Abstract
The purpose of this study was to determine the effect of supplementing the diet of type 1 diabetic rats with menhaden oil on diabetic neuropathy. Menhaden oil is a natural source for n-3 fatty acids, which have been shown to have beneficial effects in cardiovascular disease and other morbidities. Streptozotocin-induced diabetic rats were used to examine the influence of supplementing their diet with 25% menhaden oil on diabetic neuropathy. Both prevention and intervention protocols were used. Endpoints included motor and sensory nerve conduction velocity, thermal and mechanical sensitivity, and innervation and sensitivity of the cornea and hindpaw. Diabetic neuropathy as evaluated by the stated endpoints was found to be progressive. Menhaden oil did not improve elevated HbA1C levels or serum lipid levels. Diabetic rats at 16-wk duration were thermal hypoalgesic and had reduced motor and sensory nerve conduction velocities, and innervation and sensitivity of the cornea and skin were impaired. These endpoints were significantly improved with menhaden oil treatment following the prevention or intervention protocol. We found that supplementing the diet of type 1 diabetic rats with menhaden oil improved a variety of endpoints associated with diabetic neuropathy. These results suggest that enriching the diet with n-3 fatty acids may be a good treatment strategy for diabetic neuropathy.
Keywords: diabetic peripheral neuropathy, fish oil, epidermal nerve fibers, corneal nerve fibers, type 1 diabetes
it is generally accepted that increased consumption of n-3 fatty acids lowers the risk of cardiovascular disease (De Caterina 2011). The main source of n-3 fatty acids in the Western diet is fish, especially oily fish (De Caterina 2011). Over several decades a large number of studies have found an inverse association between fish consumption and morbidity and mortality from coronary heart disease (Calder 2004; De Caterina 2011; Kris-Etherton et al. 2002). Blood levels of n-3 fatty acids also appear to correlate inversely with death from cardiovascular causes (Albert et al. 2002; De Caterina 2011; Siscovick et al. 1995). Less is known about the effects of n-3 fatty acids on diabetic complications such as neuropathy.
Peripheral neuropathy afflicts over 50% of patients with diabetes and is responsible for the majority of nontrauma-related amputations. To date, tight glycemic control is the only strategy shown to prevent or delay the development of neuropathy in patients with type 1 diabetes (Ang et al. 2014). However, the Diabetes Control and Complications Trial study showed that tight glycemic control is difficult to achieve and sustain over time and, perhaps, insufficient to fully prevent diabetic neuropathy (Ang et al. 2014). Thus there is a significant unmet need for an effective and safe treatment for diabetic neuropathy. Our group has found that fish oil, a natural source of n-3 fatty acids, supplementation of type 2 diabetic rats improved diabetic neuropathy (Coppey et al. 2012). In patients with type 2 diabetes, long-term treatments with eicosapentaenoic acid, an n-3 fatty acid, had beneficial effects on diabetic neuropathy (Okuda et al. 1996). To further explore the benefits of dietary n-3 fatty acid enrichment on diabetic neuropathy, we performed a preclinical study using type 1 diabetic rats and both a prevention and intervention protocol.
MATERIALS AND METHODS
Materials.
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical (St. Louis, MO).
Animals.
Male Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN) rats 10–11 wk of age were housed in a certified animal care facility, and food (no. 7001; Harlan Teklad, Madison, WI) and water were provided ad libitum. All institutional (ACURF Approval No. 1290701) and National Institutes of Health guidelines for use of animals were followed. As a preliminary study we examined the progression of diabetes-induced neuropathic changes over a period of 12 wk. For this study rats at 12 wk of age were separated into two groups. One of these groups was treated with streptozotocin (55 mg/kg in 0.1 M citric acid buffer, pH 4.5 ip). Diabetes was verified 96 h later by evaluating blood glucose levels with the use of glucose-oxidase reagent strips (Aviva Accu-Chek; Roche, Mannheim, Germany). Rats having blood glucose level of 300 mg/dl (11.1 mM) or greater were considered to be diabetic. The other group was treated with vehicle and was termed the control group. All diabetic rats were treated with 2–3 U of Lantus insulin every other day to maintain body weight (Oltman et al. 2011). At 4, 8, and 12 wk, rats from both the control and diabetic groups were examined for neuropathic endpoints.
For the study to examine the effect of enrichment of the diet with menhaden oil on diabetic neuropathy, rats at 12 wk of age were separated into five groups. Three of these groups were treated with streptozotocin, and diabetes was verified as described above. One group of diabetic rats (diabetic nontreated) remained on the standard diet for the entire 16 wk of the study. A second group of diabetic rats (prevention group) was placed on a diet containing 25% kcal fat derived from menhaden oil immediately after verification of hyperglycemia (Research Diets, New Brunswick, NJ). The third group of diabetic rats (intervention group) remained on the standard diet for 8 wk and then was placed on the menhaden oil enriched diet for the final 8 wk of the study. The other two groups of rats not treated with streptozotocin were fed a standard diet (control group) or placed on the menhaden oil enriched diet (control treated) for the 16-wk period of the study. The fatty acid compositions of the standard diet and the diet enriched with menhaden are provided in Table 1.
Table 1.
Fatty acid %composition of diets measured by gas chromatography
| Diet | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 20:5 | 22:6 |
|---|---|---|---|---|---|---|---|
| Control (3) | 22 ± 3 | 2 ± 1 | 8 ± 1 | 28 ± 3 | 33 ± 4 | < 1 | < 1 |
| 25% Menhaden oil (3) | 17 ± 3 | 13 ± 3 | 3 ± 1 | 12 ± 2 | 13 ± 2 | 13 ± 2 | 7 ± 1 |
Data are presented as the means ± SE. Parentheses indicate the number of experimental determinations. 16:0, Palmitic acid; 16:1, palmitoleic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; 20:5, eicosapentaenoic acid; 22:6, docosahexaenoic acid.
Behavioral response.
