Abstract
Since estradiol attenuates cannabinoid-induced increases in energy intake, energy expenditure, and transmission at proopiomelanocortin (POMC) synapses in the hypothalamic arcuate nucleus (ARC), we tested the hypothesis that neuronal nitric oxide synthase (nNOS) plays an integral role. To this end, whole animal experiments were carried out in gonadectomized female guinea pigs. Estradiol benzoate (EB; 10 μg sc) decreased incremental food intake as well as O2 consumption, CO2 production, and metabolic heat production as early as 2 h postadministration. This was associated with increased phosphorylation of nNOS (pnNOS), as evidenced by an elevated ratio of pnNOS to nNOS in the ARC. Administration of the cannabinoid receptor agonist WIN 55,212-2 (3 μg icv) into the third ventricle evoked hyperphagia as early as 1 h postadministration, which was blocked by EB and restored by the nonselective NOS inhibitor N-nitro-l-arginine methyl ester hydrochloride (l-NAME; 100 μg icv) when the latter was combined with the steroid. Whole cell patch-clamp recordings showed that 17β-estradiol (E2; 100 nM) rapidly diminished cannabinoid-induced decreases in miniature excitatory postsynaptic current frequency, which was mimicked by pretreatment with the NOS substrate l-arginine (30 μM) and abrogated by l-NAME (300 μM). Furthermore, E2 antagonized endocannabinoid-mediated depolarization-induced suppression of excitation, which was nullified by the nNOS-selective inhibitor N5-[imino(propylamino)methyl]-l-ornithine hydrochloride (10 μM). These effects occurred in a sizable number of identified POMC neurons. Taken together, the estradiol-induced decrease in energy intake is mediated by a decrease in cannabinoid sensitivity within the ARC feeding circuitry through the activation of nNOS. These findings provide compelling evidence for the need to develop rational, gender-specific therapies to help treat metabolic disorders such as cachexia and obesity.
Keywords: estradiol, nNOS, POMC, cannabinoid, energy balance
naturally occurring and synthetic cannabinoids aid in the regulation and central control of energy homeostasis and peripheral metabolic processes by managing bodily functions such as food intake, gastrointestinal motility and secretion, fat and carbohydrate disposition, mitochondrial respiration, and core body temperature (for review see Borgquist and Wagner 2013). Both exo- and endocannabinoids stimulate hyperphagia (Cota et al. 2003; Fride et al. 2005) and hypothermia (Fitton and Pertwee 1982; Hillard et al. 1999). This control occurs through intricate interactions between the gut, liver, pancreas, brain stem, hypothalamus, and limbic forebrain via endogenous ligands such as anandamide and 2-arachidonoyl glycerol (2-AG) and exogenous agonists such as WIN 55,212-2 (Borgquist and Wagner 2013). CB1 receptor agonists increase the amount of time spent eating and decrease the time spent resting, whereas CB1 receptor antagonists decrease the amount of time spent eating and increase the time spent grooming (Escartín-Pérrez et al. 2009). We have previously shown that cannabinoids exert their actions on feeding behavior and metabolism in a sexually differentiated manner, with males being more sensitive to cannabinoid CB1 receptor activation than females (Farhang et al. 2009). This could be attributed in part to lower hypothalamic cannabinoid receptor binding site density in female vs. male rats (Riebe et al. 2010) as well as the pleiotropic actions of cannabinoids at anorexigenic proopiomelanocortin (POMC) synapses in the hypothalamic arcuate nucleus (ARC) (Farhang et al. 2009).
It is well known that estradiol decreases energy intake, core body temperature, and body weight in various species (Butera and Czaja 1984; Johnson et al. 1994; Palmer and Gray 1986; Stephenson and Kolka 1999). Considerable evidence suggests that this may be due, at least in part, to rapid membrane-initiated estrogen signaling mechanisms. Membrane estrogen receptor (mER)α found within the hippocampus uses calveolin-1 protein to functionally couple with the metabotropic glutamate receptor mGluR1a (Boulware et al. 2007). 17β-Estradiol (E2) activates a protein kinase C (PKC) pathway through activation of mGluR1a to increase intracellular Ca2+ and progesterone synthesis (Dewing et al. 2008; Kuo et al. 2010). Similarly, studies in rodents have demonstrated that the estrogenic changes in energy homeostasis involve the activation of Gq-coupled mERs (Roepke et al. 2010; Santollo et al. 2013; Washburn et al. 2013). This can lead to a decrease in the ability of orexigenic cannabinoids to evoke hyperphagia (Kellert et al. 2009; Washburn et al. 2013). At the cellular level, E2 rapidly reduces the ability of the cannabinoid receptor agonist WIN 55,212-2 to decrease the frequency of miniature excitatory postsynaptic currents (mEPSCs) impinging on POMC neurons (Kellert et al. 2009; Washburn et al. 2013). These effects are reversed when E2 is co-perfused with the competitive estrogen receptor (ER) antagonist ICI 182,780 (Jeffery et al. 2011). Although estradiol has been shown to downregulate hypothalamic CB1 receptors (Riebe et al. 2010), the effectiveness of WIN 55,212-2 to decrease mEPSC frequency was restored when phosphatidylinositol 3-kinase (PI3K) or PKC inhibitors were co-perfused with E2 (Jeffery et al. 2011; Washburn et al. 2013). In the hypothalamic paraventricular nucleus (PVN), the Src/kinase pathway mediates an ERβ-induced increase in phosphorylation levels of neuronal nitric oxide synthase (nNOS) by activating an intervening PI3K/Akt pathway (Gingerich and Krukoff 2008). This is similar to the estrogenic regulation of NOS in endothelial cells, where caveolin-1-dependent translocation of ERα to the plasma membrane is critical for its ability to interact with Src and, ultimately, PI3K. This interaction, in turn, enables Akt-dependent phosphorylation/activation of endothelial NOS (Haynes et al. 2003; Kim and Bender 2009; Sud et al. 2010). In addition, both nNOS expression and phosphorylation/activation in the hypothalamus vary over the course of the estrous cycle, with the highest levels observed during proestrus (Parkash et al. 2010; Sica et al. 2009). Moreover, fasting is reported to decrease nNOS levels in the ARC and ventromedial nucleus (VMN), an effect that is reversed on refeeding (Otukonyong et al. 2000). Therefore, it appears that an estradiol-induced increase in activated nNOS should lead to nitric oxide production, which would act to antagonize cannabinoid-mediated presynaptic inhibition of excitatory input onto POMC neurons.
The purpose of the present study was to test the hypothesis that the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis is due, at least in part, to the activation of nNOS in the ARC. To this end, we conducted whole animal experiments in ovariectomized female guinea pigs to determine estrogen-induced changes in energy balance and nNOS activation in the ARC and VMN microdissected from hypothalamic slices. We also examined whether NOS inhibition could restore cannabinoid hyperphagia in estrogen-treated animals. In vitro electrophysiological recordings were performed to explore whether nNOS inhibition could similarly rescue the cannabinoid-induced presynaptic inhibition of excitatory input onto ARC POMC neurons in E2-treated slices and whether nNOS substrate loading could act in lieu of E2 to diminish this presynaptic inhibition of transmitter release in vehicle-treated slices.
MATERIALS AND METHODS
Animals.
All animal procedures described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences. Female Topeka guinea pigs (450–700 g; 50–75 days old) were acquired from Elm Hill Breeding Labs (Chelmsford, MA) or bred in our animal care facility, maintained under controlled temperature (69–73°F) and a coordinated light cycle (12:12-h on-off), and provided with food and water ad libitum.
Surgical procedures.
