μL reaction, containing 50 mM of each dNTP and 0.05 μg oligo (dt) primer (Gibco-BRL). The 50-μL PCR reaction comprised 10 μL of the cDNA, 5 μL 10×PCR buffer (Biotecnologías Universitarias, México) 1 mM MgCl, 0.2 mM of each dNTP, 0.05 μM of each primer, and 2.5 units of Taq DNA polymerase (Biotecnologías Universitarias, México).
Twenty microliters of each PCR reaction was electrophoresed on a 2% agarose gel and visualized with ethidium bromide. A single band was detected in all cases, as expected. To determine whether all reactions were in the exponential phase of amplification and ensure that the changes in expression were not artifacts (eg, 18S rRNA in the stationary phase), we performed RNA, cycling, and temperature curves for each gene.
Densitometric analysis
PCR bands were quantified by densitometric scanning of several autoradiograms at various exposures and expressed as the ratio of the signal of the target gene (cytokines) to that of 18S Ribosomal RNA gene (constitutively expressed control gene).
Experimental design and statistical analysis
We designed the study as a 2-factor experiment. The independent variables were infection (Yes, No) and gender (Male or Female). The dependent variables were the number of parasites and the expression of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IFN-γ, and TNF-α in the tissue sample, expressed as the ratio of the optical density of the corresponding gel to that of 18S rRNA.
The complete design was repeated 3 times (5 animals/treatment); the tissues for each experiment were obtained from normal males (n=15) and females (n=15) and infected males (n=15) and infected females (n=15) mice. Statistical analysis of variance components was performed using the Prism 2.01 (GraphPad Software Incorporated). When applied, post hoc individual contrasts of group means were analyzed by Tukey test using the sum of residual and 3-factor interaction variance to test for significant differences.
Results
Parasite loads
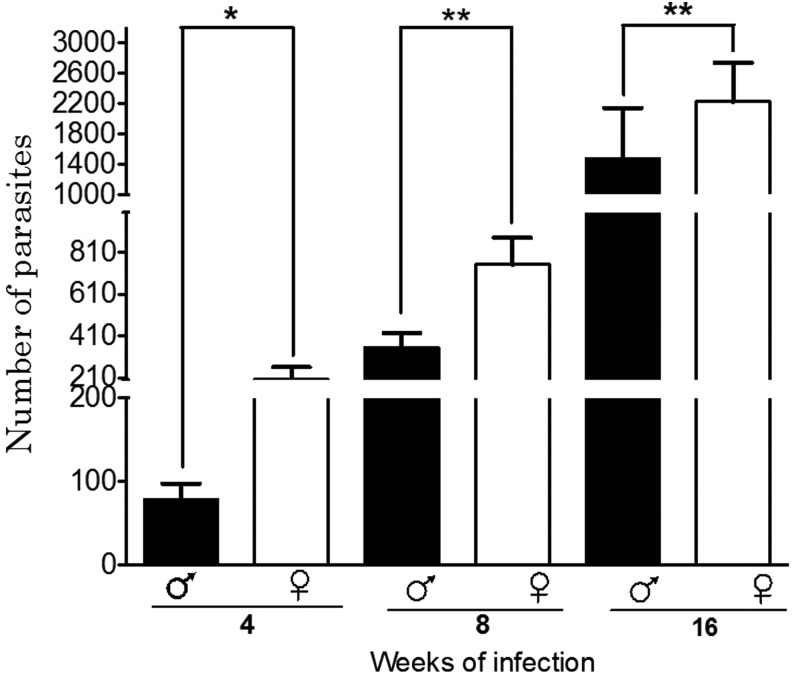
As expected, the parasite load differed between males and females, wherein females harbored more parasites, starting as early as 4 weeks after infection (Fig. 1). Also, the increase in the number of parasites from the peritoneal cavity correlated with the time of infection, peaking at 1,450±321 per male at 16 weeks postinfection, whereas females had 1.3 times as many parasites (2,240±642) (Fig. 1). No parasites were detected outside of the peritoneal cavity.
FIG. 1.
Parasite loads in male and female mice infected with Taenia crassiceps cysticerci at various times of infection. Mice were sacrificed after 4, 8, and 16 weeks of infection. *P<0.05, **P<0.01, both compared between genders.
