Abstract
Modulation of cutaneous reflexes is important in the neural control of walking, yet knowledge about underlying neural pathways is still incomplete. Recent studies have suggested that the cerebellum is involved. Here we evaluated the possible roles of the cerebellum in cutaneous reflex modulation and in attenuation of self-induced reflexes. First we checked whether leg muscle activity during walking was similar in patients with focal cerebellar lesions and in healthy control subjects. We then recorded cutaneous reflex activity in leg muscles during walking. Additionally, we compared reflexes after standard (computer triggered) stimuli with reflexes after self-induced stimuli for both groups. Biceps femoris and gastrocnemius medialis muscle activity was increased in the patient group compared with the control subjects, suggesting a coactivation strategy to reduce instability of gait. Cutaneous reflex modulation was similar between healthy control subjects and cerebellar patients, but the latter appeared less able to attenuate reflexes to self-induced stimuli. This suggests that the cerebellum is not primarily involved in cutaneous reflex modulation but that it could act in attenuation of self-induced reflex responses. The latter role in locomotion would be consistent with the common view that the cerebellum predicts sensory consequences of movement.
Keywords: cerebellum, ataxia, phase-dependent modulation, locomotion
“cutaneous reflexes” are seen as (changes in) muscle activity in reaction to nonnoxious stimulation of a cutaneous nerve. During gait such reflexes are partly, but certainly not solely, influenced by the ongoing (background) activity in the same muscle (for review see Zehr and Duysens 2004). This is most clearly seen in the case of a phase-dependent reflex reversal (Duysens et al. 1990; Yang and Stein 1990). In the tibialis anterior muscle (TA), stimulation of the sural nerve during the early swing phase results in facilitatory reflex activity, while a similar stimulus during the late swing phase results in a suppression of the background activity, despite similar background levels in these two phases (Duysens et al. 1990; Yang and Stein 1990). Although this phase-dependent reflex modulation is studied extensively (for review see Zehr and Duysens 2004), precise knowledge about the underlying neural pathways is still incomplete (Bagna and Bouyer 2011; Behrendt et al. 2013; Ruff et al. 2014).
In this study, we aimed to evaluate the role of the cerebellum in the modulation of cutaneous reflexes. The cerebellum has an important role in the control of (adaptability of) gait in humans (for review see Ilg and Timmann 2013; Morton and Bastian 2007), and observations in rats suggest a role in phase-dependent modulation of cutaneous reflexes as well (Bronsing et al. 2005; Pijpers et al. 2008). Selective impairment of the C1 module in the cerebellum of rats severely affected the modulation of cutaneous reflexes during walking. Whether humans also rely on the cerebellum for this modulation is unknown. Therefore, we analyzed and compared cutaneous reflex activity both in cerebellar patients with stable focal lesions after cerebellar tumor resection and in healthy control subjects. We hypothesized that phase-dependent reflex modulation patterns would be less pronounced in the patients.
Additionally, the cerebellum is thought to be involved in the prediction of sensory consequences of actions (for review see Bastian 2011; Wolpert et al. 1998). A well-known example is the proposition that the cerebellum is involved in the central cancellation of tickle sensation (Blakemore et al. 1998, 2001). Such predictions are important in the control of movement since afferent feedback has a delay and disruption of cerebellar activity results in movement errors (Miall et al. 2007). Furthermore, this predictive control is also important in active proprioception (Bhanpuri et al. 2013). For locomotion, it has also been suggested that sensory input resulting from one's own movements can be suppressed. In particular, it was found that sensory stimuli from the foot are not easily perceived in the period just after landing, when one may expect an abundance of afferent input from the foot (Duysens et al. 1995). Reflexes during gait are also suppressed when their occurrence can be predicted because they are elicited voluntarily (Baken et al. 2006). It was hypothesized that the cerebellum would be important for this suppression. To test these hypotheses, we directly compared cutaneous reflexes of cerebellar patients with those of healthy control subjects in reaction to both externally triggered and self-induced stimuli.
MATERIALS AND METHODS
Participants.
Eleven patients with stable focal lesions after cerebellar tumor resection (CBL; age 24.0 ± 7.1 yr; 3 men, 8 women; Table 1) and ten healthy participants (age 23.9 ± 3.7 yr; 5 men, 5 women) participated in this study. All CBL suffered from cerebellar tumors [medulloblastoma (n = 4), pilocytic astrocytoma (n = 5), Lhermitte-Duclos disease (n = 1), or hemangioblastoma (n = 1)]. Seven CBL received adjuvant radiotherapy, and three of them received adjuvant chemotherapy (treatment details in Table 2). In most CBL, no extracerebellar damage was seen, either on MR imaging or on clinical examination. In a few CBL (Table 2) there were mild signs of extracerebellar damage, as assessed on MRI images, but in no case was there a clinical repercussion: three CBL had mild residually enlarged supratentorial ventricular system and a ventriculoperitoneal shunt catheter passing through the right frontal lobe, and three had small-sized, asymptomatic cavernous angiomas (Table 2). No deficits in muscle force or sensation were observed in any of the CBL during the neurological screening. The reflexes were normal, and there were no long tract signs. In the three CBL who had received chemotherapy, there were no signs or symptoms of polyneuropathy (and no data suggestive of polyneuropathy in the medical files at the time chemotherapy was given). CBL were in a stable condition (>2 yr postop; range 8.7–30.2 yr) and were able to walk independently. Severity of ataxia was rated with the International Cooperative Ataxia Rating Scale (ICARS) (Trouillas et al. 1997), and scores ranged from 0 to 19 (6.6 ± 5.6; Table 1). All participants gave written informed consent. The experiments were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee.
Table 1.