To better resolve how estradiol influences cannabinoid sensitivity within the hypothalamic feeding circuitry, we conducted two survival surgeries. Stereotaxic guide cannula implantations, as well as ovariectomies, were performed as previously described after inducing anesthesia with ketamine-xylazine (33 and 6 mg/kg sc, respectively) while maintaining the anesthetic plane with 1.5–2% isoflurane. Animals were subject to stereotaxic surgery 13 days before experimentation, during which we inserted a 22-gauge guide cannula 1 mm above the third ventricle. Animals were secured in a stereotaxic frame by fitting the incisors over a tooth bar and inserting blunt ear bars into the ear canals. The surgery was performed using aseptic techniques. First, the scalp was opened by making a 2- to 2.5-cm incision down the midline of the skull beginning at the front of the orbits toward the occipital lobe with a no. 10 scalpel blade. A single hole was drilled and the dura layer cut so that a guide cannula could be slowly lowered at an angle 4° from the vertical plane (to avoid puncturing the midsagittal sinus) to its desired location using the following coordinates (in mm, measured from bregma and the top of the cerebral cortex): mediolateral, −0.7 mm; anterioposterior, −2.1; dorsoventral, −9.8; tooth bar, −5.5 (Luparello et al. 1964; Tindal 1965). Three 3.2-mm bone anchor screws were then inserted into predrilled holes to secure the guide cannula placement. Dental cement/acrylic was used to affix the stainless steel screws and cannula to the skull. Finally, a stylette was inserted into the guide cannula to prevent cerebrospinal fluid from entering the shaft of the cannula. Animals were allotted 7 days of recovery before the ovariectomies and an additional 6 days of recovery before the start of the in vivo experiments.
Drugs.
Unless otherwise indicated all drugs were purchased through Tocris Cookson (Bioscience, Minneapolis, MN). For the behavioral experiments, estradiol benzoate (EB; Steraloids, Newport, RI) was initially prepared as a 1 mg/ml stock solution in punctilious ethanol. A known quantity of this stock solution was added to a volume of sesame oil sufficient to produce a final concentration of 100 μg/ml following evaporation of the ethanol. The cannabinoid receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN 55,212-2) was dissolved in cremephor-ethanol-0.9% saline (CES; 1:1:18 vol/vol/vol) at a concentration of 1.5 μg/μl and delivered in a total volume of 2 μl. The NOS inhibitor N-nitro-l-arginine methyl ester hydrochloride (l-NAME) was dissolved in CES at a concentration of 50 μg/μl and given in a total volume of 2 μl.
For the electrophysiological experiments, the voltage-gated Na+ channel blocker tetrodotoxin (TTX) with citrate (Alomone Labs, Jerusalem, Israel) was dissolved in Ultrapure H2O to a stock concentration of 1 mM and diluted further with artificial cerebrospinal fluid (aCSF) to a working concentration of 500 nM. The GABAA receptor antagonist 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR-95531) was dissolved in Ultrapure H2O to a stock concentration of 10 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 10 μM. E2 (Steraloids) was dissolved in punctilious ethanol to a stock concentration of 1 mM and diluted further with aCSF to a working concentration of 100 nM. WIN 55,212-2 was dissolved in dimethyl sulfoxide (DMSO) to 1 mM stock concentrations, and the stock concentrations were diluted further with aCSF to the working concentration of 1 μM. l-NAME was dissolved in Ultrapure H2O to a stock concentration of 30 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 300 μM. The selective nNOS inhibitor N5-[imino(propylamino)methyl]-l-ornithine hydrochloride (NPLA) was dissolved in Ultrapure H2O to a stock concentration of 10 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 10 μM. The NOS substrate l-arginine was dissolved in Ultrapure H2O to a stock concentration of 30 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 30 μM. Aliquots of the stock solutions were stored at −20°C until needed.
Feeding and metabolic studies.
The analyses for energy balance were performed in a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments; Columbus, OH) as previously described and validated (Farhang et al. 2010; Qiu et al. 2014; Washburn et al. 2013). Food intake was monitored around the clock for 5 days in ovariectomized female guinea pigs, each with a guide cannula implanted into the third ventricle of the brain 13 days prior to acclimation in the CLAMS chambers and 16 days before the start of the monitoring period. A meal was defined as an event in which an animal consumed ≥10 mg of food. Once the animal had eaten at least this threshold amount, the computer logged this event as a meal in the experimental data file the instant the animal withdrew its head from the food dish. We calculated meal frequency as the number of meals consumed per unit time, and meal size as the amount of food eaten in a given hour divided by the number of meals in the same hour. We also measured O2 consumption, CO2 production, and the metabolic heat production as indexes of energy expenditure. Each morning at 8:00 AM, the animals were given either the CB1 receptor agonist WIN 55,212-2 (3 μg icv) in the third ventricle, alone or in combination with the NOS inhibitor l-NAME (100 μg icv), or their CES vehicle. Every other day, animals were injected with EB (10 μg sc) or its sesame oil vehicle. All doses of EB, E2, WIN 55,212-2, and l-NAME used in these in vivo studies were determined after careful consultation of the literature, or derived from our previously published work (Cope et al. 2010; García-Juárez et al. 2012; Hama and Sagen 2011; Kellert et al. 2009; Landi et al. 2002; Merroun et al. 2014; Reis et al. 2010).
Western blot analysis.
Unless otherwise stated, all primary antibody solutions were prepared in Tris-buffered saline (TBS) containing 0.1% Tween 20 and Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) at a 1:1,000 dilution. Primary antibodies directed against the following antigens were used: glyceride-3-phosphate dehydrogenase (GAPDH; 1:10,000; Millipore, Billerica, MA), total nNOS (Santa Cruz, Dallas, TX), and NOS phosphorylated at the serine residue located in the 1,412th position of the amino acid sequence (pnNOS; ABCAM, Cambridge, MA). At the end of the 5-day experimental period, animals were anesthetized with 32% isoflurane and rapidly decapitated. After brain removal, two to three coronal slices (1 mm in thickness) spanning the rostral-caudal extent of the ARC were prepared using a guinea pig brain matrix (Ted Pella, Redding, CA) and stored in RNAlater (Ambion, Austin, TX) for 2–3 h. The ARC and, for comparison, the VMN were then microdissected from the slices. ARC and VMN microdissections were homogenized in cold lysis buffer (50 mM Tris·HCl, pH 7.4, 0.5 M EDTA, and 0.5 M EGTA) containing protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO). Protein levels were quantified by using a Bradford assay (Bio-Rad Laboratories, Hercules, CA) to establish equal loading into the gel. Proteins were separated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked for 1 h with Odyssey blocking buffer and incubated overnight with primary antibodies at 4°C. They were then washed four times with TBS with Tween (TBST) for 10 min, followed by incubation with Odyssey infrared-conjugated secondary antibodies diluted 1:10,000 in Odyssey blocking buffer for 2 h at room temperature. After four 10-min washes with TBST followed by four 10-min washes with TBS, membranes were scanned using an Odyssey infrared imager (LI-COR Biosciences). All membranes were probed with GAPDH as a loading control. Levels of pnNOS and nNOS expression were determined by calculating the ratio of phosphoprotein density to total protein density for each experimental group and then normalizing the ratio to the values observed in vehicle-treated animals.
Electrophysiology.
Electrophysiological recordings from arcuate neurons with biocytin-filled electrodes were performed using an in vitro hypothalamic slice preparation as previously described (Jeffery et al. 2011). Briefly, electrode resistances varied from 3 to 8 MΩ. Membrane currents were recorded in voltage clamp with access resistances ranging from 8 to 20 MΩ and underwent analog-digital conversion via a Digidata 1322A interface coupled to pClamp 8.2 software (Axon Instruments). The access resistance, resting membrane potential (RMP), and input resistance (Rin) were monitored throughout the course of the recording. If the access resistance deviated >20% of its original value, the recording was ended.
