Abstract
Paeonia lactiflora and P. obovata are perennial herbs, each root of which has been consumed as a major oriental medicine, Paeoniae Radix and a famous folk medicine, Mountain Paeony Root, respectively. Although morphological studies have been performed comparing these two plants, there is insufficient scientific evidence that characterizes the differences in their chemical profiles and biological activities. Hence, the present study was undertaken to compare these two medicinal foods using a high-performance liquid chromatography–diode-array detector (HPLC-DAD) analysis and a gastric ulcer model in mice. HPLC analysis employed to assess the nine components revealed that P. lactiflora exhibited higher contents of phenolic compounds than P. obovata. Although a monoterpene glycoside, 6′-O-acetylpaeoniflorin was identified in P. obovata, it was not detected in P. lactiflora. Multivariate statistical analysis for HPLC data revealed that the orthogonal projections to latent structure-discriminant analysis is more appropriate than principal component analysis for differentiating the two groups. Moreover, the 50% methanol P. lactiflora extract (PL) was more effective against experimental gastric ulcer than P. obovata extract (PO) in the HCl/ethanol-induced ulcer model. In addition, PL displayed higher 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and lower nitric oxide production in a murine macrophage cell line, RAW 264.7, than PO. The DPPH radical scavenging activity of PL was as high as that of the positive control, butylated hydroxytoluene, at a concentration of 25 μg/mL.
Key Words: : anti-gastric ulcer activity, chemical profiles, Paeonia lactiflora, P. obovata
Introduction
Paeonia lactiflora and P. obovata are perennial Asian peony plants (Paeoniaceae), each root of which has been consumed as a major oriental medicine, Paeoniae Radix and a famous folk medicine, Mountain Paeony Root, respectively.1 Paeoniae Radix has been used to treat amenorrhea, dysmenorrhea, inflammation, and spasm.2,3 This crude drug is well known to stimulate blood circulation and exhibit anti-inflammatory, antiplatelet, and vasodilator activities.4,5 Monoterpene glycosides, such as albiflorin, oxypaeoniflorin, and paeoniflorin, have been isolated from P. lactiflora roots with galloyl and phenolic compounds, including benzoic acid, catechin, gallic acid, methyl gallate, paeonol, and 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG).6,7 Paeoniflorin, a major constituent of Paeoniae Radix, has been reported to exhibit diverse biological activities, including anti-inflammatory,8 spasmolytic,9 immune-regulating,10 and gastroprotective activities.11 Recently, methyl gallate, paeonol, and PGG with P. lactiflora root extract have been suggested as alternative sources of anti-Helicobacter pylori products because of their growth-inhibiting, bactericidal, and urease inhibitory effects.12 Therefore, the extract of Paeoniae Radix or its purified fractions could be useful plant resources for the development of functional foods.
Although P. lactiflora possess nine-lobed leaves and produce follicle-type dryfruit with black-colored seeds, P. obovata has trifoliate leaves and produces red- and black-colored seeds.13 In addition, whereas P. lactiflora is widely distributed and cultivated throughout eastern Asia, P. obovata grows predominantly in the wild and is difficult to cultivate. P. obovata roots have been regarded as an expensive folk medicine with potent tonic activity as well as the biological activity of Paeoniae Radix. Although morphological studies have attempted to compare these two natural resources,14 current scientific evidence is insufficient to differentiate their chemical profiles and biological activities. Hence, the present study was undertaken to compare these two medicinal herbs using a high-performance liquid chromatography–diode-array detector (HPLC-DAD) analysis, an HCl/ethanol-induced gastric ulcer mouse model, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method, and intracellular nitric oxide (NO) assay.
Materials and Methods
Chemicals
Among nine standard compounds, albiflorin, paeonol, and paeoniflorin were purchased from Wako (Tokyo, Japan), and another five compounds, gallic acid, (+)-catechin, methyl gallate, benzoic acid, and PGG, were purchased from Sigma Chemical (St. Louis, MO, USA). The other standard compound, 6′-O-acetylpaeoniflorin, was isolated from P. obovata in our laboratory. Bisphenol A was used as an internal standard (IS) for HPLC analysis and was purchased from Sigma Chemical with sucralfate. HPLC-grade acetonitrile, methanol, and water were provided by Fisher Scientific (Korea Ltd., Seoul, Korea). All of the other reagents and solvents used were of guaranteed or analytical grade.
