Abstract
In this study we tested the hypothesis that inspiring a low-density gas mixture (helium-oxygen; HeO2) would minimize mechanical ventilatory constraints and preferentially increase exercise performance in females relative to males. Trained male (n = 11, 31 yr) and female (n = 10, 26 yr) cyclists performed an incremental cycle test to exhaustion to determine maximal aerobic capacity (V̇o2max; male = 61, female = 56 ml·kg−1·min−1). A randomized, single-blinded crossover design was used for two experimental days where subjects completed a 5-km cycling time trial breathing humidified compressed room air or HeO2 (21% O2:balance He). Subjects were instrumented with an esophageal balloon for the assessment of respiratory mechanics. During the time trial, we assessed the ability of HeO2 to alleviate mechanical ventilatory constraints in three ways: 1) expiratory flow limitation, 2) utilization of ventilatory capacity, and 3) the work of breathing. We found that HeO2 significantly reduced the work of breathing, increased the size of the maximal flow-volume envelope, and reduced the fractional utilization of the maximal ventilatory capacity equally between men and women. The primary finding of this study was that inspiring HeO2 was associated with a statistically significant performance improvement of 0.7% (3.2 s) for males and 1.5% (8.1 s) for females (P < 0.05); however, there were no sex differences with respect to improvement in time trial performance (P > 0.05). Our results suggest that the extent of sex-based differences in airway anatomy, work of breathing, and expiratory flow limitation is not great enough to differentially affect whole body exercise performance.
Keywords: exercise, expiratory flow limitation, sex differences, ventilatory limitations to exercise
in healthy untrained humans a substantial reserve exists for increasing minute ventilation (V̇e) during heavy exercise (16, 23). Here, the flow-volume and pressure-volume responses are substantially lower than the mechanical constraints imposed by the properties of the airways, chest wall, and the capacity for generating pressure by the respiratory musculature. Conversely, the high metabolic and ventilatory requirements observed in highly trained males are associated with significant expiratory flow limitation (27). Expiratory flow limitation occurs when, for a given operational lung volume, flow plateaus despite increasing intrapleural pressures (26). To alleviate expiratory flow limitation, operational lung volumes typically increase, which, due to the shape of the maximal expiratory flow-volume curve, permits higher expiratory flow and greater ventilation (23). This relative hyperinflation places the lungs on the less compliant aspect of the pressure-volume curve, which increases the mechanical work of breathing and reduces the mechanical efficiency of the now shortened inspiratory muscles (17, 38). The increased work of breathing is likely linked to both the relative hyperinflation and expiratory flow limitation. The resultant higher work of breathing and fatigued diaphragm are associated with a redistribution of blood flow away from the locomotor muscles (21, 44), reductions in exercise performance (7, 22), and leg fatigue (42). The interrelationships between the respiratory system and the cardiovascular system, muscular fatigue, and exercise performance have been studied almost exclusively in male subjects.
There is now evidence suggesting that endurance-trained females are more susceptible to expiratory flow limitation during exercise (20, 32) and that for a given V̇e, women have a higher work of breathing compared with males (19, 20, 49). The higher tendency for expiratory flow limitation and a greater work of breathing in females can be attributed to sex-based differences in airway size. When matched for total lung volume, females have smaller airways and smaller luminal areas of the large conducting airways compared with males (31, 34, 45). Specifically, we have previously shown that males have greater (14–25%) luminal areas of the large and central airways (trachea, generation 0 through generation 2 and several of the segmental airways) that are not accounted for by differences in lung size (45). The chief sites of airway resistance (∼80%) in healthy individuals are the larger airways (up to the 7th generation), where turbulent airflow is predominate and the Reynolds number is high under exercising conditions of elevated ventilation (46). As such, the high resistive work of breathing in females performing heavy exercise (19) can, in large part, be explained by narrower airways.
Given that resistance to airflow is inversely proportional to airway radius to the fourth power, females with the same size lungs as males will have higher airway resistance due to a smaller airway radius and thus will have lower expiratory flows. Low-density gas mixtures such as helium-oxygen (HeO2) could aid in understanding the physiological relevance of the aforementioned sex-based differences. Helium has a density that is approximately one-third that of nitrogen. Breathing HeO2 is expected to reduce airway resistance and increase flow primarily as a result of a decrease in turbulence within the larger airways (10, 47). McClaran et al. (33) used HeO2 to increase the size of the maximal flow-volume envelope and consequently reduce the amount of expiratory flow limitation during heavy exercise in competitive male cyclists. Moreover, the work of breathing is significantly reduced when inspiring HeO2 during maximal exercise despite an increase in ventilation (5). However, the HeO2-induced reduction in the work of breathing is inconsistent between studies (4). Reducing turbulent airflow and increasing the maximal flow-volume envelope, which in turn lowers the mechanical work of breathing, has also been shown to improve treadmill running performance in health (50) and chronic obstructive pulmonary disease (14). To this end, we investigated whether experimentally alleviating pulmonary system constraints with HeO2 would differentially improve lung mechanics and exercise performance between men and women. We hypothesized that inspiring HeO2 would reduce the work of breathing and improve exercise performance to a greater degree in endurance-trained females relative to males.
METHODS
Subjects.
Twenty-one healthy subjects (11 males, 10 females) volunteered to participate in this study. All subjects were current competitive cyclists or triathletes who participated in regional, national, and international competitions with a range of racing accomplishments. Subjects were free of cardiopulmonary disease, were nonsmoking, and possessed normal pulmonary function. Prior to the study, all subjects provided written informed consent after all procedures and risks were fully explained. All procedures received institutional ethical approval and conformed to the Declaration of Helsinki.
Experimental protocol.
Testing took place over three visits (range: 1 wk to 1 mo). Exercise training regimes were kept consistent throughout testing, and subjects were instructed to refrain from caffeine for 4 h and to avoid strenuous exercise 24 h prior to testing. On the first visit, anthropometric and resting pulmonary function measures were obtained prior to an incremental cycle test to exhaustion to determine maximal aerobic capacity (V̇o2max). Once sufficiently recovered from the incremental test, a familiarization 5-km simulated time trial (TT) was performed. Based on previous work, the familiarization TT also served to ensure subsequent testing would elicit reproducible cycle exercise performances and that subjects were accustomed to performing respiratory maneuvers during strenuous exercise (48). A randomized single-blinded crossover design was used for the two experimental TTs and testing occurred at similar times of day. On the second and third visits, subjects were instrumented with an esophageal balloon for the assessment of breathing mechanics. The order of the 5-km TTs was randomized with subjects breathing either humidified compressed room air or HeO2 (20.87–21.04% O2:balance He). Ratings of sensory responses for breathlessness and leg discomfort were recorded during the incremental test and every kilometer during the TTs using a 10-point category ratio scale (9).
Pulmonary function.
