Abstract
We tested the hypothesis that nicotine, which acts peripherally to promote coughing, might inhibit reflex cough at a central site. Nicotine was administered via the vertebral artery [intra-arterial (ia)] to the brain stem circulation and by microinjections into a restricted area of the caudal ventral respiratory column in 33 pentobarbital anesthetized, spontaneously breathing cats. The number of coughs induced by mechanical stimulation of the tracheobronchial airways; amplitudes of the diaphragm, abdominal muscle, and laryngeal muscles EMGs; and several temporal characteristics of cough were analyzed after administration of nicotine and compared with those during control and recovery period. (−)Nicotine (ia) reduced cough number, cough expiratory efforts, blood pressure, and heart rate in a dose-dependent manner. (−)Nicotine did not alter temporal characteristics of the cough motor pattern. Pretreatment with mecamylamine prevented the effect of (−)nicotine on blood pressure and heart rate, but did not block the antitussive action of this drug. (+)Nicotine was less potent than (−)nicotine for inhibition of cough. Microinjections of (−)nicotine into the caudal ventral respiratory column produced similar inhibitory effects on cough as administration of this isomer by the ia route. Mecamylamine microinjected in the region just before nicotine did not significantly reduce the cough suppressant effect of nicotine. Nicotinic acetylcholine receptors significantly modulate functions of brain stem and in particular caudal ventral respiratory column neurons involved in expression of the tracheobronchial cough reflex by a mecamylamine-insensitive mechanism.
Keywords: nicotinic receptors, mecamylamine, brain stem, ventral respiratory group
cough is a manifestation of prolonged cigarette smoking, but only a subset of smokers will develop chronic productive cough and other sequelae of chronic obstructive lung disease (22). Paradoxically, cough sensitivity to capsaicin is reduced in chronic smokers with no other health conditions (27, 34), and this sensitivity is restored after smoking cessation (52). The reasons for this alteration in cough sensitivity in smokers are unknown. Approximately 50% of smokers undergo a period of increased coughing after smoking cessation (15). This observation is consistent with the idea that not all elements of cigarette smoke activate cough-promoting mechanisms. Nonnicotine components of cigarette smoke, such as particulates, probably have an important role in eliciting cough directly or promoting its production through the enhancement of airway inflammation (53). However, dose-dependent increases in cough occur following nicotine exposure in humans (19), and this drug surely contributes to coughing in smokers (26). Nicotine can induce cough via the inhaled or intravenous (iv) routes of administration (21, 27). The manifestation and extent of coughing in smokers may be the result of a balance between excitatory and inhibitory mechanisms as a result of complex actions of components of cigarette smoke.
Nicotine acts on nicotinic acetylcholine receptors (nAChR), including those on the sensory elements of the pulmonary and airway mucosa (26, 27), stimulating and/or upregulating the activity of airway sensory afferents, including bronchopulmonary sensory C-fibers and receptors with myelinated vagal afferents. Moreover, nAChR are coexpressed with transient receptor potential vanilloid channel family member 1 (TRPV1), which are primary channels responding to the cough-inducing irritant capsaicin (13, 32).
Nicotine penetrates into the central nervous system, activating neuronal nAChR and contributing to regulation and modulation of many neural functions (2, 16). The distribution of diverse types of nAChR in the brain is uneven, but nAChR are present in virtually all brain regions, including the brain stem (31). nAChR are also found on brain stem respiratory-related neurons (12, 35), and they are significantly involved in respiratory rhythm and pattern generation (20, 50).
Several brain stem areas, such as the solitary tract nucleus, medullary and pontine respiratory groups, and medullary raphe nuclei, contribute to the control of cough (23, 49). Recently, we explored the region of caudal ventral respiratory column (cVRC), characterized mainly by a presence of expiratory premotoneurons for its role in the control of coughing. We found that stimulation of unidentified neurons in this location by an excitatory amino acid agonist inhibits coughing (40). Excitatory amino acid neurotransmission is crucial in regulation of coughing in this area (11). Moreover, the cVRC may be involved in the cough-suppressive effects of systemically administered doses of codeine (43).
In preliminary experiments, central administration of nicotine did not enhance cough, but suppressed this behavior. We hypothesized that nicotine, in addition to its cough-promoting action on airway sensory afferents, would have a central inhibitory effect on cough by an action within the brain stem. Furthermore, we also speculated that one site of action of this drug would be neurons in the region of the cVRC.
MATERIALS AND METHODS
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the University of Florida Institutional Animal Care and Use Committees. Experiments were performed on 33 cats (4.53 ± 0.18 kg; 31 males and 2 females) under 6 protocols. All animals were anesthetized with pentobarbital sodium (35 mg/kg iv), and supplementary doses were administered (1–3 mg/kg iv) as needed. Atropine (0.1 mg/kg iv) was given at the beginning of the experiment to reduce secretions. The trachea, femoral artery, and vein were cannulated. A balloon catheter was inserted into the esophagus for the measurement of esophageal pressure. Animals were allowed to spontaneously breathe a gas mixture of 40% oxygen, balance nitrogen. Arterial blood pressure, esophageal pressure, end-tidal CO2 (ETCO2), and body temperature were continuously monitored. Body temperature was controlled by a heating pad and maintained at 37.5 ± 0.5°C. Periodically samples of arterial blood were removed for blood gas and pH analysis. Electromyograms (EMG) of respiratory muscles were recorded with bipolar insulated fine-wire hook electrodes. EMGs were recorded bilaterally from the expiratory transversus abdominis and external oblique muscles (ABD), inspiratory parasternal (PS) muscles and unilaterally from the laryngeal abductor posterior cricoarytenoid (PCA) and laryngeal adductor thyroarytenoid muscles [for details, see Poliacek et al. (40)]. In animals with intravertebral [intra-arterial (ia)] injections, a cannula was introduced into the left axillary artery, and the tip was positioned near the origin of the vertebral artery. All other branches of the axillary artery in the region were clamped. Animals with microinjections of neurochemicals into the medulla were placed prone in a stereotaxic apparatus, and the dorsal surface of the medulla was exposed surgically. The surface of the brain stem was covered by warm paraffin oil.
