Abstract
Tinnitus has been associated with enhanced central gain manifested by increased spontaneous activity and sound-evoked firing rates of principal neurons at various stations of the auditory pathway. Yet, the mechanisms leading to these modifications are not well understood. In a recent in vivo study, we demonstrated that stimulus-timing-dependent bimodal plasticity mediates modifications of spontaneous and tone-evoked responses of fusiform cells in the dorsal cochlear nucleus (DCN) of the guinea pig. Fusiform cells from sham animals showed primarily Hebbian learning rules while noise-exposed animals showed primarily anti-Hebbian rules, with broadened profiles for the animals with behaviorally verified tinnitus (Koehler SD, Shore SE. J Neurosci 33: 19647–19656, 2013a). In the present study we show that well-timed bimodal stimulation induces alterations in the rate-level functions (RLFs) of fusiform cells. The RLF gains and maximum amplitudes show Hebbian modifications in sham and no-tinnitus animals but anti-Hebbian modifications in noise-exposed animals with evidence for tinnitus. These findings suggest that stimulus-timing bimodal plasticity produced by the DCN circuitry is a contributing mechanism to enhanced central gain associated with tinnitus.
Keywords: dorsal cochlear nucleus, multisensory integration, neural plasticity, rate-level functions, tinnitus
tinnitus, the pathological condition of phantom sound perception, has been often associated with enhanced central gain of the auditory pathway following an auditory insult. Increased spontaneous activity and tone-evoked firing rates have been reported in fusiform cells of the dorsal cochlear nucleus (DCN) (Brozoski et al. 2002; Kaltenbach et al. 2004; Dehmel et al. 2012b; Koehler and Shore 2013a) and in principal cells of the inferior colliculus (Bauer et al. 2008) and auditory cortex (Yang et al. 2007; Paul et al. 2009; Gu et al. 2010). Recent in vivo and in vitro studies of tinnitus animal models have suggested that specific synaptic plasticity mechanisms as well as changes in intrinsic excitability (Pilati et al. 2012a; Li et al. 2013) play an important role in mediating these changes.
Fusiform cells in the DCN integrate auditory input relayed by the cochlear auditory nerve fibers (ANFs) and somatosensory input relayed by the granule cells parallel fibers (Shore et al. 2000; Zhou and Shore 2004; Shore 2005; Zhan and Ryugo 2007). Importantly, the parallel fiber synapses on fusiform and cartwheel cells of the DCN have been hypothesized to carry proprioceptive information, e.g., the position of the pinna relative to sound source location (Kanold and Young 2001) and to mediate suppression of internally generated body sounds, such as self-vocalization (Zhou and Shore 2004). In vitro studies investigating these synapses have demonstrated spike-timing-dependent synaptic plasticity (STDP) leading to Hebbian learning rules in fusiform cells and anti-Hebbian learning rules in cartwheel cells (Tzounopoulos et al. 2004), and have clarified some of the mechanisms mediating these modifications (Tzounopoulos et al. 2007; Zhao and Tzounopoulos 2011). In vivo studies have shown that stimulus-timing-dependent plasticity, a macroscopic correlate of STDP, can induce a variety of learning rules in DCN fusiform cells, ranging from Hebbian to anti-Hebbian in accordance with their degree of inhibition as indicated by their receptive field type (Koehler and Shore 2013b). In the context of in vitro studies, a learning rule is a display of the percent change in the amplitude of the synaptic excitatory postsynaptic potential (EPSP) as a function of the timing of the spike relative to the EPSP at the parallel fiber synapse on the fusiform cell's apical dendrite. A learning rule is termed “Hebbian” (or “anti-Hebbian”) if the STDP-induced synaptic modifications are consistent with (or opposite to) the theory of synaptic plasticity proposed by Donald Hebb in 1949 (Hebb 1949) [see also relevant early studies on STDP (Bell et al. 1997; Magee and Johnston 1997; Markram et al. 1997)]. Equivalent learning rules can be found in in vivo investigations when the change in the firing rate of the fusiform cells is displayed against the bimodal stimulation interval, i.e., the difference between the onset of the auditory and somatosensory stimulation of DCN fusiform cells (Koehler and Shore 2013b).
Plastic adjustments of the parallel fiber synapses on DCN cells have been also implicated in tinnitus pathology (Dehmel et al. 2012b). Following noise exposure, degradation of ANF input to DCN together with strengthened somatosensory inputs (Shore et al. 2008) relayed by enhanced numbers of terminals from somatosensory brainstem nuclei (Zeng et al. 2009, 2012) is likely to change the plasticity mechanisms at these synapses leading to changes in central excitability (Koehler and Shore 2013a).
The role of intrinsic cochlear nucleus (CN) circuitry in balancing the excitation/inhibition received by the fusiform cells is a well-established factor in determining the shape of fusiform cell rate-level functions (RLFs) (Davis and Young 2000; Zhou et al. 2012). The ANFs innervating fusiform cell basal dendrites demonstrate monotonic and sigmoid-like RLFs with either low thresholds and saturation plateaus for low to medium sound levels or high thresholds and nonsaturating responses (Sachs and Abbas 1974; Yates et al. 1990; Muller et al. 1991). In contrast, fusiform cells show more complex RLFs with monotonic and nonmonotonic shapes and various dynamic ranges and saturation levels (Evans and Nelson 1973; Young and Brownell 1976; Young 1980; Rhode et al. 1983; Stabler et al. 1996; Ding and Voigt 1997). This complexity is assumed to arise mainly from the inhibition provided by both the DCN circuitry via projections of the vertical cells as well as projections of the D-multipolar/stellate cells from the ventral domain of the CN (VCN) onto fusiform cells.
Studies in the hippocampus showed that STDP protocols can induce long-term mirror changes in the degree of neural excitability of the post- and presynaptic neurons (Daoudal and Debanne 2003; Xu et al. 2005; Debanne and Poo 2010). Consistent with these observations, in vitro studies in DCN (Doiron et al. 2011) showed increased membrane depolarization in fusiform cells following long-term potentiation induction, leading to enhanced spike probability. Furthermore, previous computational and experimental investigation showed that changes in the modulatory input can alter the gain of the neural responses (Chance et al. 2002). These observations suggest that in addition to the learning rules of fusiform cells' firing rate, their RLFs may also undergo stimulus-timing-dependent changes. Consistent with this idea, Dehmel et al. (2012b) showed persistent RLF modifications induced by bimodal stimulation, which differ in control vs. noise-exposed and tinnitus animals.
In this study, we build on these findings and further investigate two hypotheses: 1) the RLF gains and maximum amplitudes of DCN fusiform cells of normal-hearing guinea pigs change following plasticity inducing bimodal stimulation in a manner consistent with Hebbian learning rules, and 2) these changes differ in noise-exposed animals with and without tinnitus.
To test these hypotheses, we assess modifications in threshold, saturation, amplitude, dynamic range, and gain of DCN fusiform cells RLFs following bimodal stimulation after the protocol of Koehler and Shore (2013a). We confirm the first hypothesis and find that the gain and maximum amplitude of fusiform cell RLFs show Hebbian learning rules in sham-exposed animals and noise-exposed animals without tinnitus. These learning rules switch to anti-Hebbian profiles in animals with tinnitus confirmed by gap detection behavioral tests. These results show for the first time in vivo, stimulus-timing-dependent modulation of central gain following bimodal stimulation and establish clear differences between changes in fusiform cell RLFs of healthy and noise-exposed animals with and without tinnitus.
MATERIALS AND METHODS
Animals
Sixteen female, pigmented guinea pigs from Elm Hill colony (Ann Arbor, MI; 300–400 g at the study onset) were used in this study. These animals were also used in a previous study (Koehler and Shore 2013a), and the data used for this article were collected at the same time as that used in the referenced study. All procedures were performed in accordance with the National Institute of Health Guidelines for the Use and Care of Laboratory Animals (NIH Publication No. 80-23) and guidelines and approval by the University Committee on Use and Care of Animals of the University of Michigan.
Experimental Design
This study was designed to assess differences in the RLF modifications induced by stimulus-timing-dependent bimodal plasticity in noise-exposed animals that developed tinnitus compared with sham- and noise-exposed animals without tinnitus. Guinea pigs were exposed to a narrow band noise to induce tinnitus. Tinnitus development was assessed by gap-induced prepulse inhibition of acoustic startle. Acute recordings after tinnitus development (or an equivalent amount of time in sham animals) allowed recordings of unit activity from 32 electrode channels. To measure RLFs, unit activity was recorded in response to tones presented at varied levels. To assess the effect of bimodal stimulation on gain and maximal response, RLFs were measured before and 5 and 15 min after bimodal stimulation. Stimulus-dependent bimodal modulation of gain and maximal response was assessed by comparing RLFs before and after bimodal stimulation with varied bimodal intervals (BIs).
Noise Exposure
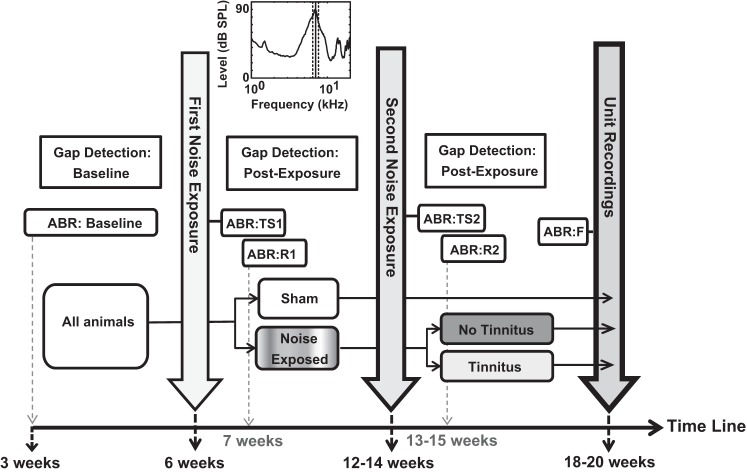
Guinea pigs were behaviorally tested biweekly before and after two sessions (each of 2 h) of noise exposure (Fig. 1) using an acoustic startle-based gap detection assay for tinnitus (see below). Guinea pigs were anesthetized with 110 mg/kg ketamine and 14 mg/kg xylazine and noise exposed to a narrow band noise (Fig. 1, top, 97 dB ¼ octave noise-band centered at 7 kHz) to induce tinnitus. The first noise exposure was at 3–6 wk of age after gap detection (baseline) testing. The left ear of each animal was exposed to the narrow band noise for 2 h while the right year was plugged with a moldable silicon ear plug. Six to eight weeks later, each guinea pig was noise exposed a second time to the same narrow band noise. The remaining six guinea pigs followed the same protocol but were instead sham exposed (no sound) at the same time. Thus the sham-exposed guinea pigs had the same experimental timeline as the noise-exposed ones except for the sham instead of noise exposure performed on this group.
