Abstract
Previous efforts in the prospective evaluation of individuals who experience attenuated psychotic symptoms have attempted to isolate mechanisms underlying the onset of full-threshold psychotic illness. In contrast, there has been little research investigating specific predictors of positive outcomes. In this study, we sought to determine biological and clinical factors associated with treatment response, here indexed by functional improvement in a pre–post examination of a 12-week randomized controlled intervention in individuals at ultra-high risk (UHR) for psychosis. Participants received either long-chain omega-3 (ω-3) polyunsaturated fatty acids (PUFAs) or placebo. To allow the determination of factors specifically relevant to each intervention, and to be able to contrast them, both treatment groups were investigated in parallel. Univariate linear regression analysis indicated that higher levels of erythrocyte membrane α-linolenic acid (ALA; the parent fatty acid of the ω-3 family) and more severe negative symptoms at baseline predicted subsequent functional improvement in the treatment group, whereas less severe positive symptoms and lower functioning at baseline were predictive in the placebo group. A multivariate machine learning analysis, known as Gaussian Process Classification (GPC), confirmed that baseline fatty acids predicted response to treatment in the ω-3 PUFA group with high levels of sensitivity, specificity and accuracy. In addition, GPC revealed that baseline fatty acids were predictive in the placebo group. In conclusion, our investigation indicates that UHR patients with higher levels of ALA may specifically benefit from ω-3 PUFA supplementation. In addition, multivariate machine learning analysis suggests that fatty acids could potentially be used to inform prognostic evaluations and treatment decisions at the level of the individual. Notably, multiple statistical analyses were conducted in a relatively small sample, limiting the conclusions that can be drawn from what we believe to be a first-of-its-kind study. Additional studies with larger samples are therefore needed to evaluate the generalizability of these findings.
Introduction
Long-chain omega-3 (ω-3) polyunsaturated fatty acids (PUFAs) have distinct and important bioactive properties compared with the other groups of fatty acids.1, 2 They reduce many risk factors associated with disease, such as cardiovascular diseases,3 diabetes4 and some cancers.5 Omega-3 PUFAs are also of interest in mental health, given their selective concentrations in synaptic neuronal membranes,2 their regulatory role in the vascular and immune functions affecting the central nervous system,2, 6, 7 as well as evidence of the risks of insufficient intakes.8, 9
Evidence from controlled clinical trials suggests that ω-3 PUFAs can have positive effects in a range of psychiatric conditions,10 including schizophrenia,11, 12, 13 and may be particularly effective in the onset phase of psychosis. A 12-week supplementation with long-chain ω-3 PUFAs (fish oil) significantly reduced the risk of progression from an at-risk state to a psychotic disorder during a 12-month period (5% for ω-3 PUFAs vs 28% for placebo), and led to significant symptomatic and functional improvements in young people experiencing subthreshold psychotic symptoms.14 For those in the active treatment group, pretreatment vs posttreatment change in the erythrocyte membrane fatty acid ω-6 to ω-3 ratio correlated with functional improvement at 12 weeks, and lower levels of nervonic acid, a long-chain monounsaturated ω-9 fatty acid important in the biosynthesis of myelin, appeared to be a possible biomarker of risk of transition to psychosis.15 However, while these results are encouraging, it is unclear whether there are specific predictive factors associated with clinical improvement in patients at ultra-high risk (UHR) for psychosis who receive fish oil.
The UHR criteria for psychosis aim to prospectively identify individuals who are prodromal for schizophrenia or other psychotic disorders.16 A recent meta-analysis of UHR studies reported the rate of onset of a psychotic disorder in this group to be 36% after 3 years.17 There are a number of factors that have been associated with transition to psychosis in UHR individuals, including age, severity of negative symptoms, subthreshold positive symptoms, low psychosocial functioning, duration of symptoms and illicit substance use.18, 19, 20, 21 These factors can help refine diagnostic criteria to improve our ability to identify those who are most at risk. However, studies in this field of research have typically focused on adverse outcomes; in particular, transition to psychosis or poor functional outcome.17, 22 Psychosocial function is an important longitudinal outcome in individuals at UHR and is independent of the presence of psychotic symptoms or transition to psychosis.21, 22 It is unclear whether there are predictors in UHR individuals that are specifically associated with positive outcomes (that is, functional improvement). To address this knowledge gap, we carried out a secondary analysis of our randomized controlled trial.14 The present study sought to determine if erythrocyte membrane fatty acids and/or clinical factors at baseline predicted functional improvement in young UHR individuals who did and did not receive 12-week supplementation with ω-3 PUFAs.