Patient characteristics
| Adjuvant Therapies |
ICARS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age, yr | Time Postop, yr | Sex | Diagnosis | Interposed Nuclei Lesioned | Radiation | Chemo | Lesion Volume, cm3 | Total/100 | P&G/34 | Kin Fun/52 |
| 1 | 22.3 | 17.7 | m | Medulloblastoma | Both | Y | 22.0 | 13 | 6 | 2 | |
| 2 | 18.5 | 10.0 | f | Medulloblastoma | Left | Y | Y | 22.6 | 2 | 1 | 1 |
| 3 | 18.6 | 13.7 | m | Medulloblastoma | Right | Y | Y | 6.3 | 19 | 5 | 10 |
| 4 | 18.4 | 15.5 | f | Medulloblastoma | Both | Y | Y | 5.4 | 5 | 1 | 3 |
| 5 | 31.4 | 19.7 | f | Astrocytoma grade III | Right | Y | 8.6 | 11 | 5 | 4 | |
| 6 | 21.6 | 19.5 | f | Astrocytoma grade II | Right | Y | 7.1 | 0 | 0 | 0 | |
| 7 | 26.9 | 24.9 | f | Pilocytic astrocytoma | Y | 58.4 | 5 | 1 | 1 | ||
| 8 | 19.6 | 11.8 | m | Pilocytic astrocytoma | Left | 1.7 | 6 | 3 | 2 | ||
| 9 | 20.2 | 8.7 | f | Pilocytic astrocytoma | 8.2 | 3 | 0 | 0 | |||
| 10 | 28.8 | 13.9 | f | Lhermitte-Duclos disease | 58.0 | 3 | 1 | 1 | |||
| 11 | 39.9 | 30.2 | f | Hemangioblastoma | no MRI | no MRI | 6 | 4 | 2 | ||
Patients were mildly ataxic and in a stable condition (>2 yr postop). For patient 11 no MRI data were acquired. Empty fields indicate that no lesions were present or that no adjuvant therapy was received. f, Female; m, male; Y, yes; ICARS, International Cooperative Ataxia Rating Scale; P&G, Posture and Gait subscore; Kin Fin, Kinetic Functions subscore.
Table 2.
Treatment details
| No. | Diagnosis | Time Post RT, yr | Target Areas RT | Dose RT | Hypopituitarism | Time Post CT, yr | Total Duration CT, mo | Scheme CT | VP Shunt | Extracerebellar Sequela |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Medulloblastoma | 17.6 | CSP + boost FP & SP | 35.2 CSP +10 SP +20 FP | Y | Y | Y* | |||
| 2 | Medulloblastoma | 9.9 | CSP + boost FP | 35.2 CSP +20 FP | 8.7 | 12 | HIT-2000 | Y | Y† | |
| 3 | Medulloblastoma | 13.1 | CSP + boost FP & SP | 35.2 CSP +10 SP +20 FP | Y | 13.0 | 8 | HIT-91 | Y | Y† |
| 4 | Medulloblastoma | 15.2 | CSP + boost FP & SP | 35.2 CSP +10 SP +20 FP | Y | 15.2 | 3 | HIT-91 | Y‡ | |
| 5 | Astrocytoma grade III | 18.1 | FP | 60 Gy | Y | |||||
| 6 | Astrocytoma grade II | 19.4 | FP | 50.4 Gy | ||||||
| 7 | Pilocytic astrocytoma | 24.7 | FP | 60 Gy | Y† | |||||
| 8 | Pilocytic astrocytoma | |||||||||
| 9 | Pilocytic astrocytoma | Y | ||||||||
| 10 | Lhermitte-Duclos disease | Y | ||||||||
| 11 | Hemangioblastoma | Y |
RT, radiotherapy; CT, chemotherapy; CSP, craniospinal; SP, spinal; FP, fossa posterior; CT, chemotherapy; VP, ventriculo-peritoneal; HIT-2000, cisplatinum, vincristine, CCNU; HIT-91, ifosfamide, etoposide (VP16), metotrexate, ara-C, cisplatinum.
Thalamic cavernous angioma, asymptomatic;
hydrocephalus;
cavernous angioma parietal white matter, asymptomatic, cavernous angioma intramedullary spinal cord, level D12, 1.8 × 2.6 mm, asymptomatic.
Experimental setup and protocol.
Procedures and setup were similar to earlier experiments (e.g., Hoogkamer et al. 2012). Participants walked for ∼10 min at 1.11 m/s (4.0 km/h) on an instrumented dual-belt treadmill (Forcelink, Culemborg, The Netherlands). An electrical stimulus was repeatedly applied at the sural nerve near the ankle of the right leg. The stimulus consisted of a train of five rectangular pulses of 1-ms duration with a frequency of 200 Hz (Grass S48 stimulator, in series with an SIU5 isolator and a CCU1 constant-current unit, Grass Instruments).
The stimulation electrode was attached near the lateral malleolus, where the sural nerve is closest to the skin surface (approximately halfway between the lateral malleolus and the Achilles tendon). We determined the exact position of the electrode according to the optimal irradiation of the stimulus, corresponding to the innervation area of the sural nerve. The stimulus electrode was attached to the skin with tape and stabilized by a strap around the ankle, to keep conditions stable throughout the experiment. We set the stimulus intensity to twice the perception threshold (Duysens et al. 1996). We recorded bipolar EMG in the biceps femoris (BF), TA, and gastrocnemius medialis (GM) of both legs by using surface electrodes to sample at 1,000 Hz (ZeroWire, Aurion). Additionally, ground reaction forces were recorded at 1,000 Hz.
Participants were tested in two different conditions. In the “externally triggered” condition the stimuli were automatically triggered by the software. In the “self-induced” condition the stimuli were manually triggered by the participants, similar to the study by Baken et al. (2006). The order in which participants performed the “externally triggered” and “self-induced” conditions was random. In both conditions, custom-written MATLAB software was used to enable a reproducible stimulation at 16 equidistantly distributed phases in the gait cycle. Stimulation was timed relative to the instant of heel strike of the right (stimulated) leg, determined by a vertical force threshold of 10% of body weight. In the “externally triggered” condition the software directly triggered the electrical stimuli; in the “self-induced” condition auditory beeps were generated. We instructed participants to push a handheld button in reaction to the beep. This action triggered a stimulus. We asked the participants to aim for a constant interval between the beep and the button response, and we emphasized that it was not necessary to respond as fast as possible (as in a reaction time test) (Baken et al. 2006). This was important since the aim was to have the participants perform a voluntary movement, unaffected by start-react effects. The algorithm presented stimuli/beeps in each phase in random order, such that there was at least one complete stride without a stimulus between consecutive stimuli. To reach the target of 10 stimuli in each of the 16 phases, 15 beeps were presented per phase (to account for variability in reaction time).
Data analyses.
Data analysis procedures were similar to those of Hoogkamer et al. (2012). Raw force data were filtered with a fourth-order recursive, zero phase-shift, Butterworth low-pass filter with a cutoff frequency of 10 Hz. Instants of heel strike and toe-off were determined based on anterior-posterior and medio-lateral maxima in the center of pressure trajectory (Roerdink et al. 2008). Stride time and stance percentages were calculated based on the instants of heel strike and toe-off. Variability of these parameters was assessed with the coefficient of variation (CV), the ratio between the standard deviation and the mean values. We defined gait cycles from right heel strike (0%) to the next right heel strike (100%). We excluded strides when a foot incidentally was placed on two belts or when two feet were on the same belt.