Fig. 1.
Schematic of the experimental protocol. All animals received baseline gap detection and auditory brainstem response (ABR) evaluations. The animals were then sham or noise-exposed (noise spectrum shown at top). Gap detection and ABR measurements were performed to assess postexposure effects. A second noise exposure was followed by ABRs and gap detection. Gap detection responses confirmed tinnitus pathology in 60% of the animals (tinnitus group). The remaining 40% that did not satisfy the tinnitus criteria were assigned to the no tinnitus group.
Auditory Brainstem Response Recordings
Auditory brainstem response (ABR) thresholds were evaluated (while the guinea pigs were under anesthesia induced as described above) before beginning gap detection (Fig. 1, ABR:Baseline), immediately before and after the first and second noise exposure to assess threshold shifts (Fig. 1, ABR:TS1 and ABR:TS2), 1 wk after noise exposure to evaluate the recovery of thresholds (Fig. 1, ABR:R1 and ABR:R2), and immediately before unit recordings (Fig. 1, ABR:F). Before ABRs recordings, all animals were intramuscularly injected with an antibacterial solution to prevent infections (enro-floxacin; 10 mg/kg body wt, Baytril, Bayer). After ABRs, they were injected with a 10-ml sterile saline solution (0.9% sodium chloride; Hospira). ABRs were collected using BioSigRP software and RA4LI/RX8/RZ2 hardware [Tucker-Davis Technologies (TDT), Alachua, FL]. The speaker (Beyer DT 48, calibrated using SigGenRP software and RX8/PA5 hardware; TDT) was connected with the 2-cm long plastic tube inserted in the animal's ear canal. ABRs were recorded for 10-ms tone pips (2-ms ramp, 11 stimuli/s; frequencies 4–16 kHz) starting at 90 dB SPL and decremented in 10-dB steps. Each level was repeated 512 times, and the lower levels around the threshold were evaluated a second time to increase the accuracy of threshold determination. ABR waveforms were visually inspected across levels, and the threshold was determined as the lowest level of sound that resulted in one or more ABR waves being distinguishable by eye from background noise. The second set of recordings for the low levels was checked for consistency of threshold assignment.
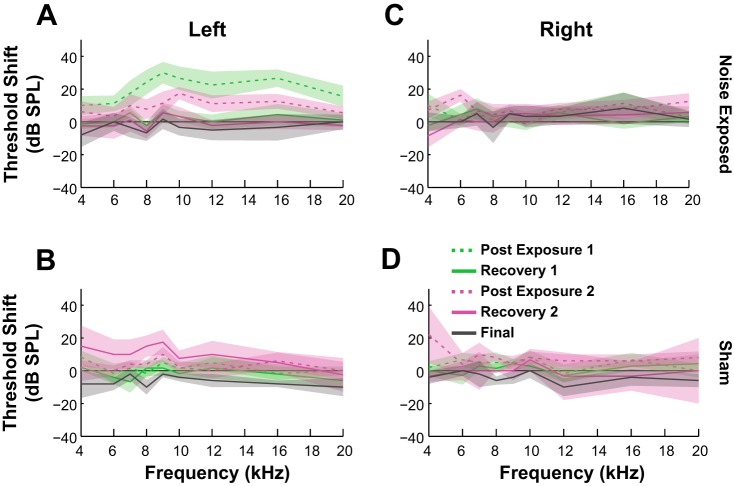
Figure 2 shows the average threshold shift recorded in the left (exposed) ear of the noise-exposed animals (Fig. 2A) compared with the unexposed ear (Fig. 2B) and in contrast with the similar measurements in sham-exposed animals (Fig. 2, C and D). The threshold shift is observed only in the noise-exposed ear. There was no important difference between the ABR threshold recoveries in noise-exposed (with or without tinnitus) and sham animals.
Fig. 2.
Evaluation of ABR threshold shift and spontaneous activity. ABR thresholds were elevated for noise-exposed guinea pigs in the exposed (A) but not the unexposed (B) ear or both ears of the sham-exposed animals (C and D). Thresholds were measured after the first noise exposure (green) and the second exposure (pink) and before the acute eletrophysiological unit recordings (gray solid line). Dashed lines represent thresholds evaluated immediately after noise exposure while solid lines indicate the measurements after recovery 1 (R1, solid green line; R2, solid pink line). Shaded bands indicate 95% confidence intervals.
Tinnitus Assessments Through Gap Detection Testing and Spontaneous Rate Evaluation
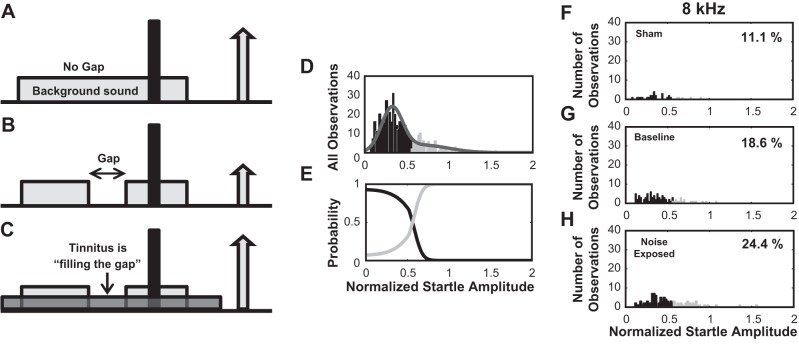
The gap detection assay is based on the method developed by Turner et al. (2006) in rat and modified for guinea pig (Dehmel et al. 2012a,b; Koehler and Shore 2013a). Briefly, female guinea pigs were placed in wire cages within single-walled, sound-isolated booths (Kinder Behavioral Testing System) on top of piezoelectric force measurement plates to measure the body movement in response to a loud startle stimulus (broadband noise, 115 dB, 200-20 kHz). The method relies on the assumption that the tinnitus percept masks the gap and leads to impaired gap detection (Fig. 3, A–C). In each gap trial, either a 50-ms silent gap or a prepulse was embedded in the background noise 50 ms before the onset of the startle stimulus. No-gap trials consisted instead of only background noise (no gap or pulse embedded; Fig. 3, A–C). The 60-dB background noise used was either broadband noise or band-pass-filtered noise with a 2-kHz band and lower cutoff frequencies of 4, 8, 12, 16, or 20 kHz. Intervals between trials were randomly varied between 18 and 24 s. On each gap detection test day, twenty trials were presented for each frequency band, half with and half without a silent gap preceding the startle stimulus. The normalized startle response for each frequency band and each test day was evaluated as the ratio Ag/Ang where Ag is the mean amplitude of the startle response from 10 trials with gap and Ang is the mean amplitude in 10 trials with no gap. Evidence for tinnitus at each frequency band was evaluated based on a Gaussian mixture model (Statistics Toolbox, Matlab release 2012b) applied to the distribution of normalized startle trials from all observations from all animals (Fig. 3D). The normalized startle responses were assumed to be drawn from one of two distributions, the no-tinnitus distribution or the tinnitus distribution characterized by elevated normalized startle amplitudes (Fig. 3E). The normalized startle observations were assigned to the tinnitus group when the probability that the observation belonged to the elevated distribution exceeded 0.55. The threshold provided by the Gaussian mixture model was used to partition the normalized startle response observations from sham and noise-exposed animals into tinnitus and no tinnitus observations. Animals from the noise-exposed group that demonstrated more tinnitus observations than baseline were considered to have tinnitus in the tested frequency and assigned to the “Exposed-Tinnitus” (ET) group (see Fig. 3, F–H, for an example). The remaining noise-exposed animals that failed this criteria were assigned to “Exposed-No Tinnitus” (ENT) group. Animals with no noise exposure were assigned to the “Sham” group. Similar to the gap detection protocol, prepulse inhibition was assessed in all animals and no differences were found in any of the animal groups before and after noise exposure. As an adjunct to gap detection, the distribution of spontaneous rates recorded from fusiform cells was analyzed to investigate whether increased spontaneous rates can be observed in the tinnitus animals at frequencies close to the noise exposure frequencies.
Fig. 3.
Gap detection assay for behavioral evaluation of tinnitus. A: healthy, sham-exposed animals respond with a robust startle to the presentation of a sound pulse (10 ms, 115 dB, represented by the black, tall bar) embedded in a continuous background sound (60 dB). B: when a silent gap (50 ms) is introduced in the background sound, the animal uses the gap to predict the incoming startle pulse and responds with decreased startle amplitude. C: noise-exposed guinea pigs that developed tinnitus fail to detect the gap due to their tinnitus percept and respond with a strong startle to the pulse presentation. D–E: Gaussian mixture model was employed to partition the distribution of startle observations into normal and tinnitus distributions. D: example of histogram of the normalized startle distribution (gray line) partitioned into a distribution with no evidence for tinnitus (black bars) and a distribution with evidence for tinnitus (light gray bars). E: probability functions that the normalized startle amplitudes belong to tinnitus (light gray curve) or no-tinnitus (black curve) distributions. F–H: example of histogram partitioned distributions of normalized startle observations evaluated at 8 kHz in sham-exposed (F), baseline (G), and noise-exposed animals (H). Percentage of observations classified as tinnitus is shown in F–H.