Materials and methods
Sample
The present sample comprised 81 UHR individuals (27 males, 54 females; mean age=16.4, s.d.=2.1 years) who were recruited as part of a double-blind randomized controlled trial of ω-3 PUFAs vs placebo. This sample is described in detail elsewhere.14 In brief, individuals were aged 13–25 years and met the inclusion criteria proposed by Yung et al.16 for one or more of the three operationally defined groups of risk factors for psychosis: attenuated positive psychotic symptoms (group 1); transient psychosis (group 2); and genetic risk plus a significant decrease in functioning (group 3). Study exclusion criteria were a history of a previous psychotic disorder or manic episode (both treated or untreated), substance-induced psychotic disorder, acute suicidal or aggressive behavior, a current DSM-IV diagnosis of substance dependence (except cannabis dependence), any neurological disorder, IQ<70, structural brain changes apparent on magnetic resonance imaging, previous treatment with an antipsychotic or mood-stabilizing agent (>1 week), having taken ω-3 supplements within 8 weeks of being included in the trial, laboratory values >10% outside the normal range for transaminases, thyroid hormones, C-reactive protein or bleeding parameters, and any severe intercurrent illness that may have put the person at risk or influenced the results of the trial or affected their ability to take part in the trial.
All patients were consecutive admissions to a specialized psychosis detection and treatment unit at the Department of Child and Adolescent Psychiatry, Medical University Vienna, between May 2004 and May 2006. The study was approved by the local ethics committee. All participants provided written informed consent, including parental consent for those less than 18 years of age. Patients were assessed within 1 week of their initial presentation, and all UHR individuals were free of antipsychotic medication.
Assessments
The Global Assessment of Functioning (GAF) score was used as the measure of functioning. The GAF is an analog scale (0 through 100) to subjectively rate the social, occupational and psychological functioning of individuals, for example, how well or adaptively one is meeting various problems in living.23 The Positive and Negative Syndrome Scale (PANSS) 24 was used to assess positive symptoms, negative symptoms and general psychiatric symptoms. The Montgomery-Asberg Depression Rating Scale25 was used to examine depressive symptoms. Raters were experienced clinicians who were trained in the administration of these instruments. Inter-rater reliability estimates for all study instruments were excellent (all intra-class correlation coefficients >0.92). After randomization, participants received weekly assessments for 4 weeks, and then at 8 and 12 weeks (end of intervention) and subsequent follow-up at 6 and 12 months. For further detailed information on UHR criteria, randomization, blinding and study measures, see Amminger et al.14
Analysis of erythrocyte membrane fatty acid composition
The erythrocyte fatty acid composition of the phosphatidylethanolamine phospholipid fraction was quantified using capillary gas chromatography. Phosphatidylethanolamine is the most common phospholipid on the inner side of cell membranes in the brain. Values for the following classes of fatty acids were obtained: saturated fatty acids (14:0, 16:0, 17:0, 18:0), monounsaturated fatty acids (18:1ω-9, 20:1ω-9, 20:3ω-9, 22:1ω-9, 24:1ω-9), trans fatty acids (18:1ω-7tr, 18:1ω-9tr) and PUFAs (18:2ω-6, 18:3ω-6, 20:3ω-6, 20:4ω-6, 22:2ω-6, 22:4ω-6, 18:3ω-3, 20:5ω-3, 22:5ω-3, 22:6ω-3). A detailed description of the laboratory methods is provided elsewhere.26 More specifically, the current study investigated ω-3 PUFAs including α-linolenic acid (ALA, 18:2ω-3), eicosapentaenoic acid (EPA, 20:5ω-3), docosapentaenoic acid (DPA, 22:5ω-3) and docosahexaenoic acid (DHA, 22:6ω-3) due to their relevance to schizophrenia6, 27 and psychosis9 and the nature of the intervention in this study. In addition, we also examined key ω-6 (linoleic acid, LA, 18:2ω-6; arachidonic acid, AA, 20:4ω-6) and ω-9 (nervonic acid, NA, 24:1ω-9) fatty acids that have been consistently reported to be altered in people with schizophrenia.28, 29, 30, 31
Treatment
The active treatment was a supplement of yellow gelatin 0.5 g capsules containing concentrated marine fish oil. The daily dose of four capsules provided 700 mg of eicosapentaenoic acid (EPA, 20:5ω-3), 480 mg of docosahexaenoic acid (DHA, 22:6ω-3) and 7.6 mg of vitamin E. The daily amount of other ω-3 fatty acids (18:3ω-3, 18:4ω-3, 20:4ω-3, 21:5ω-3, 22:5ω-3) provided with the study medication was 220 mg. The daily dose of ~1.2 g ω-3 PUFAs was based on trials in schizophrenia31, 32 and first-episode psychosis.33 Coconut oil was chosen as placebo because it does not contain polyunsaturated fatty acids and has no impact on ω-3 fatty acid metabolism. Placebo capsules were carefully matched in appearance and flavor with the active treatment; they also contained the same amount of vitamin E as the ω-3 capsules, and 1% fish oil to mimic taste. Adherence to the study medication was monitored by pill count, self-report and erythrocyte fatty acid quantification. Antipsychotic medication or mood stabilizers were not permitted. Patients could receive antidepressants for moderate-to-severe depression (as indicated by a Montgomery-Asberg Depression Rating Scale score of ⩾21) and benzodiazepines for anxiety, agitation and/or insomnia. Existing prescriptions of psychiatric medications were revaluated at baseline and continued if clinically indicated.