The EMG signals were amplified and high-pass filtered (cutoff frequency 3 Hz), full-wave rectified, and low-pass filtered (cutoff frequency 300 Hz). To evaluate muscle activity and TA-GM coactivation over the gait cycle, EMG traces were time-normalized into 100 samples per gait cycle. For these analyses only the strides without stimuli and reflex responses were included. For each muscle the average muscle activity over these strides was calculated and then normalized to its maximum value during the gait cycle. Finally, the normalized traces of both legs were averaged. TA-GM coactivation was calculated sample by sample based on the normalized muscle activity in these muscles using
where aH represents the activity of the muscle that has the highest activity during the considered sample (i.e., either TA or GM) and aL represents the activity of the other muscle at the same time sample (Mari et al. 2014).
We quantified the reflex responses by calculating the mean of the EMG data over the reflex time window. Reflex time windows were manually set around the middle latency reflexes (or “P2 reflexes”), starting 70–80 ms after the stimulation (Baken et al. 2005; Duysens et al. 1993; Haridas et al. 2005; Yang and Stein 1990). The time windows were estimated based on visual inspection of the subtracted EMG traces (see below) (Duysens et al. 1996). A single time window, relative to the stimulus, per muscle was used for all conditions. For muscles showing little or no response, we set a time window based on the response in other muscles (e.g., Duysens et al. 1996, 2010; Tax et al. 1995; Van Wezel et al. 1997). Time window mean values were designated to the appropriate phases based on the onset of the response (start of the time window).
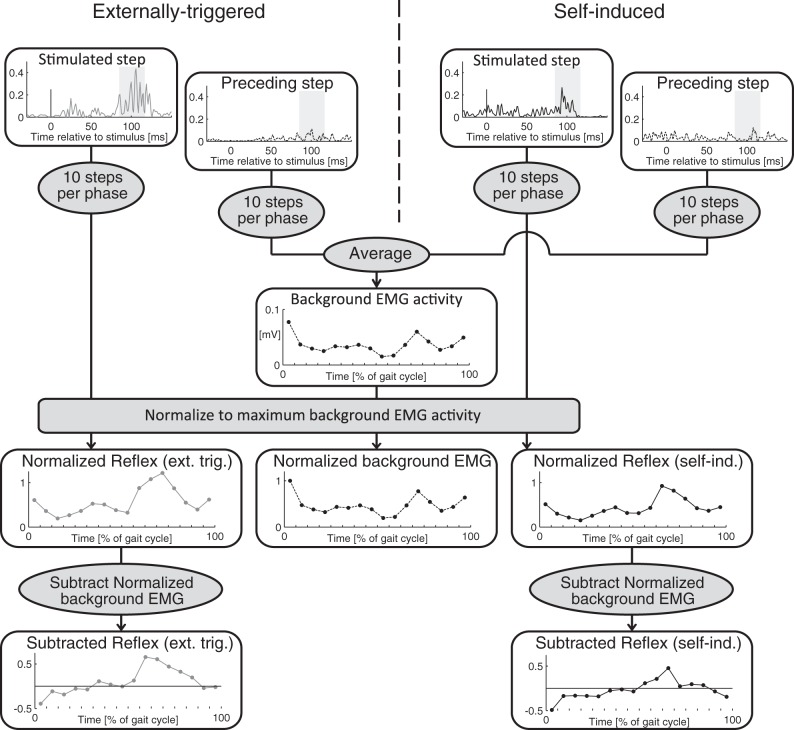
For each of the 16 phases of the gait cycle we performed the following calculations to get the reflex and background EMG data of that phase: we averaged the values of 10 stimulated strides (Fig. 1). For each stimulus, we estimated the background EMG activity by calculating the mean EMG over the same relative time window of the preceding stride (without stimulation). Next, we calculated the average value of the 10 unstimulated strides. Finally, we averaged the background values from the two conditions.
Fig. 1.
Schematic overview of the EMG data analysis procedures. Top: rectified, low-pass-filtered EMG activity. In each panel the time axis is based on the timing of the stimulus (stimulated step) or the same relative timing in the preceding gait cycle (unstimulated steps). Solid lines represent data for stimulated steps; dashed lines represent data for unstimulated steps. Gray shading on EMG traces represents the time window over which average EMG activity values were calculated. For each phase of the gait cycle we averaged the values of 10 steps. Background EMG traces were then calculated as the average of the background traces of the “externally triggered” condition (left) and the “self-induced” condition (right). Then, background and reflex EMG data were normalized to the maximum background EMG activity, displayed in the 3 panels with normalized activity (middle). Gray lines represent traces for the externally triggered condition (left); black lines represent traces for the self-induced condition (right). In a last step, we then calculated the “net” reflex amplitude by subtracting the background EMG activity from the reflex EMG activity (bottom).
Background and reflex EMG data were then normalized to the maximum background EMG activity (phase average) for each participant and muscle. In a last step, we then calculated the “net” reflex amplitude by subtracting the background EMG activity from the reflex EMG activity (Fig. 1). The range of this subtracted reflex curve was used as an index of reflex modulation.
To assess habituation in the reflex responses, we analyzed the mean absolute reflex responses to self-induced stimuli over all phases of the gait cycle, per stimulus number. For each participant, these responses were then normalized with respect to their average. Eventually, group means were calculated and ordered according to appearance during the trial.
MRI data acquisition and processing.
Image acquisition was performed with a Philips 3T Achieva MRI scanner (Philips, Best, The Netherlands) with a 32-channel matrix head coil. For all but one CBL, a three-dimensional MPRAGE high-resolution T1-weighted image (repetition time = 970 ms, echo time = 4.60 ms, flip angle = 8°, 230 1-mm slices, in-plane resolution = 0.97 × 0.98, 384 × 384 matrix) was acquired.
Lesions on the MPRAGE images were manually traced with MRIcroN software (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html). Lesion traces were spatially normalized to an atlas of the cerebellum (Diedrichsen et al. 2009) using the SUIT toolbox (http://www.icn.ucl.ac.uk/motorcontrol/imaging/suit.htm; Diedrichsen 2006; Diedrichsen et al. 2011) in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). When spatial normalization with the SUIT toolbox was inaccurate (some cases with large lesions at the outer border of the cerebellum), lesions were spatially normalized based on the whole brain image with manual corrections in atlas space when needed. Total lesion size was assessed (Table 1), and lesions in the interposed (Table 1) and other deep cerebellar nuclei (Table 3) were listed (see Fig. 2 for superposition image of all lesions). A probability threshold of 20% was used to determine whether the normalized lesions overlapped with the specific nuclei (Diedrichsen et al. 2011) and lobules.