Electrophysiology
Surgical methods.
Guinea pigs were anesthetized [subcutaneous injection of ketamine (40 mg/kg; Putney, Portland, OR) and xylazine (10 mg/kg; Lloyd) followed by local subcutaneous injections of lidocaine, 4 mg/kg at the incision site], and their eyes were protected with ophthalmic ointment. The animals' heads were rigidly fixed in a Kopf stereotaxic frame by means of hollow ear bars placed in their ear canals and a bite bar. A temperature-controlled heating pad and anal probe maintained body temperature at 38 ± 0.5 °C. A rostral-caudal incision was made and the skin was retracted to reveal the skull. After craniotomy, a small amount of cerebellum was aspirated (leaving the paraflocculus intact) to allow for visual placement of the recording electrode in the DCN. The 4-shank, 32-channel silicon substrate electrode [model A32 (A4 × 8), site spacing = 100 um, shank pitch = 250 um, impedance = 1–3 MΩ; NeuroNexus, Ann Arbor, MI] was inserted with the tips 0.8–1 mm below the DCN surface with the shanks in rostral-caudal position in an approximately isofrequency layer. A second concentric bipolar stimulating electrode (FHC, Bowdoin, ME) was dipped in Fluorogold and stereotaxically placed in the left spinal trigeminal nucleus (Sp5; 0.28 ± 0.03 cm lateral from midline, 0.25 ± 0.02 cm caudal from transverse sinus, and 0.9 ±0.1 cm below the surface of the cerebellum). The location of the stimulating electrode was confirmed post mortem by histological localization of the Fluorogold stain.
Auditory and somatosensory stimulation.
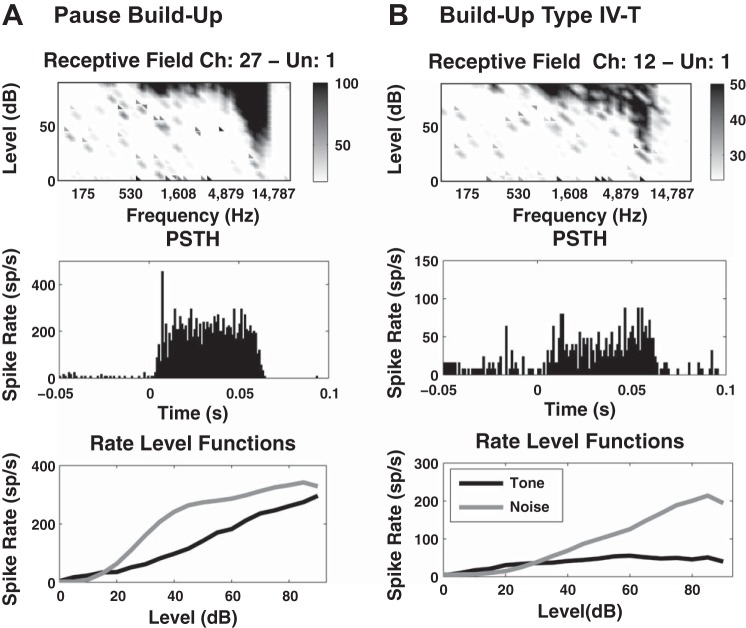
The best frequency (BF) of each fusiform cell was assessed based on its receptive field, constructed by counting the number of spikes produced in response to a total of 7,600 sound bursts over a frequency range of 100–24 kHz (in 0.2-octave steps) and an intensity range of 0–90 dB (in 5-dB steps). Poststimulus histograms, tone, and noise RLFs were collected to determine the unit type based on previously described criteria (Evans and Nelson 1973; Young and Brownell 1976; Young 1980; Rhode et al. 1983; Stabler et al. 1996; Ding and Voigt 1997) (see 2 representative examples in Fig. 4, A and B). A distribution of best frequencies was constructed for all the cells recorded in each animal, across the four shanks of the recording probe. The protocol frequency was set to coincide with the BF of at least 70% of cells that showed a receptive field. This parameter was constant for the duration of individual experiments (animals) but varied across experiments (in the range of 4–14.6 kHz) depending on the precise position of the probe in the isofrequency domain of DCN. The probe was positioned to target a protocol frequency within the frequency bandwith for which the animals were tinnitus confirmed. For the present article, only cells with BF in a 20% interval (linear scale) around the protocol frequency were considered for data analysis.
Fig. 4.
Examples of fusiform cell types. The type of the fusiform cells was determined based on their receptive field (top), poststimulus histograms (PSTH; middle), and tone and noise evoked RLF responses (bottom) and according to classical scheme of classification (Stabler et al. 1996). A: example of a pause build-up unit type I with best frequency (BF) = 11,200 Hz and threshold (Thr) = 25 dB. B: example of a build-up unit type IV-T with BF = 8,500 Hz and Thr = 20 dB.
RLFs were collected from fusiform cells of all animals in response to pseudorandom presentations of tones at protocol frequency and intensities ranging from 0 to 85 dB in 5-dB steps. For each intensity level, 50 trials were presented and recorded, each 200 ms in duration. In each trial, the first 100 ms were silent followed by 50-ms tone presentation (cosine window-gated tone signal of 50 ms, with a 2-ms rise/fall) ending with another 50 ms of silence. The tones were generated using OpenEx and Rx8 DSP system (TDT) and delivered through hollow left ear bar directly into the ear canal by a shielded speaker (DT770; Beyer) driven by a HB7 amplifier (TDT). Sound levels were adjusted using a programmable attenuator (PA5; TDT) previously adjusted to deliver calibrated levels (dB SPL) at frequencies between 200 and 24 kHz.
Sp5 brainstem stimulation was provided by biphasic (100 us/phase) current pulses at 1 kHz delivered through the bipolar electrode (see Shore et al. 2008 for more details on the effects and methodology of this stimulation). The current amplitude was set to the highest level that did not elicit any movement artifact and ranged between 50 and 70 uA.
Evaluation of stimulus-timing-dependent plasticity.
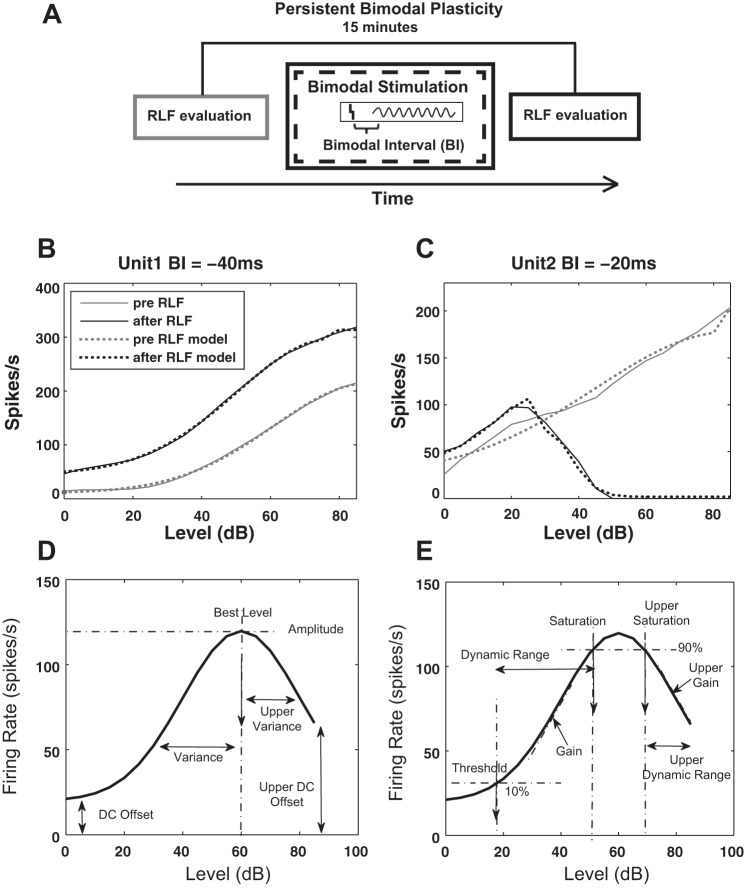
In vivo stimulus-timing-dependent plasticity was assessed using a bimodal plasticity induction protocol previously described (Koehler and Shore 2013a,b). Briefly, spontaneous activity and RLFs were recorded at three time points, before and 5 and 15 min after the bimodal stimulation protocol. The 15-min time point was selected based on the observation that this time interval is optimal for revealing the plasticity effects induced by the bimodal stimulation (Dehmel et al. 2012b; Koehler and Shore 2013a,b). The bimodal stimulation protocol consisted of 300 trials (200 ms/trial) of 50 ms tone presented at the protocol frequency (intertone interval = 100 ms) combined with Sp5 activation presented at 2 Hz with a pulse width of 100 μs and current amplitude in the range of 50–70 μA (set for each animal to the highest value that did not elicit movement artifact). The difference between the tone and Sp5 stimulus onset time was defined as the BI. Negative BIs indicated sound leading Sp5 stimulation while positive BIs corresponded to Sp5 leading sound stimulation (see Fig. 5A for a schematic of the protocol). During the experiment, subsequent tests for different BI values were randomized and presented 20 min apart. Following the same protocol, additional control recordings were performed to assess RLF plasticity in response to unimodal tone and Sp5 electrical stimulation, respectively. The parameters of unimodal stimulation were the same as those used in bimodal stimulation except that only a single stimulation modality was employed during these blocks.
Fig. 5.