In addition to active/placebo treatment, all the patients were offered nine sessions of needs-based psychological and psychosocial interventions concurrently with the research follow-up interviews. These interventions focused on presenting symptoms and pertinent issues, such as social relationships and vocational and family issues. Additional appointments for crisis management were offered to clients in accordance with the original PACE (Personal Assistance and Crisis Evaluation) Clinic concept.34 Treating clinicians also undertook a case management role providing assistance with accommodation, education or employment and family education and support.
Data analysis
The main objective of analysis was to determine predictors of pretreatment (baseline) vs posttreatment (12–week) change in GAF score in UHR patients who received ω-3 PUFAs or placebo. To eliminate treatment effects and to allow the determination of factors specifically relevant to ω-3 supplementation, both treatment groups were investigated separately. For individuals with missing data at the 12-week follow-up, the GAF change score was calculated using last-observation-carried-forward approach. The last-observation-carried-forward approach has low risk of bias in studies with low drop-out rates. In the present study, 93% (37/40) of the ω-3 PUFA group and 95% (38/40) of the placebo group completed the intervention in accordance with the study protocol. A second type of missing data occurred in this trial following transition to psychosis. Treatment with antipsychotic medication was commenced in participants who made the transition to psychosis, and no further data were collected after transition (that is, for nine individuals). The outcome of interest—the values that participants would have had if active treatment for psychosis had not been initiated and the intervention and observation had continued—is thus effectively counterfactual. In these circumstances, missingness is not random and must be explicitly modeled.35 A conservative approach was taken to model posttransition outcomes, as previously described (Amminger et al.,14 p.149). It was assumed that functioning would have been maintained at the transition levels if antipsychotic medications had not been administered, but would not have further deteriorated.
Univariate analysis
Linear regression models were used to examine the relationship between a baseline variable and change in GAF score from baseline to the end of intervention (the dependent variable). Fatty acids relevant to schizophrenia (that is, ALA, EPA, DPA, DHA, LA, AA, NA)27, 28, 29, 30 and factors previously shown to predict transition to psychosis (that is, age, negative symptoms, positive symptoms, psychosocial functioning, duration of symptoms, illicit substance use)18, 19, 20, 21 were evaluated as independent variables. Predictors with P-values <0.1 were then entered in a final prediction model. A sensitivity analysis was performed including only those individuals who completed the intervention and made no transition to psychosis (ω-3 group, n=36; placebo group, n=31). These tests were carried out with the statistical package SPSS (version 20.0, Chicago, IL, USA).
Categorical analysis
Functional improvement was also investigated as a categorical variable, using a ⩾15 point increase in GAF score as the cutoff to indicate treatment response. This cutoff was based on the group median for the baseline-to-12-week GAF score changes observed in the ω-3 PUFA group. For comparisons of categorical variables, we calculated χ2 statistics, applying Fisher's exact test when cell sizes were small. Independent-samples t-tests were used to compare group differences in baseline-to-12-week GAF score changes. Cohen's d was calculated to estimate the effect sizes of statistically significant group differences. A significance level of 0.05 (two-tailed) was used for all statistical tests. These tests were also carried out with the statistical package SPSS (version 20.0).
Multivariate analysis
To complement the univariate analysis, we conducted a multivariate analysis using a supervised machine learning technique known as Gaussian Process Classification (GPC) as implemented in the Pattern Recognition for Neuroimaging Toolbox (PRoNTo; http://www.mlnl.cs.ucl.ac.uk/pronto/). GPC is a Bayesian extension of standard logistic regression that allows the classification of individual observations into distinct groups using the rules of probability.36 This technique comprises a ‘training' phase, in which well-characterized training data are used to develop an algorithm, which captures the key differences between groups, and a ‘testing' phase in which the algorithm is used to predict the group to which a new observation belongs to. A ‘leave-one-out' cross-validation method is used to estimate the accuracy, sensitivity and specificity of the algorithm, providing an indication of its generalizability. The statistical significance of the accuracy can be determined by permutation testing. A significance level of 0.05 (two-tailed) was used for all statistical tests. Except for the GPC analysis, tests were carried out with the statistical package SPSS (version 20.0).