Histomorphology of the brain
To determine whether the infection induced any brain pathology, we performed a histopathological analysis of brains from control and infected mice. Acute (4 weeks) and chronic (8 and 16 weeks) infection did not induce inflammation in the brain, particularly in the areas that we analyzed. In both genders, there was no inflammation or high leukocyte infiltration in any brain sample, despite changes in proinflammatory cytokine expression, even in mice with a high parasite load in the peritoneal cavity, regardless of gender (data not shown).
Cytokine expression in POA
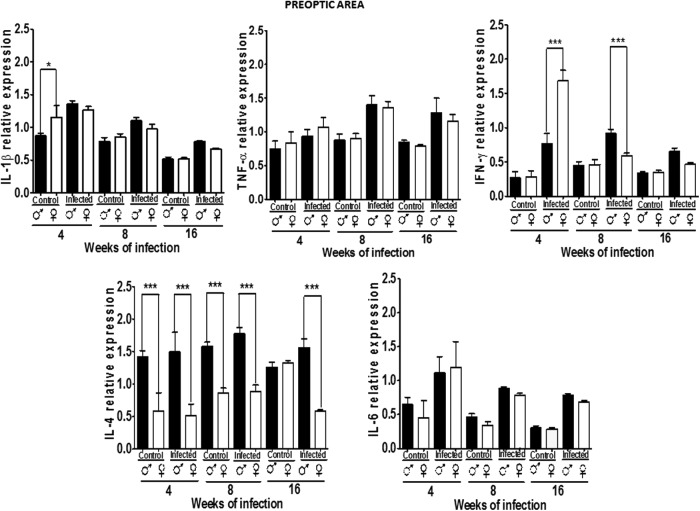
Figure 2 shows the relative expression patterns of the proinflammatory cytokines, IL-1β, TNF-α, and IFN-γ and the Th2 cytokines IL-4 and IL-6 in the POA in male and female mice, either uninfected or infected. All cytokines were detected in control and infected mice in both sexes in all brain areas analyzed, varying by gender and infection status. IL-2, IL-10, and IL-12 were undetectable in every area of the brain in control and infected mice. In the positive control (spleen of infected and control animals), these cytokines were always detected (not shown), suggesting that these findings are not artifacts.
FIG. 2.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the preoptic area (POA) of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. *P<0.05, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
The expression of IL-1β was higher in females versus males at 4 weeks in control animals. However, among infected animals, males had higher IL-1β levels, rising significantly compared with control males, beginning at 4 weeks and remaining until week 16. TNF-α expression in the POA was not dimorphic, rising 8 weeks after infection (P<0.05) (Fig. 2).
Although IFN-γ expression did not differ between genders in control animals, parasitic infection induced gender-specific patterns. IFN-γ was upregulated in infected males and females at 4 weeks, being higher in females. Interestingly after 8 weeks of infection and until 16 weeks, males showed higher levels of this cytokine than females (P<0.05). IL-4 expression was dimorphic at 4 and 8 weeks in control and infected animals, with males expressing more IL-4. But, this pattern disappeared after 16 weeks postinfection, at which point IL-4 expression rose in control females, but declined in infected females (P<0.05).
IL-6 expression in the POA did not differ between genders in control mice, but was upregulated on infection in males and females at the same rate (P<0.05) (Fig. 2).