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method as previously described (Oltman et al. 2008). Briefly, the rat was placed in the observation chamber on top of the thermal testing apparatus and allowed to acclimate to the warmed glass surface (30°C) and surroundings for a period of 15 min. The mobile heat source was maneuvered so that it was under the heal of the hindpaw and then activated, a process that activates a timer and locally warms the glass surface, when the rat withdrew its paw, the timer and the heat source were turned off and the time was recorded. The timer was defaulted to go off after 25 s to avoid injury to the rat. Following an initial recording, which was discarded, two measurements were made for each hindpaw, with a rest period of 5 min between each measurement. The mean of the measurements reported in seconds was used as the thermal nociceptive response. Tactile responses were evaluated by quantifying the withdrawal threshold of the hindpaw in response to stimulation with flexible von Frey filaments as previously described (Drel et al. 2007). The data are reported in grams. Corneal sensation was measured using a Cochet-Bonnet filament esthesiometer (Luneau Ophtalmogie) as previously described (Davidson et al. 2012). The testing began with the nylon filament extended to the maximal length (6 cm). The end of the nylon filament was touched to the cornea. If the rat blinked (positive response), the length of the filament was recorded. If the rat did not blink, then the nylon filament was shortened by 0.5 cm and the test was repeated until a positive response was recorded. This process was repeated for each eye three times. The data are reported as centimeters.
Motor and sensory nerve conduction velocity.
On the day of terminal studies, rats were weighed and anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg; Abbott Laboratories, North Chicago, IL). Motor nerve conduction velocity was determined as previously described using a noninvasive procedure in the sciatic-posterior tibial conducting system (Coppey et al. 2000). The left sciatic nerve was stimulated first at the sciatic notch and then at the Achilles tendon. Stimulation consisted of single 0.2-ms supramaximal (8 V) pulses through a bipolar electrode (Grass S44 Stimulator; Grass Medical Instruments, Quincy, MA). The evoked potentials were recorded from the interosseous muscle with a unipolar platinum electrode and displayed on a digital storage oscilloscope (model 54600A; Hewlett Packard, Rolling Meadows, IL). Motor nerve conduction velocity was calculated by subtracting the distal from the proximal latency measured in milliseconds from the stimulus artifact of the take-off of the evoked potential and the difference was divided into the distance between the two stimulating electrodes measured in millimeters using a Vernier caliper. Sensory nerve conduction velocity was determined using the digital nerve as described by Obrosova et al. (2004). Briefly, hindlimb sensory nerve conduction velocity was recorded in the digital nerve to the second toe by stimulating with a square-wave pulse of a 0.05-ms duration using the smallest intensity current that resulted in a maximal amplitude response. The sensory nerve action potential was recorded behind the medial malleolus. The sensory nerve conduction velocity was calculated by measuring the latency to the onset/peak of the initial negative deflection and the distance between stimulating and recording electrodes. The motor and sensory nerve conduction velocities are reported in meters per second.
Corneal innervation.
Subbasal corneal nerves were imaged using the Rostock cornea module of the Heidelberg Retina Tomograph confocal microscope as previously described (Davidson et al. 2012). The anesthetized rat was secured to a platform that allows adjustment and positioning of the rat in three dimensions. A drop of GenTeal (lubricant eye gel) was applied onto the tip of the lens and advanced slowly forward until the gel contacted the cornea allowing optical but not physical contact between the objective lens and corneal epithelium (Davidson et al. 2012). Six random high-quality images without overlap of the subepithelial nerve plexus around the perimeter of the central cornea were acquired by finely focusing the objective lens to maximally resolve the nerve layer just under the corneal epithelium. The investigator acquiring these images was masked with respect to identity of the animal condition. For these studies a single parameter of corneal innervation was quantified. Corneal nerve fiber length defined as the total length of all nerve fibers and branches (in millimeters) present in the acquired image standardized for area of the image (in square millimeters) was determined for each image by tracing the length of each nerve in the image, summing the total length, and dividing by the image area (Davidson et al. 2012). The corneal fiber length for each animal was the mean value obtained from the six acquired images and expressed as millimeters per millimeters squared. Based on receiver operating characteristic curve analysis, corneal nerve fiber length is the optimal parameter for diagnosing patients with diabetic neuropathy and has the lowest coefficient of variation (Quattrini et al. 2007; Tavakoli et al. 2010).
After completion of all in vivo analyses, corneas were dissected from the eyes and trimmed around the scleral-limbal region as previously described (Yorek et al. 2014). The cornea was fixed for 1 h in 2% paraformaldehyde in phosphate-buffered saline. The tissue was blocked with 0.2% Triton X-100 and 1% BSA for 1 h and then permeabilized in 10% Triton X-100 and 1% bovine serum albumin for 1 h. The cornea was then incubated with neuronal class III β-anti-tubulin 1:500 in incubation buffer overnight at 4°C (Covance, Dedham, MA). After being washed with incubation buffer, the tissue was incubated with Alexa Fluor 546 goat anti-rabbit IgG 1:2,000 in incubation buffer for 2 h at room temperature (Invitrogen, Eugene, OR). After being washed, the cornea was placed epithelium up on a microscope slide. Excess water was carefully aspirated and three radial cuts were made at 120° intervals, nearly to the center of the cornea. The tissue was carefully covered with a coverslip, mounted with ProLong Gold, and sealed with clear nail polish. A 3 × 3 matrix of Z-stack images was collected using a Zeiss LM710 confocal microscope with ZEN Black software. An analysis of corneal nerve images was completed with Imaris software version 7.6.4 X64 (Bitplane, Zurich, Switzerland). For epithelial corneal nerves to determine the total surface area covered by corneal innervation, a two-dimensional surface was rendered on the fluorescent staining of a maximum projection image. The measurements are reported as a percentage of total area.
Intraepidermal nerve fiber density in the hindpaw.
Immunoreactive intraepidermal nerve fiber profiles, which are primarily sensory nerves, were visualized using confocal microscopy. Samples of skin of the right hindpaw were fixed, dehydrated, and embedded in paraffin. Sections (7 μm) were collected and immunostained with anti-PGP9.5 antibody (rabbit anti human, AbD Serotic; Morphosys, Raleigh, NC) overnight followed by treatment with secondary antibody Alexa Fluor 546 goat anti-rabbit (Invitrogen, Eugene, OR). Profiles were counted by two individual investigators that were masked to the sample identity. All immunoreactive profiles within the epidermis were counted and normalized to epidermal length (Davidson et al. 2010, 2012).
Biological and oxidative stress markers.
