Abstract
During slow-wave sleep, neurons of the thalamocortical network are engaged in a slow oscillation (<1 Hz), which consists of an alternation between the active and the silent states. Several studies have provided insights on the transition from the silent, which are essentially periods of disfacilitation, to the active states. However, the conditions leading to the synchronous onset of the silent state remain elusive. We hypothesized that a synchronous input to local inhibitory neurons could contribute to the transition to the silent state in the cat suprasylvian gyrus during natural sleep and under ketamine-xylazine anesthesia. After partial and complete deafferentation of the cortex, we found that the silent state onset was more variable among remote sites. We found that the transition to the silent state was preceded by a reduction in excitatory postsynaptic potentials and firing probability in cortical neurons. We tested the impact of chloride-mediated inhibition in the silent-state onset. We uncovered a long-duration (100–300 ms) inhibitory barrage occurring about 250 ms before the silent state onset in 3–6% of neurons during anesthesia and in 12–15% of cases during natural sleep. These inhibitory activities caused a decrease in cortical firing that reduced the excitatory drive in the neocortical network. That chain reaction of disfacilitation ends up on the silent state. Electrical stimuli could trigger a network silent state with a maximal efficacy in deep cortical layers. We conclude that long-range afferents to the neocortex and chloride-mediated inhibition play a role in the initiation of the silent state.
Keywords: slow oscillation, long-range afferents, chloride-mediated inhibition, intracellular recordings
slow oscillation is the hallmark of the slow-wave sleep but also of several anesthetics, including ketamine-xylazine (Contreras and Steriade 1995; Steriade et al. 1993b, 1993d, 2001). This slow rhythm consists of an alternation of active and silent states. During the active states, strong synaptic barrages contribute to the depolarization of the membrane potential of all electrophysiological types of cortical neurons close to the firing threshold (Steriade et al. 2001). During silent states, the membrane potential is hyperpolarized by 10–20 mV in cortical (Chauvette et al. 2011; Steriade et al. 2001) and thalamic neurons (Contreras and Steriade 1995; Steriade et al. 1993d). The long-lasting hyperpolarization characterizing the silent state is attributed to disfacilitation (absence of synaptic activities) and the domination of potassium-mediated leak current (Contreras et al. 1996; Timofeev et al. 1996, 2001). Given the facts that the slow oscillation survives thalamic lesions (Steriade et al. 1993c) and cortical isolation (Sanchez-Vives and McCormick 2000; Timofeev et al. 2000) and that it was absent in the thalamus of decorticated animals (Timofeev and Steriade 1996), it was assumed that the cortex has all the machinery to generate the slow oscillation. Later studies, however, pointed to a possible active contribution of the thalamus in the generation of the slow oscillation (Crunelli and Hughes 2010; Hughes et al. 2002). Recent studies demonstrated that a full thalamocortical network is essential to maintain the full extent of the slow oscillation (David et al. 2013; Lemieux et al. 2014).
The silent-state onset of the slow oscillation is much more synchronous than the active-state onset, and it was hypothesized that this high level of synchrony required network mechanisms to recruit and synchronize cortical inhibitory neurons (Volgushev et al. 2006). The ability to induce slow waves with electrical stimulations in naturally sleeping rats (Vyazovskiy et al. 2009) and by transcranial magnetic stimulation in humans (Huber et al. 2007; Massimini et al. 2009) further suggests that active mechanisms are involved in the initiation of the silent state.
The most likely candidate to initiate a silent state is the chloride inhibition provided by GABAA receptors. A large number of these receptors are located close to the soma of pyramidal neurons (Gu et al. 1993; Hendry et al. 1990) and carry larger inhibitory conductances at the soma than GABAB receptors (Connors et al. 1988). A subgroup of cortical interneurons preferentially fires at the end of active states (Puig et al. 2008). A recent modeling study has explored the impact of GABAA conductances on state transitions during the slow oscillation and found that increasing the inhibitory connection of interneurons on pyramidal neurons enhanced the synchrony of silent state. Reducing the strength of this connection had the reverse effect on the synchrony of silent-state onset (Chen et al. 2012).
In this study, we hypothesized that the chloride-dependent inhibition initiating the silent state and long-range afferents are required to synchronize their activities throughout a neocortical area. To demonstrate this hypothesis, we present data from multisite local field potential (LFP) and multiple intracellular recordings of the delays of the silent-state onset in the intact neocortical network and in the isolated neocortical slab. We also show the relative contribution of chloride-mediated inhibition in the termination of active states.
MATERIALS AND METHODS
Surgery.