Results
Of the 81 patients who were enrolled in the study, 80 (98.8%) provided blood samples at baseline. Of those, 40 received ω-3 PUFAs and 40 received placebo for 12 weeks. Table 1 shows the distribution of demographic and illness characteristics in these treatment groups. No statistically significant differences in these variables were observed between the groups. By the end of the intervention (12 weeks), one (2.5%) individual from the ω-3 PUFA group and eight (20.0%) individuals from the placebo group made a transition to a threshold psychotic disorder diagnosis (Fisher's exact test: P=0.029). The mean change from baseline to end point in GAF score was significantly different between the groups (ω-3 PUFA: M=13.50, s.d.=11.56; placebo: M=7.50, s.d.=13.63) (t=−2.12, df=78, P=0.037).
Table 1. Baseline characteristics by treatment group.
| Characteristic and measure |
Omega-3 group (N=40) |
Placebo group (N=40) |
Difference between groups |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | r | P | |
| Sex (male) | 14 | 35.0 | 12 | 30.0 | 0.23 | 0.63 |
| Substance abuse (lifetime) | 12 | 30.0 | 16 | 40.0 | 0.88 | 0.35 |
| |
Mean |
s.d. |
Mean |
s.d. |
t (df=78) |
P |
| Age (years) | 16.90 | 2.42 | 16.07 | 1.68 | −1.78 | 0.08 |
| Duration of symptoms (months) | 12.40 | 9.95 | 12.90 | 11.51 | −0.21 | 0.84 |
| PANSS | ||||||
| Positive symptoms | 15.03 | 3.41 | 14.10 | 3.06 | −1.28 | 0.21 |
| Negative symptoms | 14.08 | 5.39 | 13.45 | 6.42 | −0.47 | 0.64 |
| GAF score | 61.00 | 12.10 | 59.88 | 13.08 | −0.40 | 0.69 |
| Erythrocyte fatty acids, % of total | ||||||
| α-Linolenic acid | 0.19 | 0.05 | 0.20 | 0.06 | 0.81 | 0.42 |
| Eicosapentaenoic acid | 0.49 | 0.18 | 0.47 | 0.14 | −0.72 | 0.47 |
| Docosapentaenoic acid | 2.20 | 0.44 | 2.16 | 0.41 | −0.44 | 0.66 |
| Docoshexaenoic acid | 2.77 | 0.82 | 2.49 | 0.63 | −1.73 | 0.09 |
| Linoleic acid | 6.16 | 0.76 | 6.36 | 1.65 | 0.70 | 0.48 |
| Arachidonic acid | 15.79 | 2.22 | 15.25 | 1.94 | −1.16 | 0.25 |
Abbreviations: GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale.
Univariate analysis
Linear regression analyses were undertaken with age, illicit substance use, PANSS scores (that is, positive, negative), duration of symptoms, GAF score and PUFAs (ALA, EPA, DPA, DHA, LA, AA, NA) as independent factors and change in GAF score pre–post intervention as the dependent variable. Within the ω-3 group, higher scores on PANSS negative symptoms (P=0.034) and higher levels of erythrocyte membrane ALA (P=0.019) at baseline predicted functional improvement. In the placebo group, lower PANSS positive scores (P<0.001) and lower GAF scores at baseline (P=0.007) were predictive of functional improvement. In the final regression models for the treatment groups (Table 2), ALA and negative symptoms explained 14% and 10% of the variance of functional change in the ω-3 PUFA group, respectively, whereas positive symptoms and functioning explained 23% and 11% of the variance in the placebo group, respectively. The results of the sensitivity analysis, including participants without a transition to psychosis who completed the intervention (ω-3 group, n=36; placebo group, n=31), were consistent with the primary analysis (ω-3 group: negative symptoms, P=0.004; ALA, P=0.047) (placebo group: positive symptoms, P<0.001; GAF score, P=0.006).
Table 2. Final regression of baseline predictors of functional improvement (change in GAF score) in individuals at ultra-high risk of psychosis, by treatment group.
| Coefficients | B | Standard error | Beta | T | Significance |
|---|---|---|---|---|---|
| Omega-3 group | |||||
| ALA | 75.8 | 30.8 | 0.35 | 2.46 | 0.019 |
| Negative symptoms (PANSS) | 0.68 | 0.31 | 0.32 | 2.20 | 0.034 |
| Placebo group | |||||
| Positive symptoms (PANSS) | −2.90 | 0.68 | −0.65 | −4.28 | <0.001 |
| GAF score | −39 | 0.16 | −0.37 | −2.44 | 0.020 |
Abbreviations: ALA, alpha-linolenic acid; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale.