Composite three-barrel micropipettes with a carbon filament microelectrode were used for pressure microinjection of (−)nicotine (5 mM; Sigma-Aldrich) and mecamylamine (15 mM; Sigma-Aldrich) dissolved in artificial cerebrospinal fluid. In one experiment, a single-barrel micropipette was employed in nicotine microinjection. The tip of the micropipette/microelectrode was positioned under stereotaxic control into the region of cVRC unilaterally (2–3 mm caudal to the obex, 3–3.3 mm lateral to the midline, 3.0–4.0 mm below the dorsal medullary surface). The injected volume was monitored by observation of movement of the meniscus in the micropipette barrel with a microscope. Injection sites were labeled by fluorescent latex beads as reported previously (43). The composite micropipette-microelectrodes allowed recording of expiratory neuronal activities for physiological confirmation of micropipette tip location within the cVRC. In addition to that, appropriate positions of the micropipette tips were confirmed by the occurrence of a labeled spot in or near the caudal retroambigual nucleus [see also Poliacek et al. (40, 43)].
Tracheobronchial cough was elicited by mechanical stimulation of the intrathoracic airways with a thin polyethylene catheter or a custom mechanical stimulation device composed of one to three nylon fiber loops. This stimulator was inserted into the trachea (moved back and forth and rotated at a frequency of 1–2 Hz) for periods of 10 s or in five cats 20 s to elicit repetitive coughing. Cough was defined by a large burst of inspiratory-related PS EMG activity, immediately followed by a burst of expiratory ABD EMG activity and by a related inspiratory-expiratory waveform of esophageal pressure. These criteria separated cough from other airway defensive behaviors, such as augmented breath, and mainly from the expiration reflex that is inducible from the trachea as well (41).
All EMGs were amplified, filtered (300–5,000 Hz), rectified, and integrated (time constant 200 ms). The number of coughs in response to mechanical stimulation of the trachea [cough number (CN), average number of coughs per 10-s stimulation], amplitudes of PS, ABD EMG moving averages, and amplitudes of esophageal pressure during appropriate phases of cough and breathing, respiratory rate, blood pressure, heart rate, and ETCO2 were analyzed in control preinjection and postinjection periods. In addition, we analyzed under nicotine ia injection and nicotine microinjection protocols the duration of inspiratory (CTI) and expiratory phases of cough (CTE), the duration of PS and ABD EMG activity during the cough reflex, the duration of overlap of these two activities in inspiratory-expiratory cough transition, the duration from the PS to ABD maximum, the duration of the total cough cycle (CTtot), and the active portion of coughing (CTI + CTE1). The cough inspiratory phase was defined as the period from the onset of PS EMG activity until its maximum during cough. The cough expiratory phase was defined as the interval from the maximum of PS activity to the onset of the next PS EMG burst (58). This phase consists of active (CTE1; from the maximum of PS activity to the offset of cough-related ABD activity) and quiescent (CTE2; from the offset of cough ABD activity to the next PS muscles activation) periods (58). The amplitudes of PCA and thyroarytenoid activities were analyzed separately in each of four laryngeal cough phases (42).
Monitored cardio-respiratory parameters were measured in related periods during two to three consecutive breathing cycles. These parameters were taken just before the last preinjection cough trial (in the control cough period) and right before the first, or possibly the second, cough trial after the injection (microinjection or intravertebral application of the agent).
After the microinjection experiments, the caudal medulla was removed for histological processing. The tissue was fixed in 4% paraformaldehyde followed by 30% sucrose solution. The frozen medulla was then cut into transverse slices (thickness 100 or 200 μm) by a freezing microtome. Sections were examined under light and UV microscopy for detection and localization of injection sites. We identified all 14 (5 of these for mecamylamine and nicotine microinjections) locations of microinjections, 7 in and 7 near the nucleus retroambigualis as in our laboratory's previous reports (40, 43). In all 13 locations where composite microelectrode-micropipettes were used, we recorded single or multiunit expiratory neuronal activity. In two of our cats used for microinjections of nicotine, we also performed vehicle artificial cerebrospinal fluid microinjections. The data from these control microinjections corresponded well with our laboratory's previous analysis of artificial cerebrospinal fluid microinjections within the cVRC area (40, 43); therefore, we do not report data from the vehicle microinjections again.
Results are expressed as mean values ± SE (standard error). For statistical analysis, repeated measures or ordinary ANOVA with Student-Newman-Keuls and/or Dunnett's posttest, Friedman and Kruskal-Wallis test with Dunn posttest, paired t-test, or Wilcoxon matched pairs test were employed as appropriate. For analysis of effective nicotine doses that reduced individual coughing parameters by 50% (ED50), Mann-Whitney test of log dose was used. The differences of variables were considered significant if P < 0.05.
Protocols.
Four protocols were based on injection of agents in the vertebral artery. (−)Nicotine hydrogen tartrate salt, nicotine (+) stereoisomer in the form of (+)nicotine-(+)di-p-toluoyltartrate salt, and mecamylamine hydrochloride were used. Dose responses (saline vehicle injection followed by increasing cumulative doses) of nicotine (5 cats, nicotine doses 0.1, 0.33, 1, 3.3, 10, 33 μg/kg, free base), its stereoisomer (+)nicotine (5 cats, 0.1, 0.33, 1, 3.3, 10, 33, 100, 330 μg/kg), nicotine (the same dosages as for nicotine only) after a pretreatment with intravertebral dose of 24 μg/kg of mecamylamine (5 cats, 1 of them did not cough and was used only for cardiorespiratory analysis) (Fig. 1, Table 1), and nicotine (the same dosages as for nicotine only) after iv pretreatment with mecamylamine (0.24 mg/kg) and a waiting period of 25 min (5 cats) were measured. ED50 were obtained by regression analysis of dose-response relationships. Under the fifth protocol, eight cats were microinjected with (−)nicotine (5 mM, 20–54 nl). The sixth protocol was conducted on two of these cats and three additional animals. In these five cats, microinjections of mecamylamine alone (15 mM, 33–40 nl) were conducted. Then mecamylamine was microinjected (15 mM, 35–40 nl) again, and this intervention was followed 1 min later by microinjection of nicotine (5 mM, 28–40 nl). Intravertebral injections lasted ∼20 s; microinjections were initiated and completed in 5–10 s.
