Cancer predisposition genes (CPGs) describe genes in which germline mutations result in increased risk of cancer. Over 100 CPGs have already been discovered and transformative advances in DNA analysis are leading to many new CPG discoveries. These fast, affordable methods for analysing the DNA sequence can also be utilised in diagnostics to substantially increase clinical testing of CPGs. In turn, this has potential to provide substantial cost-effective health benefits with respect to cancer treatment in people with the disease and cancer prevention in healthy individuals. In this review, I outline the clinical benefits of testing for CPGs and how increased testing can be achieved.
Cancer predisposition genes
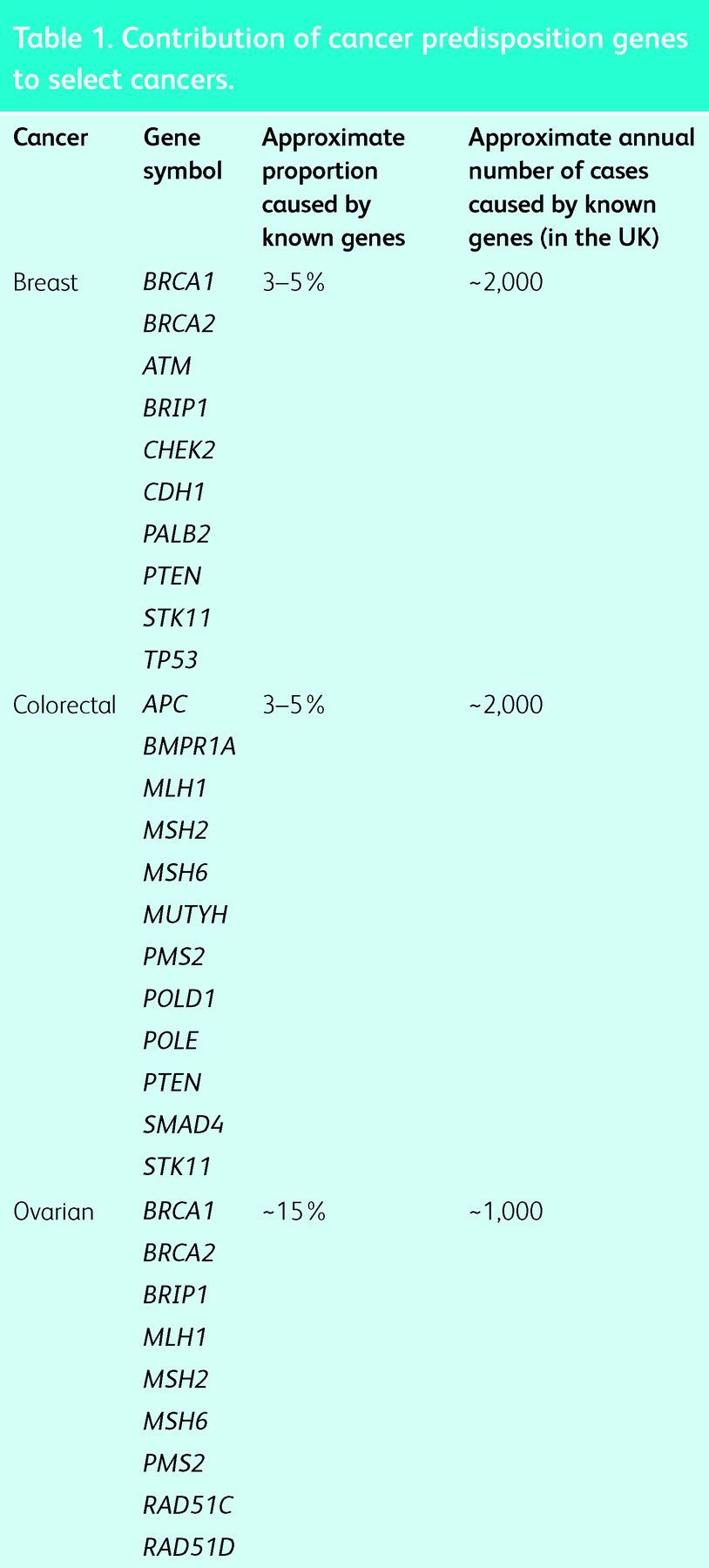
There are two ways in which gene mutations contribute to cancer. Oncogenic mutations that occur after birth, within a specific cell, are a hallmark of cancer and are called ‘somatic cancer mutations’.1 Mutations that are present in every cell, either because they have been inherited or occur at conception, are called ‘germline mutations’. Genes in which germline mutations lead to increased risks of cancer are called CPGs.2 It is currently estimated that, overall, approximately 3% of cancers are the result of germline mutations in CPGs. However, the contribution to individual cancers is variable. A high proportion of childhood embryonal cancers, such as retinoblastoma and pleuropulmonary blastoma, are caused by germline mutations in RB1 and DICER1, respectively.3,4 By contrast, CPG mutations make a small contribution to some adult cancers, such as prostate and lung cancer. However, germline CPG mutations in multiple genes predispose to other adult cancers, such as breast, colorectal and ovarian cancer (Table 1). For some cancers, the overall contribution of CPGs is sizeable, with approximately 15% of ovarian cancer, approximately 20% of medullary thyroid cancer and >30% of phaeochromocytoma resulting from CPG mutations.5–7
Table 1.
Contribution of cancer predisposition genes to select cancers.
Clinical utility of cancer predisposition genes
Identifying whether a cancer is the result of an underlying CPG mutation has significant impact for the cancer patient and, potentially, their relatives. As such, CPG testing has become standard for many genes, although typically only in highly selected cases. From the patient perspective, simply having a better understanding of why their cancer occurred is usually highly valued.
Cancer diagnosis and management