Table 3.
Overview of lesioned lobules and nuclei
| Vermis |
Paravermis |
Hemispheres |
Nuclei |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | VI | CI | CII | VIIb | VIIIa | VIIIb | IX | X | I–IV | V | VI | CI | CII | VIIb | VIIIa | VIIIb | IX | X | I–IV | V | VI | CI | CII | VIIb | VIIIa | VIIIb | X | F | I | D |
| 1 | Y | Y | Y | Y | L | B | B | B | B | B | B | L | L | B | B | B | ||||||||||||||
| 2 | Y | Y | Y | Y | Y | Y | Y | B | B | L | L | B | B | B | B | B | L | L | B | L | B | |||||||||
| 3 | Y | Y | Y | Y | R | R | R | R | B | R | B | |||||||||||||||||||
| 4 | Y | Y | Y | Y | Y | Y | Y | B | B | B | R | B | B | |||||||||||||||||
| 5 | Y | Y | Y | Y | R | R | R | R | R | R | R | |||||||||||||||||||
| 6 | Y | Y | Y | Y | Y | Y | R | R | R | B | R | R | R | R | B | |||||||||||||||
| 7 | L | L | L | L | L | L | L | L | L | L | ||||||||||||||||||||
| 8 | Y | Y | Y | Y | Y | L | L | L | L | L | L | |||||||||||||||||||
| 9 | R | R | R | R | R | |||||||||||||||||||||||||
| 10 | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | ||||||||||||||
| 11 | no MRI | no MRI | no MRI | no MRI | ||||||||||||||||||||||||||
For patient 11 no MRI data were acquired; Y, yes; L, left; R, right; B, both; F, fastigial nuclei; I, interposed nuclei; D, dentate nuclei.
Fig. 2.
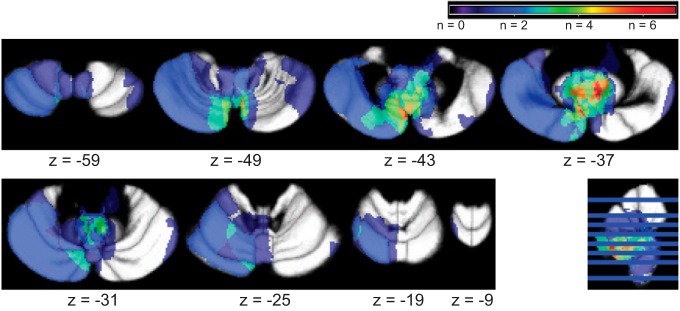
Superposition of the regions of cerebellar lesions of all patients. Maximum overlap (7 patients) was within the left paravermal lobule VIIb.
Statistical analyses.
Results in the text are presented as mean values ± SD. To compare muscle activity and coactivation during the normal (unstimulated) strides between groups, we calculated the mean values of these parameters during four periods of the gait cycle: initial double stance, single stance, terminal double stance, and swing. A group × period ANOVA was performed for each muscle and for the TM-GM coactivation. To evaluate potential differences in phase-dependent modulation of subtracted reflex responses between groups, we used Mann-Whitney U-tests (Baken et al. 2006; Duysens et al. 2010; Van Wezel et al. 1997). To evaluate the cerebellar role in attenuation of self-induced reflex responses, we compared subtracted reflex EMG in the TA muscle after externally triggered and self-induced stimuli within groups (Wilcoxon matched pairs test). In addition to these tests (uncorrected for multiple comparisons), we performed a group × condition ANOVA on average values over the period where suppression was relevant (43.75–93.75% of the gait cycle). To assess correlations between muscle activity, reflex attenuation, temporal gait parameters, and ICARS (sub)scores, Spearman's rank correlation coefficients were calculated. Additionally, we used Wilcoxon tests to assess whether muscle activity and reflex attenuation was different between patient subgroups (subdivisions based on lesion characteristics; Table 1). A traditional level of significance was used for all statistical tests (α = 0.05).
RESULTS
Muscle activity during gait.
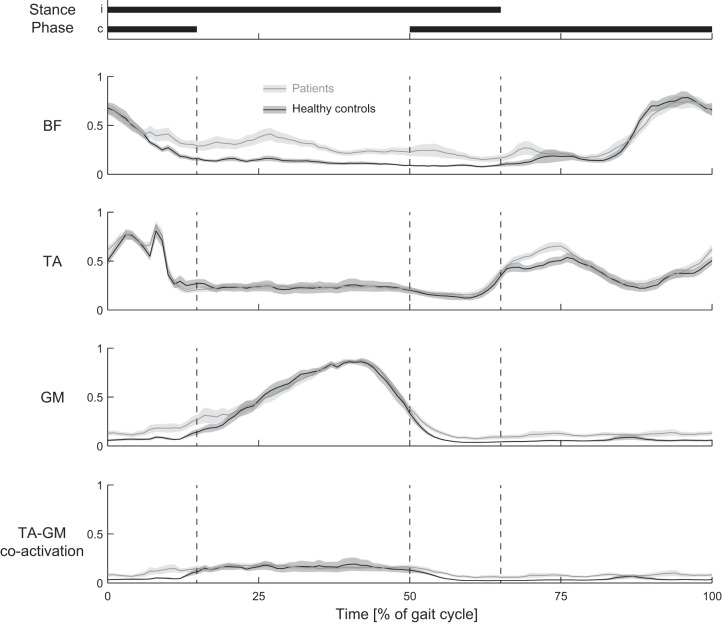
Stride time (CBL 1.05 ± 0.03 s vs. healthy control subjects 1.07 ± 0.07 s, P = 0.55) and relative stance time (64.6 ± 0.7% vs. 64.6 ± 0.5%, P = 0.60) were similar between groups, but variability of those parameters was higher in the CBL group than in the control group (stride time variability: 2.6 ± 0.8% vs. 1.6 ± 0.2%, P < 0.001; relative stance time variability: 2.3 ± 0.7% vs. 1.4 ± 0.2%, P < 0.001). CBL walked with increased activity in the BF muscles, specifically during the single-stance period (Fig. 3; main effect for group: P = 0.002; group × period interaction: P = 0.027; post hoc comparisons: initial double stance P = 0.67, single stance P = 0.002, terminal double stance P = 0.08, swing P = 1.0). For the GM muscle a significant main effect for group indicated that the CBL group walked with a higher activation than the healthy control group (P = 0.042). The group × period interaction was not significant (P = 0.07). For the TA muscle the main effect for group and the group × period interaction were not significant (P = 0.43 and P = 0.61, respectively). The main effect for period was significant for all muscles (P < 0.001).