To evaluate whether E2 modulates cannabinoid-induced presynaptic inhibition of excitatory input onto ARC neurons via a nNOS pathway, we first monitored mEPSC frequency and amplitude from a holding potential of −75 mV using an internal solution in which Cs+ was substituted for K+. We perfused slices with aCSF containing TTX (500 nM) and SR-95531 (10 μM) to block GABAA receptor-mediated synaptic input, alone or in combination with either E2 (100 nM) or its EtOH (0.01% vol/vol) vehicle. These agents were bath applied for 3–4 min before the attainment of 3–4 min worth of baseline mEPSC frequency and amplitude. E2 or EtOH was then perfused along with WIN 55,212-2 (1 μM) for an additional 3–4 min, and 3–4 more minutes of data were collected. In some experiments, slices were pretreated with the nNOS inhibitor l-NAME (300 μM) 4 min before and then along with perfusion of E2 to assess the role of NOS in mediating estrogenic modulation of cannabinoid-induced changes in mEPSC frequency and/or amplitude. Alternatively, we pretreated slices with the NOS substrate l-arginine (30 μM) to ascertain whether nitric oxide mimicked the effect of estradiol on cannabinoid-induced changes in mEPSC frequency and/or amplitude. The threshold for event detection was set at least 3 pA below the baseline holding current as assessed from the headstage output and was continuously monitored throughout each 3- to 4-min recording period. This was done to ensure that the smaller amplitude events were not inadvertently omitted from the analysis. Synaptic events were detected and analyzed using Clampfit 8.2 (Axon Instruments) in combination with the SigmaPlot (IBM/SPSS, New York, NY) and StatGraphics (StatPoint, Warrenton, VA) programs. When we analyzed the data to determine mEPSC frequency and amplitude for the ≥100 contiguous synaptic events per condition, we poured over each 250-ms sweep in the entire range to ensure that each event that we included in the analysis bore the classic kinetic profile of a fast EPSC. We used this information to evaluate cannabinoid-induced alterations in mEPSC frequency and amplitude as assessed from cumulative probability plots.
Next, we set out to determine whether endocannabinoids retrogradely inhibit excitatory input via the depolarization-induced suppression of excitation (DSE; Kreitzer and Regehr 2001; Ohno-Shosaku et al. 2002). Before executing the DSE protocol, we monitored spontaneous EPSCs (sEPSCs) from a holding potential of −75 mV for 3–4 min to establish baseline frequency and amplitude in the presence of SR-95531 to block GABAA receptor-mediated synaptic input, alone or in combination with 1) E2 or its EtOH vehicle to ascertain the ability of the steroid to modulate the extent of the DSE, 2) the CB1 receptor antagonist AM-251 (1 μM) to assess the role of the CB1 receptor, or 3) E2 combined with the nNOS-selective inhibitor NPLA (10 μM) to assess the role of nNOS signaling in the estrogenic modulation. The doses of the drugs used in these electrophysiological experiments were chosen on the basis of our prior work as well as the published work of others (Ho et al. 2007; Moore and Handy 1997; Palmer et al. 1988; Radomski et al. 1990; Washburn et al. 2013; Yu et al. 1997; Zhang et al. 1997). To elicit DSE, cells were given a 60-mV depolarization (0.75–3 s in duration). These pulses were delivered every 60 s for up to 15 consecutive trials. Data were analyzed by looking at the average poststimulation amplitude and frequency acquired from at least 3 separate trials over 5-s bins up to 20-s normalized to that observed under basal conditions.
Immunohistochemistry.
After electrophysiological recording, slices were fixed with 4% paraformaldehyde in Sorensen's phosphate buffer (pH 7.4) for 90–180 min (Kellert et al. 2009). They then were immersed overnight in 20% sucrose dissolved in Sorensen's buffer and frozen in Tissue-Tek embedding medium (Miles, Elkhart, IN) the next day. Coronal sections (20 μm) were cut on a cryostat and mounted on slides. These sections were washed with 0.1 M sodium phosphate buffer (pH 7.4) and then processed with streptavidin-AF488 (Molecular Probes, Eugene, OR) at a 1:300 dilution. After the biocytin-filled neuron was localized via fluorescence microscopy, the slides containing the appropriate sections were processed with polyclonal antibodies directed against either β-endorphin (Immunostar, Hudson, WI; 1:400 dilution), α-melanocyte-stimulating hormone (α-MSH; Immunostar; 1:200 dilution), or cocaine-amphetamine-regulated transcript (CART; Phoenix Pharmaceuticals, Burlingame, CA; 1:2,000 dilution) using fluorescence immunohistochemistry (Kellert et al. 2009).
Statistical analyses.
Comparisons between two groups were made with the Mann-Whitney U-test. Comparisons between more than two groups were performed using either the one-way multifactorial or rank-transformed multifactorial analysis of variance (ANOVA) followed by the least significant difference (LSD) test or, alternatively, via the Kruskal-Wallis test followed by analysis of the median-notched, box-and-whisker plot. Comparisons of the mEPSC interval distributions were evaluated via the Kolmogorov-Smirnov test. Differences were considered statistically significant if the probability of error was <5%.
RESULTS
Experiment 1: effects of EB and the cannabinoid receptor agonist WIN 55,212-2 on nNOS activation in the ARC and VMN.
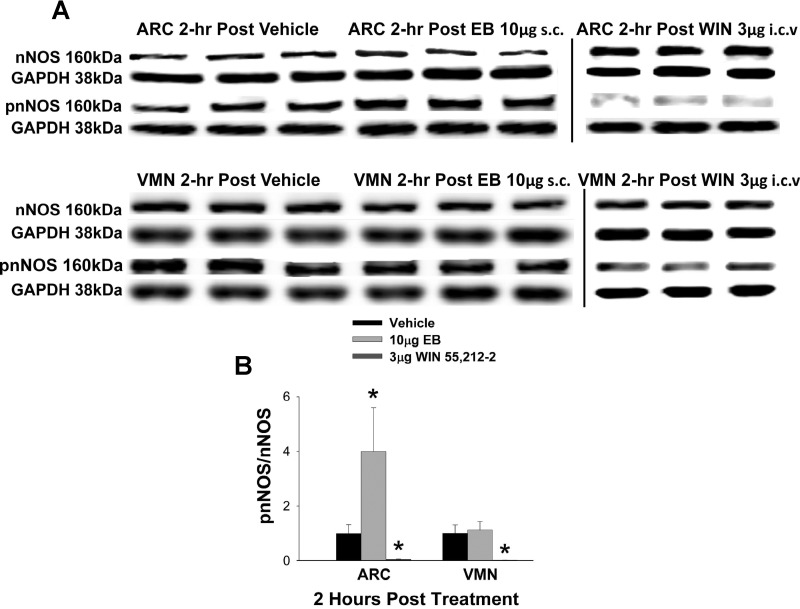
Since we know that estradiol disrupts cannabinoid signaling at POMC synapses via a pathway that involves PI3K and PKC (Jeffery et al. 2011; Washburn et al. 2013), and that it also activates nNOS in the PVN via a Src/PI3K/Akt pathway (Gingerich and Krukoff 2008), we proposed that nNOS activation represents a final common pathway through which the steroid dampens cannabinoid-induced changes in energy homeostasis. As shown in Fig. 1A, Western blotting yielded clear pnNOS and total nNOS bands registering at 160 kDa in both the ARC and VMN. EB (10 μg sc) administered 2 h before experimentation increased the intensity of the pnNOS band, resulting in a nearly 4-fold elevation in the pnNOS/NOS ratio in samples from the ARC (Fig. 1B). By contrast, the cannabinoid receptor agonist WIN 55,212-2 (3 μg icv) markedly decreased the intensity of the pnNOS band (Fig. 1A) and diminished the pnNOS/nNOS ratio in both the ARC and VMN (Fig. 1B; rank-transformed multifactorial ANOVA/LSD: Fdrug = 60.58, P < 0.0001, df = 2; Fregion = 1.54, P < 0.22, df = 1; Finteraction = 1.69, P < 0.20, df = 2; n = 5).
