metabolic syndrome is a cluster of conditions including insulin resistance, dyslipidemia, hypertension, and central obesity, and it results in an increased risk of type 2 diabetes mellitus and cardiovascular diseases such as atherosclerosis. These conditions are rising year to year and are a leading cause of deaths worldwide. Therefore, potential therapeutic approaches to attenuate the pathogenesis of metabolic syndrome are urgently needed. Autophagy is a bulk intracellular degradation pathway that involves the formation of a double-membrane autophagosome, which enwraps and delivers cargo to the lysosome for their degradation. Autophagy is a catabolic process that is activated when cells lack nutrients and energy. Autophagy also serves as a cellular protective mechanism by removing damaged organelles including mitochondria and toxic protein aggregates under many pathophysiological conditions. Emerging evidence indicates that autophagy plays critical roles in cardiovascular diseases (5). Studies from cardiomyocyte-specific Atg5 knockout mice revealed that basal autophagy is essential in removing damaged organelles and proteins in cardiomyocytes to maintain their normal functions (8). Autophagy protects against ischemia-induced heart injury, but autophagy can also be detrimental in pressure overload-induced heart failure and during reperfusion (5). Impaired autophagy is also associated with increased apoptosis, mitochondrial injury, intracellular Ca2+ dysregulation, and cardiac contractile dysfunction. Furthermore, autophagy is also implicated in the regulation of atherosclerosis by regulating reactive oxygen species production, endoplasmic reticulum stress, inflammation, and oxidized lipoproteins in atherosclerotic plagues (5).
Overnutrition due to excessive food consumption and its resultant obesity is a major cause of metabolic syndrome. Under metabolic syndrome conditions, it is well known that excessive nutrient intake leads to activation of the mammalian target of rapamycin (mTOR) and suppression of the AMP-activated protein kinase (AMPK), the key sensors of cellular nutrients and energy, respectively. mTOR and AMPK regulate autophagy on the Unc-51 like kinase 1 (ULK1, the yeast Atg1 homolog) complex, the most upstream component of the core autophagy machinery that is composed of ULK1, Atg13, FIP200, and Atg101 (7). Under excessive nutrient conditions, mTOR inhibits autophagy by directly phosphorylating ULK1 (S757), resulting in decreased ULK1 kinase activity (2, 4). Lack of cellular energy leads to the activation of AMPK, which phosphorylates ULK1 at different sites (S317, S467, S555, T574, S637, and S777) and activates ULK1 to promote autophagy (2, 4). Under this energy depletion condition, AMPK also suppresses mTOR by phosphorylating TSC2 and RAPTOR, two essential regulators of mTOR. Moreover, AMPK also regulates the function of the Beclin 1 complex by directly phosphorylating VPS34 and Beclin 1, which is essential for autophagosome formation by providing phosphatidylinositol 3-phosphate (PI3P) (3). The multiple layers of regulation on ULK1, mTOR, and VPS34-Beclin 1 complex by AMPK thus ensures the activation of autophagy when cellular energy is depleted. Interestingly, phosphorylation of ULK1 on S757 by mTOR disrupts the interaction between AMPK and ULK1, suggesting that mTOR activity acts upstream to regulate the association between AMPK and ULK1 (2). Under nutrient-rich conditions, mTOR is activated, which phosphorylates ULK1 (S757) and prevents the interaction between AMPK and ULK1 to keep ULK1 inactivated and thus blocks autophagy (Fig. 1).
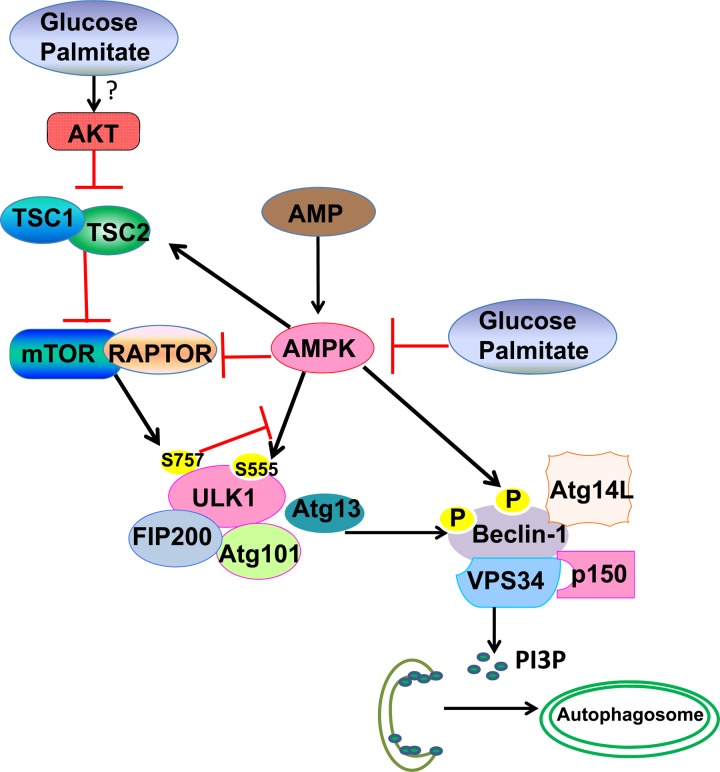
Fig. 1.