Fig. 3.
Comparisons of muscle activity and coactivation traces between cerebellar patients and healthy control subjects: muscle EMG activity for cerebellar patients (gray line) and healthy control subjects (black line). Shaded areas show SE. Durations of the stance period for the ipsilateral (i) and contralateral (c) legs are displayed at top. BF, biceps femoris; TA, tibialis anterior; GM, medial gastrocnemius. Bottom: coactivation between TA and GM for cerebellar patients (gray line) and healthy control subjects (black line). Coactivation was calculated sample by sample based on the normalized muscle activity (mean of both legs) using TA-GM coactivation = [(aH + aL)/2] × (aL/aH), where aH represents the activity of the muscle that has the highest activity during that phase (i.e., either TA or GM) and aL represents the activity of the other muscle during that phase (Mari et al. 2014).
Coactivation between the TA and GM muscles appeared higher in the CBL group, specifically during the double-stance periods and during the swing, but no significant main effect for group (P = 0.06) or group × period interaction (P = 0.32) was observed (Fig. 3). Overall coactivation was small in both groups (<0.2). To assess whether the increased coactivation or the increased BF activity in the CBL group was related to ataxia severity or gait variability, we correlated these muscle activation measures with ICARS (sub)scores and variability of either stride time or relative stance time. None of those parameters was significantly correlated (|ρ| < 0.5; P > 0.10, for all). In addition, we compared TA-GM coactivation and BF activity between subgroups based on lesion location (affected nuclei) or radiation therapy application. No differences were observed (all P > 0.10).
Phase-dependent reflex modulation.
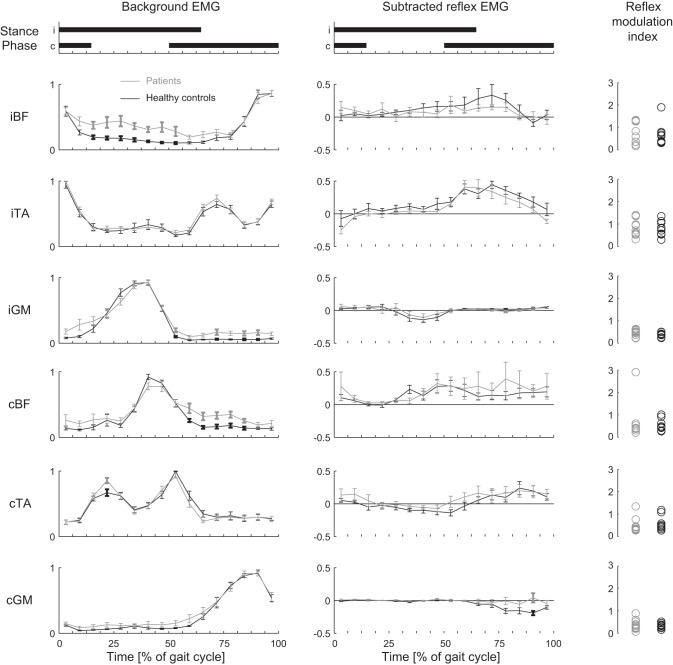
Given the difference in background EMG one would expect to see larger BF responses in the cerebellar group. This appears to occur for the contralateral (c)BF (Fig. 4) but not for the ipsilateral side, where there was no increase in BF responses during most of the stance phase despite the consistent increase in background activity. However, specifically for the BF interindividual variability was large (see also individual indexes of reflex modulation; Fig. 4, right). As such no significant group differences were observed in subtracted reflex traces of the BF muscles. In the GM one would have expected some larger responses in the second half of the step cycle (increased background) in the patients, but this was not observed, at least not in the ipsilateral (i)GM (Fig. 4). On the contralateral side there was significantly less suppression during the late single stance in the patient group, while background did not differ (87.5–93.75% of the gait cycle of the stimulated limb). In TA the subtracted reflex EMG traces were similar between groups (Fig. 4). In both groups the iTA traces showed a reflex reversal, with clear facilitatory reflex activity during the early swing phase and suppressive activity around heel strike. For all muscles the indexes of reflex modulation were similar between groups (Fig. 4, right).
Fig. 4.
Subtracted reflex traces and modulation indexes are similar between cerebellar patients and healthy control subjects: normalized background EMG activity (left) and normalized subtracted reflex EMG activity (center) for cerebellar patients (CBL; gray line) and healthy control subjects (black line). Error bars show SE. Bold data points and error bars indicate phases when CBL differ significantly from healthy control subjects in a given phase (uncorrected for multiple comparisons). Durations of the stance period for the ipsilateral and contralateral legs are displayed at top. Right: reflex modulation index in different muscles for CBL (gray circles) and healthy control subjects (black circles). Note that subtracted reflex traces and modulation indexes are similar between CBL and control subjects, irrespective of differences in background EMG.
Attenuation of self-induced reflex responses.
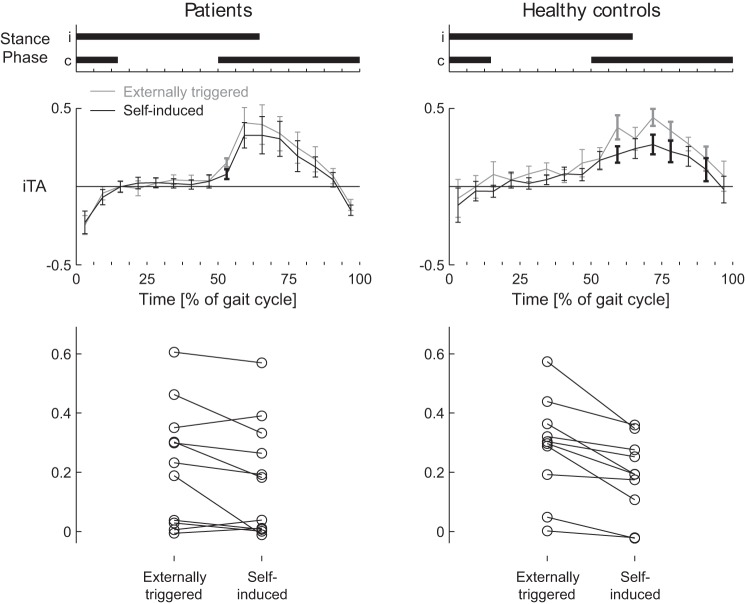
To evaluate attenuation of self-induced reflex responses (Fig. 5), we compared subtracted reflex EMG in the iTA muscle after externally triggered and self-induced stimuli, since the mean reduction in reflex responses is known to be the strongest in the iTA (Baken et al. 2006). Background EMG activity was similar between groups for this muscle (as mentioned above; Fig. 3). However, the suppression of reflex responses showed a tendency to differ between the two groups (Fig. 5). In the control group, reduced subtracted reflex responses were seen in the stance-swing transition and during the swing phase. Specifically, this reflex activity was attenuated for several phases when self induced (56.25–62.5%, 68.75–81.25%, and 87.5–93.75% of the gait cycle). This attenuation was less pronounced in the CBL group, where reflex activity to externally triggered and self-induced stimuli only differed during the early stance phase of the contralateral leg (50–56.25% of the gait cycle; Fig. 5). To evaluate whether this attenuation was different between groups we calculated the mean subtracted reflex activity over the period where suppression could occur (namely, 43.75–93.75% of the gait cycle) for both conditions in both groups (Fig. 5) and performed a repeated-measures ANOVA. The majority of the CBL and all healthy control subjects showed attenuation of self-induced reflex responses (Fig. 5, bottom) and a significant main effect for condition was observed (P < 0.001), indicating that in general reflexes to self-induced stimuli were smaller than reflexes to externally triggered stimuli. However, the main effect for group was not significant (P = 0.69) and also the group × condition interaction did not reach significance (P = 0.14). Hence, attenuation of self-induced reflex responses only appeared different between groups when considering individual phases but not when considered over the whole period. The amount of suppression varied between CBL, but there was no correlation with ICARS (sub)scores, lesion location (affected nuclei), or radiation therapy application for this relatively small group (all P > 0.10).