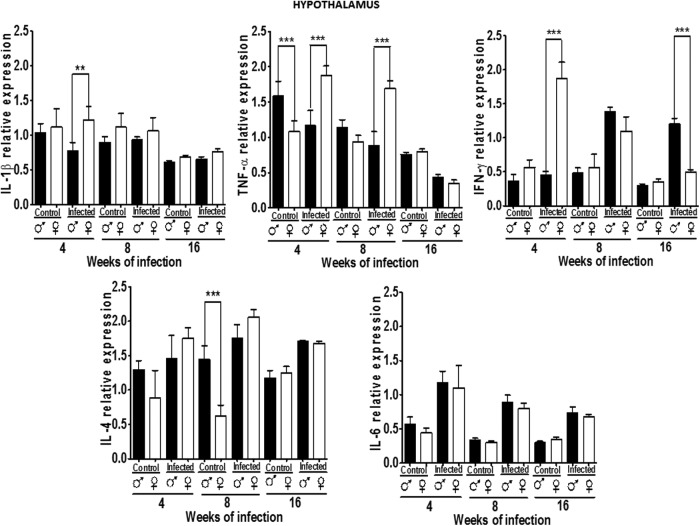
Expression of cytokines in the HYP
IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 were analyzed in the HYP from uninfected and infected mice. IL-1β expression was constant in both genders and was not dimorphic, a pattern that was unaltered on infection. We found only significant differences in infected mice at 4 weeks postinfection, where females showed higher levels of IL-1β than males. TNF-α expression in the HYP was dimorphic in control and infected animals during the early stages of infection (4 weeks), though in an opposite pattern: in control mice, males have higher expression, whereas in infected mice, females had higher expression. However, at 8 weeks no differences between genders are observed, but again, females had higher expression of TNF-α 8 weeks postinfection. No gender differences exist in TNF-α relative expression at 16 weeks in either control or infected mice (Fig. 3). IFN-γ expression was not gender-specific in control animals, but infection induced a sex-associated pattern at 4 weeks of infection, IFN-γ was upregulated in females versus males after 4 weeks postinfection (pi). Interestingly this pattern changed in infected mice since males showed higher levels of IFN-γ than females (P<0.05).
FIG. 3.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the hypothalamus (HYP) of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. **P<0.01, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
IL-4 expression was dimorphic at 4 and 8 weeks in infected animals, but not in control ones, a pattern that disappeared at 16 weeks postinfection. In females, infection upregulated IL-4 (P<0.05) whereas in males, this was not observed. IL-6 expression in the HYP did not differ between genders in control mice, but increased at the same rate on infection in males and females (P<0.05) (Fig. 3).
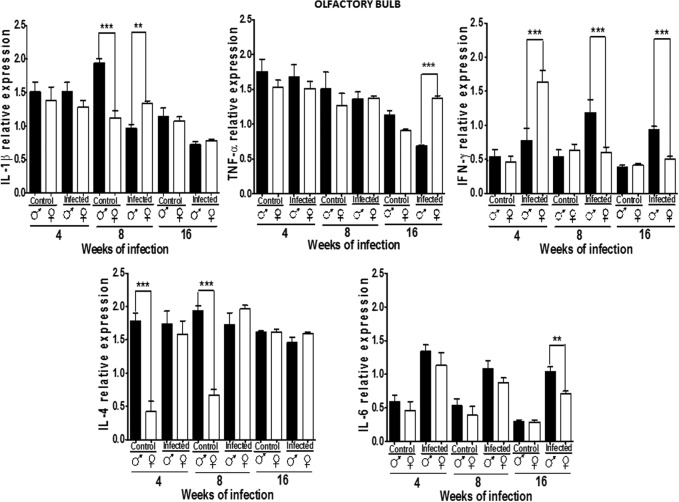
Cytokine expression pattern in the OB
All 5 cytokines were detected in the OB in both genders and in control and infected mice. IL-1β expression was constant in males and females. A dimorphic pattern was observed at 8 weeks, where control males showed higher levels than females; infection did not alter this pattern, except at 8 weeks, when females upregulated IL-1β. IL-1β decreased significantly in males starting at 4 weeks and this pattern remained until 16 weeks. TNF-α expression in the OB was not dimorphic, although it decreased in males and rose in females at 16 weeks pi (P<0.05) (Fig. 4).
FIG. 4.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the olfactory bulb (OB) of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. **P<0.01, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
IFN-γ expression was not gender-specific in control animals. But, infection induced a sex-associated pattern at 4 weeks of infection, IFN-γ was upregulated in females and males, decreasing in females after 8 and 16 weeks postinfection, but maintained in males (P<0.05). IL-4 expression was dimorphic at 4 and 8 weeks in control animals, but this pattern disappeared at 16 weeks of infection. In females, infection upregulated IL-4, which was not observed after 16 weeks (P<0.05).
IL-6 expression in the OB did not differ in control males and females, but increased at the same rate on infection in both genders (P<0.05) (Fig. 4).