Nonfasting blood glucose was determined. Hemoglobin A1C levels were determined using a Glyco-tek affinity column kit (Helena Laboratories, Beaumont, TX). Serum samples were collected for determination of free fatty acid, triglyceride and free cholesterol using commercial kits from Roche Diagnostics, (Mannheim, Germany), Sigma Chemical (St. Louis, MO), and Bio Vision (Mountain View, CA), respectively. Serum samples were also collected for analysis of fatty acid composition. Lipids were extracted from diets and serum with a 2:1 (vol/vol) mixture of chloroform and methanol followed by phase separation with a solution of 154 mM NaCl and 4 mM HCl. Fatty acid composition, were measured after the lipid fraction was transesterified in 14% boron trifluoride in methanol and the fatty acid methyl esters extracted into heptane before separation by gas-liquid chromatography (Yorek et al. 1984a,b). Individual fatty acids peaks as percentage of total fatty acids present were identified by comparison to known fatty acid standards.
Data analysis.
Results are presented as means ± SE. Comparisons between control and nontreated diabetic rats were conducted using Student t-test (Prism software; GraphPad, San Diego, CA). Comparison among control, nontreated, and treated diabetic rats were conducted using one-way ANOVA and Bonferroni posttest comparison (Prism software; GraphPad). P < 0.05 was considered significant.
RESULTS
Effect of type 1 diabetes duration of 4–12 wk on neuropathy.
Table 2 presents data for the progression of diabetic neuropathy over the period of 4–12 wk after the induction of hyperglycemia in 12-wk-old rats. From 4 to 12 wk, hemoglobin A1C levels trended to increase. Both motor and sensory nerve conduction velocities were significantly decreased compared with control rats after 4 wk of diabetes. Diabetic rats were thermal hypoalgesic after 8 wk of hyperglycemia. In this study after 4 wk of diabetes, we did not observe any indication of thermal hyperalgesia. In the cornea a significant decrease of subepithelial corneal nerves was detected using corneal confocal microscopy after 8 wk of diabetes, and a significant decrease in cornea sensitivity was detected using a Cochet-Bonnet filament esthesiometer after 12 wk of diabetes. Based on these results we chose the time frame for the intervention protocol to be 8 wk of nontreated diabetes followed by 8 wk of treatment.
Table 2.
Effect of duration of type 1 diabetes in Sprague-Dawley rats on weight gain, blood glucose, hemoglobin A1C, MNCV, SNCV, thermal and cornea sensitivity, intraepidermal nerve fiber density, and cornea nerve fiber density in the subepithelial layer
| Determination | Control 4 wk (6) | Diabetic 4 wk (6) | Control 8 wk (6) | Diabetic 8 wk (6) | Control 12 wk (6) | Diabetic 12 wk (5) |
|---|---|---|---|---|---|---|
| Start weight, g | 293 ± 5 | 300 ± 4 | 295 ± 3 | 306 ± 4 | 297 ± 3 | 296 ± 3 |
| End weight, g | 364 ± 7 | 280 ± 16* | 407 ± 7 | 300 ± 26* | 437 ± 5 | 236 ± 14* |
| Blood glucose, mg/dl | 149 ± 8 | 558 ± 19* | 154 ± 7 | 559 ± 29* | 142 ± 8 | 572 ± 17* |
| Hb A1C, % | 7.8 ± 0.3 | 15.1 ± 0.7* | 6.9 ± 0.1 | 16.0 ± 0.7* | 5.7 ± 0.1 | 17.8 ± 1.1* |
| MNCV, m/s | 51.1 ± 1.7 | 36.5 ± 2.0* | 57.2 ± 2.6 | 38.0 ± 2.8* | 52.0 ± 1.6 | 35.4 ± 2.6* |
| SNCV, m/s | 33.4 ± 1.1 | 26.5 ± 0.8* | 35.7 ± 0.9 | 26.0 ± 1.0* | 34.2 ± 1.2 | 26.8 ± 1.2* |
| Thermal nociception, s | 10.2 ± 0.4 | 9.8 ± 0.4 | 12.8 ± 0.3 | 18.4 ± 0.5* | 9.9 ± 0.9 | 20.2 ± 1.3* |
| Intraepidermal nerve fibers, profiles/mm | 15.2 ± 0.9 | 17.2 ± 0.7 | 14.8 ± 0.7 | 16.1 ± 1.1 | 16.0 ± 0.7 | 13.1 ± 0.6* |
| Cornea sensitivity, cm | 5.9 ± 0.1 | 5.5 ± 0.2 | 5.8 ± 0.1 | 5.5 ± 0.1 | 5.9 ± 0.1 | 4.8 ± 0.3* |
| Cornea confocal microscopy, mm/mm2 | 7.8 ± 0.6 | 6.3 ± 0.7 | 8.0 ± 0.6 | 5.9 ± 0.4* | 7.9 ± 0.8 | 5.0 ± 0.7* |
Data are presented as the means ± SE. Number of animals in each group is shown in parenthesis. Hb A1C, hemoglobin A1C; MNCV and SNCV, motor and sensory nerve conduction velocity.
P < 0.05, compared with control.
Effect of type 1 diabetes and dietary treatment with menhaden oil on serum fatty acid composition.
Data in Table 3 show the fatty acid composition of the serum of control rats treated with or without menhaden oil, nontreated diabetic rats, and diabetic rats treated with menhaden oil following a prevention or intervention protocol. Compared with serum from control rats, there is little change in the fatty acid composition in the serum from diabetic rats. Treating control rats for 16 wk with menhaden oil caused a significant decrease in oleic acid, linoleic acid, and arachidonic acid in the serum and a significant increase in eicosapentaenoic acid and docosahexaenoic acid compared with control rats. Treating diabetic rats with menhaden oil caused a significant decrease in stearic acid, linoleic acid, and arachidonic acid in the serum and a significant increase in eicosapentaenoic acid and docosahexaenoic acid compared with control or nontreated diabetic rats. There was no difference in the fatty acid composition of serum in diabetic rats treated with menhaden oil for 8 (intervention) or 16 (prevention) wk. As expected the fatty acid unsaturation index was significantly increased in serum from control or diabetic rats treated with menhaden oil compared with control or nontreated diabetic rats (Table 3). The n-6 to n-3 fatty acid ratios in serum from control, control + menhaden oil, nontreated diabetic, diabetic + menhaden oil (prevention), and diabetic + menhaden oil (intervention) rats was 16.3 ± 1.2, 1.5 ± 0.1, 14.2 ± 1.9, 1.5 ± 0.1, and 1.8 ± 0.1, respectively.