General
Optical rotations were performed on a Perkin-Elmer 343 polarimeter. FT-IR spectra were recorded with a Bruker Vertex 80v FT-IR Spectrophotometer. 1H and 13C NMR spectra were assessed with a Bruker Avance-500 spectrometer at 500 and 125 MHz, respectively. HRFAB/MS was obtained with a JEOL JMS-700 mass spectrometer.
Plant materials
The P. lactiflora Pall. roots were collected in October 2011 and the P. obovata Maxim. roots were collected from 1999 to 2011. The list of collected samples is presented in Table 1. After the plants were cleaned with water, the samples were identified by Prof. Jong Hee Park from the College of Pharmacy, Pusan National University, Korea. The voucher specimen (No. 37204–No. 37225) was deposited in the Herbarium of the College of Pharmacy, Pusan National University.
Table 1.
A List of Paeonia lactiflora and Paeonia obovata Samples Collected from Korea
| No. | Species | Collection locations (cultivation year) | Abbreviations |
|---|---|---|---|
| 1 | Paeonia lactiflora cv. Uiseong | Uiseong, Gyeongbuk (5) | PL1 |
| 2 | P. lactiflora cv. Sagok | Uiseong, Gyeongbuk (5) | PL2 |
| 3 | P. lactiflora cv. Taebaek | Uiseong, Gyeongbuk (5) | PL3 |
| 4 | P. lactiflora cv. Migang | Uiseong, Gyeongbuk (5) | PL4 |
| 5 | P. lactiflora cv. Geopung | Uiseong, Gyeongbuk (5) | PL5 |
| 6 | P. lactiflora | PNU Herb Garden, Busan | PL6 |
| 7 | P. lactiflora | Sancheong, Gyeongnam (2) | PL7 |
| 8 | P. lactiflora | Sancheong, Gyeongnam (1) | PL8 |
| 9 | P. lactiflora | Sancheong, Gyeongnam (6) | PL9 |
| 10 | P. lactiflora | Boseong, Jeonnam (4) | PL10 |
| 11 | P. lactiflora | Boseong, Jeonnam (10) | PL11 |
| 12 | P. lactiflora | Boseong, Jeonnam (10) | PL12 |
| 13 | Paeonia obovata | Mt. Gaji, Gyeongnam | PO1 |
| 14 | P. obovata | Mt. Sinbul, Gyeongnam | PO2 |
| 15 | P. obovata | Mt. Juwang, Gyeongbuk | PO3 |
| 16 | P. obovata | Mt. Moonbok, Ulsan | PO4 |
| 17 | P. obovata | Mt. Seorak, Gangwon | PO5 |
| 18 | P. obovata | Mt. Odae, Gangwon | PO6 |
| 19 | P. obovata | Mt. Duta, Gangwon | PO7 |
| 20 | P. obovata | Mt. Yeongchwi, Gyeongnam | PO8 |
| 21 | P. obovata | Mt. Palgong, Daegu | PO9 |
| 22 | P. obovata | Mt. Jaeyak, Gyeongnam | PO10 |
Sample preparation
The P. lactiflora and P. obovata roots were lyophilized and ground into a powder. The finely pulverized powder was weighed (3.0 g), and 30 mL of 50% methanol was added. The mixtures were extracted under sonication for 60 min, three times each, yielding 30.2–32.5% of extraction yield. The extract was centrifuged for 10 min at 4°C and 3000 ×g and filtered through a 0.45 μm PTFE syringe filter (Whatman, New York, NY, USA) before analysis. The methanol PL5 extract (PL) and PO1 extract (PO) were used for anti-ulcer, DPPH radical scavenging, and NO production reducing activity assays.
HPLC-DAD analysis
An Agilent 1100 series HPLC system equipped with an autosampler, a column oven, a binary pump and a degasser (Agilent Technologies, Palo Alto, CA, USA) was used. An aliquot (20 μL) of the standard or sample solution was directly injected on a Phenomenex Gemini column (4.6×250 mm, 5 μm) with a compatible guard column. The gradient elution using an acetonitrile–water (0.05% formic acid, v/v) solvent system was as follows: 10% acetonitrile to 20% for the first 20 min; 20% acetonitrile to 40% for a further 10 min; 40% for 15 min; acetonitrile from 40% to 50% for the next 5 min; and then 50% for another 5 min. A conditioning phase was then used to return the column to the initial state for 5 min at a flow rate of 0.8 mL/min and a column temperature of 35°C. The eluent was detected at 230 nm with a DAD. The Chemstation software (Agilent Technologies) was used to operate this HPLC-DAD system.