All experiments were conducted on cats of either sex (3–5 kg). For acute experiments, animals were anesthetized by intramuscular injection of ketamine and xylazine (10 and 2–3 mg/kg, respectively) and were subsequently intravenously supplied with ketamine (20 μg·kg−1·min−1) dissolved in 0.45% saline, 5% dextrose at a rate of 10 ml·kg−1·h−1. Electroencephalogram (EEG) was monitored throughout experiments, which lasted 8–36 h, and a supplemental dose of ketamine-xylazine was given by intravenous injection when EEG tended slightly toward an activated pattern. Lidocaine (0.5%) was injected in all pressure and incision points. Animals were paralyzed with gallamine triethiodide 2% and maintained under artificial ventilation. Ventilation rate and oxygenation were adjusted to keep an end-tidal CO2 level at 3–3.7%. Body temperature was maintained at 37.5–38.5°C throughout the experiment. To obtain intracellular recordings and cortical multiunit activity (MUA) during natural sleep, we chronically implanted two animals with a recording chamber above the suprasylvian gyrus as described previously (Steriade et al. 2001; Timofeev et al. 2001). To obtain fully deafferented neocortical networks, we isolated neocortical slabs (n = 6) as described elsewhere (Timofeev et al. 2000).
Electrophysiological recordings.
Local field potentials (LFP) and intracellular recordings were obtained from areas 5 and 7 of the suprasylvian gyrus. Most LFP were obtained with low-impedance coaxial electrodes (Rhodes Medical Instruments, Summerland, CA). For multisite LFP recordings, we used 8 coaxial electrodes arranged in a linear array at regular intervals of 1.5 mm. We also used a linear 16-channel silicon probe (100-μm interelectrode distance; NeuroNexus Technologies, Ann Arbor, MI) to record the depth profile of cortical MUA around silent-state transitions during natural slow-wave sleep. Signals were bandpass filtered (0.1 Hz–10 kHz) with an AC amplifier (A-M Systems, Sequim, WA) and digitized at a sampling rate of 20 kHz.
Dual intracellular recordings were obtained with sharp glass micropipettes (DC resistance of 30–75 MΩ) filled with potassium acetate (KAc; 2 M) or potassium chloride (KCl; 2 M). KCl was used to reverse the concentration gradient of chloride and caused a depolarization instead of a hyperpolarization when GABAA receptors were activated at resting membrane potential. Stability of intracellular recording was obtained by drainage of the cisterna magnum, bilateral pneumothorax, hip suspension, and filling the hole made in the skull with 4% agar in 0.9% saline. A high-impedance amplifier with active bridge circuitry (Neurodata IR-283 amplifiers; Cygnus Technology, Delaware Water Gap, PA) was used to record and inject current in cells. For the analysis we retained neurons showing stable membrane potential below −60 mV, action potential amplitude of at least 50 mV, and input resistance higher than 20 MΩ. Analyzed recordings, including dual intracellular recordings, lasted more than 10 min, and we analyzed at least 50 cycles of slow oscillation for each neuron or neuronal pair. All electrophysiological data were digitized with the Nicolet Vision data acquisition system (Nicolet Technologies, Madison, WI) at a sampling rate of 20 kHz.
Electrical laminar stimulations.
Laminar microstimulations were done in the slab (see Fig. 8) with a silicon probe made of 16 iridium electrodes at intervals of 150 μm (NeuroNexus Technologies). For microstimulation of neurons in the intact cortex (see Fig. 9), the distance between electrodes of the silicon probe was 100 μm.
Fig. 8.
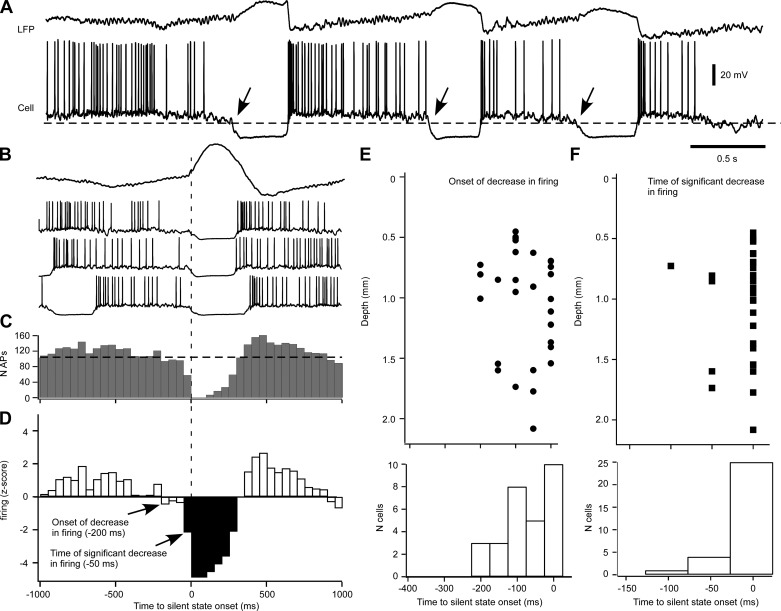
Decreased firing in intracellular recordings of neocortical cells preceding the onset of silent states. A: LFP and intracellular recordings in the cat suprasylvian gyrus during ketamine-xylazine anesthesia. Dashed line indicates the threshold of silent-state onset (arrows). B: averaged LFP (top) and 3 segments of intracellular recording ±1 s around the onset of cortical silent states. Note the decrease in firing 100–200 ms before the silent-state onset. C: histogram of cortical firing of the cell illustrated in A and B (n = 91 silent states). Bins = 50 ms. D: z-score transform of the perievent histogram in C. Average and SD were obtained from a ±5-s period around silent-state onset. Note a decrease in firing starting at 200 ms that becomes significant at 50 ms. E, top: plot of the depth profile of onsets of decrease in firing. Each circle represents a cell (n = 30). Bottom, number of cells associated to a time of decrease onset. F, top: plot of the depth profile of timing of significant decrease in firing. Each square represents a cell (n = 30). Bottom, number of cells associated to a time of significant decrease.