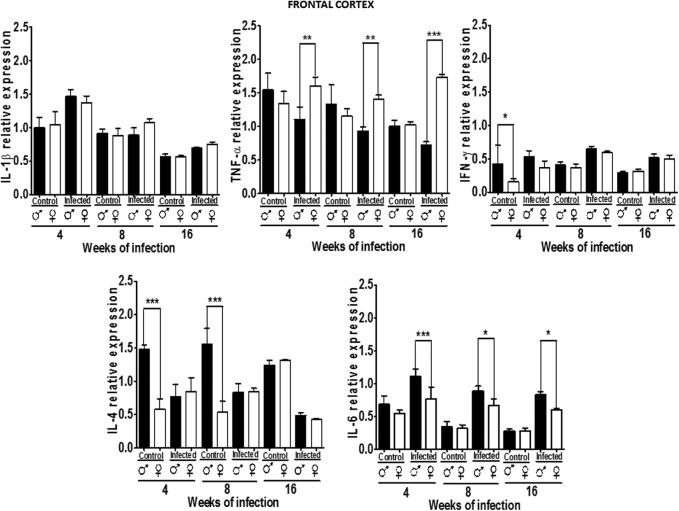
Cytokine expression pattern in the FC
Figure 5 shows the relative expression patterns of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the FC in uninfected and infected males and females. IL-1β was not dimorphic, and infection increased its expression at 4 weeks postinfection in both genders. TNF-α expression in the FC was not dimorphic, infection downregulated it in males, but increased its expression in females at every time point after infection (Fig. 5).
FIG. 5.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the frontal cortex (FC) of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. *P<0.05, **P<0.01, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
IFN-γ expression was higher in control males than females. However, infection upregulated the expression of IFN-γ in both males and in females, a pattern that was maintained until 16 weeks postinfection. IL-4 expression was dimorphic at 4 and 8 weeks in control animals, but this pattern disappeared at 16 weeks. Notably, the infected animal lost this dimorphism. In males, IL-4 expression declined and IL-6 expression rose due to infection in males and females at the same rate (P<0.05) (Fig. 5).
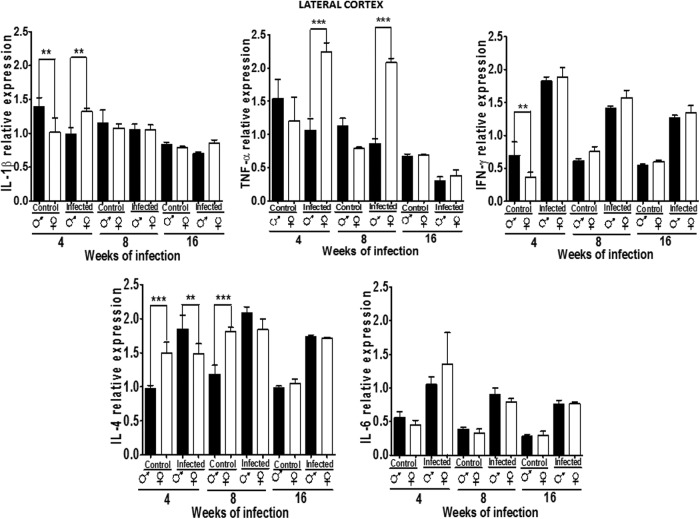
Cytokine expression pattern in the LC
In the LC, IL-1β was dimorphic at 4 weeks, wherein males had greater expression. However, in infected animals, this pattern was inverted. There were no gender- or infection-related differences at 8 or 16 weeks. TNF-α expression was not dimorphic, but infection upregulated it in females, but decreased its expression in males at 4 and 8 weeks pi (P<0.05) (Fig. 6).
FIG. 6.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the lateral cortex (LC) of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. **P<0.01, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
IFN-γ expression was similar between genders in control animals, but upregulated in infected mice in males and females (P<0.05). IL-4 expression was dimorphic at 4 and 8 weeks in control animals, and this pattern waned in week 16. Infection upregulated IL-4 in both genders at weeks 4 and 8, which was maintained only in males until 16 weeks postinfection (P<0.05). IL-6 had a similar expression pattern in male and female controls, and infection increased its expression in both genders (P<0.05) (Fig. 6).