Stock solutions for standard compounds were prepared with HPLC-grade methanol as the solvent. Working calibration solutions were prepared by successive serial dilutions of the stock solution with methanol, and the final concentrations were 100, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.8, 0.4, and 0.2 μg/mL.
Isolation of 6′-O-acetylpaeoniflorin
Compound 7 (5.1 mg, tR 31.2 min) was isolated from a 50% methanol extract of P. obovata using the YMC multiple prep-HPLC forte/R system using a YMC-Pack ODS-A column (20×250 mm, 5 μm). The step gradient elution was as follows: 15% acetonitrile to 35% for the first 35 min; 35–100% for the next 5 min; then, 100% for another 10 min. The flow rate was 8 mL/min, and the column temperature was at 25°C. The eluent was detected at 230 nm.
Animals
Male ICR mice (25–27 g) were obtained from Koatech (Seoul, Korea). The animals were housed in a controlled environment (at 24°C±2°C under a12-h light–12-h dark cycle), and access to feed and water was unrestricted. All procedures were performed using a protocol approved by the Animal Experiment Committee of Gyeongsang National University (GNU-120508-M0018) according to the national and international guidelines for the humane use of laboratory animals.
HCl/ethanol-induced gastric ulcer
The protective activity against gastric ulcers in mice was evaluated according to the method described previously.15 Briefly, the animals were divided into seven groups (five mice each) after 16 h of fasting. Sham-operated and vehicle-treated groups were given 0.2 mL (per 25 g body weight) of water. PL and PO suspended in mineral water were administered at different doses (10 and 100 mg/kg) intragastrically to the different groups through a gavage needle. Sucralfate (100 mg/kg) and 150 mM HCl/ethanol (0.2 mL/25 g body weight) were administered as positive and negative controls, respectively, after 1 h of sample treatment.
DPPH radical scavenging activity
The scavenging activity of the DPPH radicals by PL and PO was measured by previously reported method.15 Briefly, 200 μL of 0.2 mM DPPH solution in methanol (w/v) was mixed with 800 μL sample solutions of different concentrations (6.3, 12.5, 25, 50, and 100 μg/mL) and incubated at room temperature for 30 min. After incubation, absorbance of mixtures was measured at 517 nm. DPPH radical scavenging activity was calculated by using the following formula:
% activity={(Ac−As)/Ac}×100 where Ac and As are absorbance of control and samples, respectively. Butylated hydroxytoluene (BHT) and L-ascorbic acid were used as positive controls.
Determination of intracellular NO production
Nitrite levels in the RAW 264.7 cell culture media were measured using the Griess reaction to assess NO production following our previously reported method.15 The cell viability was also evaluated by using the previously described method.15
Statistical analysis
The SIMCA 13 statistical software (Umetrics, Umea, Sweden) was used for principal component analysis (PCA) and orthogonal projections to latent structure-discriminant analysis (OPLS-DA) multivariate analysis on the colorimetric and HPLC profile data. The biological assay data were obtained from more than three samples of two or three independent experiments and expressed as the mean±standard error of the mean or the mean±standard deviation (SD). The differences among samples were statistically evaluated through one-way analysis of variance (ANOVA). The values were considered statistically significant at P<.05.
Results
Differences in chemical profiles between P. lactiflora and P. obovata
A high-performance liquid chromatographic method was employed to compare the chemical profiles between the methanol extracts of P. lactiflora (PL) and P. obovata (PO). Among the nine compounds analyzed in this study (Fig. 1), two major peaks identified as paeoniflorin and PGG were present in both samples along with albiflorin, gallic acid, catechin, methyl gallate, benzoic acid, and paeonol peaks (Fig. 2B, C). Moreover, a unique peak at 29.6 min was detected only in the PO sample, and was isolated as a yellow, amorphous powder using prep-HPLC. From comparison of the spectroscopic data with previously reported data,16 the chemical structure of this compound was identified as a monoterpene glycoside, 6′-O-acetylpaeoniflorin. Although this compound had been initially isolated from P. veitchi,16 this is the first report of its presence in P. obovata. Therefore, the content of nine components, including 6′-O-acetylpaeoniflorin were determined in the PL and PO samples.
FIG. 1.
Chemical structures of compounds 1–9.
FIG. 2.
High-performance liquid chromatography (HPLC)-UV chromatograms of the standard solution and the 50% methanol Paeonia lactiflora and Paeonia obovata extracts. (A) A mixture of the standard solution (100 μg/mL), (B) the 50% methanol P. lactiflora extract, (C) the 50% methanol P. obovata extract. 1, gallic acid; 2, (+)-catechin; 3, methyl gallate; 4, albiflorin; 5, paeoniflorin; 6, 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG); 7, 6′-O-acetylpaeoniflorin; 8, benzoic acid; 9, paeonol; IS, bisphenol A.