Table 3.
Effect of menhaden oil supplementation on fatty acid %composition of serum measured by gas chromatography
| Diet | 16:0 | 18:0 | 18:1 | 18:2 | 20:4 | 20:5 | 22:6 |
|---|---|---|---|---|---|---|---|
| Control (13) | 20.9 ± 1.5 | 16.1 ± 1.0 | 11.0 ± 0.4 | 22.1 ± 0.5 | 18.6 ± 0.4 | 0.4 ± 0.1 | 2.3 ± 0.2 |
| Control + menhaden oil (7) | 21.9 ± 0.7 | 14.1 ± 0.6 | 6.3 ± 0.2* | 12.6 ± 0.6*† | 14.4 ± 0.4*† | 10.9 ± 0.8*† | 8.3 ± 1.0*† |
| Diabetic (10) | 21.3 ± 0.8 | 19.6 ± 0.5 | 8.8 ± 0.3 | 24.1 ± 1.5 | 17.8 ± 1.9 | 0.1 ± 0.1 | 3.1 ± 0.3 |
| Diabetic + menhaden oil prevention (9) | 23.3 ± 0.5 | 12.3 ± 0.8*† | 11.5 ± 0.6‡ | 14.7 ± 0.9*† | 10.1 ± 0.4*†‡ | 8.9 ± 0.6*† | 9.4 ± 0.5*† |
| Diabetic + menhaden oil intervention (9) | 24.8 ± 0.5 | 12.7 ± 0.9*† | 9.9 ± 0.7‡ | 16.8 ± 0.7*†‡ | 10.1 ± 0.4*†‡ | 7.4 ± 0.5*†‡ | 8.9 ± 0.4*† |
Data are presented as the means ± SE. Number of animals in each group is shown in parenthesis. Fatty acid unsaturation index: control, 1.54 ± 0.04; control + menhaden oil, 2.08 ± 0.01*†; diabetic, 1.56 ± 0.02; diabetic + menhaden oil prevention, 1.92 ± 0.06*†; diabetic + menhaden oil intervention, 1.88 ± 0.03*†.
P < 0.05, compared with control;
P < 0.0, compared with nontreated diabetic;
P < 0.05, compared with control + menhaden oil.
Effect of type 1 diabetes and dietary treatment with menhaden oil on weight, blood glucose, and serum lipid levels.
Data in Table 4 demonstrate that nontreated and treated diabetic rats failed to gain weight compared with control rats. Control rats fed the menhaden oil enriched diet trended to gain more weight than control rats, but the difference in the final weight was not significant. Blood glucose and hemoglobin A1C values were significantly increased in nontreated diabetic rats, and treating diabetic rats with menhaden oil did not significantly affect the hyperglycemic state. Serum triglycerides, free fatty acids, and cholesterol were all significantly increased in nontreated and treated diabetic rats. Treating control rats with menhaden oil did not affect blood glucose or serum lipid levels.
Table 4.
Effect of menhaden oil dietary enrichment in streptozotocin type 1 diabetic rats on change in body weight, blood glucose, hemoglobin A1C and serum triglycerides, free fatty acids, and cholesterol
| Determination | Control (13) | Control + Menhaden Oil (7) | Diabetic (10) | Diabetic + Menhaden Oil Prevention (9) | Diabetic + Menhaden Oil Intervention (9) |
|---|---|---|---|---|---|
| Start weight, g | 298 ± 3 | 313 ± 5 | 307 ± 2 | 317 ± 3 | 317 ± 3 |
| Final weight, g | 454 ± 9 | 475 ± 10 | 298 ± 19* | 323 ± 9* | 317 ± 13* |
| Blood glucose, mg/dl | 154 ± 11 | 145 ± 5 | 543 ± 34* | 569 ± 19* | 496 ± 36* |
| Hb A1C, % | 5.8 ± 0.1 | 6.1 ± 0.2 | 16.3 ± 0.8* | 14.3 ± 1.0* | 17.9 ± 0.9* |
| Triglycerides, mg/dl | 63 ± 7 | 44 ± 11 | 422 ± 55* | 370 ± 87* | 659 ± 121* |
| Free fatty acids, mmol/l | 0.11 ± 0.01 | 0.30 ± 0.07 | 0.73 ± 0.14* | 0.73 ± 0.11* | 0.77 ± 0.07* |
| Cholesterol, mg/ml, | 0.90 ± 0.14 | 0.91 ± 0.06 | 3.73 ± 0.61* | 2.95 ± 0.71* | 5.29 ± 1.28* |
Data are presented as the means ± SE. Parentheses indicate the number of experimental animals.
P < 0.05, compared with control.
Effect of type 1 diabetes and dietary treatment with menhaden oil on nerve conduction velocity, thermal nociception, tactile response, and intraepidermal nerve fiber density.
Motor and sensory nerve conduction velocity was significantly decreased nontreated diabetic rats compared with control rats (Fig. 1). Treating diabetic rats using a prevention or intervention protocol with a high-fat diet enriched with menhaden oil significantly improved motor and sensory nerve conduction velocity compared with nontreated diabetic rats although motor nerve conduction velocity in diabetic rats treated with menhaden oil remained significantly decreased compared with control rats. Treating control rats with menhaden oil did not affect motor or sensory nerve conduction velocity. Data in Fig. 2 demonstrate that nontreated diabetic rats are hypoalgesic to thermal stimuli compared with control rats and this was significantly improved when diabetic rats were treated using either a prevention or intervention protocol with a high-fat diet enriched with menhaden oil. Tactile response threshold was significantly decreased in nontreated diabetic rats (Fig. 2). Treating diabetic rats with a diet enriched with menhaden oil using a prevention protocol significantly improved the tactile response. The tactile response in diabetic rats treated with dietary menhaden oil using an intervention protocol was also improved but to a lesser extent than observed using the prevention protocol. Treating control rats with a diet enriched with menhaden oil did not affect thermal or tactile responses (Fig. 2). Intraepidermal nerve fiber profiles in the hindpaw of nontreated diabetic rats were significantly decreased compared with control rats (Fig. 3). Treating diabetic rats with a diet enriched with menhaden oil significantly improved intraepidermal nerve fiber density. Treating diabetic rats using a prevention protocol significantly increased intraepidermal nerve fiber profiles compared with control rats. Treating control rats with a diet enriched with menhaden oil for 16 wk also significantly increased intraepidermal nerve fiber profiles compared with control rats.