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1.0), FEV1.0/FVC, and peak expiratory flow were determined using a portable spirometer (Spirolab II, Medical International Research, Vancouver, BC) according to recommended guidelines (1) while breathing room air. Subjects were also familiarized with the graded FVC and inspiratory capacity maneuvers for subsequent testing of breathing mechanics (18).
Incremental exercise test.
All exercise was performed on a cycle ergometer (VeloTron Pro, RacerMate, Seattle, WA). Following a self-selected warm-up, the incremental exercise test started at 260 W for males and 160 W for females, with the workload increasing stepwise by 30 W every 3 min until pedaling cadence fell below 60 revolutions/min despite verbal encouragement.
Time trial exercise.
Following a self-selected warm-up similar to day 1, both TTs began at a still start with subjects in the same position. The initial gearing combination chosen by each subject on day 2 was used again at the start of day 3, but subjects were allowed to adjust the gears throughout each TT. Upper body position was also standardized such that subject's hands were to remain on the brake-hoods at all times during testing. The straight, flat 5-km TT course was created and operated using commercially available software (VeloTron 3D, version 3, RacerMate). During the TT, subjects watched a monitor displaying their distance covered and cadence. During the test the experimenter did not verbally encourage subjects. The exact bike set-up (seat and handle-bar positions) on day 2 was recorded and subjects were required to ride in the identical position on day 3 and warm-up for the same duration.
Flow, volume, and pressure.
Ventilatory and mixed expired metabolic parameters were collected using a customized metabolic cart consisting of two independent pneumotachographs (model 3813, Hans Rudolph, Kansas City, MO) to measure inspiratory and expiratory flow, and calibrated O2 and CO2 analyzers (model S-3-A/I and model CD-3A, respectively, Applied Electrochemistry, Pittsburgh, PA). Carbon dioxide could not be measured during the HeO2 trials due to He interfering with the infrared signal used by the CO2 analyzer to determine CO2 concentration. The pneumotachographs were independently calibrated using a 3-liter calibration syringe for both room air and HeO2. We wished to “unload” the respiratory system as much as possible via heliox. As such external resistance was maintained constant between the room air and heliox experiments, volumes were obtained by numerical integration of the flow signals. Due to the lower heat capacity of He and its higher thermal conductivity relative to N2, a low-resistance spirometry filter (PDS8505, Roxon, Vancouver, BC, Canada) was placed before the expired pneumotachograph, and the heater was increased to 43°C. The filter and elevated temperature were used to prevent moisture build-up on the pneumotachograph causing false measures of flow rates. The spirometry filter was present in both trials to maintain a consistent set-up and external resistance. Heliox expired ventilation was temperature corrected off-line during subsequent data analysis to take into account the vapor pressure of water at 43°C. The humidified gases were inspired via a two-way breathing valve connected to a continuously filled 200-liter meteorological balloon (1197–25, VacuMed, Ventura, CA) via a water-filled basin.
Esophageal pressure (PES) was measured with a balloon-tipped catheter (no. 47-9005-RO, Ackrad, Trumbull, CT) placed in the lower third of the esophagus (12). After the balloon was inserted, all the air was evacuated by pulling back on a syringe plunger until the plunger returned to a nonvacuum position. One milliliter of air was injected in order to partially inflate the balloon and catheter as per manufacturer specifications. Validity of the balloon pressure was checked by having the subjects perform the dynamic occlusion test (8). Mouth pressure (PM) was measured via a port in the mouthpiece, and transpulmonary pressure was taken as the difference between PM and PES. Separately calibrated pressure transducers were used to measure PES and PM (Validyne, MCI-10, Northridge, CA). All raw data during the exercise test were recorded continuously at 200 Hz (PowerLab/16SP model ML 796, ADInstruments, Colorado Springs, CO) and stored on a computer for subsequent analysis (LabChart v7.1.3, ADInstruments). Metabolic data were obtained 30 s prior to the completion of each kilometer interval. Heart rate (S610i, Polar Electro, Kempele, Finland) was recorded at rest, and throughout the incremental and TT tests.
Mechanics of breathing.
We assessed the ability of HeO2 to alleviate mechanical ventilatory constraints during the TTs in three ways. First, we determined the degree of expiratory flow limitation (18, 28), whereby FVC and graded FVC maneuvers were performed pre- and postexercise when breathing room air and when breathing heliox to account for thoracic gas compression and bronchodilation as we have previously described (18). These considerations are important for two reasons. First, the maximal expiratory flow-volume curve is based on flow and volume measured at the mouth, which does not account for thoracic gas compression. Second, exercise is associated with significant bronchodilation, which serves to increase the maximal expiratory flow-volume curve. Expiratory flow limitation during exercise can be falsely detected or overestimated if gas compression and bronchodilation are not taken into account. All maneuvers were superimposed and aligned to maximal lung volume, whereby the highest flows at a given volume represented the outer boundary of the maximal expiratory flow-volume curve. The magnitude of flow limitation was calculated as the volume of tidal breath overlapping the maximal expiratory flow-volume curve divided by the tidal volume. End-expiratory reserve lung volume (ERV) was calculated by subtracting the resting inspiratory capacity from FVC with the assumption that total lung capacity remains constant throughout exercise (28). End-inspiratory point (EIP), as an index of end-inspiratory reserve lung volume, was calculated by adding ERV to tidal volume relative to FVC. Inspiratory capacity maneuvers were considered acceptable when peak inspiratory esophageal pressure matched those obtained at rest. Operational lung volumes were expressed as %FVC as we have previously described (20). Second, we determined ventilatory capacity (V̇ecap) (12, 28) by using measured breathing parameters (duty cycle, tidal volume, and operational lung volume) and assumed that the subjects ventilated along their maximal expiratory flow-volume curve allowing for the determination of minimal expiratory time and subsequently maximal breathing frequency. Calculated V̇ecap is the product of maximal breathing frequency and tidal volume and reflects the maximal expiratory flow an individual is theoretically capable of attaining for their chosen breathing pattern. We then related the measured V̇e during the TT to V̇ecap (V̇e/V̇ecap). A reduction in V̇e/V̇ecap during the HeO2 TT would reflect a reduced fractional usage of the maximal ventilatory capacity and a reduction of mechanical constraints. Last, the work of breathing was determined from transpulmonary pressure loops using Campbell diagrams where the muscular work was separated into resistive and elastic with the sum reflecting the total mechanical work (19). When nitrogen is replaced with He as a backing gas, airflow remains more laminar, thereby allowing greater flows. As such, a reduction in mechanical constraints when breathing HeO2 would be reflected in a lowering of the resistive work of breathing. It is important to note that He has a higher viscosity than air, which will increase flow resistance when flow is laminar (i.e., the small airways). Given that the small airways contribute minimally to total resistance, any HeO2-induced increases in viscosity resistance would be masked by the substantial reduction in the main sites of resistance (i.e., the medium- and larger-sized airways) where airflow is predominantly turbulent rather than laminar.
Statistical analysis.