Calibration curves were constructed on three consecutive days by analyzing a mixture of nine standard compounds at various concentration levels and plotting the peak area against the concentration of each reference standard (Table 2). The curves exhibited a good linearity, and the correlation coefficients ranged between 0.996 and 0.999 for the compound concentration ranges of 0.2–100 and 0.4–100 μg/mL for compounds 1–3 and 4–9, respectively. The limits of detection and quantification were determined by means of serial dilution based on a signal-to-noise (S/N) ratio of 3:1 and 10:1, respectively. Limit of detection of all the compounds were less than 2 ng, which showed a high sensitivity at this chromatographic condition (Table 2).
Table 2.
Linear Ranges, Linear Equations, and Correlation Coefficients of Calibration Curves
| Compounds | Linear range (μg/mL) | Standard curve equations | r2 | LOD (ng) |
|---|---|---|---|---|
| Gallic acid (1) | 0.2–100 | y=38.153x+0.2997 | 0.9999 | ∼2 |
| (+)-Catechin (2) | 0.2–100 | y=36.724x+0.7388 | 0.9998 | ∼2 |
| Methyl gallate (3) | 0.2–100 | y=68.599x+0.7004 | 0.9998 | ∼2 |
| Albiflorin (4) | 0.4–100 | y=23.026x+5.3274 | 0.9989 | ∼2 |
| Paeoniflorin (5) | 0.4–2000 | y=27.816x–0.8562 | 0.9964 | ∼2 |
| PGG (6) | 0.4–100 | y=21.701x–11.212 | 0.9997 | ∼2 |
| 6′-O-acetylpaeoniflorin (7) | 0.4–100 | y=14.185x+11.987 | 0.9996 | ∼2 |
| Benzoic acid (8) | 0.4–100 | y=219.78x+2.0700 | 0.9998 | 0.3 |
| Paeonol (9) | 0.4–100 | y=97.798x–4.5224 | 0.9999 | ∼1 |
| Bisphenol A (IS) | 0.4–100 | y=58.276x–4.9480 | 0.9999 | ∼1 |
IS, internal standard; PGG, 1,2,3,4,6-penta-O-galloyl-β-D-glucose; LOD, limit of detection.
Nine distinct standard compounds (1–9) were isolated from a standard mixture solution (Fig. 2A), and these compounds were also identified in the PL and PO samples from a comparison with the reference compounds based on their UV spectra and retention time (Fig. 2B, C). Therefore, PL exhibited higher (+)-catechin, methylgallate, paeoniflorin, and PGG contents compared with PO. Whereas a paeoniflorin derivative, 6′-O-acetylpaeoniflorin, was identified in PO, it was not detected in PL. The benzoic acid content was generally higher in PO samples than in the PL samples. Some sample variations were also observed in the five P. lactiflora cultivars (PL1–PL5) that were cultivated under the same conditions in Uiseong. In summary, PL2 and PL5 displayed higher catechin and albiflorin contents than the other three cultivars. The relative SD values were less than 19.3% (Table 3).
Table 3.