Fig. 5.
Attenuation of self-induced reflex responses in cerebellar patients and healthy control subjects: normalized subtracted reflex EMG activity for cerebellar patients (left) and healthy control subjects (right). Durations of the stance periods for the ipsilateral and contralateral legs are displayed at top. Middle: self-induced reflexes are shown in black, externally triggered reflexes in gray. Error bars show SE. Bold data points and error bars indicate phases when self-induced reflex activity is significantly attenuated. Bottom: individual data for externally triggered and self-induced reflexes.
Habituation.
To assess whether differences in attenuation of self-induced reflexes were related to different habituation characteristics for both groups, we plotted the mean responses with respect to order of presentation (Fig. 6). Habituation was similar between groups (Fig. 6), and habituation of responses was very modest, in agreement with previous studies (Bastiaanse et al. 2006; see also Fig. 6 in Tax et al. 1995).
Fig. 6.
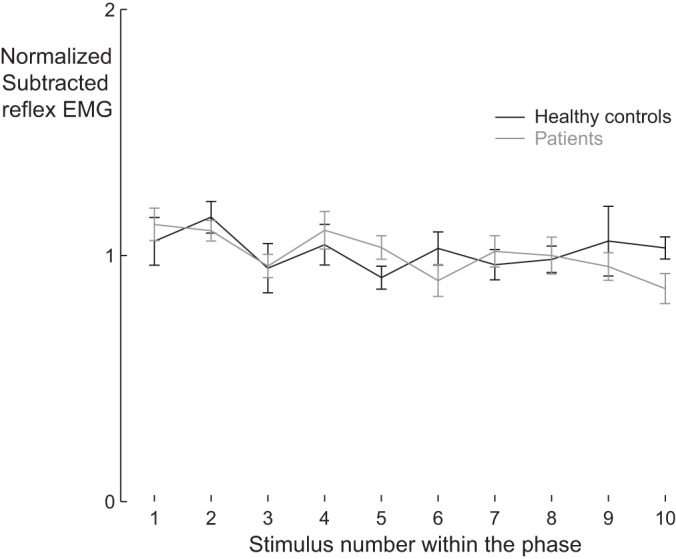
Limited habituation across trials: total mean reflex responses due to self-induced stimuli as a function of the order of appearance during the trial for cerebellar patients (gray line) and healthy control subjects (black line). We analyzed the mean absolute reflex responses to self-induced stimuli over all phases of the gait cycle, per stimulus number. For each participant, these responses were then normalized with respect to their average. Eventually, group means were calculated and plotted as a function of the order of appearance during the trial. Error bars show SE.
DISCUSSION
In this study we aimed to address the role of the cerebellum in modulation or attenuation of cutaneous reflexes during gait. We observed that patients with focal lesions in the cerebellum walk with an overall higher BF and GM muscle activity than healthy control subjects. Our results from the reflex activity analyses show relatively minor differences (with respect to the differences in background, some expected increases in reflexes were not seen in some muscles such as BF and GM). Hence the data do not suggest that the cerebellum has a major role in phase-dependent modulation of cutaneous reflexes in walking humans. On the other hand, the suppression of reflexes, which was consistently seen in control subjects, was much less apparent in the patients (fewer phases with significant suppression). Hence these data do suggest that the cerebellum might play a role in attenuating cutaneous reflexes after self-induced stimuli.
Increased GM and BF activity.
Although evaluation of the muscle activity during the steps without stimulation was not a main goal in this study, the first major finding was that the CBL group walked with an overall higher GM and BF muscle activity than healthy control subjects. The increased GM muscle activity was accompanied by an overall increased TM-GM coactivation. BF muscle activity was specifically higher during the single-stance period. Muscle activity has been observed to be increased in more severely affected cerebellar ataxia patients with diffuse cerebellar damage (Mari et al. 2014; Mitoma et al. 2000). In those patients, the increased activity in the GM and BF muscles was accompanied by an increased activity in their antagonists, the TA and vastus lateralis muscles, respectively. Such antagonist coactivation could increase leg stiffness, which can be a compensation strategy when postural threat is elevated. It is often observed in walking elderly subjects (Franz and Kram 2013; Hortobagyi et al. 2009; Peterson and Martin 2010). In our CBL group TA-GM coactivation was higher overall, similar to observations by Mari et al. (2014) for more severely affected cerebellar ataxia patients with diffuse cerebellar damage. As our main purpose was to evaluate cutaneous reflex activity, we did not measure EMG activity in the vastus lateralis. Accordingly, we could not directly assess coactivation between the BF and the vastus lateralis in our patient group.
Phase-dependent reflex modulation.
Increased coactivation could theoretically lead to increased reflex activity due to increased excitability of the motoneuron pool. Indeed, minor differences were observed for the GM muscles, but for the BF and TA muscles no significant differences were observed in the subtracted reflex traces of both legs. Overall, the reflex modulation patterns of both groups were similar to those observed in earlier studies (Baken et al. 2006; Bastiaanse et al. 2006; Duysens et al. 2010; Hoogkamer et al. 2012; Lamont and Zehr 2007; Van Wezel et al. 1997). Hence overall the results point toward subtle changes in some muscles, but overall there is no strong indication that the cerebellum plays a dominant role in the modulation of reflexes.