Concomitant medication use after randomization was low; that is, antidepressants in 5 of 40 (12.2%) patients in the ω-3 group and 3 of 40 (7.5%) patients in the placebo group, and benzodiazepines in 2 of 40 (4.9%) patients in the ω-3 group and 1 of 40 (2.5%) patients in the placebo group. No statistically significant association was observed for receiving a concomitant medication and functional improvement.
When the GAF score was dichotomized using a ⩾15 point increase as the cutoff to indicate treatment response, 55.0% (22 of 40) of individuals in the ω-3 group and 30.0% (12 of 40) of individuals in the placebo group achieved adequate functional improvement (Pearson's χ2: r=5.115, df=1, P=0.024). In the ω-3 group, the treatment response was associated with significantly higher scores of negative symptoms on the PANSS and significantly higher levels of erythrocyte membrane ALA (Table 3). The effect size for the mean difference was large for ALA (Cohen's d=1.1) and medium for negative symptoms (Cohen's d=0.7). In the placebo group, the treatment response was associated with a significantly lower PANSS positive symptom score at baseline (Table 4). The effect size for the mean difference in positive symptoms was also large (Cohen's d=0.9).
Table 3. Individuals at ultra-high risk of psychosis who received omega-3 fatty acids, by treatment response.
| Characteristic and measure | Respondera (N=22) | Non-responder (N=18) | Difference between groups | |||
|---|---|---|---|---|---|---|
| |
N |
% |
N |
% |
r |
P |
| Substance abuse (lifetime) | 8 | 36.4 | 4 | 22.2 | 0.94 | 0.33 |
| |
Mean |
s.d. |
Mean |
s.d. |
t (df=38) |
P |
| Age (years) | 17.40 | 2.94 | 16.03 | 1.43 | −1.44 | 0.16 |
| Duration of symptoms (months) | 13.23 | 10.29 | 11.39 | 9.71 | −0.58 | 0.57 |
| PANSS | ||||||
| Positive symptoms | 15.18 | 3.72 | 14.83 | 3.07 | −0.32 | 0.75 |
| Negative symptoms | 15.59 | 5.83 | 12.22 | 4.25 | −2.05 | 0.048 |
| GAF score | 58.64 | 9.54 | 63.89 | 14.01 | 1.38 | 0.18 |
| Erythrocyte fatty acids, % of total | ||||||
| α-Linolenic acid | 0.21 | 0.06 | 0.16 | 0.03 | −3.78 | 0.001 |
| Eicosapentaenoic acid | 0.49 | 0.19 | 0.49 | 0.16 | −0.004 | 1.00 |
| Docosapentaenoic acid | 2.14 | 0.36 | 2.27 | 0.52 | 0.89 | 0.38 |
| Docoshexaenoic acid | 2.64 | 0.68 | 2.92 | 0.96 | 1.07 | 0.29 |
| Linoleic acid | 6.32 | 0.64 | 5.97 | 0.86 | −1.46 | 0.15 |
| Arachidonic acid | 15.42 | 1.98 | 16.24 | 2.47 | 1.16 | 0.25 |
Abbreviations: GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale.
⩾15 point increase on the Global Assessment of Functioning scale was used to indicate response to treatment.
Table 4. Individuals at ultra-high risk of psychosis who received placebo, by treatment response.
| Characteristic and measure |
Respondera
(N=12) |
Non-responder (N=28) |
Difference between groups |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | r | P | |
| Substance abuse (lifetime) | 4 | 33.3 | 12 | 42.9 | 0.32 | 0.57 |
| |
Mean |
s.d. |
Mean |
s.d. |
t (df=38) |
P |
| Age (years) | 16.69 | 2.11 | 15.81 | 1.42 | −1.54 | 0.13 |
| Duration of symptoms (months) | 11.33 | 13.57 | 13.57 | 10.72 | 0.56 | 0.58 |
| PANSS | ||||||
| Positive symptoms | 12.33 | 2.42 | 14.86 | 3.03 | 2.55 | 0.02 |
| Negative symptoms | 13.17 | 6.71 | 13.57 | 6.41 | 0.18 | 0.86 |
| GAF score | 56.67 | 11.93 | 61.25 | 13.51 | 1.02 | 0.32 |
| Erythrocyte fatty acids, % of total | ||||||
| α-Linolenic acid | 0.20 | 0.06 | 0.20 | 0.07 | 0.19 | 0.85 |
| Eicosapentaenoic acid | 0.49 | 0.18 | 0.46 | 0.13 | −0.50 | 0.62 |
| Docosapentaenoic acid | 2.27 | 0.42 | 2.11 | 0.41 | −1.13 | 0.27 |
| Docoshexaenoic acid | 2.64 | 0.72 | 2.42 | 0.59 | −1.02 | 0.32 |
| Linoleic acid | 6.30 | 0.96 | 6.39 | 1.88 | 0.16 | 0.87 |
| Arachidonic acid | 15.56 | 0.93 | 15.11 | 2.24 | −0.91 | 0.37 |
Abbreviations: GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale.