A sample size of 9 per group was determined (G*Power; http://www.gpower.hhu.de) using α = 0.05, a power of 80%, and an expected difference of 2% in time-to-completion of the 5-km time trials (room air vs. heliox). Our estimates of sample size are primarily based on previously published cycle performance tests (48). Descriptive characteristics, pulmonary function, and maximal exercise data between males and females were compared with unpaired t-tests. All values were examined and met the assumption of being normally distributed as assessed by a Shapiro-Wilk test. Repeated-measures ANOVA procedures (Statistica v6.1, StatSoft, Tulsa, OK) were used to compare the effects of gas inspirate across the time trials. In the case of a significant F-ratio, differences were further investigated with Tukey's post hoc. To evaluate time trial exercise performance we utilized t-test procedures and the magnitude-based inferences approach and precision of estimation (90% confidence limits) to detect small effects of practical importance for cyclists (24, 25). The magnitude of the percent change in power was interpreted using 0.3% (smallest effect) of the within-athlete indoor cycling variation of power (coefficient of variation) (1.9% or 1.31 W) (39, 48) as a threshold for small differences in the change in power between the trials (25). Therefore the smallest worthwhile change in power is 0.57%, and the smallest worthwhile change in time is 0.21% (1.04 s) for a coefficient of variation of 0.7% (39, 48). The practical interpretation of an effect is deemed “unclear” when the magnitude of change is substantial and when the confidence interval (precision of estimation) could result in positive and negative outcomes. The level of significance was set at P < 0.05 for all statistical comparisons. Values are presented as means ± SD unless otherwise noted.
RESULTS
Subject characteristics and incremental exercise.
Descriptive characteristics and maximal exercise data are summarized in Table 1. Normal pulmonary function was present in all subjects relative to normative values (3). Subjects were of comparable age (males: 31 ± 5 yr; females: 26 ± 5 yr), but males were taller (180 ± 7 vs. 168 ± 7 cm) and heavier (74 ± 7 vs. 60 ± 6 kg) (P < 0.05). Females possessed aerobic capacities that were greater than males when expressed as %predicted V̇o2max (29). However, both sexes had similar competitive racing experience.
Table 1.
Resting pulmonary function and maximal exercise values
| Male | Female | |
|---|---|---|
| FVC, liters | 5.7 ± 0.7 (4.5–6.6) | 4.3 ± 0.7 (3.0–5.5)* |
| FVC, %predicted | 108 ± 8 (96–118) | 111 ± 11 (85–125) |
| FEV1.0, liters | 4.5 ± 0.6 (3.6–5.6) | 3.5 ± 0.6 (2.5–4.4)* |
| FEV1.0, % predicted | 102 ± 11 (82–118) | 106 ± 9 (94–122) |
| FEV1.0,/FVC, % | 81 ± 4 (76–85) | 82 ± 4 (76–88) |
| PEF, l/s | 11.2 ± 1.3 (9.8–13.0) | 7.5 ± 1.1 (5.6–9.0)* |
| PEF, %predicted | 112 ± 11 (99–130) | 102 ± 15 (78–123) |
Values are means ± SD (range). FVC, forced vital capacity; FEV1.0, forced expired volume in 1 s; PEF, peak expiratory flow.
Significantly different between males and females, P < 0.05.
Effect of HeO2 on the cardiopulmonary response to exercise.
Across both TTs (room air and HeO2) males had a higher V̇o2, V̇e, and tidal volume relative to females (P < 0.05) (Table 2). The frequency and timing of breathing and heart rate were unaffected by gas composition, and there were no sex differences (P > 0.05). The effect of HeO2 on V̇e was variable between subjects and was nonsignificant (P > 0.05), although there was a tendency for V̇e to increase (+3–10 l/min) in both males and females. There was no effect of sex or inspirate on sensory responses (leg discomfort, dyspnea) during TT exercise (P > 0.05).
Table 2.
Maximal exercise values
| Male | Female | |
|---|---|---|
| V̇o2max, ml·kg−1·min−1 | 60.8 ± 3.8 (55.3–67.0) | 55.8 ± 3.2 (51.8–61.3)* |
| V̇o2max, l/min | 4.5 ± 0.40 (3.8–5.0) | 3.3 ± 0.36 (2.9–3.9)* |
| V̇o2max, %predicted | 134 ± 11 (118–151) | 163 ± 12 (147–182)* |
| V̇co2, l/min | 4.9 ± 0.40 (4.1–5.4) | 3.6 ± 0.40 (3.1–4.2)* |
| RER | 1.11 ± 0.03 (1.07–1.15) | 1.08 ± 0.04 (1.03–1.13) |
| V̇e, l/min, STPD | 140 ± 12 (118–163) | 101 ± 14 (84–133)* |
| fb, breaths/min | 58 ± 14 (34–78) | 57 ± 9 (46–72) |
| VT, liters, BTPS | 2.88 ± 0.50 (2.35–4.01) | 2.10 ± 0.40 (1.44–2.75)* |
| Heart rate, beats/min | 193 ± 16 (178–233) | 185 ± 12 (168–204) |
| Heart rate, %predicted | 102 ± 8 (94–122) | 96 ± 6 (83–105) |
| Peak power, W | 361 ± 24 (320–410) | 283 ± 34 (250–340)* |
| Peak power, W/kg | 4.9 ± 0.4 (4.5–5.5) | 4.8 ± 0.4 (4.3–5.6) |
| Total time, s | 725 ± 142 (525–1013) | 836 ± 224 (601–1217) |
| Leg discomfort, Borg units | 9.0 ± 0.6 (8.0–10.0) | 7.5 ± 1.9 (5.0–10.0) |
| Breathing discomfort, Borg units | 8.5 ± 0.7 (7.0–9.0) | 7.3 ± 1.7 (5.0–9.0) |
Values are means ± SD (range). V̇o2max, maximal oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e, minute ventilation; fb, frequency of breathing; VT, tidal volume.
Significantly different between males and females, P < 0.05.
Effect of HeO2 on the mechanics of breathing.