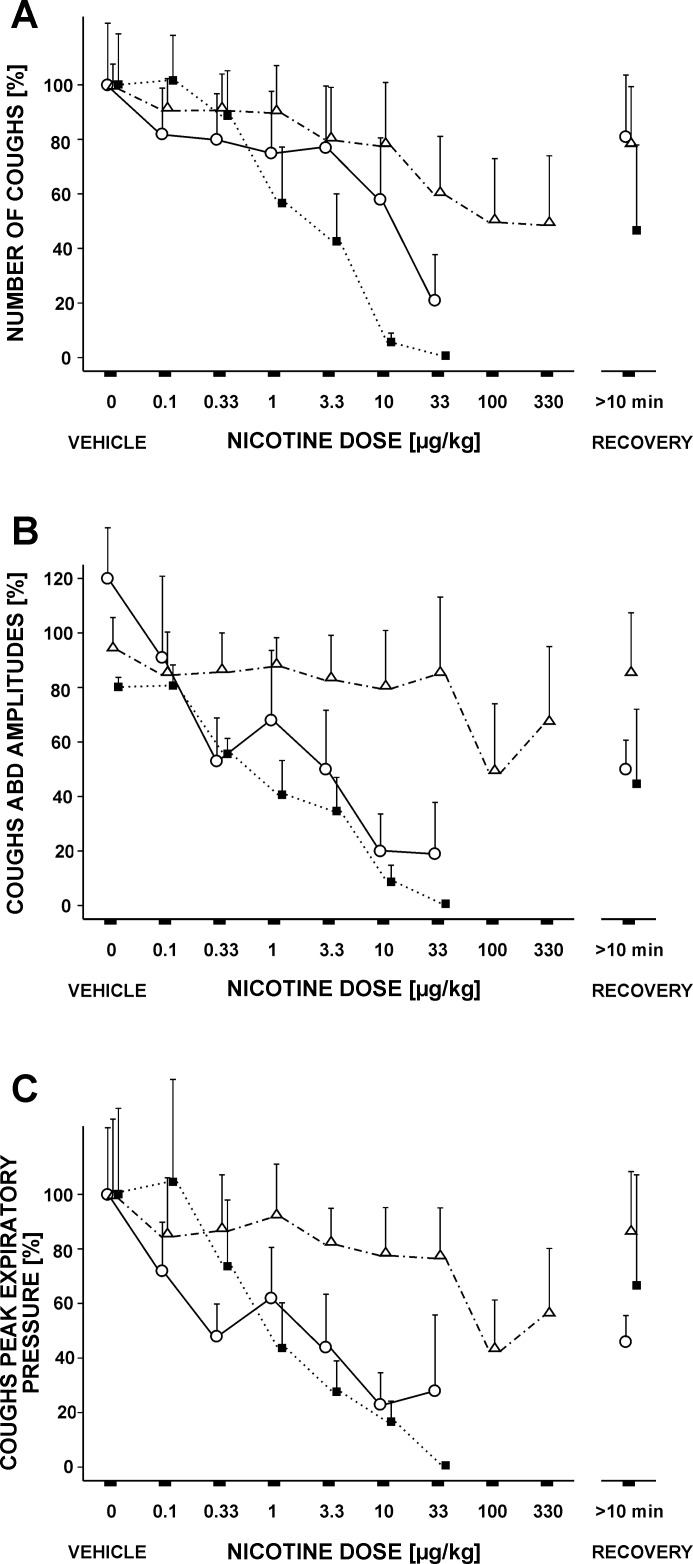
Fig. 1.
Cough reduction induced by increasing doses of nicotine [intra-arterial (ia)]. A: cough number (CN). B: amplitudes of cough abdominal muscle EMGs. C: peak cough expiratory pressures. (−)Nicotine (ia; ○, solid line, n = 5) as well as (−)nicotine (ia) after mecamylamine (ia; ■, dotted line, n = 5) markedly reduced CN at doses of 10 and 33 μg/kg and expiratory efforts of cough at doses of 3.3 μg/kg and higher. (+)Nicotine (△, dotted and dashed line; n = 4) was less effective than the (−) isomer. Values are means ± SE, for CN and esophageal pressures expressed as percentages of prenicotine values (saline for nicotine and mecamylamine for its intravertebral administration).
Table 1.
Selected coughing-related parameters and heart rates during increasing doses of (−)nicotine, (+)nicotine, and (−)nicotine after pretreatment with mecamylamine
| Dosage (−)NIC | PS Cough, % | EP I Cough, kPa | HR After NIC Admin. Only, beats/min | HR After Pretreatment with MEC and NIC, beats/min | Dosage (+)NIC | HR After (+)Stereoisomere of NIC, beats/min |
|---|---|---|---|---|---|---|
| Saline ia | 169 ± 33a | −6.8 ± 1.2a | 232 ± 10b,d | 187 ± 6 | 195 ± 8 | |
| MEC ia | 189 ± 5 | |||||
| NIC 0.1 | 191 ± 63b | −6.2 ± 0.7 | 226 ± 10b,d | 189 ± 6 | (+)NIC 0.1 | 195 ± 7 |
| NIC 0.33 | 109 ± 24 | −5.2 ± 1.2 | 227 ± 10b,d | 186 ± 5 | (+)NIC 0.33 | 196 ± 10 |
| NIC 1 | 131 ± 42 | −5.3 ± 1.1 | 231 ± 11b,d | 186 ± 6 | (+)NIC 1 | 196 ± 10 |
| NIC 3.3 | 138 ± 23 | −5.5 ± 1.6 | 220 ± 11a,d | 181 ± 6 | (+)NIC 3.3 | 195 ± 9 |
| NIC 10 | 104 ± 24 | −4.8 ± 1.5 | 205 ± 10 | 182 ± 5 | (+)NIC 10 | 195 ± 9 |
| NIC 33 | 51 ± 8 | −4.2 ± 1.4 | 200 ± 10 | 181 ± 4 | (+)NIC 33 | 188 ± 10 |
| (+)NIC 100 | 193 ± 9 | |||||
| (+)NIC 330 | 194 ± 10 | |||||
| Recovery | 154 ± 31a | −4.9 ± 2.0 | 236 ± 12c,e |
Values are means ± SE. Dosages (in μg/kg) are of (−)nicotine (NIC; n = 5 cats), (+)nicotine [(+)NIC; n = 5 cats], and (−)NIC after pretreatment with mecamylamine (MEC; n = 4 cats). ia, Intra-arterial; PS, amplitudes of parasternal muscle EMG, EP I, inspiratory amplitudes of esophageal pressure; HR, heart rate.