Contents of Nine Compounds (1–9) in Paeonia lactiflora and Paeonia obovata Samples
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| PL1 | 122.2±22.3 | 347.0±1.8 | 378.9±5.5 | 241.3±4.2 | 1740.4±35.3 | 1639.7±28.3 | ND | 15.2±0.0 | 2.4±0.0 |
| PL2 | 133.0±17.8 | 953.0±6.5 | 311.3±1.4 | 1493.3±1.9 | 2345.7±10.3 | 1824.9±10.9 | ND | 42.3±0.4 | 33.4±0.0 |
| PL3 | 109.0±0.6 | 575.3±1.6 | 412.4±1.9 | 324.1±0.1 | 2079.5±9.9 | 1998.9±6.0 | ND | 27.3±0.2 | 10.1±0.0 |
| PL4 | 119.4±20.8 | 149.2±3.8 | 332.3±12.8 | 321.1±31.4 | 1316.9±53.3 | 1264.0±59.3 | ND | 13.0±2.1 | 4.1±0.2 |
| PL5 | 165.5±0.6 | 736.9±1.1 | 351.3±0.9 | 1246.7±95.7 | 1941.4±3.5 | 1637.2±7.8 | ND | 26.8±0.0 | 12.7±0.2 |
| PL6 | 83.3±0.4 | 533.1±0.3 | 264.1±0.4 | 290.5±1.4 | 1838.5±3.0 | 1574.7±2.7 | ND | 41.9±3.7 | 21.1±0.0 |
| PL7 | 225.4±25.1 | 172.9±24.7 | 208.5±36.4 | 436.0±79.8 | 1459.2±274.6 | 1329.3±249.6 | ND | 155.0±29.2 | 4.6±0.8 |
| PL8 | 175.2±0.9 | 456.0±6.1 | 227.6±1.0 | 1342.7±5.3 | 1715.4±0.7 | 1335.8±6.7 | ND | 48.3±0.6 | 2.8±0.1 |
| PL9 | 351.4±5.2 | 125.9±1.5 | 318.6±1.5 | 353.7±5.2 | 2021.9±5.0 | 1771.0±6.0 | ND | 142.2±0.8 | 4.5±0.1 |
| PL10 | 146.2±0.5 | 485.0±3.5 | 174.4±1.0 | 561.1±9.5 | 1760.6±2.0 | 1159.9±1.4 | ND | 38.3±0.3 | 8.4±0.0 |
| PL11 | 129.3±15.8 | 261.4±41.2 | 252.6±34.9 | 270.3±31.4 | 1537.4±240.3 | 1365.8±218.5 | ND | 23.5±3.0 | 3.9±0.6 |
| PL12 | 174.7±1.9 | 784.1±7.5 | 309.8±4.1 | 352.3±17.5 | 2392.3±29.1 | 1881.5±16.4 | ND | 47.7±0.5 | 16.6±0.1 |
| Mean | 161.2±6.9 | 465.0±10.8 | 295.1±8.5 | 602.7±23.6 | 1845.8±55.6 | 1565.2±51.1 | ND | 51.8±3.4 | 10.4±0.2 |
| PO1 | 61.3±1.5 | 54.0±0.2 | 146.5±0.5 | 219.1±1.3 | 1069.2±0.4 | 706.1±1.0 | 1426.2±1.9 | 31.0±0.0 | 1.6±0.0 |
| PO2 | 119.1±1.9 | 33.5±0.8 | 69.8±0.8 | 78.7±1.3 | 104.1±0.1 | 206.4±3.9 | ND | 140.5±1.8 | 4.9±2.6 |
| PO3 | 63.0±6.3 | 37.3±5.5 | 73.5±14.6 | 485.2±82.9 | 1671.5±89.8 | 537.4±24.4 | 205.3±34.8 | 32.3±1.1 | 1.6±0.1 |
| PO4 | 25.4±3.1 | 0.3±0.0 | 59.0±5.8 | 119.1±8.1 | 81.6±10.3 | 206.0±8.4 | ND | 112.9±1.8 | 0.7±0.1 |
| PO5 | 104.1±14.9 | 89.8±8.2 | 184.2±18.8 | 660.4±1.0 | 2362.4±12.9 | 834.8±6.3 | 625.4±5.8 | 61.5±0.4 | 2.4±0.0 |
| PO6 | 114.7±4.8 | 36.0±6.1 | 25.8±4.6 | 90.4±3.9 | 637.5±43.2 | 392.6±22.1 | 92.1±17.8 | 109.0±3.6 | 1.4±0.0 |
| PO7 | 176.5±8.7 | 66.5±1.3 | 81.2±3.8 | 182.1±2.5 | 737.9±4.4 | 504.6±0.2 | 228.4±0.3 | 111.3±0.5 | 1.3±0.1 |
| PO8 | 117.7±13.5 | 52.2±0.5 | 85.1±1.5 | 199.0±31.0 | 1094.7±6.4 | 393.6±2.5 | 392.9±1.6 | 111.5±0.7 | 1.3±0.0 |
| PO9 | 54.6±0.1 | 67.2±2.6 | 92.9±0.4 | 246.6±3.2 | 1601.1±30.7 | 479.6±21.3 | 801.4±2.7 | 71.1±2.3 | 1.1±0.0 |
| PO10 | 71.4±8.0 | 58.6±1.9 | 159.6±1.3 | 262.5±4.7 | 2070.7±75.2 | 168.7±9.8 | 1847.3±34.7 | 31.7±2.4 | 5.3±0.1 |
| Mean | 90.8±1.9 | 49.5±6.3 | 97.8±8.5 | 254.3±24.8 | 1143.1±29.5 | 443.0±12.0 | 702.4±12.4 | 81.3±1.5 | 2.2±0.3 |
The denotations from 1 to 9 are the corresponding chemicals as listed in Figure 1. Contents were expressed as the mean±standard deviation (mg/100 g dried sample) (n=3).