Schematic of the bimodal protocol and example of rate-level functions (RLFs) with illustration of their modeling. A: schematic of the bimodal stimulation protocol. RLFs are evaluated before and 15 min after bimodal stimulation with a specific bimodal interval (BI) to assess persistent modifications. The BI is defined by the onset difference between the Sp5 electrical stimulation (vertical bar) and the auditory stimulation (sinusoid curve). The illustration is an example of a positive BI for which Sp5 stimulation precedes auditory stimulation. Negative BIs are defined by the opposite order of the stimuli presentation. B and C: representative examples of RLF responses from 2 different units before and after bimodal stimulation recorded in a sham animal (B) and a tinnitus animal (C). The responses before and after bimodal stimulation are indicated by black and gray solid lines. The fit of these RLFs was obtained by employing the two Gaussian-split modeling strategy and is indicated by dotted lines. D: illustration of the two-tail split Gaussian model used to fit the RLFs. The quantifiers in D have direct correspondence to (Eq. 1) as follows: the lower and upper variance are σlow and σhigh, lower and upper DC offset are DClower and DCupper, the best level is μ, and the amplitude is a. E: when a good fit is achieved, additional parameters are determined as indicated: the threshold is defined as the level corresponding to an amplitude equal to 10% of the total amplitude (evaluated as the difference between maximum and minimum amplitude), the upper and lower saturations are defined as the levels corresponding to 90% of the total amplitude, lower and upper dynamic ranges are defined by the difference between corresponding saturation and threshold, and the lower and upper gain are defined as the slope of the monotonic increasing and decreasing component of the RLFs.
Data Analysis
Spike detection and sorting.
Unit waveforms from each electrode site were digitized by a PZ2 preamp station (Fs = 12 kHz; TDT) and band-pass filtered (300–3 kHz). A voltage threshold of 2.5 SD from the background noise was used for online spike detection (RZ2 station; TDT), and the timestamps and waveform snippets were saved to a PC. Offline Sorter software (Plexon) was used to sort the spikes in clusters reflecting unit/multiunit neural activity. The clusters were identified using the EM-Dist sorting function in the space of the first three principal components of the waveforms with a fixed variance of 95% and a choice of five clusters maximum. Cluster distinctness was confirmed by pairwise cluster statistics (P > 0.05; Plexon Offline Sorter vs.2.7) and visual inspection by a trained observer. Spikes present in a 1-ms window across 80% of channels were considered artifact and removed from the analysis. The multicluster characteristics and waveform properties were consistent over the duration of the recordings.
RLF modeling.
RLFs (see representative examples in Fig. 5, B and C) were fit using a six-parameter, two-tailed split Gaussian function (see Eq. 1 and Fig. 5D) previously validated in studies investigating auditory cortex RLFs (Watkins and Barbour 2011a,b). This model allows for the upper and lower components of nonmonotonic RLFs to be fitted independently while still providing a good fit for monotonic RLFs by ignoring the parameters of their upper component. The parameters of the model (see Eq. 1 and Fig. 5D), the upper and lower variance (σlow and σhigh), the upper and lower DC offset (DClow and DChigh), the amplitude (a), and the best level (μ) were determined following an optimization protocol comprising two stages. First, a nonlinear regression fit was employed (using the function nlinfit in Matlab) to determine the parameters providing the best R2 error of the experimentally determined RLFs. These parameters were then used in the second stage of optimization when a nonlinear, constrained optimization algorithm (using the function fmincon in Matlab) was employed to further optimize their values.
| (1) |
Multiple characteristics of interest (called quantifiers herein, Q) were defined and evaluated as follows: threshold and saturation values were evaluated as the levels corresponding to 10 and 90% of the maximum driven rate (i.e., maximum discharge rate minus spontaneous rate) while gain and upper gain were evaluated as the slope of the lower and upper components of the fitted RLFs. The dynamic range was defined in respect to intensity as the difference between the saturation and threshold. The amplitude was defined as the maximum firing rate with its corresponding level designated as “best level.” Variance and upper variance as well as DC offset and upper DC offset were assigned the values of the corresponding parameters of the model (Fig. 5E).
The degree of monotonicity of the RLFs was quantified by the monotonicity index (MI; Eq. 2), a previously established measure (de la Rocha et al. 2008; Watkins and Barbour 2011a) to reflect the degree of reduced spiking at higher stimulus levels.
| (2) |
Fusiform cell RLFs were classified as nonmonotonic if MI was smaller or ≤0.5 and monotonic otherwise.
Evaluation and classification of learning rules.
Plasticity of the RLF quantifiers was evaluated for each BI by calculating the percent change, i.e., the ratio 100 × (Qpost − Qpre)/Qpre, where Q defines the specific quantifier (see above) and Qpre and Qpost are the values of the RLF quantifier evaluated before and 15 min after bimodal stimulation.
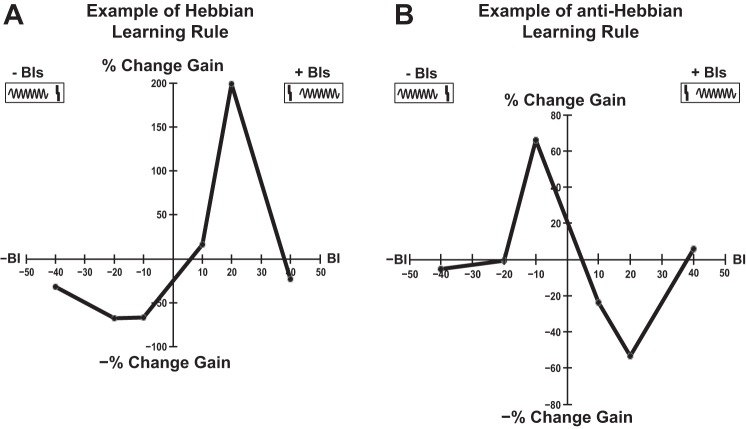
Learning rules were evaluated for each unit for each RLF quantifier as functions displaying the percent change corresponding to each BI (see Fig. 6, A and B, for examples). The learning rules (LR) evaluated for gain and maximum amplitude were classified in four categories: Hebbian, anti-Hebbian, suppressive, and enhancing based on the mean percent change in the RLF quantifier (L̄R) evaluated for negative (L̄R−) and positive BIs (L̄R+), respectively (the percent change for BI = 0 ms was ignored), and according to the conditions detailed in Table 1.
Fig. 6.
Representative examples of bimodal-induced changes in RLF gain. Examples of Hebbian (A) and anti-Hebbian (B) learning rules for the RLF gain in a sham (S) and exposed-no tinnitus (ENT) animal, respectively. Statistically significant differences between the RLFs evaluated before and after bimodal stimulation were confirmed for each BI with a two-way ANOVA test (see materials and methods for more details). Positive BIs correspond to stimuli configurations in which Sp5 electrical stimulation (A and B, top right box, solid vertical line) precedes auditory stimulation (A and B, top right box, sinusoid line). Negative BIs correspond to the opposite order of stimuli (an example is illustrated in the top left boxes of A and B).
Table 1.
Classification of the learning rules
| Condition | Learning Rule Classification |
|---|---|
| L̄R− < 0 and L̄R+ > 0 | Hebbian |
| L̄R− > 0 and L̄R+ < 0 | Anti-Hebbian |
| L̄R− > 0 and L̄R+ > 0 | Enhancing |
| L̄R− < 0 and L̄R+ < 0 | Suppressive |
| Otherwise | Unclassified |
The mean of percent change in the rate-level function quantifier of interest was evaluated for negative bimodal intervals (L̄R−) and positive bimodal intervals (L̄R+), respectively. The learning rules (LR) were classified as Hebbian, anti-Hebbian, Suppressive, and Enhancing if the specific conditions stated at left were satisfied.
Statistical Analysis
For each BI, statistical differences between the RLFs evaluated before and after bimodal stimulation were assessed with a two-way ANOVA test with pre/postbimodal stimulation and level as main factors and only the RLFs that reached statistical significance (P < 0.05) for the pre/postmain effect were retained for further analysis.
Timing rules were constructed for each unit and classified as Hebbian, anti-Hebbian, suppressive, and enhancing. Differences between mean population timing rules were tested for statistical significance using a two-way ANOVA followed by Holm-Sidak post hoc tests. The proportion of the timing rule types were compared among S, ENT, and ET animals using a 2 × 2 or 2 × 3 χ2-test. Differences between change in gain induced by unimodal acoustic, unimodal electric, and bimodal stimulation were tested using a three-way ANOVA followed by Holm-Sidak post hoc tests. All statistical tests were performed in Matlab using the Statistical toolbox. The P values of the post hoc tests were evaluated with a custom Matlab routine.
Correlations between the gain and other RLF quantifiers were evaluated using a linear regression model in Matlab, and the probability was adjusted for multiple tests using a Bonferroni correction. These results are considered in the discussion.
RESULTS
Narrow Band Noise Exposure Centered at 7 kHz Induced Elevated Normalized Startle Amplitude and Spontaneous Rates Between 4 and 12 kHz in Tinnitus Animals
Mean normalized startle amplitudes showed frequency-specific, elevated values for noise-exposed animals with confirmed tinnitus in the frequency bands of 4, 8, and 12 kHz (Fig. 7A). A two-way ANOVA revealed a significant main effect for exposure group [F(2) = 30.87, P < 0.0001], for the frequency band [F(4) = 26.74, P < 0.0001], and for interactions [F(8) = 2.21, P = 0.0275]. Holm-Sidak post hoc tests confirmed statistical significance for difference between tinnitus and sham-exposed animals in these three frequency bands, which we indicate with stars in Fig. 7A. The normalized startle response was not significantly elevated for the ET or ENT group for broadband noise or 16–18 kHz background signal (see Koehler and Shore 2013a for more details).
Fig. 7.