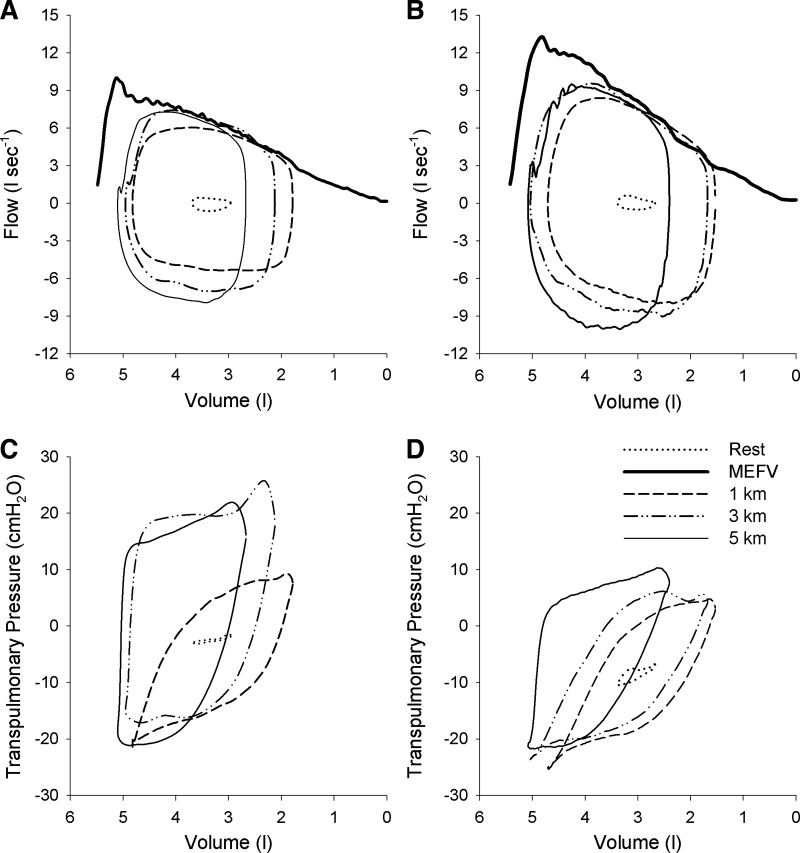
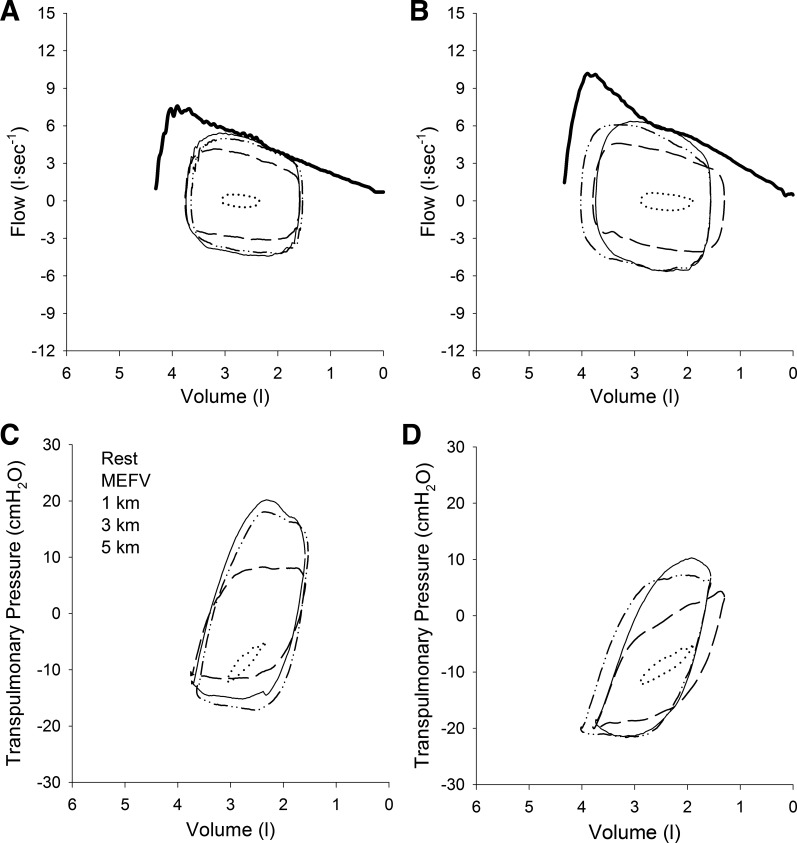
Inspiring HeO2 significantly increased resting peak expiratory flow and maximal expiratory flow at 50% of vital capacity for both males and females (Table 3) but did not change FVC. Averaged over the duration of the time trials, peak expiratory flow was higher when breathing HeO2 compared with room air in males (room air 6.65 ± 0.90 vs. HeO2 8.42 ± 1.01 l/s, P < 0.001) and females (room air 4.78 ± 0.73, HeO2 5.52 ± 0.82 l/s, P < 0.05) and the increase in peak expiratory flow during exercise was greater in males relative to females (P < 0.05). Across all trials, ERV decreased relative to rest with the onset of exercise and remained below resting values (P < 0.05). Values for ERV at each kilometer are shown in Table 4 for males and females and for both inspired gas conditions. Breathing HeO2 resulted in lowering of ERV relative to room air at end-exercise, and the changes were similar between men and women. End-inspiratory lung volume was unaffected by HeO2. The development of flow limitation was variable between subjects during TT exercise. During room air breathing, 5 males and 7 females became flow limited at some point during the 5-km time trial, and the same subjects became flow limited during HeO2 breathing. Three of the other male subjects and two female subjects demonstrated “impending” flow limitation where their tidal flow-volume loop approached the maximal flow-volume curve and developed its characteristic shape but did not fully intersect. Of those that developed flow limitation, the magnitude was similar between sexes during room air breathing and HeO2 breathing. On average, the magnitude of flow limitation was unaffected by inspiring HeO2 because flow-limited subjects increased their tidal flow-volume loops thereby taking advantage of the heliox-induced increase in the maximal expiratory flow-volume loop. This phenomenon is displayed by a representative male (Fig. 1) and female (Fig. 2) subject whose higher expiratory flow rates achieved during the HeO2 TT were generated via lower transpulmonary pressures compared with room air breathing.
Table 3.
Change in resting expiratory flow when inspiring HeO2 compared with room air breathing
| Male | Female | |
|---|---|---|
| Δ PEF, l/s | 4.04 ± 1.21 | 2.07 ± 0.68* |
| Δ PEF, % | 35 ± 11 | 28 ± 12 |
| Δ FEF50% FVC, l/s | 2.46 ± 1.13 | 1.59 ± 0.46* |
| Δ FEF50% FVC, % | 38 ± 16 | 33 ± 10 |
Values are means ± SD. Δ PEF, change in peak expiratory flow; Δ FEF50% FVC, change peak expiratory flow at 50% vital capacity measured in each condition (room air, heliox).
Significantly different between males and females, P < 0.05.
Table 4.