Fig. 1.
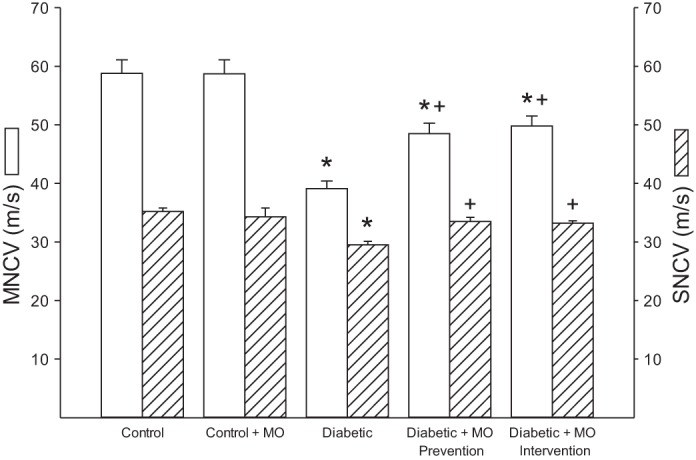
Effect of treatment of type 1 diabetic rats with menhaden oil (MO)-supplemented diet using a prevention or intervention protocol on motor and sensory nerve conduction velocity. Motor and sensory nerve conduction velocity was determined as described in materials and methods. Data are presented as the means ± SE in m/s. The number of rats in each group was the same as shown in Table 3. *P < 0.05, compared with control rats; +P < 0.05, compared with nontreated diabetic rats.
Fig. 2.
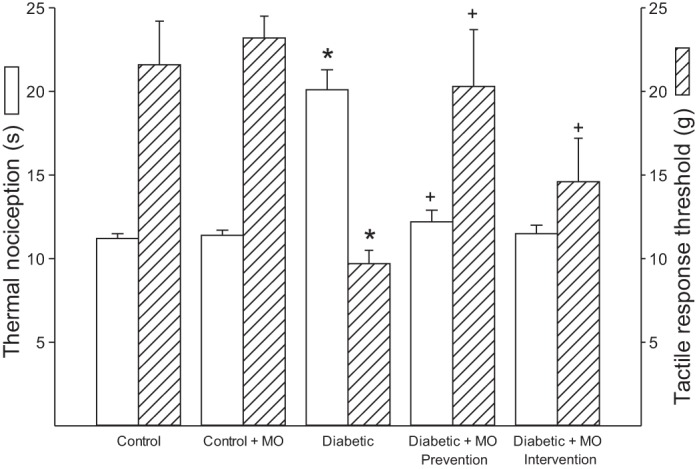
Effect of treatment of type 1 diabetic rats with MO-supplemented diet using a prevention or intervention protocol on thermal nociception and tactile response. Thermal sensitivity and tactile response threshold was determined as described in described in materials and methods. Data are presented as the means ± SE in seconds for thermal sensitivity and grams for tactile response threshold. The number of rats in each group was the same as shown in Table 3. *P < 0.05, compared with control rats; +P < 0.05, compared with nontreated diabetic rats.
Fig. 3.
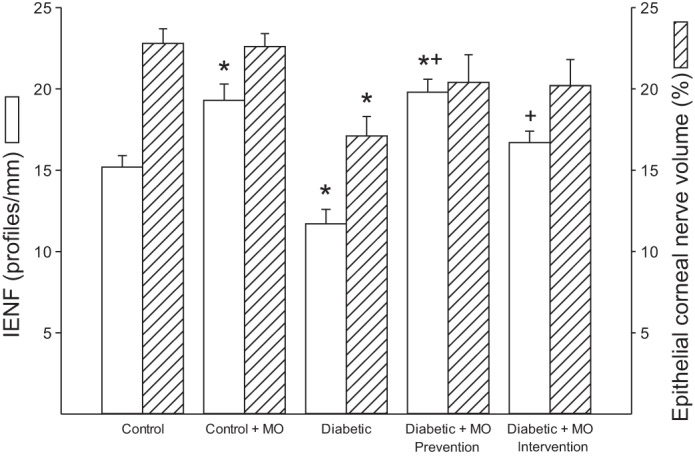
Effect of treatment of type 1 diabetic rats with MO-supplemented diet using a prevention or intervention protocol on intraepidermal nerve fiber (IENF) and epithelial corneal nerve density. Intraepidermal and epithelial corneal nerve fiber density was determined as described in materials and methods. Data are presented as the means ± SE in profiles/mm2 for intraepidermal nerve fiber density and area percent for epithelial corneal nerve fiber density. The number of rats in each group was the same as shown in Table 2. *P < 0.05, compared with control rats; +P < 0.05, compared with nontreated diabetic rats.
Effect of type 1 diabetes and dietary treatment with menhaden oil on epithelial and subepithelial corneal nerve fibers and cornea sensitivity.