⩾15 point increase on the Global Assessment of Functioning scale was used to indicate response to treatment.
Multivariate analysis
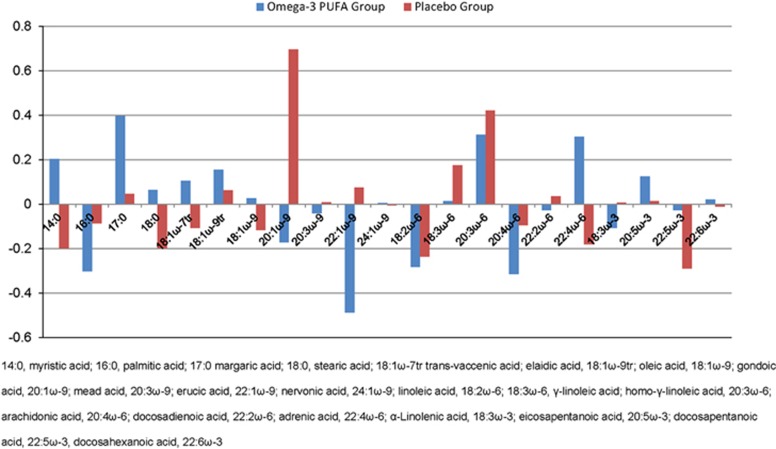
Results indicated that fatty acids significantly predicted response to treatment (⩾15 point increase in GAF scores) in the ω-3 PUFA group, with a sensitivity of 86.7%, a specificity of 86.7% and an overall accuracy of 86.7% (P<0.001). In addition, fatty acids significantly predicted response to treatment (⩾15 point increase of GAF scores) in the placebo group, with a sensitivity of 83.3%, a specificity of 75.0% and an overall accuracy of 79.2% (P<0.001). The relative contributions of the fatty acids to the above predictions are displayed in Figure 1. It can be seen that the contribution of the different fatty acids for predicting GAF change appears to vary between the two groups, with some fatty acids being more predictive in the ω-3 PUFA group and others being more predictive in the placebo group. This pattern of results suggests that some fatty acids may be predictive of response to ω-3 treatment specifically, whereas others may be predictive of clinical outcome in general. However, it should be noted that GPC is a multivariate approach and as such it does not allow one to make statistical inferences about the contribution of individual fatty acids. In other words, all fatty acids informed prediction in both groups to a greater or lesser extent, and the information on the differential contribution of different fatty acids provided in Figure 1 should be considered as descriptive only.
Figure 1.
Relative contributions of fatty acids to predict treatment response in Gaussian Process Classification by treatment group. PUFA, polyunsaturated fatty acid.
Discussion
To the best of our knowledge, this is the first study to investigate predictive factors associated with clinical and functional improvement in UHR patients who were treated with ω-3 PUFAs or placebo in addition to standard clinical care without antipsychotic medication. A noteworthy finding of this study is that higher baseline levels of ALA predicted treatment response in the ω-3 PUFA group. ALA is the shortest chain fatty acid in the ω-3 family, and this result is consistent with the clinical observation that ω-3 PUFAs were found effective in preventing transition to psychosis.14 Although the past 20 years has seen an increasing interest and remarkable knowledge gain regarding the roles of EPA and DHA, the importance of ALA is still not fully elucidated.37 A particular consideration is whether ALA has an essential physiological role in its own right, or whether its major function is to serve as a precursor of long-chain ω-3 PUFA synthesis (that is, EPA, DPA, DHA). Currently, ALA is only known to have a crucial metabolic function as a precursor.