Metabolic and respiratory responses during 5-km time trials
| 1 km | 2 km | 3 km | 4 km | 5 km | |
|---|---|---|---|---|---|
| V̇o2, ml·kg−1·min−1 | |||||
| M | |||||
| Room air | 46.7 ± 3.1 | 51.9 ± 6.1 | 54.6 ± 6.0 | 56.2 ± 6.1 | 57.2 ± 6.4 |
| HeO2 | 47.3 ± 6.2 | 50.6 ± 4.8 | 53.0 ± 4.9 | 55.3 ± 4.4 | 56.0 ± 4.4 |
| F | |||||
| Room air | 44.9 ± 3.3 | 48.0 ± 4.0 | 49.7 ± 5.1* | 50.3 ± 5.7* | 51.3 ± 5.8* |
| HeO2 | 42.9 ± 3.3* | 45.4 ± 4.4* | 47.9 ± 5.4* | 48.1 ± 5.7* | 48.9 ± 6.0* |
| HR, beats/min | |||||
| M | |||||
| Room air | 165 ± 12 | 171 ± 11 | 175 ± 8 | 179 ± 6 | 184 ± 6 |
| HeO2 | 164 ± 12 | 174 ± 10 | 178 ± 8 | 182 ± 7 | 187 ± 5 |
| F | |||||
| Room air | 163 ± 11 | 168 ± 12 | 172 ± 10 | 175 ± 10 | 181 ± 9 |
| HeO2 | 164 ± 8 | 170 ± 9 | 173 ± 9 | 175 ± 8 | 181 ± 10 |
| V̇e, l/min, STPD | |||||
| M | |||||
| Room air | 83 ± 18 | 113 ± 19 | 121 ± 18 | 126 ± 15 | 134 ± 12 |
| HeO2 | 85 ± 16 | 116 ± 19 | 122 ± 17 | 131 ± 13 | 141 ± 12 |
| F | |||||
| Room air | 65 ± 10* | 83 ± 14* | 87 ± 15* | 90 ± 16* | 94 ± 17* |
| HeO2 | 66 ± 9* | 83 ± 14* | 89 ± 17* | 92 ± 16* | 97 ± 18* |
| fb, breaths/min | |||||
| M | |||||
| Room air | 37 ± 10 | 44 ± 11 | 47 ± 12 | 50 ± 12 | 55 ± 12 |
| HeO2 | 41 ± 11 | 49 ± 12 | 53 ± 13 | 57 ± 13 | 67 ± 13 |
| F | |||||
| Room air | 39 ± 8 | 48 ± 13 | 51 ± 12 | 52 ± 9 | 54 ± 10 |
| HeO2 | 47 ± 14 | 54 ± 13 | 58 ± 12 | 63 ± 15 | 67 ± 17 |
| VT, liters, BTPS | |||||
| M | |||||
| Room air | 2.7 ± 0.5 | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.7 |
| HeO2 | 2.7 ± 0.5 | 3.1 ± 0.5 | 3.1 ± 0.5 | 3.0 ± 0.5 | 3.0 ± 0.6 |
| F | |||||
| Room air | 2.0 ± 0.4* | 2.3 ± 0.5* | 2.2 ± 0.6* | 2.2 ± 0.6* | 2.2 ± 0.5* |
| HeO2 | 1.9 ± 0.5* | 2.1 ± 0.5* | 2.1 ± 0.5* | 2.0 ± 0.5* | 2.0 ± 0.5* |
| ERV, % FVC | |||||
| M | |||||
| Room air | 37 ± 35 | 35 ± 8 | 33 ± 13 | 39 ± 8 | 39 ± 9 |
| HeO2 | 27 ± 11 | 30 ± 7 | 30 ± 8 | 33 ± 9† | 33 ± 13† |
| F | |||||
| Room air | 35 ± 6 | 33 ± 12 | 32 ± 14 | 37 ± 8 | 39 ± 9 |
| HeO2 | 34 ± 8 | 35 ± 10 | 35 ± 7 | 35 ± 6 | 35 ± 6† |
| EIP, % FVC | |||||
| M | |||||
| Room air | 88 ± 7 | 87 ± 6 | 80 ± 3 | 91 ± 5 | 88 ± 6 |
| HeO2 | 75 ± 5 | 86 ± 5 | 85 ± 6 | 85 ± 9 | 80 ± 3 |
| F | |||||
| Room air | 86 ± 4 | 82 ± 3 | 79 ± 3 | 87 ± 5 | 88 ± 1 |
| HeO2 | 84 ± 7 | 85 ± 10 | 84 ± 11 | 84 ± 8 | 82 ± 11 |
| %Flow limited | |||||
| M (n = 5) | |||||
| Room air | 0 ± 0 | 26 ± 3 | 35 ± 20 | 32 ± 10 | 48 ± 24 |
| HeO2 | 19 ± 10 | 30 ± 23 | 27 ± 27 | 32 ± 16 | 30 ± 17 |
| F (n = 7) | |||||
| Room air | 0 ± 0 | 21 ± 10 | 41 ± 33 | 36 ± 17 | 30 ± 19 |
| HeO2 | 0 ± 0 | 16 ± 7 | 0 ± 0 | 42 ± 21 | 45 ± 13 |
| Power, W | |||||
| M | |||||
| Room air | 323 ± 51 | 302 ± 42 | 309 ± 37 | 307 ± 37 | 323 ± 42 |
| HeO2 | 335 ± 50 | 305 ± 36 | 310 ± 39 | 311 ± 41 | 327 ± 46 |
| F | |||||
| Room air | 217 ± 39* | 215 ± 41* | 212 ± 40* | 215 ± 42* | 232 ± 44* |
| HeO2 | 221 ± 32* | 223 ± 42* | 223 ± 41* | 218 ± 38* | 235 ± 41* |
| Cadence, rpm | |||||
| M | |||||
| Room air | 105 ± 7 | 106 ± 7 | 106 ± 9 | 105 ± 8 | 107 ± 8 |
| HeO2 | 105 ± 7 | 107 ± 6 | 107 ± 7 | 106 ± 7 | 107 ± 8 |
| F | |||||
| Room air | 96 ± 5 | 97 ± 5 | 100 ± 6 | 98 ± 5 | 100 ± 6 |
| HeO2 | 101 ± 6 | 100 ± 7 | 96 ± 9 | 100 ± 7 | 102 ± 8 |
Values are means ± SD. V̇o2, oxygen consumption; ERV, end-expiratory reserve lung volume; EIP, end-inspiratory point; %FVC, percent forced vital capacity; M, male; F, female; HR, heart rate.
Significantly different between males and females, P < 0.05.
Significantly different between room air and HeO2, P < 0.05.
Fig. 1.
Flow-volume and pressure-volume loops during time trial exercise in representative male subject. A and B: tidal loops obtained at rest and at 1, 3, and 5 km positioned within the maximal expiratory flow volume curve (MEFV) breathing room air (A) or HeO2 (B). Note that with each successive kilometer, end-expiratory lung volume increased towards resting values. C and D: pressure-volume loops obtained at rest and at 1, 3, and 5 km while breathing room air (C) or HeO2 (D). Note the reduction in size of the transpulmonary loop when breathing HeO2 (i.e., a reduced work of breathing) despite a larger flow-volume loop.
Fig. 2.
Flow-volume and pressure-volume loops during time trial exercise in representative female subject. A and B: tidal loops obtained at rest and at 1, 3, and 5 km positioned within the maximal expiratory flow volume curve (MEFV) breathing room air (A) or HeO2 (B). Note that with each successive kilometer, end-expiratory lung volume increased towards resting values. C and D: pressure-volume loops obtained at rest and at 1, 3, and 5 km while breathing room air (C) or HeO2 (D). Note the reduction in size of the transpulmonary loop when breathing HeO2 (i.e., a reduced work of breathing) despite a larger flow-volume loop. See Fig. 1 for description of individual lines.