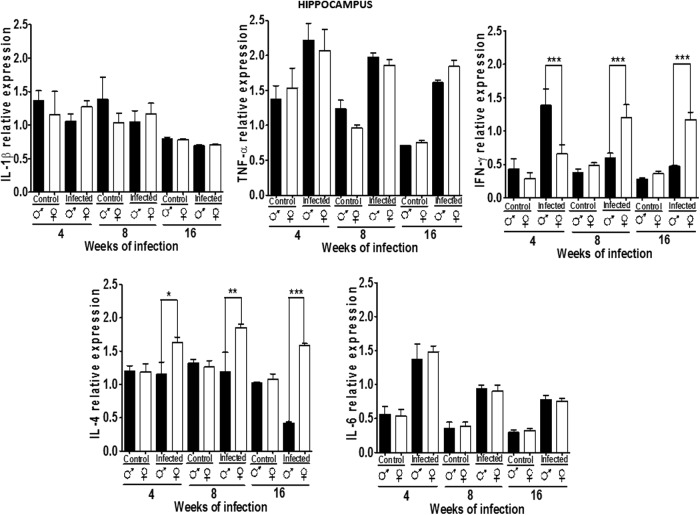
Cytokine expression of in the hippocampus
We examined IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 expression in the hippocampus (HC) in uninfected and infected mice. IL-1β did not differ between genders and did not change on infection. TNF-α was equally expressed in males and females and increased depending on the infection (P<0.05) (Fig. 7). IFN-γ expression was similar between genders in control mice, but rose on infection at every stage in females and only at 4 weeks in males (P<0.05). IL-4 did not differ between male and female control mice, but increased in females at 8 and 16 weeks postinfection (P<0.05). IL-6 was not dimorphic and was upregulated on infection in both genders (P<0.05) (Fig. 7).
FIG. 7.
Expression of IL-1β, TNF-α, IFN-γ, IL-4, and IL-6 in the hippocampus of male and female mice infected with T. crassiceps at various times of infection. Data are presented as mean±SD. *P<0.05, **P<0.01, ***P<0.001. ANOVA and Tukey test were performed to compare selected pairs of columns.
Discussion
In this study, we report novel findings on an important neuroimmune interaction in male and female mice that have been infected with T. crassiceps cysticerci, noting significant changes in cytokine expression in their brains. We found that cytokine expression was up or downregulated in the hippocampus, LC, FC, HYP, OB, and POA of infected males and females to various extents. Also, the cytokine expression patterns in the brain were gender-specific, independent of infection.
These changes in expression followed various dynamics, based on time, infection, and gender, reflecting specific alterations in the function of each region in the brain.
Changes in cytokine expression in mice that are chronically infected with T. crassiceps are likely caused by modulation in steroid levels (Larralde and others 1995; Morales and others 1996; Arteaga-Silva and others 2009), consistent with the findings that estradiol activates cytokine gene transcription in rodents (Loose-Mitchell and others 1988; Travers and others 1988; Nephew and others 1993; Bigsby and Li 1994) and in the spleen and thymus of cysticercotic mice (Morales-Montor and others 1998). In T. crassiceps cysticercosis-infected male mice, sexual behavior is abolished. The region-specific changes in cytokine expression in the brain that we observed during cysticercosis could explain behavioral disruptions, because cytokines mediate brain-specific control of neurotransmission (Camacho-Arroyo and others 2009).
Although cytokines modulate brain development, regeneration, and synapses, their origin in the brain is unclear—astrocytes produce cytokines and chemokines that attract various immune cell types that facilitate their access to the brain through the blood–brain barrier during inflammatory responses (Yoshida and others 1992; Benveniste and others 1994; Hu and others 1994; Feldhaus and others 2004). Notably, astrocytes constitutively produce and secrete cytokines, suggesting that these messengers modulate CNS function.
Our goal is to determine the implications of changes in cytokine expression in the brain in an infected host for its physiology. In the mammalian brain, neurons communicate predominantly through chemical synapses, mediated by various neurotransmitters. The steps in communication through chemical synapses—neurotransmitter synthesis, storage, release, uptake, degradation, interaction with specific receptors, and transduction—are crucial in the regulation of brain function, all of which can be regulated by cytokines (Camacho-Arroyo and others 2009). For instance, the neurotransmitter serotonin is an immune modulator that is necessary for optimal synthesis of IL-6 and TNF-α in the brain; under physiological concentrations, it can increase their production by stimulating its cytokine receptors. Conversely, extracellular serotonin concentrations above baseline physiological levels can suppress the production of IL-6 and TNF-α (Kubera and others 2005).