Fig. 1.
Estradiol benzoate (EB) and the cannabinoid receptor agonist WIN 55,212-2 (WIN) modulate neuronal nitric oxide synthase (nNOS) activation in the mediobasal hypothalamus. A: representative Western blots illustrating the levels of nNOS, phosphorylated nNOS (pnNOS), and the loading control GAPDH in the arcuate nucleus (ARC) and ventromedial nucleus (VMN) microdissected from vehicle-, EB-, and WIN-treated animals. The vertical lines represent demarcations between bands run on different gels. B: composite bar graph illustrating the pnNOS/nNOS ratio determined in ARC and VMN microdissections from vehicle-, EB-, and WIN-treated animals. Bars and vertical lines represent means and SE, respectively. *P < 0.05, values in drug-treated animals that are significantly different (rank-transformed multifactorial ANOVA/LSD; n = 5) from those in vehicle-treated controls.
Experiment 2: role of nNOS in mediating EB-induced changes in the cannabinoid regulation of energy intake.
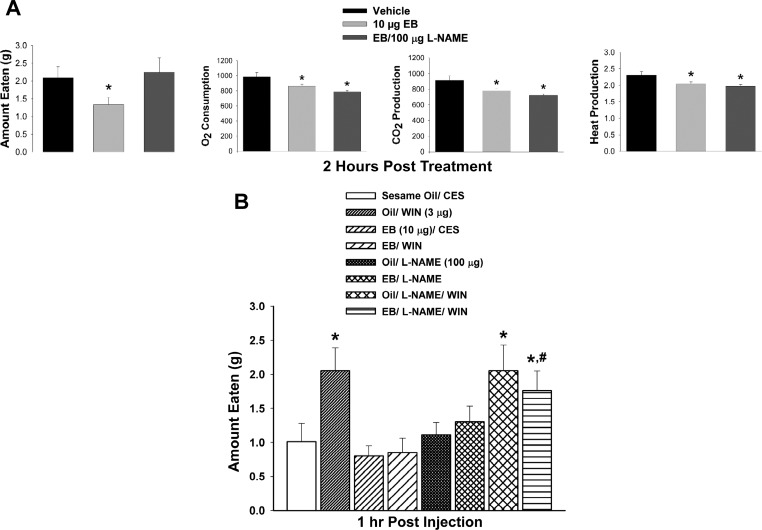
We then used in vivo behavioral studies in ovariectomized female guinea pigs to examine the effects of estradiol on energy intake and expenditure. As shown in Fig. 2A, the EB-induced activation of nNOS in the ARC was associated with reduced cumulative food intake (1-way ANOVA/LSD: F = 3.21, P < 0.05; n = 5), O2 consumption, (F = 5.10, P < 0.009; n = 5), CO2 production (F = 5.79, P < 0.006; n = 5), and metabolic heat production (F = 4.40, P < 0.02; n = 5). The decrease in energy intake, but not the various measures of energy expenditure, was blocked by the NOS inhibitor l-NAME (100 μg icv). After seeing the effects of EB per se on energy homeostasis, as well as its ability to activate nNOS in the ARC, we investigated whether prior delivery of l-NAME could restore the hyperphagic effect of the cannabinoid receptor agonist WIN 55,212-2 administered into the third ventricle in EB-treated animals. For this experiment, we used a 1-h time point because we wanted to see if EB could negatively modulate the hyperphagia caused by WIN 55,212-2 before the onset of its own hypophagic response (see Fig. 2A). As shown in Fig. 2B, the hyperphagic effect of centrally administered WIN 55,212-2 is rapidly and completely blocked by EB within 1 h after delivery and sustained at all subsequent time points observed up to 24 h postadministration (not shown). l-NAME alone had no effect on energy intake in vehicle- or EB-treated animals and did not alter the increase in energy intake caused by WIN 55,212-2 in vehicle-treated animals. It did, however, prevent the EB-induced blockade of the hyperphagic effect of the agonist, thereby enabling an increase in consumption similar to that seen in vehicle-treated animals (multifactorial ANOVA/LSD: Fsteroid = 4.29, df = 1, P < 0.04; FWIN 55,212-2 = 11.48, df = 1, P < 0.001, Finteraction = 4.09, df = 1, P < 0.05; n = 5). This suggests that nNOS is involved in the estrogenic uncoupling of cannabinoid-induced hyperphagia in our guinea pig model.
Fig. 2.
A: EB decreases energy intake and expenditure, the former of which is dependent on the activation of nNOS. The composite bar graphs illustrate whether the nNOS inhibitor N-nitro-l-arginine methyl ester hydrochloride (l-NAME; 100 μg icv) can block the changes in food intake, O2 consumption, CO2 production, and metabolic heat production caused by EB (10 μg sc). Bars and vertical lines represent means and SE, respectively. *P < 0.05, values in EB- and EB/l-NAME-treated animals that are significantly different (1-way ANOVA/LSD; n = 5) from those in vehicle-treated controls. B: the hyperphagic effect of WIN (3 μg icv) is blocked by EB and rescued by l-NAME in EB-treated animals. CES, cremephor-ethanol-0.9% saline. Bars and vertical lines represent means and SE, respectively, of the amount of food eaten in the 1 h following the various treatment conditions. *P < 0.05, values of food intake in animals treated with WIN that are significantly different (multifactorial ANOVA/LSD; n = 5) from those in vehicle-treated controls. #P < 0.05, values in l-NAME-treated animals that are significantly different (multifactorial ANOVA/LSD; n = 5) from those in vehicle-treated animals.
Experiment 3: role of nNOS in mediating E2-induced changes in the cannabinoid regulation of excitatory synaptic input onto anorexigenic POMC neurons.