P < 0.05,
P < 0.01,
P < 0.001 vs. 33 μg/kg of NIC.
P < 0.05,
P < 0.01 vs. 10 μg/kg of NIC.
Fifteen to twenty-five consecutive cough stimulation trials separated by ∼1 min were conducted to establish a stable cough baseline. Then three to five control preinjection trials were performed (each separated by ∼1 min). Another three to five trials were performed after each injection/microinjection during the period of ∼5 min. An additional three to five cough trials were performed in the 10–30 min after the last injection interval. Magnitudes of the moving averages (and for relative measures also other parameters, e.g., amplitudes of esophageal pressure) during coughing were normalized relative to the mean values for control preinjection coughs (the average magnitudes of all control coughs for each particular parameter). All parameters were averaged over each group of three to five trials. When long intervals occurred in the protocol in which mechanical stimuli were not applied to the trachea, such as the recovery period, we often observed a reduced cough response during the first trial after this interval. We eliminated this cough trial from the analysis due to unstable and reduced coughing. This transient reduction in cough excitability after a delay or gap between tracheobronchial stimulation trials occurs regularly, even in vehicle-treated animals (43).
RESULTS
(−)Nicotine applied into vertebral artery dose dependently reduced CN (Fig. 1) with the ED50 value 31 μg/kg. Cough-related amplitudes of ABD EMG (Fig. 1; ED50 = 5 μg/kg) and expiratory esophageal pressure (Fig. 1; ED50 = 8 μg/kg) decreased in a dose-dependent manner in response to (−)nicotine (ia) as well. Reductions in inspiratory cough efforts were much less pronounced, and they are documented by lower amplitudes of PS EMG and peak inspiratory esophageal pressure [for all inspiratory efforts during coughing, Poliacek et al. (39)] at high nicotine doses (Table 1).
The expiratory amplitude of the PCA EMG during coughing decreased from 116 ± 9% for control coughs after the saline injection to 70 ± 14% (P < 0.05) for coughs after the highest dose of nicotine. The inspiratory amplitudes of PCA EMG were lower at a nicotine dose of 33 μg/kg (84 ± 21%; P < 0.05) than those in control after saline injections (104 ± 19%) and in recovery periods (108 ± 24%). The reduction in thyroarytenoid activation during coughing after (−)nicotine ia was not statistically significant.
Nicotine (ia) did not induce any significant changes in cough phase durations. However, a cumulative nicotine dose of 33 μg/kg shortened the duration of cough-related ABD activity from 0.95 ± 0.23 to 0.50 ± 0.11 s (P < 0.05), and the overlap of PS and ABD activity from 0.31 ± 0.12 to 0.09 ± 0.06 s, but this difference was not statistically significant (P > 0.05).
Intravertebral nicotine induced a dose-dependent reductions in blood pressure (Fig. 2) and heart rate (Table 1), with no significant alterations in respiratory rate (range: 20.3 ± 1.7 to 25.1 ± 1.9 breaths/min), ETCO2, magnitude of thyroarytenoid EMG activity during breathing, or the inspiratory activity of the PCA EMG. The highest nicotine dose, however, increased expiratory PCA EMG activity during breathing to 43 ± 18% (P < 0.05) from 30 ± 15% after saline control injection. Immediately after ia injection of nicotine, we observed ear twitching in our animals, starting at the dose of 3.3 or 10 μg/kg [at 10, 30, or 100 μg/kg for (+)nicotine].
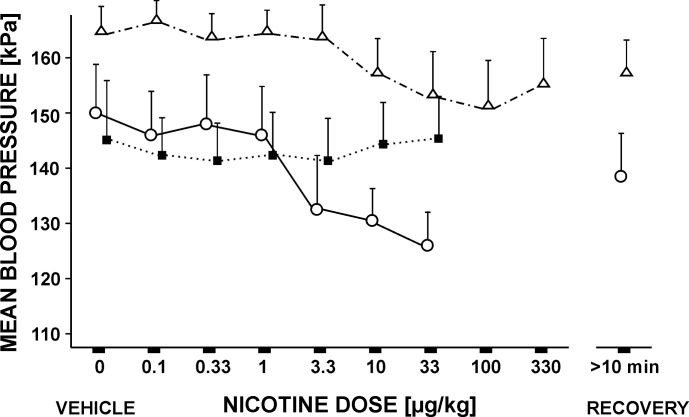
Fig. 2.
Mean arterial blood pressures during increasing doses of nicotine (ia). (−)Nicotine (ia; ○, solid line; n = 5), as well as stereoisomer (+)nicotine (ia; △, dotted and dashed line; n = 5), significantly reduced blood pressure at doses of 10 μg/kg and higher. Mecamylamine (24 μg/kg) injected in vertebral artery (■, dotted line; n = 5) prevented the depressor effect of nicotine. Values are means ± SE.
Mecamylamine did not prevent the inhibitory effect of nicotine on cough. Pretreatment with 240 μg/kg mecamylamine (iv; 7 cats) reduced CN (4.32 ± 0.38; P < 0.05 compared with control, 5.38 ± 0.35; and 25 min postinjection recovery, 5.24 ± 0.54) and amplitudes of cough ABD EMG (to 66%; P < 0.05 vs. control and recovery). Pretreatment with 24 μg/kg mecamylamine (ia) did not significantly reduce CN, amplitudes of cough PS and ABD EMGs, and cough inspiratory and expiratory esophageal pressure compared with saline controls. Neither mecamylamine iv nor ia eliminated or reduced the cough suppressant action of nicotine (Fig. 1). The potencies for (−)nicotine (ia) after mecamylamine (ia) to inhibit cough-related parameters were not significantly changed (CN ED50 = 3 μg/kg, ABD ED50 = 3 μg/kg, expiratory esophageal pressure ED50 = 3 μg/kg; Fig. 1; all P > 0.05 vs. nicotine ED50 values without mecamylamine pretreatment). Similarly, (−)nicotine injected ia 25 min after the iv pretreatment with 0.24 mg/kg of mecamylamine suppressed cough (data not shown in Figs. 1 and 2 and Tables 1 and 2) with CN ED50 = 8 μg/kg, ABD ED50 = 11 μg/kg, and expiratory esophageal pressure ED50 = 11 μg/kg (all P > 0.05 vs. nicotine without mecamylamine pretreatment and nicotine after mecamylamine ia ED50 values). No significant differences were found for the effect of (−)nicotine (ia) after mecamylamine (ia or iv) on respiratory rate and ETCO2, and the reductions of cough PS EMG and inspiratory esophageal pressure amplitudes did not reach statistical significance. However, pretreatment with mecamylamine (ia or iv) prevented (−)nicotine (ia)-induced depression of blood pressure (Fig. 2) and heart rate (Table 1). This pretreatment also blocked ear twitching observed after administration of nicotine ia. Mecamylamine alone (ia) had no significant effect on blood pressure, respiratory rate, heart rate, or ETCO2.