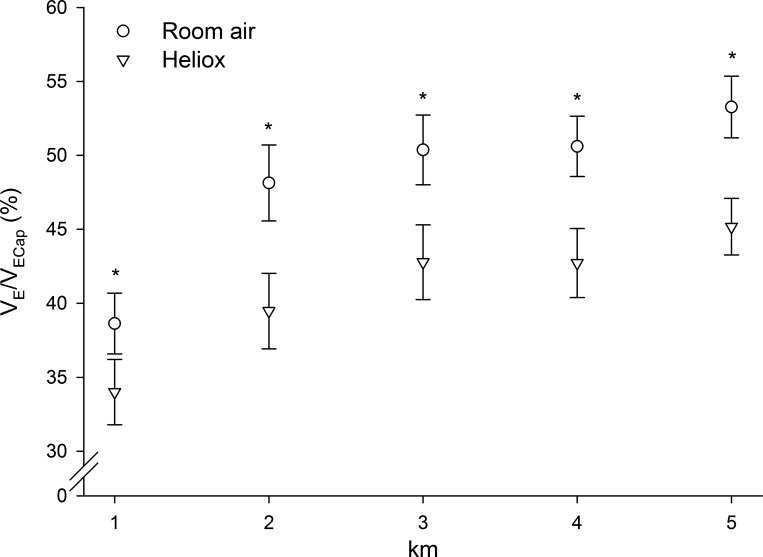
Group averages for the ventilatory fractional usage (V̇e/V̇ecap) are shown in Fig. 3. The V̇e/V̇ecap was decreased with HeO2 in each woman without exception, and 9 of 11 men. The reduction of V̇e/V̇ecap was similar between males and females (7–9%; P > 0.05), and values have been combined to show the effect of gas inspirate. Table 5 shows the effect of breathing HeO2 on the total and constituent components of the work of breathing values averaged across the entire 5-km trials. HeO2 reduced the total work of breathing and the resistive components of breathing (P < 0.05), but there was no difference between men and women (P > 0.05). Inspiratory and expiratory elastic work of breathing were increased in men but were unchanged in women.
Fig. 3.
Change in minute ventilation/ventilatory capacity (V̇e/V̇ecap) when breathing HeO2 relative to room air. Shown are mean values for the inspired gas conditions with male and female values combined. No statistical differences were detected between males and females (P > 0.05). Inspiring HeO2 reduced V̇e/V̇ecap at each kilometer (*P < 0.05) indicating a reduced fractional usage of the maximal ventilatory capacity and a minimization of mechanical constraints.
Table 5.
Work of breathing (WOB) during 5-km time trials
| 1 km | 2 km | 3 km | 4 km | 5 km | |
|---|---|---|---|---|---|
| Total WOB, J/min | |||||
| M | |||||
| Room air | 248 ± 105 | 329 ± 118 | 396 ± 156 | 425 ± 107 | 457 ± 109 |
| HeO2 | 230 ± 110 | 308 ± 123 | 315 ± 103* | 375 ± 105* | 407 ± 110* |
| F | |||||
| Room air | 170 ± 38 | 256 ± 86 | 282 ± 95 | 291 ± 100 | 338 ± 86 |
| HeO2 | 154 ± 52 | 226 ± 99 | 232 ± 106* | 244 ± 113* | 272 ± 104* |
| Resistive WOB, J/min | |||||
| M | |||||
| Room air | 131 ± 61 | 195 ± 93 | 245 ± 120 | 246 ± 117 | 273 ± 67 |
| HeO2 | 93 ± 68 | 135 ± 76* | 148 ± 66* | 180 ± 75* | 207 ± 71* |
| F | |||||
| Room air | 90 ± 28 | 139 ± 62 | 171 ± 73 | 179 ± 74 | 206 ± 69 |
| HeO2 | 68 ± 27 | 108 ± 55* | 112 ± 54* | 123 ± 68* | 139 ± 62* |
| Elastic WOB, J/min | |||||
| M | |||||
| Room air | 117 ± 49 | 134 ± 51 | 151 ± 45 | 180 ± 58 | 184 ± 65 |
| HeO2 | 137 ± 57 | 173 ± 69 | 176 ± 52 | 195 ± 85 | 200 ± 77 |
| F | |||||
| Room air | 80 ± 21 | 117 ± 41 | 111 ± 34 | 112 ± 36 | 133 ± 38 |
| HeO2 | 86 ± 27 | 118 ± 48 | 120 ± 56 | 121 ± 52 | 131 ± 51 |
Values are means ± SD.
Significantly different between room air and HeO2, P < 0.05.
Time trial performance.
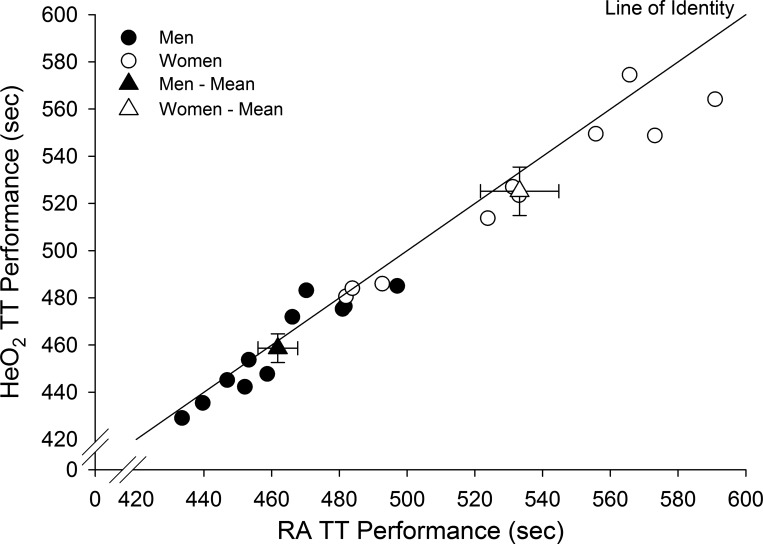
Inspiring heliox was associated with a statistically significant performance improvement of 0.7 ± 1.5% (3.2 s) for males and 1.5 ± 1.9 % (8.1 s) for females; however, there were no sex differences with respect to improvement in time trial performance. Figure 4 shows individual time trial values during conditions of room air and HeO2. Three subjects (2 men and 1 woman) were above the line of identity; all others were below the line (indicating performance improvement) or on the line (indicating no change in performance). Using the magnitude-based inferences approach (see Statistical analyses), the chances the effect of inspiring HeO2 is beneficial, trivial, or harmful are 94.6%, 5.3%, and 0.1% with regards to time trial performance, respectively. Inspiring HeO2 was also associated with statistically significant improvements in power for males 1.61 ± 0.81% (5.00 W) and females 4.03 ± 1.8 (7.90 W). There was no effect of order on TT performance. The TT with the highest power output was performed first for approximately half of the subjects; accordingly, test order did not appear to have an effect on performance. Both men and women equally improved their room air time trial relative to the familiarization time trial suggesting that the familiarization equally improved the ability to perform time trial cycle exercise.