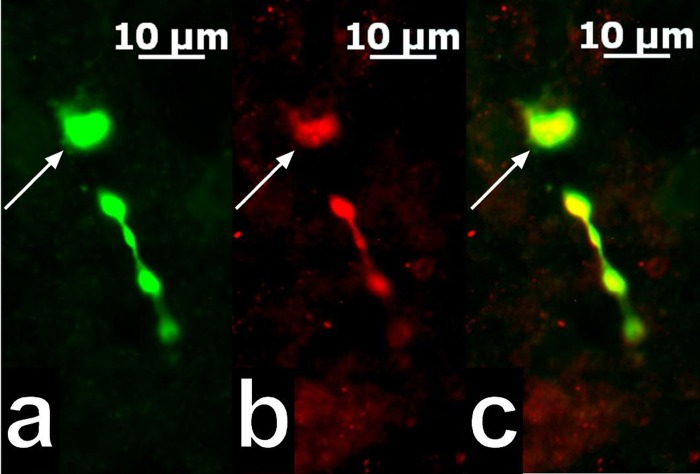
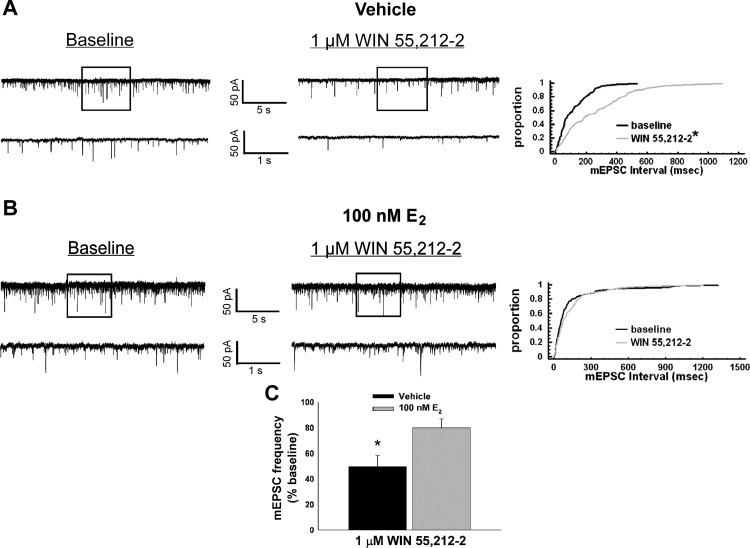
We made recordings from a total of 294 ARC neurons. These cells had a RMP of −52.5 ± 0.9 mV and a Rin of 727.2 ± 47.8 MΩ. Eighty-two of these neurons exhibited conductances like the hyperpolarization-activated cation current and A-type K+ current that are characteristic of POMC neurons (Ibrahim et al. 2003; Kellert et al. 2009). Of these, 67 were immunopositive for POMC neurons like the one shown in Fig. 3. To further our understanding of how these changes in energy homeostasis are occurring on a cellular level, we then examined how estradiol could impact cannabinoid-induced changes in excitatory synaptic input onto POMC neurons. The representative membrane current traces, corresponding quantile plots, and the composite bar graph demonstrate that WIN 55,212-2 (1 μM) reduced the number of excitatory synaptic events per unit time in recordings from vehicle-treated slices [Kolmogorov-Smirnov (K-S) statistic = 2.54558, P < 0.0001; Mann-Whitney U-test, W = 12.5, P < 0.03; n = 8] and that E2 (100 nM) appreciably attenuated this effect (Fig. 4; K-S statistic = 1.06912, P < 0.21; n = 8).
Fig. 3.
A color photomicrograph of an ARC proopiomelanocortin (POMC) neuron from which an electrophysiological recording was taken. a: Biocytin labeling of the neuron filled throughout the recording and visualized with AF488. b: Immunoreactivity for β-endorphin located in the soma and varicosities of the cell in a as visualized with AF546. c: Merged photomicrographs from a and b illustrating the ARC neuron (arrows) that was double-labeled with biocytin and β-endorphin immunoreactivity.
Fig. 4.
17β-Estradiol (E2) attenuates the cannabinoid-induced decrease in miniature excitatory postsynaptic current (mEPSC) frequency in POMC neurons. Membrane current traces and cumulative probability distributions show the decrease in mEPSC frequency caused by the CB1 receptor agonist WIN in a recording from a vehicle-treated slice (A; *P < 0.05, Kolmogorov-Smirnov test) and the diminution of the cannabinoid-induced decrease in mEPSC frequency in a recording from an E2-treated slice (B). C: a composite bar graph illustrating the estrogenic attenuation of the cannabinoid-induced decrease in mEPSC frequency. Bars and vertical lines represent means and SE, respectively, of the mEPSC frequency that was normalized to values observed under baseline conditions. *P < 0.05, Mann-Whitney U-test (n = 8).
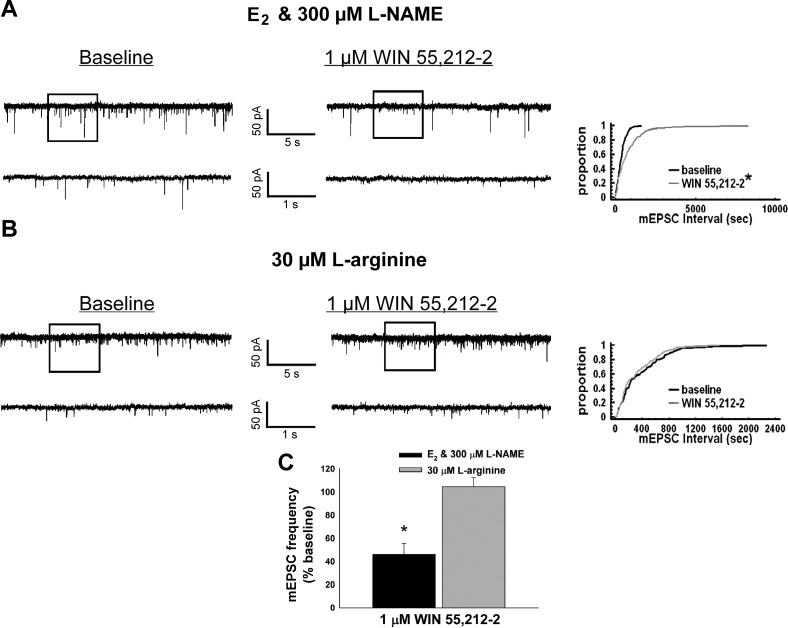
Since E2 inhibits the ability of WIN 55,212-2 to decrease mEPSC frequency, coupled with the fact that l-NAME restored the hyperphagia caused by WIN 55,212-2 in the presence of EB, we wanted to explore the role of NOS in the estrogenic attenuation of the cannabinoid-induced decrease in excitatory synaptic input. As shown in Fig. 5, A and C, WIN 55,212-2 clearly reduced mEPSC frequency in recordings from slices pretreated for 16–20 min with E2 and l-NAME (300 μM; K-S statistic = 2.06316, P < 0.0005; Mann-Whitney U-test, W = 30.0, P < 0.009; n = 5). By contrast, this effect was markedly diminished in the presence of the NOS substrate l-arginine (30 μM; K-S statistic = 0.725691, P < 0.69; Fig. 5B and C; n = 6).
Fig. 5.
The estrogenic attenuation of the cannabinoid-induced decrease in mEPSC frequency in POMC neurons is nullified by the NOS inhibitor l-NAME and mimicked by the NOS substrate l-arginine. Membrane current traces and cumulative probability distributions show the decrease in mEPSC frequency caused by WIN in a recording from a slice that was co-treated with E2 and l-NAME (A; *P < 0.05, Kolmogorov-Smirnov test) and the dampening of the cannabinoid-induced decrease in mEPSC frequency in a recording from a slice that was treated with l-arginine (B). C: a composite bar graph illustrating how the cannabinoid-induced decrease in mEPSC frequency is rescued by l-NAME in E2-treated slices and obstructed in slices treated with l-arginine. Bars and vertical lines represent means and SE, respectively, of the mEPSC frequency that was normalized to values observed under baseline conditions. *P < 0.05, Mann-Whitney U-test (n = 5–6).