Table 2.
Coughing values during microinjections of (−)nicotine into the caudal ventral respiratory column area
| Control | Microinjection, %Control | >10 min Later, %Control | |
|---|---|---|---|
| CN, no. | 3.41 ± 0.48 | 53 ± 11b | 99 ± 8e |
| MN | 5.56 ± 1.16 | 75 ± 7a | 99 ± 11 |
| PS ips, % | 90 ± 6 | 65 ± 7a | 88 ± 11d |
| MN | 70 ± 15 | 50 ± 14 | 78 ± 13d |
| PS con, % | 92 ± 4 | 66 ± 8a | 99 ± 11d |
| MN | 117 ± 22 | 107 ± 46 | 201 ± 99 |
| EP I, cmH2O | −6.37 ± 1.22 | 103 ± 11 | 108 ± 10 |
| MN | −6.97 ± 1.52 | 76 ± 11a | 93 ± 11d |
| ABD ips, % | 100 ± 15 | 51 ± 10b | 93 ± 13e |
| MN | 83 ± 18 | 46 ± 10a | 86 ± 23d |
| ABD con, % | 99 ± 12 | 48 ± 10b | 92 ± 15e |
| MN | 79 ± 15 | 43 ± 8b | 81 ± 16e |
| EP E, cmH2O | 14.24 ± 2.76 | 53 ± 10b | 79 ± 7d |
| MN | 11.13 ± 3.30 | 57 ± 15c | 104 ± 8f |
| CTI, s | 1.37 ± 0.13 | 116 ± 6 | 108 ± 10 |
| CTE, s | 2.08 ± 0.32 | 119 ± 10b | 97 ± 5d |
| CTE1, s | 0.97 ± 0.15 | 91 ± 7 | 101 ± 7 |
| CTE2, s | 1.13 ± 0.28 | 134 ± 24a | 100 ± 8 |
| TABD, s | 1.11 ± 0.24 | 71 ± 11a | 92 ± 7 |
| Overlap, s | 0.39 ± 0.11 | 47 ± 13b | 78 ± 9d |
| CTtot, s | 3.45 ± 0.38 | 118 ± 8a | 101 ± 6 |
Values are means ± SE; n = 8 cats (n = 5 cats for MN values). MN, postnicotine data after MEC microinjections; CN, number of coughs per 10 s stimulation; ips, ipsilateral; con, contralateral; ABD, amplitudes of abdominal muscle EMG; EP E, expiratory amplitudes of esophageal pressure; CTI, duration of inspiratory phase; CTE, CTE1, and CTE2, durations of cough expiratory, active expiratory, and passive expiratory phase, respectively; TABD, duration of cough abdominal muscles activity; Overlap, overlapping of PS and ABD cough related activity; CTtot, duration of the total cough cycle.
P < 0.05,
P < 0.01,
P < 0.001 vs. control.
P < 0.05,
P < 0.01,
P < 0.001 vs. (−)NIC microinjection data.
The (+)stereoisomer of nicotine (ia) had a similar suppressive effect on cough as (−)nicotine, but at higher dosages (Fig. 1). The potencies for (+)nicotine (ia) were as follows: CN ED50 = 1 mg/kg [P > 0.05 vs. all (−)nicotine ED50], ABD ED50 = 387 mg/kg, and expiratory esophageal pressure ED50 = 4 mg/kg [P < 0.05 for both ABD and esophageal pressure ED50 vs. (−)nicotine ABD and esophageal pressure ED50]. Blood pressure was reduced at similar doses with (+)nicotine as for (−)nicotine at 33 μg/kg (Fig. 2). However, (+)nicotine (ia) had no significant effect on the amplitudes of PS EMG and inspiratory esophageal pressure in cough, or on respiratory rate, heart rate (Table 1), and ETCO2.
Thirteen unilateral microinjections of nicotine (5 mM, 33 ± 2 nl) were performed in nine cVRC locations in eight cats. These unilateral microinjections significantly suppressed coughing. CN, expiratory cough efforts, and amplitudes of PS (Table 2) and inspiratory (to 89 ± 16%; P < 0.05 vs. control 102 ± 18% and recovery 104 ± 22%) and expiratory PCA EMG moving averages (to 73 ± 4%, P < 0.01 vs control) were reduced by microinjection of (−)nicotine. The reduction in thyroarytenoid EMG amplitudes during coughing did not reach statistical significance. We found mild but significant prolongations in relative durations (expressed as percentages of control values) of CTE, CTE2, and CTtot (Table 2) induced by microinjections of (−)nicotine. The overlap of PS and ABD EMG activity in the cough inspiratory-expiratory phase transitions was shortened by more than one-half, and the relative duration of ABD activation during coughing was shorter as well (Table 2). Microinjections of nicotine in cVRC had no significant effect on respiratory rate (19.0 ± 1.9 to 19.9 ± 1.9 breaths/min), heart rate (190 ± 10 to 193 ± 11 beats/min), blood pressure (121 ± 5 to 122 ± 5 mmHg), and ETCO2 in our animals.
Mecamylamine microinjections (15 mM, 38 ± 2 nl; 5 cats) had no effect on CN (all P > 0.18) or inspiratory and expiratory cough efforts (all P > 0.49). In addition, mecamylamine microinjections (15 mM, 38 ± 1 nl; 5 cats) did not significantly reduce the cough suppression induced by nicotine (5 mM, 36 ± 2 nl; 5 cats) microinjected 1 min after mecamylamine in the same location of cVRC (Table 2). The expiratory cough efforts were reduced in a similar manner to those after nicotine microinjections without mecamylamine pretreatment (Table 2). CN decreased less (Table 2), but no significant difference was found between the depressions of CN that was induced by microinjection of nicotine, with and without pretreatment with mecamylamine administered by microinjection.
