Abstract
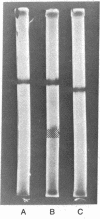
The importance of arginine residues 13 and 14 in the bee venom neurotoxin, apamin, was teste by the synthesis of replacement analogs. [13,14-di-Ndelta-trifluoroacetylornithine]Apamin was synthesized by the solid phase method on a benzhydrylamine resin. It was deprotected to [13,14-diornithine]apamin, which was then guanidinated to produce the 4-homoarginine-13,14-diarginine analog, [Har4]apamin. Neither the trifluoroacetylornithine analog nor the ornithine analog produced any detectable symptoms when injected intravenously into mice. However, the synthetic [Har4]apamin exhibited the full neurotoxic activity of native apamin and of [Har4]apamin derived from the natural toxin. This provided an internal structural control for the correctness of the primary structure of the inactive synthetic analogs and strengthened the conclusion that one, or both, of the arginine residues plays an important role in the action of apamin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODANSZKY M., ONDETTI M. A., RUBIN B., PIALA J. J., FRIED J., SHEEHAN J. T., BIRKHIMER C. A. Biologically active citrulline peptides. Nature. 1962 May 5;194:485–486. doi: 10.1038/194485a0. [DOI] [PubMed] [Google Scholar]
- Borin G., Toniolo C., Moroder L., Marchiori F., Rocchi R., Scoffone E. Synthesis of peptide analogs of the N-terminal eicosapeptide sequence of ribonuclease A. XV. Synthesis and guanidination of(Orn 10 ,Orn 12 )S-peptide. Int J Protein Res. 1972;4(1):37–45. doi: 10.1111/j.1399-3011.1972.tb03396.x. [DOI] [PubMed] [Google Scholar]
- CHERVENKA C. H., WILCOX P. E. Chemical derivatives of chymotrypsinogen. II. Reaction with O-methylisourea. J Biol Chem. 1956 Oct;222(2):635–647. [PubMed] [Google Scholar]
- Callewaert G. L., Shipolini R., Vernon C. A. The disulphide bridges of apamin. FEBS Lett. 1968 Aug;1(2):111–113. doi: 10.1016/0014-5793(68)80033-x. [DOI] [PubMed] [Google Scholar]
- Chauvet J., Acher R. Active derivatives of a polypeptide inhibitor of trypsin (Kunitz and Northrop inhibitor). Biochem Biophys Res Commun. 1967 Apr 20;27(2):230–235. doi: 10.1016/s0006-291x(67)80066-4. [DOI] [PubMed] [Google Scholar]
- Felix A. M., Jimenez M. H. Rapid fluorometric detection for completeness in solid phase coupling reactions. Anal Biochem. 1973 Apr;52(2):377–381. doi: 10.1016/0003-2697(73)90040-7. [DOI] [PubMed] [Google Scholar]
- HOFMANN K. Chemistry and function of polypeptide hormones. Annu Rev Biochem. 1962;31:213–246. doi: 10.1146/annurev.bi.31.070162.001241. [DOI] [PubMed] [Google Scholar]
- Haux P., Sawerthal H., Habermann E. Sequenzanalyse des Bienengift-Neurotoxins (Apamin) aus seinen tryptischen und chymotryptischen Spaltstücken. Hoppe Seylers Z Physiol Chem. 1967 Jun;348(6):737–738. [PubMed] [Google Scholar]
- Hruby V. J., Muscio F., Gronginsky C. M., Gitu P. M., Saba D., Chan W. Y. Solid-phase synthesis of (2-isoleucine, 4-leucine) oxytocin and (2-phenylalanine, 4-leucine) oxytocin and some their pharmacological properties. J Med Chem. 1973 Jun;16(6):624–629. doi: 10.1021/jm00264a010. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Oparil S., Tregear G. W., Koerner T., Barnes B. A., Haber E. Mechanism of pulmonary conversion of angiotensin I to angiotensin II in the dog. Circ Res. 1971 Dec;29(6):682–690. doi: 10.1161/01.res.29.6.682. [DOI] [PubMed] [Google Scholar]
- Pietta P. G., Cavallo P. F., Takahashi K., Marshall G. R. Preparation and use of benzhydrylamine polymers in peptide synthesis. II. Syntheses of thyrotropin releasing hormone, thyrocalcitonin 26-32, and eledoisin. J Org Chem. 1974 Jan 11;39(1):44–48. doi: 10.1021/jo00915a008. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, LENTZ K. E., KAHN J. R., SHUMWAY N. P. The synthesis of a tetradecapeptide renin substrate. J Exp Med. 1958 Sep 1;108(3):283–297. doi: 10.1084/jem.108.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E. Verbesserte Synthese von tert.-Butyloxycarbonyl-aminosäuren durch pH-Stat-Reaktion. Justus Liebigs Ann Chem. 1967;702:188–196. doi: 10.1002/jlac.19677020123. [DOI] [PubMed] [Google Scholar]
- Scoffone E., Marchiori F., Rocchi R., Vidali G., Tamburro A., Scatturin A., Marzotto A. Synthesis of an enzymatically active Orn 10-S-peptide of ribonuclease-S. Tetrahedron Lett. 1966 Mar;9:943–949. doi: 10.1016/s0040-4039(00)76255-1. [DOI] [PubMed] [Google Scholar]
- Spoerri P. E., Jentsch J., Glees P. Apamin from bee venom: effects of the neurotoxin on cultures of the embryonic mouse cortex. Neurobiology. 1973;3(4):207–214. [PubMed] [Google Scholar]
- Vincent J. P., Schweitz H., Lazdunski M. Structure-function relationships and site of action of apamin, a neurotoxic polypeptide of bee venom with an action on the central nervous system. Biochemistry. 1975 Jun 3;14(11):2521–2525. doi: 10.1021/bi00682a035. [DOI] [PubMed] [Google Scholar]
- van Rietschoten J., Granier C., Rochat H., Lissitzky S., Miranda F. Synthesis of apamin, a neurotoxic peptide from bee venom. Eur J Biochem. 1975 Aug 1;56(1):35–40. doi: 10.1111/j.1432-1033.1975.tb02204.x. [DOI] [PubMed] [Google Scholar]