In humans, the metabolic conversion of ALA to long-chain ω-3 PUFAs, in particular DHA, is very limited and highly variable, at least under physiological conditions.38, 39 However, in situations where the demand for long-chain ω-3 PUFAs is increased (for example, during pregnancy and lactation), an upregulation of the conversion from ALA to EPA and DHA may help to compensate for this additional need.40 In schizophrenia, pathological mechanisms, such as high oxidative stress41 or a problem in the synthesis of long-chain fatty acids (for example, a peroxisomal dysfunction or enzyme defect),42 contribute to the depletion of EPA and DHA in cell membranes. This depletion could stimulate physiological mechanisms, as is the case in pregnant women, to upregulate the conversion rate of ALA to long-chain ω-3 PUFAs. The view that ALA conversion is upregulated to compensate for the loss of, or an increased need for, long-chain ω-3 PUFAs in UHR patients is supported by a comparison of the present study sample to 142 healthy adolescents43 (Amminger et al., in preparation). This comparison showed that UHR individuals have highly significant deficits for erythrocyte membrane ALA (Cohen's d=−1.41);43 but not for DPA or DHA. These observations are consistent with the notion that an upregulation of ALA conversion may compensate for the loss of long-chain ω-3 PUFAs during the at-risk/prodromal phase (that is, resulting in low cell membrane ALA levels). Accordingly, higher levels of ALA in UHR patients could indicate a pathophysiological stage of illness where a person in the onset phase of psychosis still has capacity to compensate for the loss of long-chain ω-3 PUFAs, which may explain why higher ALA levels in the current study were associated with better outcome following EPA and DHA supplementation.
An important finding from the multivariate machine learning analysis of this study is that fatty acids predicted response to treatment in both treatment groups with high levels of sensitivity, specificity and accuracy. Notably, the observed values for the accuracy, that is, 87% in the ω-3 PUFA group and 79% in the placebo group, were higher than those typically found in neuroimaging studies of UHR individuals, which are in the 65–80% range.44 The GPC analysis, therefore, corroborates the result of the univariate analysis by showing that fatty acids predicted response to treatment in the ω-3 PUFA group at the level of the individual. In addition, the GPC analysis extends the results of the univariate analysis by showing that fatty acids also predicted treatment response in the placebo group. The apparent inconsistency between the lack of significant association found in the univariate analysis and the high levels of sensitivity, specificity and accuracy found in the multivariate analysis, can be explained by the fact that the latter takes the covariation between variables into account in addition to their individual values. For example, it could be that individual fatty acids have limited predictive power when considered separately in the context of a univariate analysis, but have good predictive power when considered jointly in the context of a multivariate analysis. Overall, this pattern of results suggests that fatty acids could potentially be used to inform prognostic evaluations and treatment decisions at the level of the individual.
An interesting finding of this study is that more severe baseline negative symptoms were associated with treatment response in the ω-3 supplemented group. We have recently examined the relationship between ω-3 PUFA (that is, ALA, EPA, DPA, DHA) levels in erythrocyte membranes and measures of psychopathology (PANSS positive, negative and general symptoms) in this UHR cohort at baseline of the current trial. ALA, EPA and DHA did not correlate with any symptom measure, whereas low levels of DPA correlated with more severe negative symptoms.45 Therefore, the present finding on negative symptoms is consistent with the view that ω-3 PUFA metabolism is relevant to the negative symptom syndrome of schizophrenia.46 This is also concordant with the notion that oxidative damage to lipids is germane to the process of neuroprogression and the expression of negative symptoms.47 However, it is also possible that in addition to the evidence showing that negative symptoms and lipid biology may be related, regression to the mean may be occurring here, since those with severe negative symptoms at the start may also be those who initially have poor functioning. Regression to the mean may also contribute to the findings for positive symptoms and GAF in the placebo group.
The findings of this study must be interpreted in view of its limitations; most importantly, the relatively small sample size. Small sample sizes can lead to spurious findings of significant results, and replication in larger samples is required. Furthermore, the number of variables in the regression analysis, although they were empirically selected, was relatively high, which increases the chance of type I errors. However, the large effect size of the mean difference for ALA in responders and non-responders in ω-3 PUFA-treated individuals provides assurance of the validity of the finding. The validity of the finding (that is, that ALA predicted treatment response) in the ω-3 group is also supported by the nature of the intervention. As mentioned above, a limitation of GPC is that it does not allow one to make statistical inferences about the contribution of individual fatty acids. Because all fatty acids were included in this multivariate analysis, prediction of clinical response in both the groups was informed by all of them to a greater or lesser extent. Finally, the use of multiple comparisons in post hoc analyses requires the findings to be seen as exploratory rather than definitive. Strengths of the study include the application of standardized assessment instruments; that all assessments were conducted by experienced raters with high inter-rater reliability; the study sample was drawn from a representative cohort of UHR individuals assembled from a front-line public psychiatric service with a geographically defined catchment area; and the robustness of key findings in the sensitivity analysis.