At first sight the present results appear to be inconsistent with animal studies. In rats, Pijpers et al. (2008) observed reduced reflex activity in the BF after selective impairment of the C1 module in the cerebellum. However, it should be mentioned that the present group consisted of mildly affected patients with long-standing lesions. Lesions in our CBL group were due to tumor resection at a young age (7.1 ± 4.3 yr), and their immature brains had a high potential for neural reorganization and compensation (Caeyenberghs et al. 2009; Gramsbergen 2007; Kolb et al. 2001). In contrast, the rats in the study mentioned above had acute lesions and had no chance to show plastic changes. Furthermore, it should be noted that in the rat study the differences in reflexes between lesioned rats and control rats became less apparent and nonsignificant at postoperative day 7. Finally, it could be that the locations of the lesions differed between the rats and the humans. In the rats the hindlimb part of the C1 module (paravermal zones of lobules I–IV, V, and VIII; medial part of the anterior interposed nucleus) was impaired (Pijpers et al. 2008). In none of the CBL were these paravermal zones of lobules I–IV, V, and VIII fully affected (Table 3). However, in most CBL the interposed nuclei were affected (Table 1). Hence, if these areas were important for this function one would have expected to see at least some effect.
A second point is that, in humans, differences between populations are difficult to demonstrate because there are considerable interindividual differences with respect to the modulation of this type of reflexes (Bagna and Bouyer 2011; Baken et al. 2006; Duysens et al. 1992; Hoogkamer et al. 2012; Ruff et al. 2014). Because of the nature of our patient sample, one should be cautious in drawing too strong conclusions. Related to the potential for compensation mentioned above, and the limited size of the lesions and the variation in their location, it could be argued that cerebellar damage in our sample was too mild to observe major effects on phase-dependent reflex modulation.
In this respect it would be interesting to evaluate phase-dependent modulation of cutaneous reflex in more severely ataxic patients, such as patients with degenerative cerebellar diseases. However, gait pattern and muscle coordination in these patients might be too much affected, indirectly changing reflex modulation and making it less feasible to assess the direct effects of the cerebellar damage on reflex modulation. Future research should also focus on other brain structures to reveal the underlying neural pathway for phase-dependent reflex modulation. So far, the most likely neural structures involved are the spinal cord (central pattern generator) and the motor cortex (for review see Duysens et al. 2004). Results from several transcranial magnetic stimulation studies suggest an important role for the motor cortex and the transcortical pathway (Christensen et al. 1999; Nielsen et al. 1997; Pijnappels et al. 1998). Recent observations of phase-dependent reflex modulation during passive viewing of walking (Behrendt et al. 2013) and during visually guided stepping (Ruff et al. 2014) further support the importance of the motor cortex in reflex modulation.
Attenuation of self-induced reflex responses.
Our third finding entails the observation that some CBL were less able to attenuate reflex activity after self-induced stimuli than healthy control subjects. There was a clear reduction in the number of phases where such suppression was observed. Attenuated reflexes after self-induced stimuli during walking were first observed by Baken et al. (2006). In their main group, self-induced reflexes were attenuated over 50% of the gait cycle, from midstance to midswing (31.25–81.25% of the gait cycle). Healthy participants in our study showed attenuated reflexes over 25% of the gait cycle, from late stance to midswing (56.25–62.5%, 68.75–81.25%, and 87.5–93.75% of the gait cycle; Fig. 5). In the patient group attenuation occurred in a single phase of the gait cycle during double stance (50–56.25% of the gait cycle). However, on a group level (considering the period where suppression could occur) the reflex attenuation was not significantly different between groups. Hence, one has to conclude that the data are suggestive for a contribution of cerebellar structures but that a definite role cannot be assigned on the basis of the present sample of mildly affected cerebellar patients. Note that we specifically refer to these reflexes as “self-induced” since it was observed in earlier work that auditory cues preceding the stimuli do not result in reflex attenuation, suggesting that the attenuation is not just caused by anticipation of the stimuli in general (Bastiaanse et al. 2006).
Cerebellar involvement in reflex suppression would be in line with findings from other studies, supporting the idea that the cerebellum provides signals to cancel the sensory response to self-induced stimulation. According to this idea, predictions of the sensory consequences of motor commands are used to partly cancel the actual sensory consequences. This would make the system more sensitive to external (unexpected) perturbations, as they result in unpredictable sensory inputs. Furthermore, such predictions are an important control feature. Without predictive control a system only depends on feedback information, which comes with delays, making correction of movements in real time impossible (Miall et al. 2007; Wolpert and Flanagan 2001). Reduced responses after self-induced stimuli or self-initiated actions have been observed in multiple different domains, such as self-induced tickling sensations (Blakemore et al. 1998, 2001), active head movements (McCrea et al. 1999), self-induced muscle stimulation (Gerilovsky et al. 2002), self-induced sounds (Knolle et al. 2013), and self-induced eyeblink reflexes (Meincke et al. 2003). In the latter study it was found that the electrically evoked R2 blink reflex is suppressed by using self-stimulation (in particular, the later sections of R2 were affected). The present study shows that such self-induced suppression can be consistently evoked in the context of cutaneous reflexes during locomotion. Note that habituation was similar between groups, which suggests that differences in attenuation of self-induced reflexes were not related to different habituation characteristics.
GRANTS
This work was supported by Research Foundation-Flanders (FWO Grant G.0756.10). J. Duysens has been funded by Research Foundation-Flanders (FWO Grant G.0901.11) and by the Interuniversity Attraction Poles Program initiated by the Belgian Science Policy Office (P7/11).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.H., F.V.C., S.P.S., and J.D. conception and design of research; W.H. performed experiments; W.H., F.V.C., and J.D. analyzed data; W.H., F.V.C., S.P.S., and J.D. interpreted results of experiments; W.H. prepared figures; W.H. and J.D. drafted manuscript; W.H., F.V.C., S.P.S., and J.D. edited and revised manuscript; W.H., F.V.C., S.P.S., and J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen Jansen and Marc Beirinckx for developing the software to control the reflex stimulation during the experiment.