ND, not detected.
Multivariate statistical analysis for colorimetric and HPLC profile data
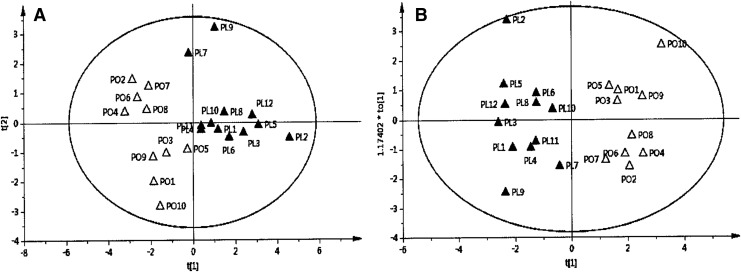
To examine whether the P. lactiflora and P. obovata roots can be differentiated using colorimetric or HPLC profile data, PCA and OPLS-DA multivariate statistical analyses were applied to these data obtained formerly. As shown in Figure 3, overall, the OPLS-DA model is more efficiently discriminated between the two groups than PCA. However, both PCA and OPLS-DA failed to clearly distinguish the two groups when using the colorimetric data (data not shown). The OPLS-DA combined with the HPLC profiles clearly classified the two groups.
FIG. 3.
Score plots by principal component analysis (PCA) (A) and orthogonal projections to latent structure-discriminant analysis (OPLS-DA) (B) for the P. lactiflora and P. obovata groups.
Anti-ulcer effect of PL and PO on the HCl/ethanol-induced gastric ulcer mouse model
HCl and absolute ethanol were used as necrotizing agents of gastric mucosa to produce gastric ulcers in mice. Gastric mucosa damage was evaluated in the area harboring the gastric ulcer. The protection percentage was calculated after comparison with the ulcer control group, which was considered as 100% damage. The administration of HCl/ethanol produced lesions on the gastric mucosa (73.3±7.43 mm2) (Fig. 4B), which were significantly reduced to 8.22±3.37 and 27.62±4.57 mm2 in the animals pretreated with 100 mg/kg PL and PO, respectively (Fig. 4E, G). The protective effect (88.8%) of the group pretreated with PL was higher than that (62.3%) of the group pretreated with PO. Sucralfate (100 mg/kg) was used as a reference drug and also significantly prevented the gastric lesions by 75.9% (17.68±1.1 mm2) as compared to the vehicle group (Fig. 4C). The anti-gastric ulcer activity of two Paeonia species was also assessed at the 10 mg/kg dose, and the PL protective activity was higher (73.6%±7.6%) than the PO activity (27.4%±5.4%) (Fig. 4D, F). The PL anti-ulcer effect of the 10 mg/kg dose was similar to that of the positive control, 100 mg/kg of sucralfate.
FIG. 4.
The protective effects of PL and PO on the HCl/ethanol-induced gastric ulcer mouse model. (A) A normal stomach, (B) a glandular stomach treated with a vehicle, (C) the stomach from the positive control group treated with sucralfate, 100 mg/kg, (D, E) the stomach from the test groups treated with 10 mg/kg and 100 mg/kg P. lactiflora extract, respectively, (F, G) the stomach from test groups treated with 10 mg/kg and 100 mg/kg of P. obovata extract, respectively, 1 h before the administration of 150 mM HCl/ethanol, (H) the determination of the gastric ulcer lesion (mm2). Data are expressed as the mean±standard error of the mean, n=5–10. *P<.05, **P<.01, and ***P<.001, significantly different from the gastric ulcer group (B). Color images available online at www.liebertpub.com/jmf
Free radical-scavenging activity
The free radical scavenging activity of PL and PO was evaluated since increases in free radical damage are observed in ulcerous and inflammatory processes.17 As a result, the PL sample displayed a potent DPPH radical scavenging activity 71.5%±1.3%, which was similar to the 72.0%±3.7% of the reference compound, 25 μg/mL of BHT (Fig. 5). However, PL displayed a more potent radical scavenging activity with the IC50 value of 16.4 μg/mL than the PO activity (36.6 μg/mL). In this case, the IC50 values for LAA and BHT were 5.3 and 13.4 μg/mL, respectively.
FIG. 5.
Differences in 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity between PL and PO (6.3–100 μg/mL). Ascorbic acid and BHT were used as references. The absorbance of the reaction was measured at 517 nm. Values represent the mean±standard deviation (SD). BHT, butylated hydroxytoluene.