Fig. 4.
Individual and group mean time trial (TT) performance times relative to the line of identity. Triangles are means ± SE. HeO2, helium oxygen; RA, room air; TT, time trial.
DISCUSSION
We tested the hypothesis that inspiring HeO2 would reduce the work of breathing, attenuate expiratory flow limitation, and improve exercise performance to a greater degree in endurance-trained females relative to males. The rationale for our study was based on observations that 1) the conducting airways are narrower in women even when matched for lung size, 2) women have a higher propensity for flow limitation than men, and 3) women have a higher total and resistive mechanical work of breathing. The primary finding of this study is that when mechanical ventilatory constraints are minimized, males and females improve time trial exercise performance to a similar degree. Our results suggest that the extent of sex-based differences in airway anatomy, work of breathing, and expiratory flow limitation are not great enough to differentially affect whole body exercise performance.
Mechanics of breathing and sensory responses.
In our study we were primarily concerned with innate sex-based differences in airway anatomy and function with respect to the properties that govern airflow under conditions of strenuous whole body dynamic exercise. As such, we replaced nitrogen with helium as the backing gas whereby airflow remains more laminar and permits greater flows. Using our approach we successfully reduced the work of breathing and V̇e/V̇ecap with the effects of HeO2 being similar between men and women. When both men and women are provided additional “room” to increase expiratory flow offered by HeO2, they do so equally although we observed considerable between-subject variation.
Any intervention that reduces central ventilatory drive, improves ventilatory mechanics, or improves respiratory muscle function has the potential to reduce exertional dyspnea (37). We found that the perceptual ratings of breathing were unaffected by gas inspirate and were not different between males and females. There are inconsistent reports on the effects of HeO2 on dyspnea (5, 6, 33), which may reflect between-study differences in exercise intensity as well as the aerobic fitness of the subjects. That we observed an absence of effect also speaks to the complexities of the interactions between sensory responses and the cardiopulmonary demands of exercise. It may be that the substantive feed-forward (i.e., so-called “central command”) and numerous feedback mechanisms (i.e., muscle reflexes: chemical, mechanical, thermoregulatory) necessary to perform strenuous exercise are dominant and mask any changes to the mechanics of breathing. Based on the recent findings of Schaeffer et al. (43), who found significant sex differences in the intensity and unpleasantness of exertional dyspnea in healthy young males and females, we predicted that HeO2 would have reduced the awareness of breathing to a greater degree in females. We attribute a lack of difference in sensory responses to the fact that HeO2 changed the mechanics of breathing similarly between the sexes.
Time trial performance.
To assess the effects of HeO2 on exercise performance we used time trial tests rather than a constant-load test. We selected a short-duration, high-intensity test of exercise performance because it has been shown that reducing respiratory muscle work by 60% with a proportional assist ventilator is associated with a 14% increase in time to exhaustion when trained cyclists exercise at near-maximal intensities (90–95% V̇o2max) (22). High-intensity exercise is required because there is no effect of reducing respiratory muscle work when subjects perform constant-load exercise at lower levels of intensity (75% V̇o2max) (15, 30). Furthermore, performance tests which require self-selection of pace (i.e., constant-distance test), such as the test used in the present study, are known to have a low source of random error and are appropriate for determining small changes in competitive performance (40). We included a familiarization performance trial and randomized the order of gas inspirate in order to reduce variation and improve our ability to detect systematic HeO2-induced changes in performance. We found that exercise performance was significantly improved in highly trained males and females when breathing HeO2 relative to room air breathing in the form of reductions in time and increases in power. We also used the magnitude-based inferences approach to show that the performance effect of HeO2 is meaningful. No differences in performance between males and females were observed. It should be emphasized that the reduction in the work of breathing with HeO2 (see Table 5) was less than that which can be achieved with a proportional assist ventilator (22). The time trial improvements we observed were statistically significant and important from a performance standpoint but were nonetheless relatively small. We attribute the moderate improvements to the fact that the muscles of respiration were unloaded to a lesser degree in our study relative to ventilator studies (22).
The statistically significant improvements in performance we observed were modest but the gains can be considered relevant and important to competitive cyclists (24, 25). The improvements we observed are consistent with another study that used 5-km time trials to evaluate exercise performance (2). What is the mechanism by which exercise performance improved with HeO2 breathing? Our study was not designed to specifically address this question but it merits brief consideration. The respiratory muscles are known to influence exercise performance via the triggering of a respiratory muscle metaboreflex (11). The substantial mechanical and metabolic demands placed on the respiratory muscles during high-intensity exercise lead to fatigue of the diaphragm and expiratory muscles. The fatiguing contractions and high metabolic work of the respiratory muscles lead to a sympathetically mediated decrease in limb blood flow and O2 transport to locomotor muscles and is associated with significant fatigue of the quadriceps muscle and decrements in exercise performance (9). The effects of high respiratory muscle work described above occur only during heavy exercise such as the protocol we employed rather during than submaximal exercise.
Finally, the HeO2-related improvement we observed could also be attributed to the increases in maximal expiratory flow rather than decreases in airway resistance and the mechanical work of breathing. The physics underlying the phenomenon of expiratory flow limitation are complex and have been described in detail elsewhere (41). Briefly, one explanation relates to the contraction of expiratory muscles and the increasingly positive pleural pressure, which results in dynamic airway compression (35). An alternate explanation relates to the Bernoulli effect whereby increases in airflow velocity cause reductions in lateral airway pressure, which in turn narrows the airway lumen. Breathing a less dense gas increases expiratory flow, in part, because Bernoulli pressure is proportional to gas density (36). In our study we observed increases in resting and exercise peak expiratory flows, which may explain the beneficial effects of HeO2. On the other hand, we observed an inconsistent effect of HeO2 on V̇e (+3–10 l/min; P > 0.05) and the change in performance was not associated with the change in V̇e.
Study limitations.
We observed a significant improvement in time trial performance and a reduction in mechanical constraints with HeO2. However, our approach permitted subjects to adjust their pacing (i.e., power output, cadence) during the time trials, which was likely influenced, in part, by perception of effort. As such the fact that expiratory flow limitation along with other respiratory parameters was variable may reflect the subjects' ability to adjust power. Our study was designed to alleviate mechanical constraints and ascertain the effects on the integrative response that governs exercise performance. To determine the mechanisms for the observed improvements in performance, future studies will need to incorporate limb blood flow and arterial oxygenation measures into the experimental design. We did not catheterize our subjects for the direct assessment of leg blood flow but many of the predisposing factors for fatigue of the respiratory muscles and ensuing metaboreflex were present in our study, including flow limitation, a high work of breathing, and metabolic acidosis. While we favor the metaboreflex explanation for our findings, future invasive studies with experimental manipulation of the work of breathing are required to confirm our hypothesis.