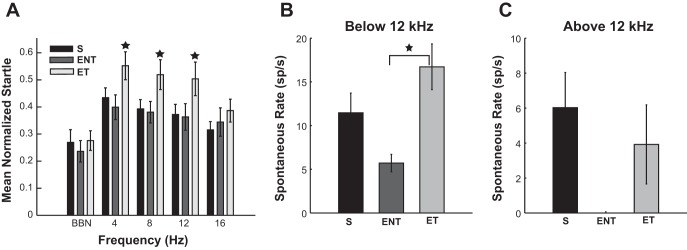
Normalized startle amplitudes and mean spontaneous rates in sham and noise-exposed animals with and without tinnitus. A: normalized startle response amplitudes were evaluated for each noise exposure frequency band for sham (black bars), ENT (dark gray bars), and exposed-tinnitus (ET; light gray bars) animals. Error bars indicate 95% confidence interval. Stars indicate statistical significantly different means in the same frequency band. B and C: mean spontaneous rate (spikes/s) evaluated for S, ENT, and ET animals for units with BF below (B) and above (C) 12 kHz. No ENT neurons have been found at CF >12 kHz. The error bars indicate SE (adapted from Koehler and Shore 2013a). BBN, broadband noise.
In addition to gap-detection indications of tinnitus, the mean spontaneous rate recorded from fusiform cells was increased in tinnitus animals at frequencies close to the noise-exposure frequencies (Fig. 7, B and C). Two-way ANOVA revealed a main effect for frequency band group [F(1) = 10.37, P = 0.0014] and for exposure group [F(2) = 4.16, P = 0.0163], while Holm-Sidak post hoc tests showed statistical significance (P = 0.0088) for the difference between noise exposed with tinnitus and no tinnitus in the frequency range <12 kHz (for more details, see Koehler and Shore 2013a).
Consistent with previous observations (Dehmel et al. 2012b), the RLF gains evaluated before bimodal stimulation were also significantly higher in tinnitus animals vs. sham animals (Wilcoxon rank sum test: P = 9.3e-05).
RLF Gain and Maximum Amplitude Modifications Follow Hebbian Learning Rules in Sham Animals and Anti-Hebbian Rules in Noise-Exposed Animals with Tinnitus
Persistent changes in the gain, maximum amplitude, and other RLF quantifiers (see materials and methods; Fig. 5, D and E) were evaluated using a bimodal stimulation protocol previously demonstrated in in vivo studies to induce plastic modifications in the neural activity of DCN neurons (Koehler and Shore 2013a,b). Briefly, initial RLF recordings were followed by bimodal (paired auditory and somatosensory) stimulation at specific intervals between their onsets (i.e., BIs). RLFs were then recorded again 15 min after bimodal stimulation (see materials and methods; Fig. 5A), the time at which maximal effects were previously observed in spontaneous and tone-evoked firing rates of fusiform cells (Koehler and Shore 2013b).
Following bimodal stimulation, persistent modifications in RLFs occurred in one or more quantifiers such as gain, threshold, maximum amplitude, saturation, dynamics range, and DC offset. For the example unit in Fig. 5B (see materials and methods), the threshold decreased by 28%, the gain increased by 21.2%, the dynamic range increased by 12%, and the maximum amplitude increased by 38.8%, following bimodal stimulation with Sp5 preceding tone stimulation by 40 ms. Qualitative changes were observed in some units (see example unit in materials and methods; Fig. 5C) in which the monotonic profile of the RLF before bimodal stimulation changed to a nonmonotonic profile following bimodal stimulation.
To evaluate whether plastic changes in the RLF following bimodal stimulation were stimulus-timing dependent, percent changes in these quantifiers were measured following bimodal stimulation with BIs of −40, −20, −10, 0, 10, 20, and 40 ms. The learning rules (see materials and methods) considered for this study were evaluated in 311 single units, of which 91 were from S animals, 144 units from ET animals, and 72 units from ENT animals. A two-way ANOVA test with main effects of pre/postbimodal stimulation and level was used to confirm statistically significant differences between the RLFs evaluated before and after bimodal stimulation.
Gain.
Hebbian learning rules were characterized by gain decreases at negative BIs (when tone presentation preceded Sp5 stimulation) and gain increases at positive BIs (when Sp5 stimulation preceded tone presentation) (see materials and methods; Fig. 6A). Anti-Hebbian profiles were characterized by gain decreases occurring at positive BIs and gain increases at negative BIs in the learning rules (see materials and methods; Fig. 6B). Suppressive and enhancing learning rules were characterized by only decreases or increases, respectively, in the RLF quantifier observed for all BIs (not shown).
The mean timing rules estimate the overall persistent modifications induced by auditory-somatosensory stimulation at specific BIs on DCN neural population activity. The change in gain in sham animals followed a Hebbian profile characterized by an increase in RLF gain for BIs (+10 and +20 ms) and a decrease in the gain for BIs of −10 and −20 ms (Fig. 8A, black curve). In contrast, the exposed animals with evidence for tinnitus show an anti-Hebbian profile of the gain learning rule (Fig. 8A, light gray curve). ENT animals displayed a learning rule somewhat in between the Hebbian and anti-Hebbian profile (see below).
Fig. 8.
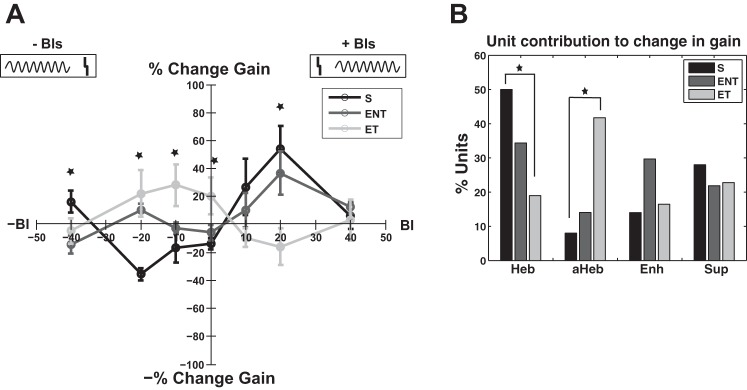
Learning rules of the change in gain in sham and noise-exposed animals with and without tinnitus. A: learning rule of the gain represented by the percent change as a function of the BI follows a Hebbian profile in sham animals (black curve) with maximum decrease (light gray curve) for BI = −20 ms and maximum increase at BI = 20 ms. In noise-exposed animals with confirmed tinnitus, the learning rule changes to an anti-Hebbian profile with a maximum enhancement (dark gray curve) at BI = −10 ms and maximum decrease at BI = 20 ms. Noise-exposed animals with no evidence for tinnitus exhibit a Hebbian-like learning rule with maximum enhancement in firing rate observed for a BI of +20 ms. Error bars indicate SE. Statistical significance is indicated with stars for each appropriate BI. Diagram at top indicates the relative order of auditory and Sp5 stimulation. The gray vertical lines represents the Sp5 stimulation, and the sinusoid gray curves represent the tone presentation. B: this change after noise damage is reflected in a significant change in the number of individual units that show Hebbian and anti-Hebbian learning rules in sham ENT and ET animals, respectively.
A two-way ANOVA with exposure group and BI factors revealed a significant main effect for BI (F = 2.39, P = 0.027) and a significant interaction between the exposure group and BI (F = 4.96, P < 0.001). Holm-Sidak post hoc tests revealed statistically significant differences in the gain change between S and ET for the following BIs: 20 ms (P = 0.0026), 0 ms (P = 0.009), −10 ms (P = 0.003), −20 ms (P = 0.0005), and −40 ms (P = 0.0028). No statistical significance was observed at BI of 10 and 40 ms. The statistical significance is indicated in Fig. 8A with stars.
The Hebbian and anti-Hebbian profiles of the mean learning rule of the gain in S animals and ET animals, respectively, are mostly due to a significantly larger number of units that exhibit individual anti-Hebbian learning rules (χ2 = 17.073; df = 1; P = 3.6e-05) and a significant decrease in the number of units with Hebbian learning rule (χ2 = 13.766; df = 1; P = 2.07e-4; Fig. 8B).
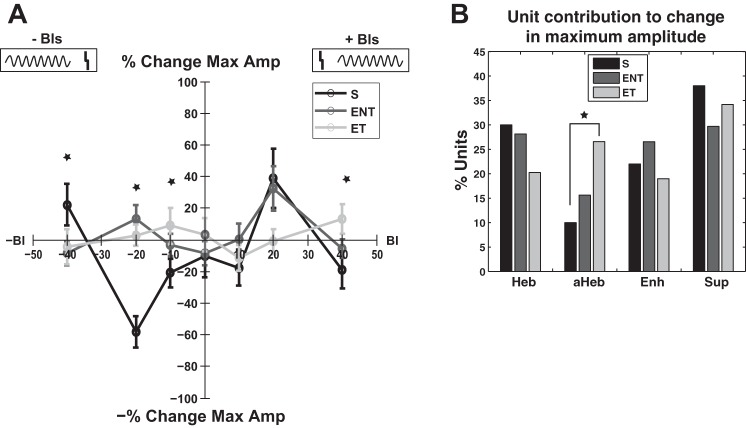
Maximum amplitude.
Similar to the learning rules for the gain, maximum amplitude of the RLFs also exhibited distinct patterns for sham vs. ENT and ET animals, respectively. Sham animals demonstrated Hebbian learning rules with maximum enhancement in amplitude observed for a BI of +20 ms and a maximum decrease in amplitude obtained for a BI of −20 ms (Fig. 9A, black curve). For ET animals, the learning rule for the maximum amplitude followed an anti-Hebbian profile with maximum changes observed for BIs of + 10 and −10 ms (Fig. 9A, light gray curve). Again, the ENT animals showed a mixed learning rule (see below).
Fig. 9.