Effect of PL and PO on intracellular NO production in lipopolysaccharide-stimulated RAW 264.7 cells
The inhibitory activity of PL and PO against lipopolysaccharide-stimulated NO production was evaluated in a murine macrophage cell line, RAW 264.7. Our results revealed that both extracts decreased NO production in a dose-dependent manner at the concentrations of 1, 10, and 100 μg/mL, and the inhibitory activity was greater in PL than in PO (Fig. 6). At the 100 μg/mL concentration, PL and PO significantly decreased NO production, up to 37.4%±1.2% and 31.9%±1.2%, respectively. In addition, neither extracts affected cell viability (data not shown).
FIG. 6.
Effect on nitric oxide (NO) production of PL and PO in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. **P<.01, ***P<.001 versus LPS-treated controls.
Discussion
With the increasing use of herbal products worldwide, the regulation of natural botanical resources is merited due to quality and safety concerns. Regulatory guidelines include the identification of botanical origin, chemical profiles, and biological efficacy.18 Although scientific data is lacking, medicinal food has occasionally been regarded as valuable and expensive due to its rarity. In the present study, we performed qualitative and quantitative analyses of P. lactiflora and P. obovata roots using colorimetry and HPLC paired with a UV photodiode array. Biological activity differences were also evaluated with an HCl/ethanol-induced gastric ulcer mouse model, DPPH radical scavenging method, and intracellular NO assay.
HPLC chromatograms revealed significant differences in the content of major constituents between the P. lactiflora and P. obovata roots. P. lactiflora exhibited higher (+)-catechin, methylgallate, paeoniflorin, and PGG contents than P. obovata. Interestingly, although a monoterpene glycoside, 6′-O-acetylpaeoniflorin was identified in P. obovata, it was not detected in P. lactiflora. In addition, the results indicated that the developed HPLC fingerprint analytical method was practical and reliable for the above purpose.
Multivariate statistical analysis for HPLC data revealed that the OPLS-DA is more appropriate than PCA for differentiating the two groups. This result was consistent with previous reports that distinguished the origins of Scutellaria baicalensis samples with OPLS-DA.19 Additionally, this study revealed that different HPLC fingerprint patterns could be the differentiating factors for each other because OPLS-DA on HPLC profile data clearly discriminated between the two sample groups.
Both types of Paeonia species harbored anti-gastric ulcer activities, and PL exhibited a greater anti-gastric ulcer than PO in the HCl/ethanol-induced gastric ulcer mouse model. Additionally, the PL sample displayed higher DPPH radical scavenging activity and lower NO production in a murine macrophage cell line, RAW 264.7, than PO. Because catechin and PGG are known to exert DPPH radical scavenging activity,20 and paeoniflorin exhibits a protective effect on the HCl and ethanol-triggered gastric mucosal injury,11 the greater radical scavenging, inhibitory NO production, and anti-ulcer activity of PL could be attributed to the higher content of these compounds compared with PO.
Although a previous study has assessed the differences in chemical profiles of P. lactiflora and P. veitchii roots, both of which have been used as Paeoniae Radix in China,21 this study is the first report that differentiates P. obovata from P. lactiflora based on their chemical profiles and biological activities. Additionally, this research contributes to the functional food validation of the methanol extracts of P. lactiflora and P. obovata having anti-ulcerogenic activity. Finally, our research suggests that further pharmacological investigation into 6′-O-acetylpaeoniflorin is necessary to define the unique value of P. obovata, which differs from P. lactiflora.