DISCUSSION
Our results have shown an important role of nicotine in central neuronal control of coughing. Administration of nicotine into the brain stem circulation or directly into the caudal medulla by microinjection suppressed mechanically induced tracheobronchial cough.
Central action of nicotine.
Peripheral excitatory (19, 26, 27, 53) and central inhibitory effects of cigarette smoke on coughing may explain why smokers, after an initial heightened cough response, are able to engage in this behavior for years without significant coughing. Presumably only when significant airway pathology emerges is this central antitussive effect of nicotine overcome, leading to the development of chronic cough.
Central administration of (−)nicotine reduced coughing. The lack of significant effect on the thyroarytenoid activity relative to the action on PCA motor drive supports a stronger role of nicotinic cholinergic modulation of laryngeal abduction compared with adduction, at least during coughing. The region of cVRC, where microinjections of (−)nicotine also suppressed cough (Table 2), is not thought to play an important role in PCA motor activity during breathing, but may be important in regulating laryngeal tone during nonbreathing behaviors, such as vocalization (51).
Cough phase timing alterations after intravertebral injections of (−)nicotine were limited to shorter ABD EMG activity. Reduced cough ABD activation could contribute to this effect by a shortening of preexpulsive activity (10). Reduced activation of PS and ABD in postnicotine coughs may contribute to shorter temporal overlap of PS and ABD bursting. These data are consistent with limited effects of nicotine on elements of the respiratory/cough central pattern generator that regulate respiratory muscle phase timing. Cholinergic neurotransmission is important for the generation of fictive breathing in rat brain stem slices (50). These apparently differing results could be reconciled by multiple factors, including species, preparation, or even behavioral differences (20, 23, 55).
Nicotine ia reduced blood pressure and heart rate [that has been reported in cats (44)] and increased the EMG activity of the PCA in the expiratory phase of breathing. This effect during breathing on PCA activity differs from that during coughing, suggesting distinct modulatory effects of nicotine on PCA activity during eupnea and cough. Delivery of nicotine ia certainly affected brain stem areas related to cardiovascular control, e.g., solitary tract nucleus, area postrema (25), or caudal ventrolateral medulla (1), where microinjections of nicotine have produced bradycardic and hypotensive responses. The lack of cardiovascular changes after our microinjections of (−)nicotine into the cVRC is consistent with an effect of the drug on only a limited area surrounding the pipette tip, rather than diffusion to nearby cardiovascular control areas.
Stereospecificity, mecamylamine effects, effectiveness.
The (−)nicotine stereoisomer was significantly more potent in suppression of coughing than (+)nicotine, but decreased blood pressure occurred at similar dosages of the two stereoisomers, and only (−)nicotine decreased heart rate. The structural and functional complexity of nAChR possibly accounts for an uneven stereospecificity at different binding sites (14, 47). Even the nAChR antagonist mecamylamine may produce equal blockade of (−) and (+)nicotine-mediated cues (33).
Mecamylamine did not reduce cough suppression induced by ia and microinjected nicotine. The dose of mecamylamine delivered ia (unlike that injected iv) or microinjection had no significant effect on coughing and cardiorespiratory parameters on its own. These observations are consistent with a limited effect of mecamylamine on the activity of brain stem neurons (12) and suggest that mecamylamine-sensitive cholinergic neuronal modulation within the brain stem is not required for cough to occur. However, nicotine does modulate the respiratory network in rat neonatal slices that include the pre-Bötzinger area, and this effect is mecamylamine sensitive, with a limited role of α7-nAChR (50). Mecamylamine is more specific to the nAChR containing β-subunit (1, 38).
The hypotensive and bradycardic responses induced by ia doses of nicotine, which were sufficient to suppress cough, were moderate in magnitude. However, these cardiovascular effects, along with ear twitching induced by nicotine, were completely prevented by mecamylamine pretreatment. This finding is consistent with prolonged mecamylamine-induced inhibition of nAChR, particularly those receptors with β-subunits (38). Cough suppression was observed following higher ia mecamylamine doses (0.24 mg/kg used for iv injections), as well as after administration of methyllycaconitine (a more specific α7-nAChR blocker at doses above 1 μg/kg ia). Thus the usefulness of these drugs as tools to determine the nAChR receptor subtype mediating nicotine antitussive effect was limited.
Nicotine penetrates into the brain very rapidly (36) via the vertebral artery and is distributed into the limited volume (9) of the medulla and pons (44, 59). It also leaves the brain rapidly (48), with up to three times higher brain concentration than that in the blood circulation (8). The effects induced by ia nicotine and elimination of blood pressure and heart rate changes by mecamylamine confirmed that effective concentrations of these drugs were attained (maximum around 0.1 mM). Mecamylamine at amounts similar to our study (intraventricular 0.01–0.2 mg and iv 0.15–0.5 mg/kg) effectively blocked effects induced by nicotine in other studies [e.g., convulsions (7); hypothermia (18); or the duration of ear twitching and blood pressure changes (6)] in cats. Moreover, in our study, cough was suppressed by ia nicotine even after its reduction by iv pretreatment with mecamylamine (0.24 mg/kg). Furthermore, 15 mM mecamylamine microinjected in cVRC was not effective in blocking the cough suppression induced by microinjection of nicotine.
Possible mechanisms.
nAChR easily desensitize (17, 37) for periods up to several minutes (even at μM concentrations for homometric α7 and at nM concentrations for α4 containing nAChR; Ref. 45) in particular after their prolonged exposure to agonists (46). The desensitization of nAChR may contribute to the effects of nicotine observed under the conditions of tobacco smoking or other lengthy delivery of nicotine (16, 24).
Centrally located TRPV1 (57) and possibly transient receptor potential ankyrin channel family member 1 are involved in presynaptic modulation of glutamate release in particular in the solitary tract nucleus (4, 54). TRPV1-mediated modulation can alter the laryngeal chemoreflex (60). The actions of nicotine on TRPV1 (56, 57), serotonin type 3, some N-methyl-d-aspartate receptors, and K channels (47) are mostly inhibitory. Moreover, these effects occurred at nicotine concentrations around 1 mM (30, 47, 57). However, micromolar concentrations of nicotine have been reported to activate heterologously expressed mouse and human transient receptor potential ankyrin channel family member 1 (56). The efficacy (and sensitivity) in stimulating of nAChR by nicotine is at least 10 times of that for other aforementioned receptors in the recombinant Xenopus expression system (47). It is unlikely that nonspecific low-efficacy effects of nicotine contributed to our findings. Determination of the mechanisms by which nicotine induced the observed cardiorespiratory and cough effects requires further study, and this is behind the scope of our report.
nAChR are significantly affected (suppressed) by pentobarbital (35, 61) in a similar manner to a number of other types of receptors. However, cats under this type of anesthesia cough vigorously, suggesting limited disruptions in the function of neuronal network producing cough.