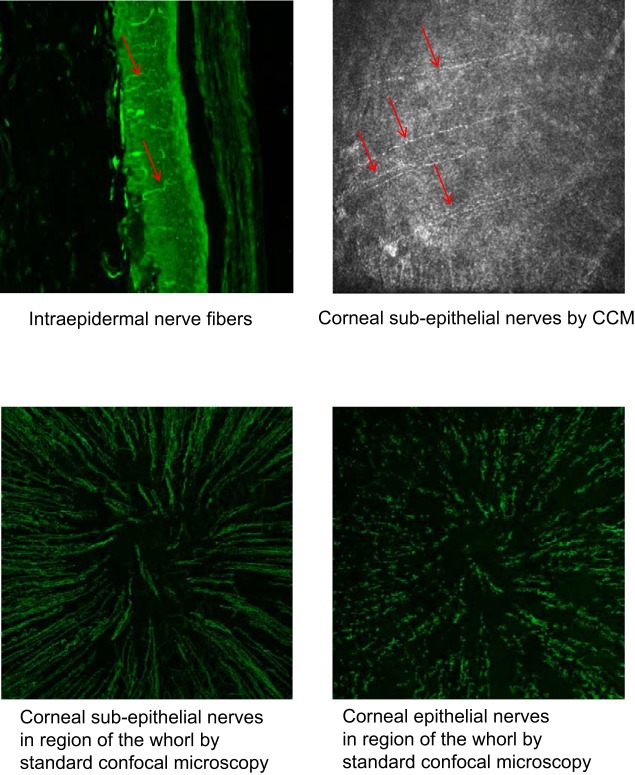
Data in Figs. 3 and 4 demonstrate that corneal nerves of the subepithelial layer (Fig. 4) and epithelium (Fig. 3) are significantly decreased in diabetic rats. Treating diabetic rats with a diet enriched with menhaden oil prevented and/or reversed the loss in corneal nerves. Treating diabetic rats with menhaden oil also prevented the diabetes-induced decrease in cornea sensitivity (Fig. 4). Treating control rats with menhaden oil had no significant effect on corneal nerve density in the subepithelial layer or epithelium or on cornea sensitivity. Figure 5 provides representative images of intraepidermal nerve fibers in the skin from a hindpaw (top left), subepithelial corneal nerves obtained using corneal confocal microscopy (top right), subepithelial corneal nerves in the region of the whorl obtained following immunohistochemical staining of the nerves with β-anti-tubulin and visualization using standard confocal microscopy (bottom left) and corneal nerves of the epithelium in the region of the whorl obtained following immunohistochemical staining of the nerves with β-anti-tubulin and visualization using standard confocal microscopy (bottom right). All images were obtained from a control animal.
Fig. 4.
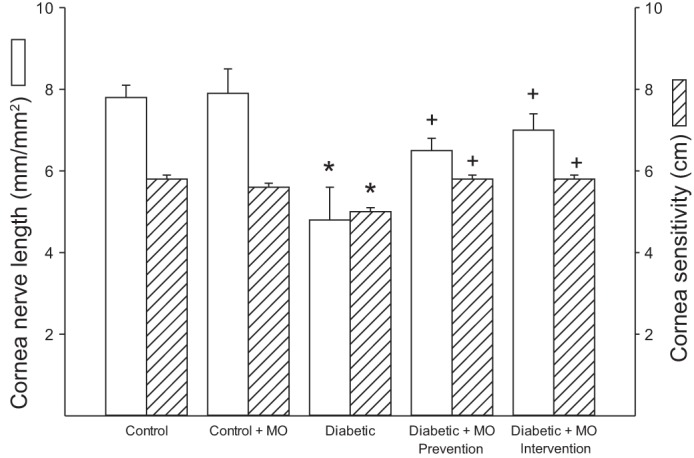
Effect of treatment of type 1 diabetic rats with MO-supplemented diet on subepithelial cornea nerve fiber length and cornea sensitivity. Subepithelial cornea nerve fiber length and cornea sensitivity was determined as described in materials and methods. Data are presented as the means ± SE in mm/mm2 for subepithelial nerve fiber length and cm for cornea sensitivity. The number of rats in each group was the same as shown in Table 2. *P < 0.05, compared with control rats; +P < 0.05, compared with nontreated diabetic rats.
Fig. 5.
Representative images for intraepidermal skin nerve fibers (top left), subepithelial cornea nerve fibers as examined by corneal confocal microscopy (top right), subepithelial nerve fibers in the region of the whorl examined in vitro using histochemical staining with β-anti-tubulin (bottom left), and epithelial cornea nerve fibers examined in vitro using histochemical staining with β-anti-tubulin (bottom right). CCM, corneal confocal microscopy.
DISCUSSION
The goal of these studies was to determine whether enriching the diet of type 1 diabetic rats with menhaden oil, a natural source of n-3 fatty acids, improves diabetic neuropathic endpoints. Fish oils are a common dietary supplement used for a variety of conditions including cardiovascular health and could be easily translated to clinical trials for diabetic peripheral neuropathy. Some of the unique features of this study were that we used a 16-wk duration for diabetes and incorporated both a prevention and intervention protocol into the study design with initiation of the intervention protocol beginning after neuropathology had developed. The hypothesis being tested was that treatment of diabetic rats with a source of n-3 fatty acids will promote a decrease in the n-6/n-3 fatty acid ratio, a sign of reduced inflammatory stress, leading to prevention and repair of diabetic neuropathy related endpoints. The endpoints examined included determination of the motor and sensory nerve conduction velocity as well as examination of nerve structure and functional changes in the skin and cornea. The determinations of nerve conduction velocities are standard endpoints for the study of diabetic neuropathy, whereas examinations of changes of structure and function of the small sensory nerve fibers in the skin or cornea have recently been promoted as markers of diabetic peripheral neuropathy and may provide a means for early detection (Loseth et al. 2008; Narayanaswamy et al. 2012; Pittenger et al. 2005; Quattrini et al. 2007).
The major findings from this study were that the development of diabetic neuropathology endpoints examined appeared at different times over the duration of 4–12 wk of nontreated diabetes. Reduction in motor and sensory nerve conduction velocity was the first deficit to appear after 4 wk of diabetes. Impairment of functional and structural deficits in the skin and cornea occurred after 8–12 wk of diabetes. Treating diabetic rats with a diet enriched with menhaden oil from the onset of hyperglycemia prevented the development of the neuropathology observed in nontreated diabetic rats. More importantly, intervention after 8 wk of nontreated diabetes with menhaden oil enriched diet reversed the neuropathological changes after only 8 wk of treatment.