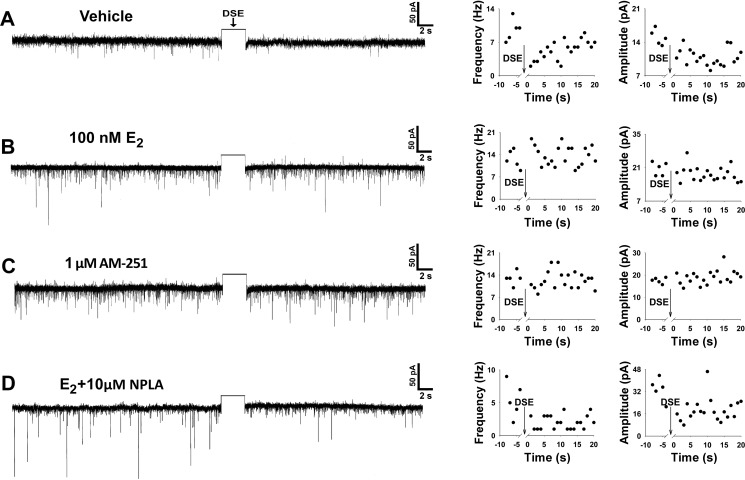
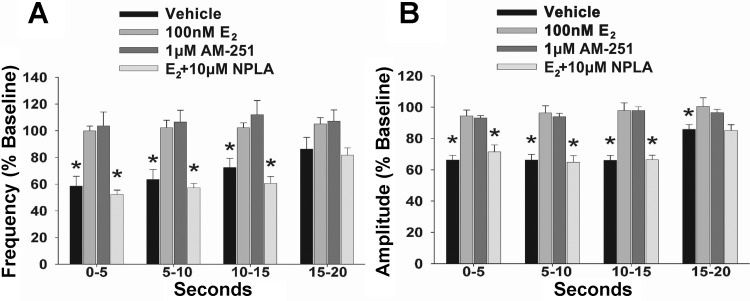
To test whether estradiol could also modulate retrograde endocannabinoid signaling, we then attempted to elicit DSE during recordings from hypothalamic slices. We found that a 60-mV depolarizing stimulus (0.75–3 s in duration) delivered to the postsynaptic neuron in vehicle-treated slices decreased the frequency and amplitude of sEPSCs impinging on it (Figs. 6A and 7; n = 11). The onset of this effect was observed within 5 s (Kruskal-Wallis/median-notched box-and-whisker analysis; frequency: test statistic = 19.3284, P < 0.0003; amplitude: test statistic = 24.2295, P < 0.0001), sustained for at least 15 s (frequency: test statistic = 17.1405, P < 0.0007; amplitude: test statistic = 21.8684, P < 0.0001), and appeared to return to baseline levels by 20 s after the stimulus (frequency: test statistic = 7.57239, P < 0.06; amplitude: test statistic = 9.97297, P < 0.02). This stimulus-induced reduction in sEPSC frequency and amplitude was negated by both E2 (Figs. 6B and 7; n = 10) and AM-251 (Figs. 6C and 7; n = 7). Furthermore, when E2 was bath applied to slices pretreated with the nNOS-selective inhibitor NPLA (10 μM), poststimulus decrements in sEPSC frequency and amplitude were subsequently restored to levels similar to those observed in recordings from vehicle-treated slices (Figs. 6D and 7; n = 7). Collectively, this indicates that E2 blocks endocannabinoid-mediated DSE by activating a nNOS pathway in POMC neurons.
Fig. 6.
E2 antagonizes endocannabinoid-mediated depolarization-induced suppression of excitation (DSE) in POMC neurons by activating a nNOS pathway. Left: representative membrane current traces illustrate the changes in spontaneous EPSC (sEPSC) frequency and amplitude elicited by DSE during recordings in slices treated with vehicle (A), E2 (B), the CB1 receptor antagonist AM-251 (C), or E2 and the nNOS-selective inhibitor N5-[imino(propylamino)methyl]-l-ornithine hydrochloride (NPLA; D). The rectangular wave under the arrow labeled “DSE” represents the truncated change in membrane current caused by the 3-s, 60-mV depolarizing voltage command. Right: graphical depictions of the DSE-induced changes in raw frequency and amplitude (relative to the last 5 s of the baseline control period) for each of the representative traces in the 4 different treatment conditions of A–D.
Fig. 7.
A and B: composite bar graphs illustrate the DSE-induced changes in sEPSC frequency and amplitude, respectively, observed during treatment with vehicle, E2, AM-251, or E2 and NPLA. Bars and vertical lines represent means and SE, respectively. *P < 0.05, values of poststimulus sEPSC frequency and amplitude that are significantly different (Kruskal-Wallis/median-notched box-and-whisker analysis; n = 7–11) from those observed under basal conditions.
DISCUSSION
Our results demonstrate that estrogen-induced decreases in energy intake are mediated by a reduction in cannabinoid sensitivity via a mechanism involving the activation of nNOS in the ARC. We base these conclusions on the following findings: 1) EB decreases energy intake and expenditure while activating nNOS in the ARC but not the VMN; 2) the hyperphagic effect of the cannabinoid receptor agonist WIN 55,212-2 is blocked by EB and rescued by the NOS inhibitor l-NAME in EB-treated animals; 3) the estrogenic attenuation of the cannabinoid-induced decrease in mEPSC frequency in POMC neurons is nullified by l-NAME and mimicked by the NOS substrate l-arginine; and 4) the estrogen-induced decrease in retrograde endocannabinoid-mediated DSE is largely negated by the nNOS-selective inhibitor NPLA.
The estradiol-induced reduction in food consumption currently observed is consistent with the literature reported for other rodent models (Butera and Czaja 1984; Dubuc 1985; Palmer and Gray 1986). This also agrees with findings in human females, in whom energy intake is lowest at the late follicular phase of the ovulatory cycle, when estrogen levels peak and are unopposed by progesterone (Johnson et al. 1994). The estrogen-induced anorexigenesis can be attributed, in part, to the negative modulation of metabotropic receptors expressed in POMC neurons. For example, estrogens rapidly impede postsynaptic Gi/o-coupled receptors such as the GABAB receptor from G protein-gated, inwardly rectifying K+ (GIRK) channels by activating a PLC/PKC/PKA pathway (Qiu et al. 2003, 2006, 2008). Estrogen also potentiates the appetite suppression caused by fenfluramine-induced serotonin release (Rivera and Eckel 2005). This results in higher activation of 5-HT2C receptors, which are Gq-coupled and in turn activate PLC to hydrolyze phosphatidylinositol bisphosphate. In POMC neurons, this leads to an enhanced firing rate via activation of canonical transient receptor potential channels (Sohn et al. 2011) and the uncoupling of GIRK channels from metabotropic Gi/o-coupled receptors (Qiu et al. 2007).
Presently, we found that estradiol rapidly blocks cannabinoid-induced hyperphagia and the decrease in mEPSC frequency. This is consistent with what we have shown previously (Kellert et al. 2009; Washburn et al. 2013). The steroid is able to elicit its negative modulatory effect within minutes after bath application to hypothalamic slices and lasts for at least 24 h when systemically administered to ovariectomized female guinea pigs (Kellert et al. 2009; Nguyen and Wagner 2006). Estradiol also rapidly occludes the AM-251-induced decrease in energy intake, and by 24 h it negates the hypophagia and increase in excitatory synaptic input onto POMC neurons caused by CB1 receptor antagonism (Kellert et al. 2009; Nguyen and Wagner 2006). In addition, we have demonstrated that the rapid estrogenic attenuation of the cannabinoid-induced increase in energy intake and the decrease in glutamatergic input onto POMC neurons are mimicked by the diphenylacrylamide compound STX, which activates a Gq-coupled membrane ER that is neither ERα nor ERβ (Qiu et al. 2006). Moreover, the ERα agonist propyl pyrazole triol (PPT) attenuates the cannabinoid-induced hyperphagia within 4 h and negates the cannabinoid-induced decrease in glutamate release onto POMC neurons in animals treated 24 h before experimentation (Washburn et al. 2013). This latter finding is in accordance with the fact that nearly 75% of guinea pig POMC neurons express ERα (Roepke et al. 2007), as opposed to only 25–30% in the mouse (de Souza et al. 2011). Thus the estrogenic disruption of metabotropic CB1 receptor signaling is most likely due to a combination of ERα and Gq-coupled mER activation in guinea pig POMC neurons.