REFERENCES
- Bagna M, Bouyer LJ. A new approach for detecting and analyzing cutaneous reflexes during locomotion. J Neurophysiol 105: 1406–1415, 2011. [DOI] [PubMed] [Google Scholar]
- Baken BC, Dietz V, Duysens J. Phase-dependent modulation of short latency cutaneous reflexes during walking in man. Brain Res 1031: 268–275, 2005. [DOI] [PubMed] [Google Scholar]
- Baken BC, Nieuwenhuijzen PH, Bastiaanse CM, Dietz V, Duysens J. Cutaneous reflexes evoked during human walking are reduced when self-induced. J Physiol 570: 113–124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanse CM, Degen S, Baken BC, Dietz V, Duysens J. Suppression of cutaneous reflexes by a conditioning pulse during human walking. Exp Brain Res 172: 67–76, 2006. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol 21: 596–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt F, Wagner H, de Lussanet MH. Phase-dependent reflex modulation in tibialis anterior during passive viewing of walking. Acta Psychol 142: 343–348, 2013. [DOI] [PubMed] [Google Scholar]
- Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci 33: 14301–14306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12: 1879–1884, 2001. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1: 635–640, 1998. [DOI] [PubMed] [Google Scholar]
- Bronsing R, van der Burg J, Ruigrok TJ. Modulation of cutaneous reflexes in hindlimb muscles during locomotion in the freely walking rat: a model for studying cerebellar involvement in the adaptive control of reflexes during rhythmic movements. Prog Brain Res 148: 243–257, 2005. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Wenderoth N, Smits-Engelsman BC, Sunaert S, Swinnen SP. Neural correlates of motor dysfunction in children with traumatic brain injury: exploration of compensatory recruitment patterns. Brain 132: 684–694, 2009. [DOI] [PubMed] [Google Scholar]
- Christensen LO, Morita H, Petersen N, Nielsen J. Evidence suggesting that a transcortical reflex pathway contributes to cutaneous reflexes in the tibialis anterior muscle during walking in man. Exp Brain Res 124: 59–68, 1999. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33: 127–138, 2006. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46, 2009. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54: 1786–1794, 2011. [DOI] [PubMed] [Google Scholar]
- Duysens J, Bastiaanse CM, Smits-Engelsman BC, Dietz V. Gait acts as a gate for reflexes from the foot. Can J Physiol Pharmacol 82: 715–722, 2004. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Murrer L, Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. J Neurophysiol 76: 301–310, 1996. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Nawijn S, Berger W, Prokop T, Altenmuller E. Gating of sensation and evoked potentials following foot stimulation during human gait. Exp Brain Res 105: 423–431, 1995. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res 90: 404–414, 1992. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Res 613: 230–238, 1993. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82: 351–358, 1990. [DOI] [PubMed] [Google Scholar]
- Duysens J, Van Wezel BM, Smits-Engelsman B. Modulation of cutaneous reflexes from the foot during gait in Parkinson's disease. J Neurophysiol 104: 230–238, 2010. [DOI] [PubMed] [Google Scholar]
- Franz JR, Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture 37: 378–384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerilovsky L, Philipova D, Georgieva S. Reduction of the muscle activity associated with self-imposed electrical stimulation of mixed nerves supplying lower limb muscles in man. J Electromyogr Kinesiol 12: 247–258, 2002. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. Neural compensation after early lesions: a clinical view of animal experiments. Neurosci Biobehav Rev 31: 1088–1094, 2007. [DOI] [PubMed] [Google Scholar]
- Haridas C, Zehr EP, Misiaszek JE. Postural uncertainty leads to dynamic control of cutaneous reflexes from the foot during human walking. Brain Res 1062: 48–62, 2005. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Massaad F, Jansen K, Bruijn SM, Duysens J. Selective bilateral activation of leg muscles after cutaneous nerve stimulation during backward walking. J Neurophysiol 108: 1933–1941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture 29: 558–564, 2009. [DOI] [PubMed] [Google Scholar]
- Ilg W, Timmann D. Gait ataxia-specific cerebellar influences and their rehabilitation. Mov Disord 28: 1566–1575, 2013. [DOI] [PubMed] [Google Scholar]
- Knolle F, Schröger E, Kotz SA. Cerebellar contribution to the prediction of self-initiated sounds. Cortex 49: 2449–2461, 2013. [DOI] [PubMed] [Google Scholar]
- Kolb B, Brown R, Witt-Lajeunesse A, Gibb R. Neural compensations after lesion of the cerebral cortex. Neural Plast 8: 1–16, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EV, Zehr EP. Earth-referenced handrail contact facilitates interlimb cutaneous reflexes during locomotion. J Neurophysiol 98: 433–442, 2007. [DOI] [PubMed] [Google Scholar]
- Mari S, Serrao M, Casali C, Conte C, Martino G, Ranavolo A, Coppola G, Draicchio F, Padua L, Sandrini G, Pierelli F. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum 13: 226–236, 2014. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82: 416–428, 1999. [DOI] [PubMed] [Google Scholar]
- Meincke U, Topper R, Gouzoulis-Mayfrank E. The electrically elicited R2 blink reflex consists of distinct subcomponents. Electromyogr Clin Neurophysiol 43: 91–95, 2003. [PubMed] [Google Scholar]
- Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5: e316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: a study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci 174: 22–39, 2000. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum 6: 79–86, 2007. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. J Physiol 501: 473–484, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 31: 355–359, 2010. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Van Wezel BM, Colombo G, Dietz V, Duysens J. Cortical facilitation of cutaneous reflexes in leg muscles during human gait. Brain Res 787: 149–153, 1998. [DOI] [PubMed] [Google Scholar]
- Pijpers A, Winkelman BH, Bronsing R, Ruigrok TJ. Selective impairment of the cerebellar C1 module involved in rat hind limb control reduces step-dependent modulation of cutaneous reflexes. J Neurosci 28: 2179–2189, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerdink M, Coolen BH, Clairbois BH, Lamoth CJ, Beek PJ. Online gait event detection using a large force platform embedded in a treadmill. J Biomech 41: 2628–2632, 2008. [DOI] [PubMed] [Google Scholar]
- Ruff CR, Miller AB, Delva ML, Lajoie K, Marigold DS. Modification of cutaneous reflexes during visually guided walking. J Neurophysiol 111: 379–393, 2014. [DOI] [PubMed] [Google Scholar]
- Tax AA, Van Wezel BM, Dietz V. Bipedal reflex coordination to tactile stimulation of the sural nerve during human running. J Neurophysiol 73: 1947–1964, 1995. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timmann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- Van Wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci 17: 3804–3814, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol 11: R729–R732, 2001. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63: 1109–1117, 1990. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist 10: 347–361, 2004. [DOI] [PubMed] [Google Scholar]