A proposed model for how glucose and palmitate impair the AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR)-Unc-51-like kinase 1 (ULK1) signaling cascade in autophagy. High nutrients may activate mTOR through Akt activation (although this remains to be further determined in the future under glucose and palmitate conditions). Glucose and palmitate inactivate AMPK, which is a positive regulator for autophagy at three layers of regulation: suppression of mTOR activity through phosphorylation of TSC2 and RAPTOR; phosphorylation of ULK1 (S555); and promotion of VPS34 kinase activity through phosphorylation of Beclin 1. Activated VPS34 increases the production of phosphatidylinositol 3-phosphate (PI3P), which promotes the biogenesis of autophagosomes. mTOR negatively regulates autophagy through direct phosphorylation of ULK1 (S757) to inactivate ULK1 activity. ULK1 directly phosphorylates Beclin 1 and enhances VPS34 kinase activity to promote autophagy. mTOR-mediated phosphorylation of ULK1 (S757) also prevents ULK1 interaction with AMPK and thus suppresses the phosphorylation of ULK1 (S555) by AMPK to activate ULK1 and autophagy. mTOR-mediated blockage of the interaction of AMPK with ULK1 is most likely the mechanism for how AMPK was uncoupled from autophagy under glucose and palmitate conditions in human aortic endothelial cells (HAECs).
In this issue of American Journal of Physiology-Cell Physiology, Weikel et al. (9) established a unique cell culture model and dissected the role of AMPK-mTOR-ULK1 in autophagy in human aortic endothelial cells (HAECs) (9). In this model, HAECs were maintained and passaged in media containing 25 mM glucose (to mimic chronic hyperglycemia conditions) and were further acutely challenged with 0.4 mM of the saturated fatty acid palmitate (a physiologically relevant concentration that is often achieved in diabetes patients during fasting or exercise). Using this model, Weikel et al. convincingly demonstrated that glucose with palmitate decreased autophagy in HAECs based on a series of autophagic flux assays including assessment of changes in p62 (an autophagy substrate protein that is normally degraded upon autophagy induction), GFP-LC3 puncta, and LC3-II in the presence of the lysosomal autophagy inhibitor bafilomycin A1. Glucose and palmitate treatment also increased apoptosis and inflammation in HAECs, which are known factors that contribute to the development of atherosclerotic plague. Interestingly, induction of autophagy by rapamycin (an mTOR inhibitor) significantly inhibited glucose and palmitate treatment-induced apoptosis and inflammation. These results indicate that induction of autophagy by rapamycin may have a beneficial effect against diabetic atherosclerosis.
How did glucose and palmitate impairment of autophagy result in apoptosis and inflammation in HAECs? They further found that glucose and palmitate did not affect mTOR activity but decreased AMPK activity resulting in decreased AMPK-mediated S555 phosphorylation of ULK1 and subsequent decreased autophagy. Intriguingly, they found that pharmacological activation of AMPK by three different known AMPK agonists (AICAR, A769662, and phenformin) or genetic overexpression of a constitutive active AMPK form restored the decreased AMPK but not the impaired autophagy induced by glucose and palmitate. These results suggest that under high nutrient (high sugar and fat) conditions, activation of AMPK is no longer able to induce autophagy, which is different from the starvation conditions where AMPK activates autophagy. These findings are quite significant because it is known that metformin and statins, two AMPK activators that are the most popular prescribed drugs for treatment of diabetes and cardiovascular diseases, are not effective in all patients (1). It is likely that the defect on the activation of autophagy by AMPK under high nutrient conditions found in this study may reflect these patients who are resistant to AMPK agonist treatment.