Clinical characteristics alone are of limited predictive value and there is a pressing need for further enhancement of predictive models. Biological predictors of the course of illness and of response to treatments are of enormous clinical value. In conjunction with benign neuroprotective interventions, such predictors have the potential to facilitate early targeted treatment of biomarker-positive UHR individuals. In conclusion, the findings in this study highlight the importance of lipid biology for the treatment of subthreshold, attenuated forms of psychosis. Additional studies with larger samples are needed to evaluate the generalizability of these findings.
Acknowledgments
This work was supported by grants from the Stanley Medical Research Institute (03T-315, 07TGF-1102). GPA is supported by a NHMRC Senior Research Fellowship 1080963. MB is supported by a NHMRC Senior Principal Research Fellowship 1059660. PDM is supported by a NHMRC Senior Research Fellowship 1060996.
The authors declare no conflict of interest.
References
- Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med. 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30:193–208. doi: 10.1016/s0920-9964(97)00151-5. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hedelin M, Löf M, Olsson M, Lewander T, Nilsson B, Hultman CM, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;26:38. doi: 10.1186/1471-244X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Berger GE, Smesny S, Amminger GP. Bioactive lipids in schizophrenia. Int Rev Psychiatry. 2006;18:85–98. doi: 10.1080/09540260600583072. [DOI] [PubMed] [Google Scholar]
- Peet M. Omega-3 polyunsaturated fatty acids in the treatment of schizophrenia. Isr J Psychiatry Relat Sci. 2008;45:19–25. [PubMed] [Google Scholar]
- Fusar-Poli P, Berger G. Eicosapentaenoic acid interventions in schizophrenia. J Clin Psychopharmacol. 2012;32:179–185. doi: 10.1097/JCP.0b013e318248b7bb. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schäfer MR, Klier CM, Slavik JM, Holzer I, Holub M, et al. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol Psychiatry. 2012;17:1150–1152. doi: 10.1038/mp.2011.167. [DOI] [PubMed] [Google Scholar]
- Yung A, Phillips L, McGorry P, McFarlane C, Francey S, Harrigan S, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Yung A, Phillips L, Yuen H, McGorry P. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Leicester S, Yung AR, Phillips LJ, Berger GE, Francey SM, et al. Early-onset of symptoms predicts conversion to non-affective psychosis in ultra-high risk individuals. Schizophr Res. 2006;84:67–76. doi: 10.1016/j.schres.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high risk ("prodromal") for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders4th edn. American Psychiatric Association: Washington, DC, USA; 1994 [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Smesny S, Milleit B, Hipler UC, Milleit C, Schäfer MR, Klier CM, et al. Omega-3 fatty acid supplementation changes intracellular phospholipase A2 activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol Psychiatry. 2014;19:317–324. doi: 10.1038/mp.2013.7. [DOI] [PubMed] [Google Scholar]
- Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L, et al. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH, et al. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–522. doi: 10.1016/s0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- Messamore E, Hoffman WF, Yao JK. Niacin sensitivity and the arachidonic acid pathway in schizophrenia. Schizophr Res. 2010;122:248–256. doi: 10.1016/j.schres.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M, Horrobin DF, E-E Multicentre Study Group A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD, Francey SM, Nelson B, Baker K, Phillips LJ, et al. PACE: a specialised service for young people at risk of psychotic disorders. Med J Australia. 2007;187:S43–S46. doi: 10.5694/j.1326-5377.2007.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- Marquand A, Howard M, Brammer M, Chu C, Coen S, Mourão-Miranda J, et al. Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage. 2010;49:2178–2189. doi: 10.1016/j.neuroimage.2009.10.072. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Attar-Bashi NM, Li D. What is the role of alpha-linolenic acid for mammals. Lipids. 2002;37:1113–1123. doi: 10.1007/s11745-002-1008-x. [DOI] [PubMed] [Google Scholar]
- Brenna JT, Salem N, Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids, ISSFAL. Alpha-linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR, et al. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12:142–154. doi: 10.1111/j.1399-5618.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.Restoring the DHA levels in the brains of Zellweger patients J Mol Neurosci 200116309–316.discussion 317–321. [DOI] [PubMed] [Google Scholar]
- Holub M.Der Einfluss von Fischöltherapie auf Vitamin E–Gehalt und Fettsäuremuster in der Erythrozytenmembran von psychiatrischen jugendlichen Patienten. Masters thesis. University Vienna, Austria2006
- Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Amminger GP, McGorry PD. Update on ω-3 polyunsaturated fatty acids in early-stage psychotic disorders. Neuropsychopharmacology. 2012;37:309–310. doi: 10.1038/npp.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethom MM, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, et al. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2010;83:131–136. doi: 10.1016/j.plefa.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]