Microinjections.
The areas affected during microinjections were discussed in a number of previous studies (11, 29, 40). Minutes after microinjections, the nicotine concentration probably stayed effective in activation and desensitization of nAChR in an area much less than 1 mm from the center of the microinjection. The extent to which intracellular binding of nicotine (3) occurred in our experiments (and contributed to observed effects) is unknown.
The cVRC (1–4 mm caudal to obex in the cat), where microinjections of nicotine suppressed cough, is composed of a high percentage of expiratory bulbospinal neurons, which appear to have few if any axon collaterals to other regions of the brain stem (5, 28). As such, we have inferred the presence of other neurons in this region mediating the alterations in the cough excitability (through axonal connections with other brain stem regions; Ref. 40). Indeed, a silent population of neurons in cVRC with axonal projections to the contralateral VRC just rostral to the obex and the proposed function of these silent neurons in the regulation of laryngeal motor activity were identified (51). These silent neurons could represent a specific substrate for the action of nicotine [and d,l-homocysteic acid (40) or codeine (43)] that was microinjected into the cVRC.
It is now well understood that microinjection of neurotransmitter receptor agonists or antagonists into several regions of the medulla can alter cough excitability. While we have shown that a specific region of the cVRC can be a site of action for systemically administered nicotine, we propose an action of this drug in other regions of the brain stem as well. The overall inhibitory effect of nicotine on cough when administered via the vertebral artery may be a synthesis of its actions at multiple sites. This interpretation may explain analogous, but sometimes more pronounced cough alterations after cVRC microinjections of nicotine vs. its ia administration (cough inspiratory efforts, relative durations of CTE, CTE2, and CTtot; see results and Tables 1 and 2). Indeed, nAChR (and/or other nicotine-sensitive receptors) on cVRC neurons are significantly involved in modulation of coughing.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-103415 and HL-89104; Florida Dept. of Health James and Esther King Biomedical Research Program 06TSP-01, VEGA 1/0126/12; and by project Center of Experimental and Clinical Respirology (CECR II) ITMS: 26220120034. We supported research activities in Slovakia/Project, which is cofinanced by European Union sources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.P., T.E.P., P.W.D., and D.C.B. conception and design of research; I.P., M.J.R., T.E.P., A.M., L.W.-C.C., and D.C.B. performed experiments; I.P., M.J.R., T.E.P., A.M., and L.W.-C.C. analyzed data; I.P., T.E.P., P.W.D., and D.C.B. interpreted results of experiments; I.P. prepared figures; I.P. and D.C.B. drafted manuscript; I.P., P.W.D., and D.C.B. edited and revised manuscript; I.P., M.J.R., T.E.P., A.M., L.W.-C.C., P.W.D., and D.C.B. approved final version of manuscript.
REFERENCES
- 1.Aberger K, Chitravanshi VC, Sapru HN. Cardiovascular responses to microinjections of nicotine into the caudal ventrolateral medulla of the rat. Brain Res 892: 138–146, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderdice MT, Weiss GB. Ionic basis for intracellular 14C-nicotine accumulation in slices from different rat brain areas. Arch Int Pharmacodyn Ther 218: 252–267, 1975. [PubMed] [Google Scholar]
- 4.Andresen MC, Hofmann ME, Fawley JA. The unsilent majority-TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol 303: R1207–R1216, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res 401: 258–266, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Armitage AK, Milton AS, Morrison CF. Effects of nicotine and some nicotine-like compounds injected into the cerebral ventricles of the cat. Br J Pharmacol Chemother 27: 33–45, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beleslin DB, Krstic SK. Nicotine-induced convulsions in cats and central nicotinic receptors. Pharmacol Biochem Behav 24: 1509–1511, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Porchet H, Jacob P III. Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects, edited by Wonnacott S, Russell H, Stolerman IP. London: Oxford University Press, 1990, p. 112–157. [Google Scholar]
- 9.Berkenbosch A, Heeringa J, Olievier CN, Kruyt EW. Artificial perfusion of the ponto-medullary region of cats. A method for separation of central and peripheral effects of chemical stimulation of ventilation. Respir Physiol 37: 347–364, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J Appl Physiol 88: 1207–1214, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bongianni F, Mutolo D, Nardone F, Pantaleo T. Ionotropic glutamate receptors mediate excitatory drive to caudal medullary expiratory neurons in the rabbit. Brain Res 1056: 145–157, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Bradley PB, Lucy AP. Cholinoceptive properties of respiratory neurones in the rat medulla. Neuropharmacology 22: 853–858, 1983. [DOI] [PubMed] [Google Scholar]
- 13.Canning BJ. Functional implications of the multiple afferent pathways regulating cough. Pulm Pharmacol Ther 24: 295–299, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Connelly MS, Littleton JM. Lack of stereoselectivity in ability of nicotine to release dopamine from rat synaptosomal preparations. J Neurochem 41: 1297–1302, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Cummings KM, Giovino G, Jaen CR, Emrich LJ. Reports of smoking withdrawal symptoms over a 21 day period of abstinence. Addict Behav 10: 373–381, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47: 699–729, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Dani JA, Radcliffe KA, Pidoplichko VI. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. Eur J Pharmacol 393: 31–38, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hall GH. Changes in body temperature produced by cholinomimetic substances injected into the cerebral ventricles of unanaesthetized cats. Br J Pharmacol 44: 634–641, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson L, Choudry NB, Karlsson JA, Fuller RW. Inhaled nicotine in humans: effect on the respiratory and cardiovascular systems. J Appl Physiol 76: 2420–2427, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Haxhiu MA, Van Lunteren E, Van de Graaff WB, Strohl KP, Bruce EN, Mitra J, Cherniack NS. Action of nicotine on the respiratory activity of the diaphragm and genioglossus muscles and the nerves that innervate them. Respir Physiol 57: 153–169, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther 234: 1–12, 1985. [PubMed] [Google Scholar]
- 22.Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25-year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev 3: 289–298, 1994. [PubMed] [Google Scholar]