Identifying a CPG mutation provides important information that can aid diagnosis and management. The surgical management might be altered; for instance, more radical surgery might be appropriate in CPG mutation carriers, who are at increased risk of further cancer. Radiation treatment might be modified or excluded because some CPGs are associated with increased radiation sensitivity. Chemotherapy might be changed because some treatments are more effective and others less effective in CPG mutation carriers. For example, platinum-based therapies are not standard treatment for breast cancer but can have utility in BRCA1/2 carriers, who are particularly sensitive to platinum-based drugs.8,9 Newer, personalised therapies that either target the CPG mutation directly or pathways that become vulnerable because of the CPG mutation, are increasingly being developed. For example, some gastrointestinal tumours result from germline gain-of-function mutations in KIT or PDGFRA that could be inhibited by imantinib.10 Similarly, poly (ADP-ribose) polymerase (PARP) inhibitors that target DNA repair pathways that become vulnerable in women with BRCA1 or BRCA2 mutations are currently in phase III trials.11
Identifying an underlying CPG mutation can also provide prognostic information. For instance, survival is significantly better for patients with BRCA2 mutation-positive ovarian cancer, but significantly worse for patients with BRCA2 mutation-positive prostate cancer.12,13 The likelihood of recurrence, a new primary and/or a second malignancy can all be increased in CPG mutation carriers, who require ongoing review and consideration of tailored surveillance and/or risk-reducing interventions. Management of noncancer-associated problems can also be important; for example, certain WT1 mutations result in insidious renal dysfunction that requires monitoring and early intervention.
Cancer screening and prevention
CPGs are unusual because they can serve as a biomarker of future disease. Identifying a CPG mutation provides a window of opportunity to implement surveillance and/or risk-reducing measures that mitigate or prevent cancer. The type of screening is naturally determined by the type of cancer, but usually involves imaging to detect a lesion before it presents clinically. Prevention usually involves surgical removal of the at-risk tissue and is necessarily reserved for nonessential organs in individuals at very high-risk, such as the stomach in CDH1 mutation carriers, the thyroid in RET mutation carriers and the breast and ovaries in BRCA1 mutation carriers.14–16 Chemoprevention is an attractive strategy, but to date there have been few applications. A notable exception is individuals with increased risk of colorectal cancer in whom the cancer risk is significantly reduced by daily aspirin.17 Although it is often overlooked, there is high patient and economic value in using CPG mutation testing to identify relatives without the familial mutation. Such individuals are released from anxiety for themselves and their offspring, and do not require costly screening and interventions.