Learning rules of the change in maximum amplitude in sham and noise-exposed animals with and without evidence for tinnitus. A: learning rule of the maximum amplitude represented by the percent change as a function of the BI follows a Hebbian profile in sham animals (black curve) with maximum decrease for BI = −20 ms and maximum increase at BI = 20 ms. In noise-exposed animals with confirmed tinnitus (light gray curve), the learning rule changes to an anti-Hebbian profile with a maximum enhancement at BI = −10 ms and maximum decrease at BI = 20 ms. Noise-exposed animals with no evidence for tinnitus present show a Hebbian-like learning rule (dark gray curve) with maximum changes observed for BIs of ±20 ms. Error bars indicate SE. Statistical significance is indicated with stars for each appropriate BI. Diagram at top indicates the relative order of auditory and Sp5 stimulation. The gray vertical lines represents the Sp5 stimulation and the sinusoid gray curves represent the tone presentation. B: this change is reflected in a significant change in the number of individual units that show Hebbian and anti-Hebbian learning rules in sham, ENT, and ET animals, respectively.
A two-way ANOVA with exposure group and BI factors revealed a statistical significant main effect for the BI [F(6) = 4.11, P = 0.0005] and for interactions [F(12) = 3.55, P < 0.0001]. Holm-Sidak post hoc tests revealed statistically significant differences in the maximum amplitude change between S and ET were for the following BIs: 40 ms (P = 0.0221), −10 ms (P = 0.0369), −20 ms (P < 0.0001), and −40 ms (P = 0.0254). Statistical significance between S and ENT was observed only for BI of −20 ms (P < 0.0001). The differences between changes in maximum amplitude between S and ET showed a statistical trend for BI = 20 ms (P = 0.0668). The statistical significance is indicated in Fig. 9A with stars for each BI.
The plasticity of the maximum amplitude is associated with changes in the number of units that show individual anti-Hebbian learning rule profiles for their corresponding change in the maximum amplitude (χ2 = 5.232; df = 1; P = 0.022; Fig. 9B).
We also evaluated the learning rules for several other RLF quantifiers including threshold, dynamic range, DC offset (as a measure of spontaneous activity), and saturation and found that none of these quantifiers exhibited clear mean Hebbian or anti-Hebbian profiles.
Gain and Maximum Amplitude Plasticity in the Noise-Exposed Animals Without Tinnitus Follow Mainly Hebbian-Like Learning Rules
The learning rule for the gain and maximum amplitude in ENT animals was Hebbian-like, with characteristics similar to a Hebbian learning rule for positive intervals (for which tone presentation precedes Sp5 stimulation; see Figs. 8A and 9A, dark gray curves) and reduced alterations for negative intervals (for which Sp5 stimulation precedes tone presentation). For both positive and negative BIs, the gain plasticity in ENT animals is bounded by the magnitude of gain plasticity observed in S and ET animals. These characteristics suggest that noise exposure modifies fusiform cell activity and that these modifications are likely to become more severe or change in nature when the tinnitus condition is established after noise exposure. These observations are consistent with some in vitro studies showing early changes in the gain of DCN fusiform cell following noise exposure (Pilati et al. 2012a) and in vivo studies showing increased steepness of RLFs in fusiform cells in tinnitus animals (Dehmel et al. 2012b).
Statistical significance between the change in gain in S and ENT was revealed by Holm-Sidak post hoc tests for the following BIs: 20 ms (P = 0.0135), 0 ms (P = 0.0258), −10 ms (P = 0.0116), and −20 ms (P = 0.0025). No statistical significance was observed at BI of −40, 10, or 40 ms.
Similarly, changes in maximum amplitude presented the extreme positive values for both positive and negative BIs (Fig. 8, dark gray curve). Differences in maximum amplitude change between S and ENT group, estimated by Holm-Sidak post hoc tests, were statistically significant only for BI of −20 ms (P < 0.0001), although BI = 20 ms also showed a statistical trend (P = 0.081). The statistical significance is indicated in Figs. 8 and 9A with stars for each BI.
Bimodal Stimulation Modulates RLF Nonmonotonicity
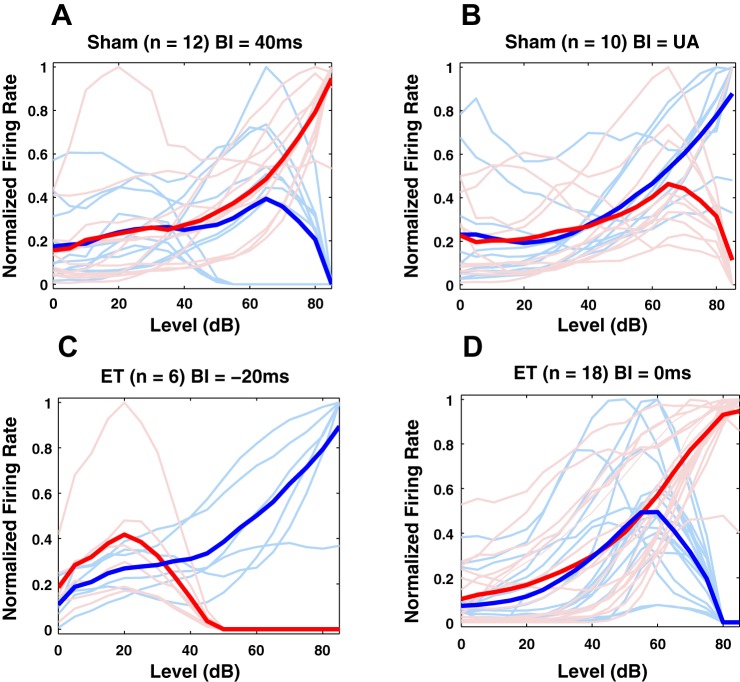
Unimodal acoustic and bimodal stimulation but not unimodal Sp5 stimulation can modulate the nonmonotonic component of the RLFs in DCN fusiform cells. Before any bimodal stimulation, 18% of the S units and 26% of the ET units showed nonmonotonic RLFs. Thirty-six units recorded in ET animals and 22 units in S animals showed transitions between monotonic and nonmonotonic RLF profiles. Depending on the BI, two consistent trends were observed: a monotonic RLF with or without saturation plateau was converted to a nonmonotonic RLF and a nonmonotonic RLF changed to a monotonic RLF after stimulation. Figure 10 shows representative examples of RLF transitions from nonmonotonic to monotonic profiles in S animals (Fig. 10A) and ET animals (Fig. 10D) and the reversed transition in Fig. 10, B and C. The RLFs of individual units are displayed before bimodal stimulation with thin blue curves and after bimodal stimulation with thin red curves and their average in thick lines of the same colors.
Fig. 10.
Representative examples of qualitative modifications from monotonic to nonmonotonic profiles of RLFs of dorsal cochlear nucleus fusiform cells. A: in sham animals bimodal stimulation with BI = 40 ms induces a consistent change from an initial nonmonotonic RLF profile (blue curve) to a monotonic profile after bimodal stimulation (red curve). B: in contrast, a bimodal stimulation with BI = 10 ms induces opposite modifications where RLFs change from an initial monotonic profile to a nonmonotonic one after stimulation. UA, unimodal acoustic. C: similar pattern is observed in noise-exposed animals with evidence for tinnitus (ET), in this case also accompanied by significant changes in the lower gain of the RLFs. D: similar to A, example of RLFs modifications from a nonmonotonic to a monotonic profile observed in ET animals after bimodal stimulation with BI = 0 ms.
Interestingly, specific stimulation modalities trigger specific modifications in the nonmonotonic RLF profile. For instance, for BI = 40 ms, out of 13 fusiform units that showed nonmonotonic modulation following unimodal acoustic stimulation in sham animals, 12 units (92.3%) showed a consistent and significant pattern (χ2 = 18.28, df = 1; P = 1.9e-05) in which their initial monotonic profile changed to a nonmonotonic function (see Fig. 10A). Similarly, for tinnitus animals, bimodal stimulation at −20 ms induces a systematic change in the RLF profiles from nonmonotonic to monotonic profiles (see Fig. 10C). Similar trends were observed in sham animals for BI = 10 ms (χ2 = 15.086; df = 1; P = 0.1e-03; Fig. 10B), for unimodal acoustic stimulation, and for tinnitus animals for BI = 0 ms (χ2 = 32.21; df = 1; P = 1.38e-08; Fig. 10D) and BI = −10 ms (not shown here).
Bimodal Stimulation Is More Effective than Unimodal Stimulation
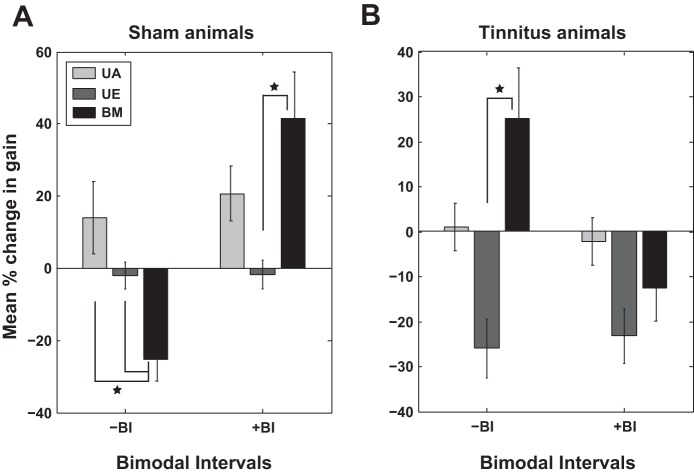
Here, we compare the persistent effects induced by bimodal stimulation at BIs that show most prominent changes in lower gain and amplitude (±10 ms and ±20 ms) with the changes induced by unimodal acoustic and electrical stimulation (Fig. 11). As the changes for positive and negative BIs, respectively, are consistent in sign and do not differ significantly (data not shown), we pooled the data together for the most relevant positive BIs (of +10 and ±20 ms), which we indicate by +BI, and negative BIs (of −10 and −20 ms), which we designate as −BI. As the results for both gain and maximum amplitude show similar trends, we discuss in detail here only the gain (Fig. 11). Consistent with previous observations of firing rate changes of DCN fusiform cells after a bimodal plasticity-inducing protocol (Koehler and Shore 2013b), the changes in the gain were significantly stronger after bimodal stimulation protocol than those observed with unimodal stimulation.