Perhaps the most intriguing question is why AMPK is uncoupled from autophagy induction under high glucose and palmitate conditions but not starvation conditions? As discussed above, mTOR, a key cellular nutrient sensor, can phosphorylate ULK1 (S757) to prevent the interaction between AMPK and ULK1 and block autophagy. Thus, nutrients can suppress autophagy by at least two layers of regulation: direct inhibition of autophagy on ULK1 activity via mTOR-mediated phosphorylation of ULK1 and indirect inhibition of autophagy by promoting the dissociation of ULK1 from AMPK via mTOR-mediated phosphorylation of ULK1 (S757). Since mTOR is usually activated under high nutrient conditions, this would provide the answer that the uncoupling of AMPK from ULK1 activation under high glucose and palmitate conditions is simply because AMPK can no longer access and interact with ULK1 due to mTOR activation. While Weikel et al. have considered this possibility, they did not find increased mTOR activity under high glucose and palmitate conditions by assessing the levels of phosphorylated mTOR (S2448) and mTOR substrate protein p70S6K (T389). However, only one time point of mTOR activation was assessed in this study. It is possible that the failure to detect increased mTOR activity could be due to the lack of a time-course study under these conditions because it is well known that high nutrients lead to the activation of mTOR, which is sensitive to the flux of nutrient changes during prolonged cell culture. Furthermore, the direct interaction of ULK1 and AMPK was not determined under high glucose and palmitate conditions by Weikel et al. Nevertheless, it is known that saturated and unsaturated fatty acids can differentially regulate autophagy in different cell types, and only palmitate, but not the unsaturated oleate, increases cellular synthesis of ceramide (6, 10). Indeed, Weikel et al. found that high glucose and palmitate treatment increased cellular levels of ceramide and sphingosine. Overexpression of acid ceramidase, a lysosomal enzyme that catalyzes the conversion of ceramide to sphingosine, increased the level of phosphorylated ULK1 in HAECs that were treated with excess glucose and palmitate. These results suggest that accumulation of ceramide may contribute to the decreased phosphorylated ULK1 under high glucose and palmitate conditions. Surprisingly, while ectopic expression of acid ceramidase restored ULK1 phosphorylation, it did not improve autophagy under high glucose and palmitate conditions, suggesting that high glucose and palmitate may also alter other factors in addition to ULK1 to impair autophagy. These results also imply that the ceramide signaling pathway may not be a suitable and ideal target for improving diabetic cardiovascular diseases. Therefore, it seems that a better target to improve diabetic cardiovascular diseases would be to combine the AMPK agonist with an mTOR inhibitor since decreased mTOR can directly activate autophagy by inhibiting mTOR-mediated ULK1 phosphorylation and increasing the access of AMPK to ULK1. The observation that rapamycin attenuated high glucose and palmitate induced-apoptosis and inflammation in HAECs in this study supports this notion.
Taken together, this important work expands our understanding on how excess nutrients increase cardiovascular risk by impairing autophagy. The uncoupling of AMPK on autophagy under excess nutrient conditions discovered in this study also suggests that combinations of AMPK-independent (such as an mTOR inhibitor) and AMPK-dependent approaches need to be considered for treating nutrient-induced cellular dysfunction. However, several important questions still need to be answered. How is AMPK uncoupled from autophagy? Is the uncoupling of AMPK from autophagy also true in vivo under metabolic syndrome stress? Future work should be performed to further dissect the details of mTOR status and its impact on the uncoupling of AMPK from autophagy.
GRANTS
The research work in Wen-Xing Ding's lab was supported in part by the National Institute on Alcohol Abuse and Alcoholism Grant R01 AA020518, National Institute of Diabetes and Digestive and Kidney Disease Grant R01 DK102142, National Center for Research Resources (5P20RR021940), the National Institute of General Medical Sciences (8P20 GM103549), T32 ES007079, and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20 GM103418).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
W.-X.D. prepared figure; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
REFERENCES
- 1.Cairns SA, Shalet S, Marshall AJ, Hartog M. A comparison of phenformin and metformin in the treatment of maturity onset diabetes. Diabete Metab 3: 183–188, 1977. [PubMed] [Google Scholar]
- 2.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152: 290–303, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA. Cardiovascular autophagy: concepts, controversies, and perspectives. Autophagy 9: 1455–1466, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Mei S, Ni HM, Manley S, Bockus A, Kassel KM, Luyendyk JP, Copple BL, Ding WX. Differential roles of unsaturated and saturated fatty acids on autophagy and apoptosis in hepatocytes. J Pharmacol Exp Ther 339: 487–498, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22: 132–139, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Glucose and palmitate uncouple AMPK from autophagy in human aortic endothelial cells. Am J Physiol Cell Physiol (October29, 2014). doi: 10.1152/ajpcell.00265.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 282: 9777–9788, 2007. [DOI] [PubMed] [Google Scholar]