Cancer predisposition gene testing methods
DNA sequencing technology has evolved rapidly over recent years. Traditionally, gene testing relied on development of individual tests for each CPG using costly, time-consuming methods. Now it is possible to sequence multiple genes in parallel, faster and more affordably than a single gene test with traditional methods, using a technique known as next-generation sequencing (NGS).18 In turn, this means many more genes and many more people can have gene testing, and the approach is of particular value if the cancer predisposition is heterogeneous (ie multiple different genes predispose to the cancer). Gene panels of 5–100 CPGs are currently available. In time, it is likely that these will be superseded by whole-genome sequencing, which would also enable genetic information about other medical conditions or drug responses to be harvested.
Increasing cancer predisposition gene testing – an ‘oncogenetic’ model
The laborious, expensive nature of gene testing previously meant that: (i) strict eligibility criteria to limit testing were required, and (ii) testing infrastructure was developed primarily to serve the complex needs of unaffected individuals who had time to wait for the results. Faster, more affordable testing now enables eligibility criteria to be relaxed and for results to be delivered within the time frame required to impact cancer management. However, changes to the testing process are required to achieve this, particularly in relation to providing greater flexibility for cancer patients to access testing. In the UK, almost all CPG testing is currently performed through genetics services irrespective of cancer status, although gene testing in patients with some diseases is already performed by nongeneticists. An ‘oncogenetic’ model of CPG testing, whereby testing in patients with cancer can be performed through the cancer team, with support as required from genetics, is being piloted by several services, including the Mainstreaming Cancer Genetics programme (www.mcgprogramme.com). If the test is positive, the patient is automatically sent an appointment by the genetics service to address future issues for the patient and implications for the family. The cancer team use the information to tailor cancer management as appropriate. If the test is negative, it is still useful information for the cancer team, but typically does not require input from genetics, unless there is an extensive or complex family history. Any patient that requires more detailed discussions can be referred to genetics in the usual fashion. This model enables more streamlined, more flexible and more efficient testing for people who already have cancer. All gene tests in healthy individuals without cancer are undertaken by genetics services, because detailed discussions about whether and when to have the test are typically required (Fig 1).
Fig 1.
Proposed new pathways to deliver cancer predisposition gene testing.
Interpreting and utilising gene information
Gene variants and/or mutations occur frequently in our genomes and are usually innocuous. Distinguishing which are benign and which are pathogenic can be challenging even for genes that were discovered many years ago and have been extensively studied, such as BRCA1. Current gene test reports are often complex, provide unclear information (eg a variant of unknown significance was discovered) and are not linked to clear management guidance. As such, inconsistent, ad hoc and inappropriate interventions are implemented. For mainstreaming to be successful, expert genetic analysis and triage to clear management protocols is essential. Although it is appropriate and anticipated that all clinicians will become genome aware, it is neither feasible nor necessary for all clinicians to be genome experts. Rather, the role and training of clinical geneticists will need to be reformed such that they can provide expert interpretation of the clinical implications of complex gene tests, with automated pipelines producing routine results.
Conclusion
Mainstreaming gene testing offers unprecedented opportunities to improve the equity and quality of care provided to patients with cancer and the wider population. The paradigm shift of providing genetic testing within mainstream medical services will require education of physicians, allied health practitioners, patients and the general public. Remodelling of existing processes and additional infrastructure, including specialised laboratories with bioinformatics and clinical interpretive expertise, will be required. However, if correctly implemented, the potential for improvement in cancer treatment and cancer prevention is high.
Key points.