Neurotransmission by glutamate and γ-aminobutyric (GABA), key excitatory and inhibitory neurotransmitters, respectively, is modulated by cytokines. In vitro, IL-1β increases GABA-A-mediated inward chloride currents in synaptosomes and cultured neurons of the amygdala and cerebral cortex in rats (Miller and others 1991; Hu and others 2000). Moreover, in vivo, systemically injected IL-1β upregulates c-fos in GABAergic, enkephalinergic, neurotensinergic, and CRHergic neurons of the amygdaloid complex (Miller and Fahey 1994; Yu and Shinnick-Gallagher 1994; Mazzoni and Kenigsberg 1997). Our result of increased IL-1β expression in various areas of the brain in an infected host support the finding that c-fos expression rises in the hippocampus during chronic infection with T. crassiceps (Morales-Montor and others 2004b).
IL-6 also participates in neurochemical communication and undergoes changes in its expression in the brain during infection; this cytokine potentiates evoked GABA release from mediobasal hypothalamic explants and posterior pituitary cells in culture (De Laurentiis and others 2000). Moreover, long-term exposure of the brain to IL-6, as observed in certain degenerative disorders and infections, impedes adult hippocampal neurogenesis (Vallieres and others 2002). Thus, our results support and extend a model in which chronically infected animals that experience increased IL-6 expression in the brain have impaired short-term memory (Morales-Montor and others 2014).
IFN-γ was also altered during cysticercosis. Interferons regulate neural transmission—application of IFN-γ during peak synaptogenesis reduces the frequency of spontaneous excitatory activity, but increases that of spontaneous inhibitory postsynaptic currents, upsetting the balance between excitatory and inhibitory neurotransmission (Brask and others 2004).
TNF-α regulates important functions in the brain, which might correlate with the alterations in TNF-α expression in infected host brains that we observed. For instance, TNF-α increases Ca2+ current density through L-type voltage-dependent channels, but it decreases currents that are induced by glutamate, NMDA, AMPA, and kainite in cultured neurons (Furukawa and Mattson 1998; Liu and others 2002). TNF-α also modulates synaptic maturation and neuronal branching in the hippocampus by controlling the expression of synaptic vesicle-associated proteins (Golan and others 2004). Moreover, TNF-α alters the electrophysiological properties of myenteric neurons through cyclooxygenase metabolites and protein tyrosine phosphorylation (Son and others 2004).
In conclusion, cytokines interact with the brain in a systemic and complex manner, influencing the development, function, and hormone production in the host. Thus, many clinical situations might be attributed to cytokine activity in the brain, and therapeutic manipulation of the immune system might affect brain function. Further work is needed to determine the exact function of cytokines and determine whether other factors are associated with the parasite or host in the feminization that occurs during experimental male murine T. crassiceps-induced cysticercosis.
Acknowledgments
Financial support: Grant # IN-214011 from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) from Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México (UNAM), and Grant 176803, from Programa de Fondos Sectoriales CB-SEP, Consejo Nacional de Ciencia y Tecnología (CONACyT), both to Jorge Morales Montor. Karen Nava-Castro has a postdoctoral fellowship from CONACyT. Rosalía Hernandez-Cervantes and Nelly Tiempos Guzmán are PhD students at Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México, and both have scholarships from CONACyT. Lenin Pavón is supported by PROMEP NETWORK No. 14411150.
Author Disclosure Statement
No competing financial interests exist.