Fig. 11.
Comparison of the change in the lower gain induced by unimodal vs. bimodal stimulation. A: in sham animals, the change in gain after bimodal (BM) stimulation (black) is compared with that induced by UA stimulation (light gray) and unimodal electrical (UE) stimulation of the Sp5 (dark gray), respectively. B: similar comparisons are evaluated in the noise-exposed animals with evidence for tinnitus. Statistically significant differences are indicated by black stars. Error bars indicate SE.
A three-way ANOVA with BI, exposure group, and stimulation modality factors revealed a significant main effect for stimulation modality [F(2) = 5.69, P = 0.0036], for exposure group [F(1) = 8.21, P = 0.0043], and interactions between BI and exposure group [F(1) = 20.42, P < 0.001]. Holm-Sidak post hoc tests indicated that in sham animals, for −BI, the change in gain induced by bimodal stimulation is statistically significantly different than the change induced by auditory stimulation (P = 0.0294) and unimodal electrical stimulation (P = 0.0002; Fig. 11A, left). In the same group of animals, for +BI, the change in gain mediated by bimodal stimulation was significantly larger than the change mediated by unimodal electrical stimulation (P = 0.0048; Fig. 11A, right). In animals with evidence of tinnitus, the change in gain due to bimodal stimulation was significantly higher than that induced by unimodal electrical stimulation (P = 0.0004) and shows a significant trend compared with unimodal acoustic stimulation (P = 0.066; Fig. 11B, left). The changes for the +BI in tinnitus animals were not statistically significant.
DISCUSSION
This study focused on understanding the persistent modifications of DCN fusiform cell RLFs following bimodal stimulation of the auditory and somatosensory pathways to the DCN. Similar to previous in vivo studies of tone-evoked firing activity of DCN fusiform cells (Dehmel et al. 2012b; Koehler and Shore 2013a), the gain and maximum amplitude of the RLFs were stimulus-timing dependent, following Hebbian learning rules in sham animals. These changes were characterized by decreases in gain for negative bimodal stimulation intervals when the auditory precedes the Sp5 stimulation and increases for positive BIs where the opposite order of stimuli (i.e., the Sp5 stimulation precedes the auditory stimulation) is presented. These rules remained mostly Hebbian-like in the noise-exposed animals without evidence for tinnitus but became anti-Hebbian for noise-exposed animals with behaviorally confirmed tinnitus. A consistency between the learning rules of the maximum amplitude across all the animal groups and the ones determined in previous studies (Koehler and Shore 2013a,b) using tone-evoked firing activity is expected because in both studies the stimulation frequencies were close to or matching the BF of the DCN fusiform cells. It is often the case that for sound levels 20 dB above threshold the RLFs saturate. It is less intuitive, however, that the learning rules for the gain would follow a similar trend since, for many of the BIs investigated, the change in the maximum amplitude was not the dominant mechanism mediating the change in gain. This might suggest that the overall effects of plasticity-inducing bimodal stimulation are synergistic in nature and provide consistent decreases or enhancements of various quantifiers of fusiform cells firing activity at specific BIs.
Molecular Mechanisms Mediating Changes Following Noise Exposure and Tinnitus
Several factors could influence noise exposure- and tinnitus-associated changes in the stimulus-timing-dependent learning rules in the DCN. First, a reduction in glycinergic inhibition after noise exposure and tinnitus (Suneja et al. 1998; Wang et al. 2009) may shift the balance in the excitatory-inhibitory intrinsic DCN circuit controlling the sound-evoked responses. Second, findings that the cholinergic input can mediate conversion of the STDP learning rules at parallel synapses on the fusiform cells from Hebbian to anti-Hebbian (Zhao and Tzounopoulos 2011), together with findings of carbachol-induced suppression of DCN neural activity following noise damage (Zhang and Kaltenbach 2000), suggest that acetylcholine up- or downregulation could facilitate the shift in the learning rule profiles in tinnitus animals. Importantly, a redistribution of the somatosensory terminals on granule cells that preferentially innervate fusiform cells following noise exposure may diminish the influence of the cartwheel cells. This would lead to a reduction in the Hebbian learning rules in fusiform cells (Doiron et al. 2011; Zeng et al. 2012). Similar to observations in other brain regions where changes in N-methyl-d-aspartate receptror (NMDAr) type and density have been associated with changes in long-term potentiation/long-term depression expression (Cho et al. 2009; Peng et al. 2010; Dalton et al. 2012), a redistribution of the NMDAr-2B (Marianowski et al. 2000; Sato et al. 2000) at the synaptic terminals of the parallel fibers onto the fusiform and cartwheel cells may change the learning rule profiles in the DCN of the noise-exposed and tinnitus animals. Third, an important contribution to the plasticity of fusiform cell responses is provided by the degree of intrinsic neural excitability. In particular, transient K+ currents have been shown to regulate the discharge pattern of the fusiform cells by adjusting their sensitivity to different temporal patterns of inhibition and excitation (Kanold and Manis 2001). Furthermore, a reduction in the Kv7.2/3 channel activity has been recently shown to correlate with tinnitus-specific hyperactivity (Li et al. 2013). Interestingly, pharmacological manipulations that shift the voltage dependence of this channel towards more negative voltages prevented tinnitus development. Modulation of the neural intrinsic excitability is also subject to homeostatic plasticity, which develops over longer time scale of days, weeks, or months. The mechanisms mediating these changes are not entirely understood. CN neurons have been shown to redistribute AMPA and glycine receptors in response to monoaural conductive hearing loss (Whiting et al. 2009) and to change their spiking patterns to burst firing following acoustic overexposure (Pilati et al. 2012b). However, more research is needed to clarify the mechanisms inducing homeostatic plasticity in the DCN. Finally, the distinct modifications in the RLFs evaluated in noise-exposed animals with and without tinnitus may be correlated with different inputs received by the DCN fusiform cells from the VCN. Following noise exposure, primary-like neurons in the VCN show decreased maximum amplitudes and gains in their RLFs while the chopper cells show elevations in the same characteristics (Cai et al. 2009). Given that VCN chopper cells are most commonly classified as T-stellate/multipolar cells, with collateral projections into the deep layer of the DCN (Oertel et al. 2011; Campagnola and Manis 2014), it is possible that their altered firing activity leads to changes in the DCN fusiform cells response and their associated plasticity.
Modulation of the Upper Gain
Some of these mechanisms may also be responsible for modulation of inhibitory input received by the fusiform cells leading to changes in the RLF profiles. Twenty-two units in S animals and 36 in ET animals showed transitions from a monotonic to nonmonotonic profiles and vice versa following stimulation at specific BIs (Fig. 10). A limited number of fusiform cells (only 5 units) showed transitions from nonmonotonic RLFs to different nonmonotonic profiles. Previous studies have shown that neurons in the auditory cortex can modify their lower and upper gains in an independent manner to adjust for the statistics of the tones of lower respectively higher intensities (Watkins and Barbour 2011a,b). Changes in RLFs have also been identified in the inferior colliculus, which contribute to the ability of the auditory neurons to adjust their coding according to the statistical distribution of the sound level presented (Dean et al. 2005). A significant contribution to the inhibitory input controlling the upper gain is supplied by the type II neurons (vertical cells) from the DCN and by the wideband inhibitors (D-multipolar cells) from VCN. These cells are likely to be little effected by the bimodal stimulation protocol employed in this study mostly because the somatosensory input provided by the parallel fibers of the granule cells is not relayed by direct projections onto these neurons. Still, some changes could be induced by the auditory component in the bimodal stimulation, which together with potential changes in the cartwheel cell firing activity mediated by bimodal stimulation, may explain the limited plasticity observed in the upper gain.
Bimodal vs. Unimodal Stimulation
In the majority of observations. bimodal stimulation induced stronger modifications in gain and maximum amplitude than either unimodal acoustic or Sp5 stimulation (Fig. 11). This is consistent with previous observations of persistent modifications of tone-evoked firing rates (Koehler and Shore 2013b) and supports the idea that STDP is most likely mediating the synaptic modifications in the fusiform cell activity associated with bimodal stimulation.
Tinnitus and Hyperacusis
Tinnitus and hyperacusis pathology can occur simultaneously in patients. For instance, 40 to 80% of tinnitus patients also report hyperacusis (Bartnik et al. 1999; Jastreboff and Jastreboff 2000; Dauman and Bouscau-Faure 2005) while a majority (86%) of subjects treated for hyperacusis also report tinnitus (Anari et al. 1999). This comorbidity suggests that some mechanisms mediating the two pathologies may be common, an idea reinforced by recent findings of enhanced auditory sensitivity in tinnitus patients (Hebert et al. 2013). While the two pathologies can be easily assessed and distinguished in humans based on direct symptomatology reports and answers to scientifically designed questionnaires, the equivalent evaluations in animal models are challenging. One way of discriminating between the elevated gains due to tinnitus and hyperacusis could be to rely on the assumption that hyperacusis animals should show elevated startle amplitudes and/or elevated normalized prepulse inhibition (Chen et al. 2013) across multiple frequencies of the sound stimulation, as hyperacusis is not frequency specific. However, no such pattern could be established in our data. Although this observation is not conclusive, due to limitations of the range of sound frequencies and intensities tested in our experiments, it does not provide support to the hypothesis of hyperacusis comorbidity in our tinnitus animals.