Arguably, the most seminal finding of the present study is that estradiol can rapidly antagonize endocannabinoid-mediated DSE. This contrasts with studies in transgenic mice suggesting that endocannabinoid regulation of energy homeostasis is independent from the melanocortin system (Hentges et al. 2005; Sinnayah et al. 2008). In our guinea pig model, we clearly observe that endocannabinoids can inhibit POMC neurons via activation of presynaptic CB1 receptors on glutamatergic nerve terminals, which plays an integral role in the hyperphagia induced in this species. Further support for the use of this guinea pig animal model comes from reports demonstrating that guinea pigs were more sensitive than rats or mice during the development of appetite-suppressing drugs such as fenfluramine (Mennini et al. 1991) and fluoxetine (Anelli et al. 1992). Moreover, guinea pigs, like humans, do not have the capability of synthesizing their own vitamin C (Horton et al. 1975; Odumosu 1981). Coupled with the fact that guinea pig POMC neurons also express ERα to a greater extent than is observed in rats or mice (de Souza et al. 2011; Roepke et al. 2007), this would suggest that these cells may serve as a comparatively more important locus in this species for the regulatory interplay between estradiol and endocannabinoids that helps control energy balance in the female. The DSE that we encountered in POMC neurons is clearly observed within 5 s and lasts at least 15 s after the depolarizing stimulus. This is congruent with that found in the cerebellum, where a brief depolarization of cerebellar Purkinje cells at physiological temperature was found to induce a transient suppression of excitatory synaptic transmission at both parallel fiber synapses and climbing fiber synapses and lasts for 10–20 s (Kreitzer and Regehr 2001). Furthermore, in these cells the DSE is prevented by the CB1 receptor antagonist AM-251 (Kreitzer and Regehr 2001). Similarly, a depolarization of CA1 pyramidal neurons in hippocampal slices for 7–10 s produces a DSE that follows a similar time course (Ohno-Shosaku et al. 2002). The results found in the cerebellum and hippocampus support our findings that DSE is prevalent in short-term neuronal signaling in the hypothalamus and is mediated by endocannabinoids that are released from depolarized postsynaptic neurons to suppress the excitatory transmission through activation of the CB1 receptor.
Lastly, our results demonstrate that estrogen suppresses energy intake by rapidly disrupting cannabinoid signaling at POMC synapses via a nNOS pathway. This is in agreement with the fact that nitric oxide mediates the estrogenic potentiation of gonadotropin-releasing hormone secretion in hypothalamic explants caused by the activation of 2-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid/kainate receptors (Matagne et al. 2005). There is also considerable evidence to suggest that estradiol-stimulated nitric oxide production represents the final step in this signal transduction pathway. For example, estrogen-induced activation of NOS is dependent on upstream PI3K/Akt activity, as has been shown in the vascular endothelium (Haynes et al. 2003) and centrally in the hypothalamic PVN (Gingerich and Krukoff 2008). The Src/PI3K/Akt signaling pathway leading to increased nNOS activation in the PVN is initiated by ERβ (Gingerich and Krukoff 2008). The nitric oxide thus formed may very well serve as a retrograde messenger that increases the strength of excitatory synapses during long-term potentiation (Di et al. 2009; Fenselau et al. 2011; Hardingham and Fox 2006; Schuman and Madison 1991; Volgushev et al. 2000). In addition, the rapid estrogenic attenuation of the CB1 receptor-mediated decrease in glutamate release onto POMC neurons involves the activation of PI3K and PKC pathways (Jeffery et al. 2011; Washburn et al. 2013). Estrogens have also been found to activate PI3K to functionally uncouple GABAB receptors from GIRK channels in POMC neurons (Malyala et al. 2008), and the PI3K inhibitors wortmannin and LY-294002 significantly reduced estrogen-mediated GABAB receptor desensitization in these cells (Malyala et al. 2008). This estrogen activation can be very rapid, as shown by the finding that in NG108-15 neurons, estrogens stimulate the phosphorylation of Akt within 30 min in a PI3K-sensitive manner (Akama and McEwen 2003). These observations serve to reinforce the importance of PI3K as an upstream messenger of estrogenic signaling and subsequent activation of nNOS. While nitric oxide may very well be synthesized in the POMC neurons themselves, it may also be the case that nNOS itself is suppressing the expression of enzymes involved in endocannabinoid biosynthesis such as sn-1-selective diacylglycerol lipase and/or N-acyl-phosphatidylethanolamine-selective phospholipase D (Borgquist and Wagner 2013). Alternatively, it could be that nNOS is localized to the upstream bouton, where it enhances the expression of endocannabinoid degrading enzymes like monoacylglycerol lipase, or even the rate of endocannabinoid removal from the synaptic cleft (Bisogno et al. 2001; Hashimotodani et al. 2007; Straiker et al. 2009). This latter scenario is given additional credence because of the finding that in transgenic mice, POMC neurons per se do not express nNOS (Leshan et al. 2012). These possibilities will ultimately serve as the basis for future experiments along these lines.
In conclusion, our results indicate that the estradiol upregulates nNOS activity in POMC neurons to disrupt the activation of presynaptic CB1 receptors by endocannabinoids. Our data helps to elucidate how rapid estrogenic signaling alters cellular responsiveness to cannabinoids to help bring about estrogen-induced changes in energy balance. Given that the cannabinoid regulation of energy homeostasis is sexually differentiated (Farhang et al. 2009), this supports the need for rational, gender-based therapies for the treatment of HIV/AIDS- and cancer-related cachexia as well as obesity.
GRANTS
This study was supported by National Institutes of Health Grants DA024314 and HD058638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B. and E.J.W. conception and design of research; A.B., C.M., and E.J.W. performed experiments; A.B., C.M., and E.J.W. analyzed data; A.B. and E.J.W. interpreted results of experiments; A.B. and E.J.W. prepared figures; A.B. and E.J.W. drafted manuscript; A.B. and E.J.W. edited and revised manuscript; A.B., C.M., and E.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Steve Do for technical assistance and Dr. Martin Kelly for insightful comments after reading this manuscript.