- 23.Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol 160: 289–300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19: 96–101, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Kubo T, Misu Y. Changes in arterial blood pressure after microinjection of nicotine into the dorsal area of the medulla oblongata of the rat. Neuropharmacology 20: 521–524, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther 20: 355–364, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lee LY, Gu Q, Lin YS. Effect of smoking on cough reflex sensitivity: basic and preclinical studies. Lung 188, Suppl 1: S23–S27, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol 57: 1101–1117, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Lipski J, Bellingham MC, West MJ, Pilowski P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods 26: 169–179, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhu W, Zhang ZS, Yang T, Grant A, Oxford G, Simon SA. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J Neurophysiol 91: 1482–1491, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, Saga T, Saji H. Quantification of human nicotinic acetylcholine receptors with 123I-5IA SPECT. J Nucl Med 45: 1458–1470, 2004. [PubMed] [Google Scholar]
- 32.Mazzone SB. Sensory regulation of the cough reflex. Pulm Pharmacol Ther 17: 361–368, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer LT, Rosecrans JA, Aceto MD, Harris LS. Discriminative stimulus properties of the optical isomers of nicotine. Psychopharmacology (Berl) 68: 283–286, 1980. [DOI] [PubMed] [Google Scholar]
- 34.Millqvist E, Bende M. Capsaicin cough sensitivity is decreased in smokers. Respir Med 95: 19–21, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Morin-Surun MP, Champagnat J, Denavit-Saubie M, Moyanova S. The effects of acetylcholine on bulbar respiratory related neurones. Consequences of anaesthesia by pentobarbital. Naunyn Schmiedebergs Arch Pharmacol 325: 205–208, 1984. [DOI] [PubMed] [Google Scholar]
- 36.Nordberg A, Hartvig P, Lundqvist H, Antoni G, Ulin J, Långström B. Uptake and regional distribution of (+)-(R)- and (−)-(S)-N-[methyl-11C]-nicotine in the brains of rhesus monkey. An attempt to study nicotinic receptors in vivo. J Neural Transm Park Dis Dement Sect 1: 195–205, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Papke RL, Kem WR, Soti F, Lopez-Hernandez GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329: 791–807, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther 297: 646–656, 2001. [PubMed] [Google Scholar]
- 39.Poliacek I, Corrie LW, Rose MJ, Wang C, Bolser DC. Influence of microinjections of d,l-homocysteic acid into the Botzinger complex area on the cough reflex in the cat. J Physiol Pharmacol 59, Suppl 6: 585–596, 2008. [PMC free article] [PubMed] [Google Scholar]
- 40.Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol 102: 1014–1021, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poliacek I, Rose MJ, Corrie LW, Wang C, Jakus J, Barani H, Stransky A, Polacek H, Halasova E, Bolser DC. Short reflex expirations (expiration reflexes) induced by mechanical stimulation of the trachea in anesthetized cats. Cough 4: 1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poliacek I, Stransky A, Jakus J, Barani H, Tomori Z, Halasova E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res 52: 749–762, 2003. [PubMed] [Google Scholar]
- 43.Poliacek I, Wang C, Corrie LWC, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol 108: 858–865, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porsius AJ, Van Zwieten PA. The central actions of nicotine on blood pressure and heart rate after administration via the left vertebral artery of anaesthetized cats. Distribution of nicotine into the brain after central application. Arzneimittelforschung 28: 1628–1631, 1978. [PubMed] [Google Scholar]
- 45.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Reitstetter R, Lukas RJ, Gruener R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289: 656–660, 1999. [PubMed] [Google Scholar]
- 47.Schreiner BSP, Lehmann R, Thiel U, Ziemba PM, Beltrán LR, Sherkheli MA, Jeanbourquin P, Hugi A, Werner M, Gisselmann G, Hatt H. Direct action and modulating effect of (+)- and (−)-nicotine on ion channels expressed in trigeminal sensory neurons. Eur J Pharmacol 728: 48–58, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Sershen H, Lajtha A. Cerebral uptake of nicotine and of amino acids. J Neurosci Res 4: 85–91, 1979. [DOI] [PubMed] [Google Scholar]
- 49.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther 17: 369–376, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Shao XM, Feldman JL. Pharmacology of nicotinic receptors in preBötzinger Complex that mediate modulation of respiratory pattern. J Neurophysiol 88: 1851–1858, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiba K, Umezaki T, Zheng Y, Miller AD. The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp Brain Res 115: 513–519, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Sitkauskiene B, Stravinskaite K, Sakalauskas R, Dicpinigaitis PV. Changes in cough reflex sensitivity after cessation and resumption of cigarette smoking. Pulm Pharmacol Ther 20: 240–243, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson CS, Coote K, Webster R, Johnston H, Atherton HC, Nicholls A, Giddings J, Sugar R, Jackson AJ, Press NJ, Brown Z, Butler K, Danahay H. Characterization of cigarette smoke-induced inflammatory and mucus hypersecretory changes in rat lung and the role of CXCR2 ligands in mediating this effect. Am J Physiol Lung Cell Mol Physiol 288: L514–L522, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Sun B, Bang SI, Jin YH. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport 20: 1002–1006, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Szereda-Przestaszewska M, Kopczyñska B. Apnoeic responses to intracarotid nicotine challenge in anaesthetized cats. J Physiol Pharmacol 54: 151–162, 2003. [PubMed] [Google Scholar]
- 56.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JAJ, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12: 1293–1299, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Vetter I, Lewis RJ. Natural product ligands of TRP channels. In: Transient Receptor Potential Channels, edited by Islam S. New York: Springer, 2011, p. 41–85. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC. Spatiotemporal regulation of the cough motor pattern. Cough 5: 12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wellens DLF, Wouters LJMR, De Reese RJJ, Beirnaert P, Reneman RS. The cerebral blood distribution in dogs and cats. An anatomical and functional study. Brain Res 86: 429–438, 1975. [DOI] [PubMed] [Google Scholar]
- 60.Xia L, Bartlett D Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol 176: 21–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yost CS, Dodson BA. Inhibition of the nicotinic acetylcholine receptor by barbiturates and by procaine: do they act at different sites? Cell Mol Neurobiol 13: 159–172, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]