References
- Arteaga-Silva M, Vargas-Villavicencio JA, Vigueras-Villasenor RM, Rodriguez-Dorantes M, Morales-Montor J. 2009. Taenia crassiceps infection disrupts estrous cycle and reproductive behavior in BALB/c female mice. Acta Trop 109(2):141–145 [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Kwon J, Chung WJ, Sampson J, Pandya K, Tang LP. 1994. Differential modulation of astrocyte cytokine gene expression by TGF-beta. J Immunol 153(11):5210–5221 [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. 1996. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 17(1):64–102 [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. 2000. The cytokine-HPA axis feed-back circuit. Z Rheumatol 59 (Suppl 2):II/26–30 [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Li A. 1994. Differentially regulated immediate early genes in the rat uterus. Endocrinology 134(4):1820–1826 [DOI] [PubMed] [Google Scholar]
- Brask J, Kristensson K, Hill RH. 2004. Exposure to interferon-gamma during synaptogenesis increases inhibitory activity after a latent period in cultured rat hippocampal neurons. Eur J Neurosci 19(12):3193–3201 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Loxley HD, Christian HC, Philip JG. 1996. Activation of the HPA axis by immune insults: roles and interactions of cytokines, eicosanoids, glucocorticoids. Pharmacol Biochem Behav 54(1):285–298 [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Lopez-Griego L, Morales-Montor J. 2009. The role of cytokines in the regulation of neurotransmission. Neuroimmunomodulation 16(1):1–12 [DOI] [PubMed] [Google Scholar]
- De Laurentiis A, Pisera D, Lasaga M, Diaz M, Theas S, Duvilanski B, Seilicovich A. 2000. Effect of interleukin-6 and tumor necrosis factor-alpha on GABA release from mediobasal hypothalamus and posterior pituitary. Neuroimmunomodulation 7(2):77–83 [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. 2009. Cytokines and CNS development. Neuron 64(1):61–78 [DOI] [PubMed] [Google Scholar]
- Dinarello CA. 2007. Historical insights into cytokines. Eur J Immunol 37(S1):S34–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus B, Dietzel ID, Heumann R, Berger R. 2004. Effects of interferon-gamma and tumor necrosis factor-alpha on survival and differentiation of oligodendrocyte progenitors. J Soc Gynecol Investig 11(2):89–96 [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. 1998. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70(5):1876–1886 [DOI] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. 2004. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex 14(1):97–105 [DOI] [PubMed] [Google Scholar]
- Gourbal BE, Lacroix A, Gabrion C. 2002. Behavioural dominance and Taenia crassiceps parasitism in BALB/c male mice. Parasitol Res 88(10):912–917 [DOI] [PubMed] [Google Scholar]
- Gourbal BE, Righi M, Petit G, Gabrion C. 2001. Parasite-altered host behavior in the face of a predator: manipulation or not? Parasitol Res 87(3):186–192 [DOI] [PubMed] [Google Scholar]
- Hu S, Martella A, Anderson WR, Chao CC. 1994. Role of cytokines in lipopolysaccharide-induced functional and structural abnormalities of astrocytes. Glia 10(3):227–234 [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. 2000. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7(3):153–159 [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Kenis G, Kim YK, Lason W. 2005. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor alpha and interleukin-6. Psychiatry Res 134(3):251–258 [DOI] [PubMed] [Google Scholar]
- Larralde C, Morales J, Terrazas I, Govezensky T, Romano MC. 1995. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J Steroid Biochem Mol Biol 52(6):575–580 [DOI] [PubMed] [Google Scholar]
- Larralde C, Sotelo J, Montoya RM, Palencia G, Padilla A, Govezensky T, Diaz ML, Sciutto E. 1990. Immunodiagnosis of human cysticercosis in cerebrospinal fluid. Antigens from murine Taenia crassiceps cysticerci effectively substitute those from porcine Taenia solium. Arch Pathol Lab Med 114(9):926–928 [PubMed] [Google Scholar]
- Liu B, Li H, Brull SJ, Zhang JM. 2002. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol 88(3):1393–1399 [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell DS, Chiappetta C, Stancel GM. 1988. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol 2(10):946–951 [DOI] [PubMed] [Google Scholar]