The serum fatty acid profile was not significantly different between control and diabetic rats. However, after 8–16 wk of treatment with menhaden oil both control and diabetic rats had a significantly different serum fatty acid profile compared with untreated rats and reflects a new steady state with higher levels of the n-3 fatty acids eicosapentaenoic and docosahexaenoic acids. With higher levels of n-3 fatty acids accounting for a greater percentage of the fatty acids in serum, there was a significant decrease in oleic, linoleic, and arachidonic acids in control rats treated with menhaden oil compared with untreated control rats and a significant decrease in stearic, linoleic, and arachidonic acids in diabetic rats treated with menhaden oil compared with untreated diabetic rats. Notable differences between control and diabetic rats treated with menhaden oil were that levels of oleic acid in serum of diabetic rats were significantly higher in diabetic rats compared with control rats treated with menhaden oil and levels of arachidonic acid in control rats treated with menhaden oil were significantly higher compared with diabetic rats treated with menhaden oil. The reason for these differences is not entirely clear, but it appears that treating diabetic rats with menhaden oil tended to maintain levels of oleic acid in the serum at the expense of arachidonic acid. These changes in the serum fatty acid composition with menhaden oil treatment of diabetic rats resulted in a significant lowering of the n-6/n-3 fatty acid ratio. Lowering of the n-6/n-3 fatty acid ratio is a marker for reduction in inflammatory stress and could partially explain some of the beneficial effects of enriching the diet with menhaden oil on diabetic neuropathy. This suggests that the potential for inflammatory mediators being produced is significantly reduced in diabetic rats fed the menhaden oil enriched diet (Valenzuela and Videla 2011). n-3 Fatty acid enrichment is well known to have anti-inflammatory effects including increase in adiponectin production, an anti-inflammatory adipokine, suppressing the activation of Toll-like receptor-4 (Kalupahana et al. 2011; Liu et al. 2013; Moreno-Aliaga et al. 2010; Siriwardhana et al. 2012; Tishinsky et al. 2012). Increased inflammatory stress has long been considered a contributing factor to the development and progression of diabetic neuropathy and a target for therapeutic intervention (Cameron and Cotter 2008; Sytze Van Dam et al. 2013; Vincent et al. 2011). However, results from this study should not be interpreted as n-6 fatty acids being a risk factor for diabetic neuropathy. Several studies have demonstrated that treating diabetic rats with evening primrose oil, a source of γ-linolenic acid (an n-6 fatty acid), improves diabetic neuropathy as demonstrated by correction of impaired nerve conduction velocity, nerve blood flow, and neurovascular function (Cameron and Cotter 1997; Dines et al. 1995; Head et al. 2000; Omran 2012; Tomlinson et al. 1989). Omran et al. (2012) demonstrated that diabetic rats treated with evening primrose oil showed fewer morphologic alterations with a decrease in myelin breakdown. It is thought that treating diabetic rats with evening primrose oil provides a source for γ-linolenic acid, which is decreased by diabetes, thereby improving the synthesis of eicosanoids important for vasodilation especially prostacyclin (Cameron and Cotter 1997; Omran 2012). Vascular dysfunction is thought to be a contributing factor to diabetic neuropathy, and we have previously demonstrated that impaired vascular relaxation to acetylcholine by epineurial arterioles, blood vessels that supply the sciatic nerve, precedes slowing of nerve conduction velocity (Coppey et al. 2000). In this study we did not determine the effect of menhaden oil on vascular function or blood flow, a goal for future studies. However, we previously demonstrated that treating diet-induced obese mice with menhaden oil corrected vasodilation to acetylcholine by arteries of the gracilis muscle (Lamping et al. 2013).
Metabolites of eicosapentaenoic acid and docosahexaenoic acid, referred to as resolvins (resolution-phase interaction products) and neuroprotectin D1, have antioxidant, antiinflammatory, and neuroprotection properties (Ariel and Serhan 2007; Kohli and Levy 2009). Resolvins are oxygenated metabolites of eicosapentaenoic acid (E series resolvins) and docosahexaenoic acid (D series resolvins). In nonvascular tissue, 15-lipoxygenase-1 is responsible for the generation of resolvins and neuroprotectin D1 and eicosapentaenoic acid and docosahexaenoic acid are good substrates for 15-lipoxygenase-1. Resolvin formation can be increased by consuming increased amounts of eicosapentaenoic acid or docosahexaenoic acid (Ariel and Serhan 2007; Kohli and Levy 2009). Regeneration of corneal nerves damaged by refractive surgery can be increased with treatment of docosahexaenoic acid through synthesis of neuroprotectin D1 (Cortina et al. 2010; Gordon and Bazan 2013). This group also reported that neuroprotectin D1 increases neurite outgrowth by trigeminal ganglia from Swiss Webster mice (Cortina et al. 2013). Robson et al. (2010) reported that n-3 fatty acids promote neurite outgrowth by dorsal root ganglia and the effect of docosahexaenoic acid was still prominent in aged tissue. In addition to being a substrate for formation of bioactive metabolites and having anti-inflammatory properties, n-3 fatty acids have been shown to affect a range of molecular pathways including alteration of physical properties of cellular membranes, modulation of membrane channels and proteins, and regulation of gene expression via nuclear receptors and transcription factors (Mozaffarian and Wu 2011). Membrane alteration with n-3 fatty acids has been shown to affect Akt signaling, impacting neuronal survival (Akbar et al. 2005).
In the current study we found that enrichment of the diet with menhaden oil of a rat model of type 1 diabetes improved endpoints associated with diabetic neuropathy. Our study did not address the mechanism(s) that may be responsible for the beneficial effects of menhaden oil on diabetic neuropathy. However, it is possible that enriching the diet of diabetic rats with menhaden oil contributed to an increase in resolvin and neuroprotectin D1 production and neural protection/regeneration. Future studies will examine the effect menhaden oil supplementation has on reducing inflammatory stress and promoting the formation of neuroprotective compounds such as resolvins and neuroprotectin D1.
In summary, we have demonstrated that dietary enrichment with menhaden oil, a natural source of n-3 fatty acids, in a rat model for type 1 diabetes prevented, but more importantly reversed, numerous pathological endpoints associated with diabetic neuropathy. These results are in agreement with previous studies performed with a type 1 and 2 diabetic rat models (Coppey et al. 2012; Gerbi et al. 1999). This suggests that dietary enrichment with n-3 fatty acids may be beneficial treatment for diabetic peripheral neuropathy.