The specific heat capacity of helium is higher than that of nitrogen. As such, respiratory heat loss could have been higher during the heliox trials and this could have contributed to the observed exercise performance effect. We have no measure of body temperature or heat storage and are unable calculate the potential effect of differences in temperature on exercise performance. However, in our study we delivered inspired gas via a humidified system, which decreases the evaporative component of respiratory heat loss in both conditions. This would have minimized the potential effect of a helium-associated difference in heat loss. The absolute differences in temperature were likely to have been relatively small between conditions but we recognize this as a limitation to the interpretation of the performance changes we observed.
Perspectives.
Our findings suggest that mechanical ventilatory constraints negatively impact exercise performance equally in endurance-trained males and females. Healthy females exhibit greater mechanical constraints during exercise than males (13, 20, 32), owing to their smaller lungs and airways (34, 45). Specifically, our recent work (12) and that of McClaran et al. (32) show that females develop significant expiratory flow limitation which can constrain the ventilatory response during high-intensity exercise. The higher overall work of breathing in females during dynamic exercise is associated with a substantially greater resistive work during inspiration and expiration (19). In the present study we reasoned that reducing mechanical ventilatory constraints would result in greater exercise performance gains in females. We observed a similar improvement between males and females. This can be attributed to at least two possibilities. First, there is no male-female difference. The sex differences in airway anatomy, high work of breathing, and expiratory flow limitation are of insufficient magnitude to negatively impact exercise performance. Second, the reductions in ventilatory constraints offered by HeO2 were modest relative to that offered by a proportional assist ventilator. It may be that in order to determine if sex differences exist with respect to respiratory system influences on exercise performance, a greater degree of unloading is required.
GRANTS
The Natural Sciences and Engineering Research Council (NSERC) of Canada supported this study. S. S. Wilkie and P. B. Dominelli were supported by NSERC postgraduate scholarships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. conception and design of research; S.S.W. and P.B.D. performed experiments; S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. analyzed data; S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. interpreted results of experiments; S.S.W., P.B.D., and A.W.S. prepared figures; S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. drafted manuscript; S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. edited and revised manuscript; S.S.W., P.B.D., B.C.S., M.S.K., and A.W.S. approved final version of manuscript.
REFERENCES
- 1.American Thoracic Society. Standardization of Spirometry, 1994 Update American Thoracic Society. Am J Respir Crit Care Med 152: 1107–1136, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Hopkins WG, Marcora SM. Similar sensitivity of time to exhaustion and time-trial time to changes in endurance. Med Sci Sports Exerc 40: 574–578, 2008. [DOI] [PubMed] [Google Scholar]
- 3.ATS. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 166: 518–624, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Babb TG. Breathing He-O2 increases ventilation but does not decrease the work of breathing during exercise. Am J Respir Crit Care Med 163: 1128–1134, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Babb TG. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol 109: 15–28, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol 82: 746–754, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol 93: 201–206, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 10.Brice AG, Welch HG. Metabolic and cardiorespiratory responses to He-O2 breathing during exercise. J Appl Physiol 54: 387–392, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591: 3017–3034, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominelli PB, Guenette JA, Wilkie SS, Foster GE, Sheel AW. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc 43: 1666–1674, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 763–771, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher CG, Younes M. Effect of pressure assist on ventilation and respiratory mechanics in heavy exercise. J Appl Physiol 66: 1824–1837, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Grimby G, Saltin B, Wilhelmsen L. Pulmonary flow-volume and pressure-volume relationship during submaximal and maximal exercise in young well-trained men. Bull Physiopathologie Respir 7: 157–172, 1971. [PubMed] [Google Scholar]
- 17.Grimby G, Goldman M, Mead J. Respiratory muscle action inferred from rib cage and abdominal V-P partitioning. J Appl Physiol 41: 739–751, 1976. [DOI] [PubMed] [Google Scholar]
- 18.Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170: 279–286, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Guenette JA, Querido JS, Eves ND, Chua R, Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol 297: R166–R175, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82: 1573–1583, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol 89: 131–138, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J Appl Physiol 64: 135–146, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins WG. A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a P value. Sportscience 11: 16–20, 2007. [Google Scholar]
- 25.Hopkins WG, Batterham AM, Marshall SW, Hanin JH. Progressive statistics. Sportscience 13: 55–70, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Hyatt R. Pressure-flow-volume interrelationships in man. Am Rev Respir Dis 80: 138–140, 1959. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73: 874–886, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 116: 488–503, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan B, Zintel T, McParland C, Gallagher CG. Lack of importance of respiratory muscle load in ventilatory regulation during heavy exercise in humans. J Physiol 490: 537–550, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63: 2042–2047, 1987. [DOI] [PubMed] [Google Scholar]
- 32.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 84: 1872–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 33.McClaran SR, Wetter TJ, Pegelow DF, Dempsey JA. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol 86: 1357–1366, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol 22: 95–108, 1967. [DOI] [PubMed] [Google Scholar]
- 36.Mink S, Ziesmann M, Wood LD. Mechanisms of increased maximum expiratory flow during HeO2 breathing in dogs. J Appl Physiol 47: 490–502, 1979. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol 167: 116–132, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 2: 592–607, 1950. [DOI] [PubMed] [Google Scholar]
- 39.Palmer GS, Dennis SC, Noakes TD, Hawley JA. Assessment of the reproducibility of performance testing on an air-braked cycle ergometer. Int J Sports Med 17: 293–298, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Paton CD, Hopkins WG. Tests of cycling performance. Sports Med 489–496, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen OF, Butler JP. Expiratory flow limitation. Compr Physiol 1: 1861–1882, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571: 425–439, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D. Physiological mechanisms of sex differences in exertional dyspnea: role of neural respiratory motor drive. Exp Physiol 99: 427–441, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537: 277–289, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol 107: 1622–1628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheel AW, Romer LM. Ventilation and respiratory mechanics. Compr Physiol 2: 1093–1142, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Spitler DL, Horvath SM, Kobayashi K, Wagner JA. Work performance breathing normoxic nitrogen or helium gas mixtures. Eur J Appl Physiol 43: 157–166, 1980. [DOI] [PubMed] [Google Scholar]
- 48.Sporer BC, McKenzie DC. Reproducibility of a laboratory based 20-km time trial evaluation in competitive cyclists using the Velotron Pro ergometer. Int J Sports Med 28: 940–944, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Wanke T, Formanek D, Schenz G, Popp W, Gatol H, Zwick H. Mechanical load on the ventilatory muscles during an incremental cycle ergometer test. Eur Respir J 4: 385–392, 1991. [PubMed] [Google Scholar]
- 50.Wilson GD, Welch HG. Effects of varying concentrations of N2/O2 and He/O2 on exercise tolerance in man. Med Sci Sports Exerc 12: 380–384, 1980. [PubMed] [Google Scholar]