An alternative would be to compare qualitatively the changes observed in the RLFs of the DCN fusiform cells with the changes observed in the loudness functions evaluated in humans (Cai et al. 2009). It has been proposed that tinnitus is a result of increased neural noise or increased central gain (Norena 2011; Schaette and Kempter 2012), while hyperacusis is a result of increased nonlinear gain (Zeng 2013). In the present study we show that consistent with the first proposal (tinnitus), the RLF gains evaluated before bimodal stimulation were significantly higher in tinnitus animals vs. sham animals. Furthermore, bimodal stimulation at BI = −10 and −20 ms further elevated the RLF gain in tinnitus animals (Fig. 8A). Consistent with the latter model (hyperacusis), ENT and ET animals showed changes in gain primarily mediated by changes in saturation and dynamic range for certain BIs. These results suggest that stimulus-timing-dependent plasticity may play a role in modulating central gain related to both tinnitus and hyperacusis.
Tinnitus Treatment Perspectives
Detailed understanding of the learning rule transitions from sham- to noise-exposed to tinnitus animals, together with a comprehension of the dominant mechanisms mediating these modifications, is expected to facilitate the development of novel therapeutic strategies to treat tinnitus and hyperacusis. For instance, as indicated by the learning rules in Fig. 8, bimodal stimulation at BI = 20 ms decreases the gain of the fusiform cell RLFs in tinnitus animals as well as their spontaneous firing rates (see Fig. 7D in Koehler and Shore 2013a). As the circuitry involved in this modulation is largely conserved in primates and humans (Moore and Osen 1979; Rubio et al. 2008; Rhode et al. 2010), it is likely that such stimulation would have similar effects, depressing the abnormal firing activity in the auditory pathway, thus potentially alleviating tinnitus. Moreover, understanding how these rules may change in the presence of various drugs or as a function of specific individual genetic background may provide additional treatment avenues. In this respect, this study provides an important contribution by clarifying the RLF modifications following noise exposure and the establishment of tinnitus pathology.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants P01-DC-0078, R01-DC-004825 (to S. E. Shore), and T32-DC-000011 (to S. D. Koehler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A.S. and S.D.K. analyzed data; R.A.S., S.D.K., and S.E.S. interpreted results of experiments; R.A.S. prepared figures; R.A.S. drafted manuscript; R.A.S., S.D.K., and S.E.S. approved final version of manuscript; S.D.K. and S.E.S. conception and design of research; S.D.K. performed experiments; S.D.K. and S.E.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sanford Bledsoe for excellent comments and suggestions for manuscript improvements and Chris Ellinger and Jim Wiler for excellent technical support. We also thank two anonymous reviewers of our manuscripts, as we consider their comments significantly improved the presentation framework of the paper.
REFERENCES
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound: questionnaire data, audiometry and classification. Scand Audiol 28: 219–230, 1999. [DOI] [PubMed] [Google Scholar]
- Bartnik G, Fabijanska A, Rogowski M. Our experience in treatment of patients with tinnitus and/or hyperacusis using the habituation method. In: Proceedings of the Sixth International Tinnitus Seminar. London: The Tinnitus and Hyperacusis Centre, 1999, p. 415–417. [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86: 2564–2578, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387: 278–281, 1997. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22: 2383–2390, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Ma WL, Young ED. Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. J Assoc Res Otolaryngol 10: 5–22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola L, Manis PB. A map of functional synaptic connectivity in the mouse anteroventral cochlear nucleus. J Neurosci 34: 2214–2230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron 35: 773–782, 2002. [DOI] [PubMed] [Google Scholar]
- Chen G, Lee C, Sandridge SA, Butler HM, Manzoor NF, Kaltenbach JA. Behavioral evidence for possible simultaneous induction of hyperacusis and tinnitus following intense sound exposure. J Assoc Res Otolaryngol 14: 413–424, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KK, Khibnik L, Philpot BD, Bear MF. The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex. Proc Natl Acad Sci USA 106: 5377–5382, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology 62: 797–806, 2012. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem 10: 456–465, 2003. [DOI] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol (Stockh) 125: 503–509, 2005. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol 83: 926–940, 2000. [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci 28: 9151–9163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8: 1684–1689, 2005. [DOI] [PubMed] [Google Scholar]
- Debanne D, Poo MM. Spike-timing dependent plasticity beyond synapse–pre- and post-synaptic plasticity of intrinsic neuronal excitability. Front Synaptic Neurosci 2: 21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci 6: 42, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus–possible basis for tinnitus-related hyperactivity? J Neurosci 32: 1660–1671, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Voigt HF. Intracellular response properties of units in the dorsal cochlear nucleus of unanesthetized decerebrate gerbil. J Neurophysiol 77: 2549–2572, 1997. [DOI] [PubMed] [Google Scholar]
- Doiron B, Zhao Y, Tzounopoulos T. Combined LTP and LTD of modulatory inputs controls neuronal processing of primary sensory inputs. J Neurosci 31: 10579–10592, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EF, Nelson PG. The responses of single neurones in the cochlear nucleus of the cat as a function of their location and the anaesthetic state. Exp Brain Res 17: 402–427, 1973. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104: 3361–3370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. NewYork: Wiley & Sons, 1949. [Google Scholar]
- Hebert S, Fournier P, Norena A. The auditory sensitivity is increased in tinnitus ears. J Neurosci 33: 2356–2364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol 11: 162–177, 2000. [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett 355: 121–125, 2004. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. A physiologically based model of discharge pattern regulation by transient K+ currents in cochlear nucleus pyramidal cells. J Neurophysiol 85: 523–538, 2001. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci 21: 7848–7858, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S, Shore S. Stimulus Timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci 33: 19647–19656, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One 8: e59828, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci USA 110: 9980–9985, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275: 209–213, 1997. [DOI] [PubMed] [Google Scholar]
- Marianowski R, Liao WH, Van Den Abbeele T, Fillit P, Herman P, Frachet B, Huy PT. Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. I. Influence of early auditory deprivation. Hear Res 150: 1–11, 2000. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275: 213–215, 1997. [DOI] [PubMed] [Google Scholar]
- Moore JK, Osen KK. The cochlear nuclei in man. Am J Anat 154: 393–418, 1979. [DOI] [PubMed] [Google Scholar]
- Muller M, Robertson D, Yates GK. Rate-versus-level functions of primary auditory nerve fibres: evidence for square law behaviour of all fibre categories in the guinea pig. Hear Res 55: 50–56, 1991. [DOI] [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev 35: 1089–1109, 2011. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R. The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276: 61–69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AK, Lobarinas E, Simmons R, Wack D, Luisi JC, Spernyak J, Mazurchuk R, Abdel-Nabi H, Salvi R. Metabolic imaging of rat brain during pharmacologically-induced tinnitus. Neuroimage 44: 312–318, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Zhao J, Gu QH, Chen RQ, Xu Z, Yan JZ, Wang SH, Liu SY, Chen Z, Lu W. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus 20: 646–658, 2010. [DOI] [PubMed] [Google Scholar]
- Pilati N, Ison MJ, Barker M, Mulheran M, Large CH, Forsythe ID, Matthias J, Hamann M. Mechanisms contributing to central excitability changes during hearing loss. Proc Natl Acad Sci USA 109: 8292–8297, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati N, Large C, Forsythe ID, Hamann M. Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hear Res 283: 98–106, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Roth GL, Recio-Spinoso A. Response properties of cochlear nucleus neurons in monkeys. Hear Res 259: 1–15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Smith PH, Oertel D. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol 213: 426–447, 1983. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Gudsnuk KA, Smith Y, Ryugo DK. Revealing the molecular layer of the primate dorsal cochlear nucleus. Neuroscience 154: 99–113, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J Acoust Soc Am 56: 1835–1847, 1974. [DOI] [PubMed] [Google Scholar]
- Sato K, Shiraishi S, Nakagawa H, Kuriyama H, Altschuler RA. Diversity and plasticity in amino acid receptor subunits in the rat auditory brain stem. Hear Res 147: 137–144, 2000. [DOI] [PubMed] [Google Scholar]
- Schaette R, Kempter R. Computational models of neurophysiological correlates of tinnitus. Front Syst Neurosci 6: 34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci 21: 3334–3348, 2005. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27: 155–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol 419: 271–285, 2000. [DOI] [PubMed] [Google Scholar]
- Stabler SE, Palmer AR, Winter IM. Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. J Neurophysiol 76: 1667–1688, 1996. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol 154: 473–488, 1998. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120: 188–195, 2006. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7: 719–725, 2004. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron 54: 291–301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164: 747–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Level-tuned neurons in primary auditory cortex adapt differently to loud versus soft sounds. Cereb Cortex 21: 178–190, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Rate-level responses in awake marmoset auditory cortex. Hear Res 275: 30–42, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience 163: 1264–1276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci 25: 1750–1760, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226: 244–253, 2007. [DOI] [PubMed] [Google Scholar]
- Yates GK, Winter IM, Robertson D. Basilar membrane nonlinearity determines auditory nerve rate-intensity functions and cochlear dynamic range. Hear Res 45: 203–219, 1990. [DOI] [PubMed] [Google Scholar]
- Young ED. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain Res 200: 23–37, 1980. [DOI] [PubMed] [Google Scholar]
- Young ED, Brownell WE. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol 39: 282–300, 1976. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci 29: 4210–4217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci 32: 15791–15801, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res 295: 172–179, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Ryugo DK. Projections of the lateral reticular nucleus to the cochlear nucleus in rats. J Comp Neurol 504: 583–598, 2007. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Modulation of spontaneous activity by acetylcholine receptors in the rat dorsal cochlear nucleus in vivo. Hear Res 140: 7–17, 2000. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci 31: 3158–3168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res 78: 901–907, 2004. [DOI] [PubMed] [Google Scholar]
- Zhou M, Tao HW, Zhang LI. Generation of intensity selectivity by differential synaptic tuning: fast-saturating excitation but slow-saturating inhibition. J Neurosci 32: 18068–18078, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]