Acknowledgments
The authors would like to thank Researcher Sang Seok Lee at the Organic Agriculture Research Institute, Gyeongsangbuk-do Agricultural Research & Extension Services for providing five P. lactiflora cultivars cultivated in Uiseong, Gyeongbuk Province. This research was financially supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Inter-ER Cooperation Projects (Grant no. A004500005), and Gyeongsang National University Hospital Biomedical Research Institute (GNUHBRIF-2012-02).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lee TB: Colored Flora of Korea I. Hyangmoon Press, Seoul, 1985, pp. 368–369 [Google Scholar]
- 2.Zhang W, Dai S: Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int Immunopharmacol 2012;14:27–31 [DOI] [PubMed] [Google Scholar]
- 3.Wu SH, Wu DG, Chen YW: Chemical constituents and bioactivities of plants from the genus Paeonia. Chem Biodivers 2010;7:90–104 [DOI] [PubMed] [Google Scholar]
- 4.Hongmei X, Qingyun L, Min D, Peng D, Xiaomei Z: The effect of anti-thrombus on total glucosides of Chishao. J Anhui Tradit Chin Med Coll 2000;19:46–47 [Google Scholar]
- 5.Jin SN, Wen JF, Wang TT, Kang DG, Lee HS, Cho KW: Vasodilatory effects of ethanol extract of Radix Paeoniae Rubra and its mechanism of action in the rat aorta. J Ethnopharmacol 2012;142:188–193 [DOI] [PubMed] [Google Scholar]
- 6.Kaneda M, Iitaka Y, Shibata S: Chemical studies on the oriental plant drugs XXXIII: the absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from chinese paeony root. Tetrahedron 1972;28:4309–4317 [Google Scholar]
- 7.Kim JS, Kim YJ, Lee JY, Kang SS: Phytochemical studies on Paeoniae Radix (2)–Phenolic and related compounds. Korean J Pharmacogn 2008;39:28–36 [Google Scholar]
- 8.Jiang D, Chen Y, Hou X, Xu J, Mu X, Chen W: Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J Ethnopharmacol 2011;137:914–920 [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Hafez AA, Meselhy MR, Nakamura N, et al. : Anticonvulsant activity of paeonimetabolin-I adducts obtained by incubation of paeoniflorin and thiol compounds with Lactobacillus brevis. Biol Pharm Bull 1999;22:491–497 [DOI] [PubMed] [Google Scholar]
- 10.Yun CS, Choi YG, Jeong MY, Lee JH, Lim S: Moutan Cortex Radicis inhibits inflammatory changes of gene expression in lipopolysaccharide-stimulated gingival fibroblasts. J Nat Med 2012;67:576–589 [DOI] [PubMed] [Google Scholar]
- 11.Asai M, Kawashima D, Katagiri K, Takeuchi R, Tohnai G, Ohtsuka K: Protective effect of a molecular chaperone inducer, paeoniflorin, on the HCl- and ethanol-triggered gastric mucosal injury. Life Sci 2011;88:350–357 [DOI] [PubMed] [Google Scholar]
- 12.Ngan LT, Moon JK, Shibamoto T, Ahn YJ: Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J Agric Food Chem 2012;60:9062–9073 [DOI] [PubMed] [Google Scholar]
- 13.Lee YN: New Flora of Korea I. Kyohaksa Press, Seoul, 2006, pp. 394–395 [Google Scholar]
- 14.Bae JY, Ahn M-J, Park JH: Pharmacognostical studies on the ‘SanJagYak’. Korean J Pharmacog 2010;41:6–8 [Google Scholar]
- 15.Bae J-Y, Rhee Y-S, Han SY, et al. : Comparison between water and ethanol extracts of Rumex acetosa for protective effects on gastric ulcers in mice. Biomol Ther 2012;20:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu SH, Luo XD, Ma YB, Hao XJ, Wu DG: A new monoterpene glycoside from Paeonia veitchii. Chin Chem Lett 2002;5:430–431 [Google Scholar]
- 17.Pedernera AM, Guardia T, Calderón CG, Rotelli AE, de la Rocha NE, Genaro SD, Pelzer LE: Anti-ulcerogenic and anti-inflammatory activity of the methanolic extract of Larrea divaricata Cav. in rat. J Ethnopharmacol 2006;105:415–420 [DOI] [PubMed] [Google Scholar]
- 18.Liu WJH: Introduction to traditional herbal medicines and their study. In: Traditional Herbal Medicine Research Methods (Liu WJH, ed.). John Wiley & Sons, Singapore, 2011, pp. 7–20 [Google Scholar]
- 19.Kang J, Choi MY, Kang S, et al. : Application of a 1H nuclear magnetic resonance (NMR) metabolomics approach combined with orthogonal projections to latent structure-discriminant analysis as an efficient tool for discriminating between Korean and Chinese herbal medicines. J Agric Food Chem 2008;56:11589–11595 [DOI] [PubMed] [Google Scholar]
- 20.Bang MH, Song JC, Lee SY, Park NK, Baek NI: Isolation and structure determination of antioxidants from the root of Paeonia lactiflora. J Korean Soc Agric Chem Biotechnol 1999;42:170–175 [Google Scholar]
- 21.Xu S, Yang L, Tian R, et al. : Species differentiation and quality assessment of Radix Paeoniae Rubra (Chi-shao) by means of high-performance liquid chromatographic fingerprint. J Chromatogr A 2009;1216:2163–2168 [DOI] [PubMed] [Google Scholar]