REFERENCES
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci 23: 2333–2339, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli M, Bizzi A, Caccia S, Codegoni AM, Fracasso C, Garattini S. Anorectic activity of fluoxetine and norfluoxetine in mice, rats and guinea-pigs. J Pharm Pharmacol 44: 696–698, 1992. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Maccarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agrò A, Hillard C, Di Marzo V. The uptake by cells of 2-arachidonylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem 268: 1982–1989, 2001. [DOI] [PubMed] [Google Scholar]
- Borgquist A, Wagner EJ. On the cannabinoid regulation of energy homeostasis: past, present and future. In: Endocannabinoids: Molecular, Pharmacological, Behavioral and Clinical Features, edited by Murillo-Rodríguez E, Onaivi ES, Darmani NA, and Wagner EJ. Oak Park, IL: Bentham Science, 2013, p. 60–91. [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27: 9941–9950, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res 322: 41–48, 1984. [DOI] [PubMed] [Google Scholar]
- Cope JL, Chung E, Ohgami Y, Quock RM. Antagonism of the antinociceptive effect of nitric oxide by inhibition of enzyme activity or expression of neuronal nitric oxide synthase in the mouse brain and spinal cord. Eur J Pharmacol 626: 234–238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 27: 289–301, 2003. [DOI] [PubMed] [Google Scholar]
- de Souza FS, Nasif S, López-Leal R, Levi DH, Low MJ, Rubinstein M. The estrogen receptor α colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol 660: 181–187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych PE. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology 149: 5934–5942, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci 29: 393–401, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc PU. Effects of estrogen on food intake, body weight and temperature of male and female obese mice. Proc Soc Exp Biol Med 180: 468–473, 1985. [DOI] [PubMed] [Google Scholar]
- Escartín-Pérrez RE, Cendejas-Trejo NM, Cruz-Martínez AM, González-Hernández B, Mancilla-Díaz JM, Florán-Garduño B. Role of cannabinoid CB1 receptors on macronutrient selection and satiety in rats. Physiol Behav 96: 646–650, 2009. [DOI] [PubMed] [Google Scholar]
- Farhang B, Diaz S, Tang SL, Wagner EJ. Sex differences in the cannabinoid regulation of energy homeostasis. Psychoneuroendocrinology 34S: S237–S246, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 59: 190–200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau H, Heinke B, Sandkühler J. Heterosynaptic long-term potentiation at GABAergic synapses of spinal lamina I neurons. J Neurosci 31: 17383–17391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of Δ9-tetrahydrocannabinol. Br J Pharmacol 75: 409–414, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Bregman T, Kirkham TC. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp Biol Med (Maywood) 230: 225–234, 2005. [DOI] [PubMed] [Google Scholar]
- García-Juárez M, Beyer C, Gómora-Arrati P, Lima-Hernández FJ, Domínguez-Ordoñez R, Eguibar JR, Etgen AM, González-Flores O. The nitric oxide pathway participates in lordosis behavior induced by central administration of leptin. Neuropeptides 46: 49–53, 2012. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Krukoff TL. Activation of ERβ increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology 55: 878–885, 2008. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain Res 1412: 44–54, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci 26: 7395–7404, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci 27: 1211–1219, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem 278: 2118–2123, 2003. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25: 9746–9751, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, Dicamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther 289: 1427–1433, 1999. [PubMed] [Google Scholar]
- Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons? Physiol Behav 92: 507–519, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BJ, West CE, Turley SD. Diurnal variation in the feeding pattern of guinea pigs. Nutr Metab 18: 294–301, 1975. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology 144: 1331–1340, 2003. [DOI] [PubMed] [Google Scholar]
- Jeffery GS, Peng KC, Wagner EJ. The role of phosphatidylinositol-3-kinase and AMP-activated kinase in the rapid estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Pharmaceuticals 4: 630–651, 2011. [Google Scholar]
- Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol Behav 56: 523–527, 1994. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol 622: 15–24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol 308: 3–8, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych PE. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30: 12950–12957, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi M, Croci T, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Manara L. Modulation of gastric emptying and gastrointestinal transit in rats through intestinal cannabinoid CB1 receptors. Eur J Pharmacol 450: 77–83, 2002. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG. Leptin action via hypothalamic nitric oxide synthase-1 neurons controls energy balance. Nat Med 18: 820–823, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luparello TJ, Stein M, Park CD. A stereotaxic atlas of the hypothalamus of the guinea pig. J Comp Neurol 122: 201–217, 1964. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol 506: 895–911, 2008. [DOI] [PubMed] [Google Scholar]
- Matagne V, Lebrethon MC, Gérard A, Bourguignon JP. Kainate/estrogen receptor involvement in rapid estradiol effects in vitro and intracellular signaling pathways. Endocrinology 146: 2313–2323, 2005. [DOI] [PubMed] [Google Scholar]
- Mennini T, Bizzi A, Caccia S, Codegoni A, Fracasso C, Frittoli E, Guiso G, Padura IM, Taddei C, Uslenghi A, Garattini S. Comparative studies on the anorectic activity of d-fenfluramine in mice, rats and guinea pigs. Naunyn Schmiedebergs Arch Pharmacol 343: 483–490, 1991. [DOI] [PubMed] [Google Scholar]
- Merroun I, Errami M, Hoddah H, Urbano G, Porres JM, Aranda P, Llopis J, López-Jurado M. Influence of intracerebroventricular or intraperitoneal administration of cannabinoid receptor agonist (WIN 55,212-2) and inverse agonist (AM 251) on the regulation of food intake and hypothalamic serotonin levels. Br J Nutr 101: 1569–1578, 2014. [DOI] [PubMed] [Google Scholar]
- Moore PK, Handy RL. Selective inhibitors of neuronal nitric oxide synthase–is no NOS really good NOS for the nervous system? Trends Pharmacol Sci 18: 204–211, 1997. [DOI] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology 84: 123–137, 2006. [DOI] [PubMed] [Google Scholar]
- Odumosu A. Vitamin C and weight reducing drugs on brain ascorbic acid in guinea pigs. Acta Vitaminol Enzymol 3: 96–102, 1981. [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci 22: 3864–3872, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otukonyong EE, Okutani F, Takahashi S, Murata T, Morioka N, Kaba H, Higuchi T. Effect of food deprivation and leptin repletion on the plasma levels of estrogen (E2) and NADPH-d reactivity in the ventromedial and arcuate nuclei of the hypothalamus in the female rats. Brain Res 887: 70–79, 2000. [DOI] [PubMed] [Google Scholar]
- Palmer K, Gray JM. Central vs peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav 37: 187–189, 1986. [DOI] [PubMed] [Google Scholar]
- Palmer RMJ, Rees DD, Ashton DS, Moncada S. l-Arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 153: 1251–1256, 1988. [DOI] [PubMed] [Google Scholar]
- Parkash J, de Tassigny XD, Bellefontaine N, Campagne C, Mazure D, Buée-Scherrer V, Prevot V. Phosphorylation of N-methyl-d-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle. Endocrinology 151: 2723–2735, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23: 9529–9540, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26: 5649–5655, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen receptor. Steroids 73: 985–991, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharmacol 72: 885–896, 2007. [DOI] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab 19: 682–693, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RMJ, Moncada S. An l-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA 87: 5193–5197, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis WL, Saad WA, Camargo LA, Elias LL, Antunes-Rodrigues J. Central nitrergic system regulation of neuroendocrine secretion, fluid intake and blood pressure induced by angiotensin-II. Behav Brain Funct 6: 64, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35: 1265–1269, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav 86: 331–337, 2005. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151: 4926–4937, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148: 4937–4951, 2007. [DOI] [PubMed] [Google Scholar]
- Santollo J, Marshall A, Daniels D. Activation of membrane-associated estrogen receptors decreases food and water intake in ovariectomized rats. Endocrinology 154: 320–329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intracellular messenger nitric oxide in long-term potentiation. Science 254: 1503–1506, 1991. [DOI] [PubMed] [Google Scholar]
- Sica M, Martini M, Viglietti-Panzica C, Panzica G. Estrous cycle influences the expression of neuronal nitric oxide synthase in the hypothalamus and limbic system of female mice. BMC Neurosci 10: 78, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS One 3: e2202, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams CM, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71: 488–497, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol 86: 22–28, 1999. [DOI] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. Monacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol 76: 1220–1227, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud N, Wiseman DA, Black SM. Caveolin 1 is required for the activation of endothelial nitric oxide synthase in response to 17β-estradiol. Mol Endocrinol 24: 1637–1649, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tindal JS. The forebrain of the guinea pig in stereotaxic coordinates. J Comp Neurol 124: 259–266, 1965. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Balaban P, Chistiakova M, Eysel UT. Retrograde signaling with nitric oxide at neocortical synapses. Eur J Neurosci 12: 4255–4267, 2000. [DOI] [PubMed] [Google Scholar]
- Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology 97: 160–175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 138: 5055–5058, 1997. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-l-arginine. J Med Chem 40: 3869–3870, 1997. [DOI] [PubMed] [Google Scholar]