- Mazzoni IE, Kenigsberg RL. 1997. Microglia from the developing rat medial septal area can affect cholinergic and GABAergic neuronal differentiation in vitro. Neuroscience 76(1):147–157 [DOI] [PubMed] [Google Scholar]
- Miller LG, Fahey JM. 1994. Interleukin-1 modulates GABAergic and glutamatergic function in brain. Ann N Y Acad Sci 739:292–298 [DOI] [PubMed] [Google Scholar]
- Miller LG, Galpern WR, Dunlap K, Dinarello CA, Turner TJ. 1991. Interleukin-1 augments gamma-aminobutyric acidA receptor function in brain. Mol Pharmacol 39(2):105–108 [PubMed] [Google Scholar]
- Morales J, Larralde C, Arteaga M, Govezensky T, Romano MC, Morali G. 1996. Inhibition of sexual behavior in male mice infected with Taenia crassiceps cysticerci. J Parasitol 82(5):689–693 [PubMed] [Google Scholar]
- Morales-Montor J, Arrieta I, Del Castillo LI, Rodriguez-Dorantes M, Cerbon MA, Larralde C. 2004a. Remote sensing of intraperitoneal parasitism by the host's brain: regional changes of c-fos gene expression in the brain of feminized cysticercotic male mice. Parasitology 128 (Pt 3):343–351 [DOI] [PubMed] [Google Scholar]
- Morales-Montor J, Escobedo G, Rodriguez-Dorantes M, Tellez-Ascencio N, Cerbon MA, Larralde C. 2004b. Differential expression of AP-1 transcription factor genes c-fos and c-jun in the helminth parasites Taenia crassiceps and Taenia solium. Parasitology 129 (Pt 2):233–243 [DOI] [PubMed] [Google Scholar]
- Morales-Montor J, Larralde C. 2005. The role of sex steroids in the complex physiology of the host-parasite relationship: the case of the larval cestode of Taenia crassiceps. Parasitology 131 (Pt 3):287–294 [DOI] [PubMed] [Google Scholar]
- Morales-Montor J, Picazo O, Besedovsky HO, Hernandez-Bello R, Lopez-Griego L, Becerril LE, Moreno J, Pavón L, Nava-Castro K, Camacho-Arroyo I. 2014. Helminth infection alters mood and short-term memory as well as levels of neurotransmitters and cytokines in the mouse hippocampus. Neuroimmunomodulation 21(4):195–205 [DOI] [PubMed] [Google Scholar]
- Morales-Montor J, Rodriguez-Dorantes M, Mendoza-Rodriguez CA, Camacho-Arroyo I, Cerbon MA. 1998. Differential expression of the estrogen-regulated proto-oncogenes c-fos, c-jun, and bcl-2 and of the tumor-suppressor p53 gene in the male mouse chronically infected with Taenia crassiceps cysticerci. Parasitol Res 84(8):616–622 [DOI] [PubMed] [Google Scholar]
- Nephew KP, Webb DK, Akcali KC, Moulton BC, Khan SA. 1993. Hormonal regulation and expression of the jun-D protooncogene in specific cell types of the rat uterus. J Steroid Biochem Mol Biol 46(3):281–287 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Dorantes M, Cerbon MA, Larralde C, Morales-Montor J. 2007. Modified progesterone receptor expression in the hypothalamus of cysticercotic male mice. Acta Trop 103(2):123–132 [DOI] [PubMed] [Google Scholar]
- Smith JK, Esch GW, Kuhn RE. 1972. Growth and development of larval Taenia crassiceps (cestoda). I. Aneuploidy in the anomalous ORF strain. Int J Parasitol 2(2):261–263 [DOI] [PubMed] [Google Scholar]
- Son DS, Arai KY, Roby KF, Terranova PF. 2004. Tumor necrosis factor alpha (TNF) increases granulosa cell proliferation: dependence on c-Jun and TNF receptor type 1. Endocrinology 145(3):1218–1226 [DOI] [PubMed] [Google Scholar]
- Travers MT, Wakeling AE, Knowler JT. 1988. The isolation of recombinant RNA species responsive to oestrogen and tamoxifen in rat uterus and MCF-7 cells. Mol Cell Endocrinol 57(3):179–186 [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. 2002. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22(2):486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kakihana M, Chen LS, Ong M, Baird A, Gage FH. 1992. Cytokine regulation of nerve growth factor-mediated cholinergic neurotrophic activity synthesized by astrocytes and fibroblasts. J Neurochem 59(3):919–931 [DOI] [PubMed] [Google Scholar]
- Yu B, Shinnick-Gallagher P. 1994. Interleukin-1 beta inhibits synaptic transmission and induces membrane hyperpolarization in amygdala neurons. J Pharmacol Exp Ther 271(2):590–600 [PubMed] [Google Scholar]