A proportion of cancer cases are caused by mutations in cancer predisposition genes (CPGs)
Identification of a CPG mutation provides information that can be used to optimise the management of individuals with cancer
Identification of a CPG mutation provides opportunities to reduce cancer incidence in healthy individuals
New sequencing technologies can be used to deliver fast, affordable mutation testing that will enable testing of more genes in more individuals
Flexible testing processes that allow increased, simplified access to gene testing for patients with cancer are being developed
Acknowledgments
I am grateful to the many colleagues with whom I have discussed the topics addressed in this review and to Daniel Riddell for assistance in preparing the manuscript. I acknowledge support of the NIHR RM/ICR Biomedical Research Centre and the Wellcome Trust Award 098518/Z/12/Z.
References
- 1.Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science 2011;331:1553–8. 10.1126/science.1204040 [DOI] [PubMed] [Google Scholar]
- 2.Rahman N. Realizing the promise of cancer predisposition genes. Nature, 2014;505:302–8. 10.1038/nature12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmann DR. RB1 gene mutations in retinoblastoma. Hum Mutat 1999;14:283–8. 10.1002/(SICI)1098-1004(199910)14:43.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 4.Slade I, Bacchelli C, Davies H, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet 2011;48:273–8. 10.1136/jmg.2010.083790 [DOI] [PubMed] [Google Scholar]
- 5.Gayther SA, Pharoah PD. The inherited genetics of ovarian and endometrial cancer. Curr Opin Genet Dev 2010;20:231–8. 10.1016/j.gde.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elisei R, Romei C, Cosci B, et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J Clin Endocrinol Metab 2007;92:4725–9. 10.1210/jc.2007-1005 [DOI] [PubMed] [Google Scholar]
- 7.Jafri M, Maher ER. The genetics of phaeochromocytoma: using clinical features to guide genetic testing. Eur J Endocrinol 2012;166:151–8. 10.1530/EJE-11-0497 [DOI] [PubMed] [Google Scholar]
- 8.Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res 2012;14:R110. 10.1186/bcr3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner NC, Tutt AN. Platinum chemotherapy for BRCA1-related breast cancer: do we need more evidence? Breast Cancer Res 2012;14:115. 10.1186/bcr3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachet JB, Landi B, Laurent-Puig P et al. Diagnosis, prognosis and treatment of patients with gastrointestinal stromal tumour (GIST) and germline mutation of KIT exon 13. Eur J Cancer 2013;doi:10.1016/j.ejca.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–34. 10.1056/NEJMoa0900212 [DOI] [PubMed] [Google Scholar]
- 12.Vencken PM, Reitsma W, Kriege M et al. Outcome of BRCA1- compared with BRCA2-associated ovarian cancer: a nationwide study in the Netherlands. Ann Oncol 2013;24:2036–42. 10.1093/annonc/mdt068 [DOI] [PubMed] [Google Scholar]
- 13.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748–57. 10.1200/JCO.2012.43.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells SA, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab 2013;98:3149–64. 10.1210/jc.2013-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozen P, Macrae F. Familial adenomatous polyposis: the practical applications of clinical and molecular screening. Fam Cancer 2006;5:227–35. 10.1007/s10689-005-5674-2 [DOI] [PubMed] [Google Scholar]
- 16.Seevaratnam R, Coburn N, Cardoso R, et al. A systematic review of the indications for genetic testing and prophylactic gastrectomy among patients with hereditary diffuse gastric cancer. Gastric Cancer 2012;15:S153–63. 10.1007/s10120-011-0116-3 [DOI] [PubMed] [Google Scholar]
- 17.Burn J, Mathers JC, Bishop DT. Chemoprevention in Lynch -syndrome. Fam Cancer 2013; 12:707–18. 10.1007/s10689-013-9650-y [DOI] [PubMed] [Google Scholar]
- 18.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol 2008;26:1135–5. 10.1038/nbt1486 [DOI] [PubMed] [Google Scholar]