GRANTS
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (BX001680-01), Rehabilitation Research and Development (RX000889-01), Iowa City Veterans Affaris Center of Excellence for the Prevention and Treatment of Visual Loss (C9251-C), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK081147.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
DISCLAIMER
The content of this manuscript are new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
AUTHOR CONTRIBUTIONS
Author contributions: L.J.C., E.P.D., A.O., and M.A.Y. performed experiments; L.J.C., E.P.D., A.O., and M.A.Y. analyzed data; L.J.C., E.P.D., A.O., and M.A.Y. approved final version of manuscript; E.P.D. and M.A.Y. prepared figures; M.A.Y. conception and design of research; M.A.Y. interpreted results of experiments; M.A.Y. drafted manuscript; M.A.Y. edited and revised manuscript.
REFERENCES
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA 102: 10858–10863, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113–1118, 2002. [DOI] [PubMed] [Google Scholar]
- Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 14: 528, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28: 176–183, 2007. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanism explored. Clin Sci 107: 1–11, 2004. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes 46: S31–S37, 1997. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets 9: 60–67, 2008. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that provide circulation to the sciatic nerve. Int J Exp Diabetes Res 1: 131–143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab 2012: 950517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci 51: 804–810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci 54: 4109–4116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci 53: 1182–1187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet induced obesity in Sprague Dawley rats causes vascular and neural dysfunction. Diabetes Met Res Rev 26: 306–318, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 Fatty acids in cardiovascular disease. N Engl J Med 364: 2439–2450, 2011. [DOI] [PubMed] [Google Scholar]
- Dines KC, Cameron NE, Cotter MA. Comparison of the effects of evening primrose and triglycerides containing gamma-linolenic acid on nerve conduction and blood flow in diabetic rats. J Pharmacol Exp Ther 273: 49–55, 1995. [PubMed] [Google Scholar]
- Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Valeriy V, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol 569: 48–58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbi A, Maixent JM, Ansaldi JL, Pierlovisi M, Coste T, Pelissier JF, Vague P, Raccah D. Fish oil supplementation prevents diabetes-induced nerve conduction velocity and neuroanatomical changes in rats. J Nutr 129: 207–213, 1999. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG. Mediator lipidomics in ophthalmology: Targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res 38: 995–1005, 2013. [DOI] [PubMed] [Google Scholar]
- Head RJ, McLennan PL, Raederstorff D, Muggli R, Burnard SL, McMurchie EJ. Prevention of nerve conduction deficit in diabetic rats by polyunsaturated fatty acids. Am J Clin Nutr 71: 386S–392S, 2000. [DOI] [PubMed] [Google Scholar]
- Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr 2: 304–316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 158: 960–971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106: 2747–2757, 2002. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Nuno DW, Coppey LJ, Holmes AJ, Hu S, Oltman CL, Norris AW, Yorek MA. Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular function. Diabetes Obes Metab 15: 144–152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HQ, Qiu Y, Mu Y, Zhang XJ, Liu L, Hou XH, Zhang L, Xu XN, Ji AL, Cao R, Yang RH, Wang F. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr Res 33: 849–858, 2013. [DOI] [PubMed] [Google Scholar]
- Loseth S, Stalberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol 255: 1197–1202, 2008. [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Lorente-Cebrian S, Martinez JA. Regulation of adipokine secretion by n-3 fatty acids. Proc Nutr Soc 69: 324–332, 2010. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease. J Am Coll Cardiol 58: 2047–2067, 2011. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy H, Facer P, Misra VP, Timmers M, Byttebier G, Meert T, Anand P. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci 19: 1490–1496, 2012. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjali K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes 53: 711–720, 2004. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Mizutani M, Ogawa M, Sone H, Asano M, Asakura Y, Isaka M, Suzuki S, Kawakami Y, Field JB, Yamashita K. Long-term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. J Diabetes Complications 10: 280–287, 1996. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Dake B, Yorek MA. Role of the effect of inhibition of neutral endopeptidase on vascular and neural complications in streptozotocin-induced diabetic rats. Eur J Pharmacol 650: 556–562, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril or rosuvastatin. Obesity (Silver Spring) 16: 82–89, 2008. [DOI] [PubMed] [Google Scholar]
- Omran OM. Histopathological study of evening primrose oil effects on experimental diabetic neuropathy. Ultrastruct Pathol 36: 222–227, 2012. [DOI] [PubMed] [Google Scholar]
- Pittenger GL, Mehrabyan A, Simmons K, Amandarice Dublin C, Barlow P, Vinik AI. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord 3: 113–121, 2005. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56: 2148–2154, 2007. [DOI] [PubMed] [Google Scholar]
- Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurons throughout development and in aged animals. Neurobiol Aging 31: 678–687, 2010. [DOI] [PubMed] [Google Scholar]
- Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 65: 211–222, 2012. [DOI] [PubMed] [Google Scholar]
- Siscovick DS, Raghunathan TE, King I, Wwinmann S, Wicklund KG, Albright J, Bovbjerg V, Abrogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 274: 1363–1367, 1995. [DOI] [PubMed] [Google Scholar]
- Sytze Van Dam P, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol 719: 180–186, 2013. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 33: 1792–1797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishinsky JM, Gulli RA, Mullen KL, Dyck DJ, Robinson LE. Fish oil prevents high-saturated fat diet-induced impairments in adiponectin and insulin response in rodent soleus muscle. Am J Physiol Regul Integr Comp Physiol 302: R598–R605, 2012. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Robinson JP, Compton AM, Keen P. Essential fatty acid treatment-effects on nerve conduction, polyol pathway and axonal transport in streptozotocin diabetic rats. Diabetologia 32: 655–659, 1989. [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Videla LA. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ration in development of nonalcoholic fatty liver associated with obesity. Food Funct 2: 644–648, 2011. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 7: 573–583, 2011. [DOI] [PubMed] [Google Scholar]
- Yorek MA, Bohnker RR, Dudley DD, Spector AA. Comparative utilization of n-3 polyunsaturated fatty acids by cultured human Y-79 retinoblastoma cells. Biochim Biophys Acta 795: 277–285, 1984a. [DOI] [PubMed] [Google Scholar]
- Yorek MA, Strom DK, Spector AA. Effect of membrane polyunsaturation on carrier-mediated transport in cultured retinoblastoma cells: alterations in taurine uptake. J Neurochem 42: 254–261, 1984b. [DOI] [PubMed] [Google Scholar]
- Yorek M, Obrosov A, Shevalye H, Lupachyk S, Harper M, Kardon R, Yorek M. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst 2014 Nov 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]