Fig. 9.
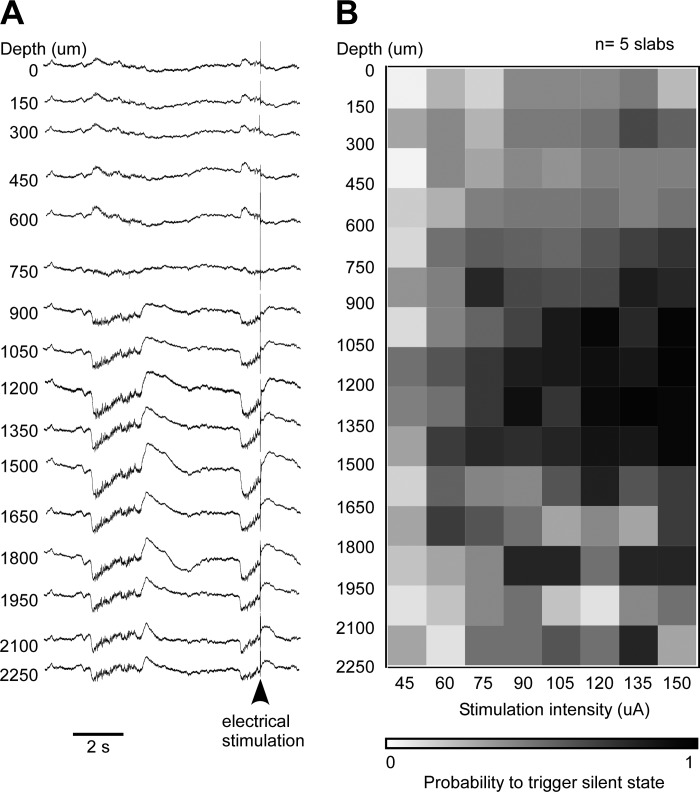
Depth profile of the efficacy to trigger a silent state. A: laminar profile of LFP activity in the neocortical slab showing a spontaneous transition to the silent state and an evoked transition. B: depth-current intensity profile of the probability to trigger a silent state, averaged from 5 experiments (at least 10 stimuli at each electrode's pair and stimulus intensity). The probability matrix is color coded and ranges from 0% (white) to 100% efficacy (black). Note the higher efficiency between 900 and 1,500 μm.
Data analysis and statistical tests.
Data were analyzed off-line using Igor Pro (version 4; Wavemetrics, Portland, OR). We extracted the timing of silent-state onset with the LFP recorded at each cortical site. The onset was defined as the time corresponding to the half-amplitude of the sigmoid fitting of the transition to the silent state. For each transition to the silent state, we calculated the averaged time of silent state and calculated the delay for each of the six to eight sites to this averaged time. We represented the delay of each site to the averaged silent-state onset with a box plot. To evaluate the level of synchrony of the silent-state onset for each condition (intact cortex and neocortical slab), we extracted the median delay to the averaged silent-state onset of each site and calculated the variance of delays. Population data are presented as frequency histograms. Comparisons of variances were made with a F-test.
To determine the timing of the silent-state onset in intracellular recordings, we defined it as the crossing of a threshold of the membrane potential (Vm). A frequency histogram of Vm in bins of 25 ms was built for each cell. The Vm of all neurons had a bimodal distribution that was split at the trough between the active (more depolarized Vm) and the silent (more hyperpolarized Vm) state modes. The threshold for the silent-state onset was defined as the mean Vm of the active state minus one standard deviation (1 SD) of this distribution (see Fig. 2B, right). The minimal duration for silent state was defined as 100 ms. For dual recordings with KAc, the reference cell was randomly selected. For dual recordings with one pipette filled with KCl, the cell recorded with KAc was always selected as the reference cell. The delay of the silent-state onset was calculated as the time of the onset in the test cell minus the time of onset in the reference cell.
Fig. 2.
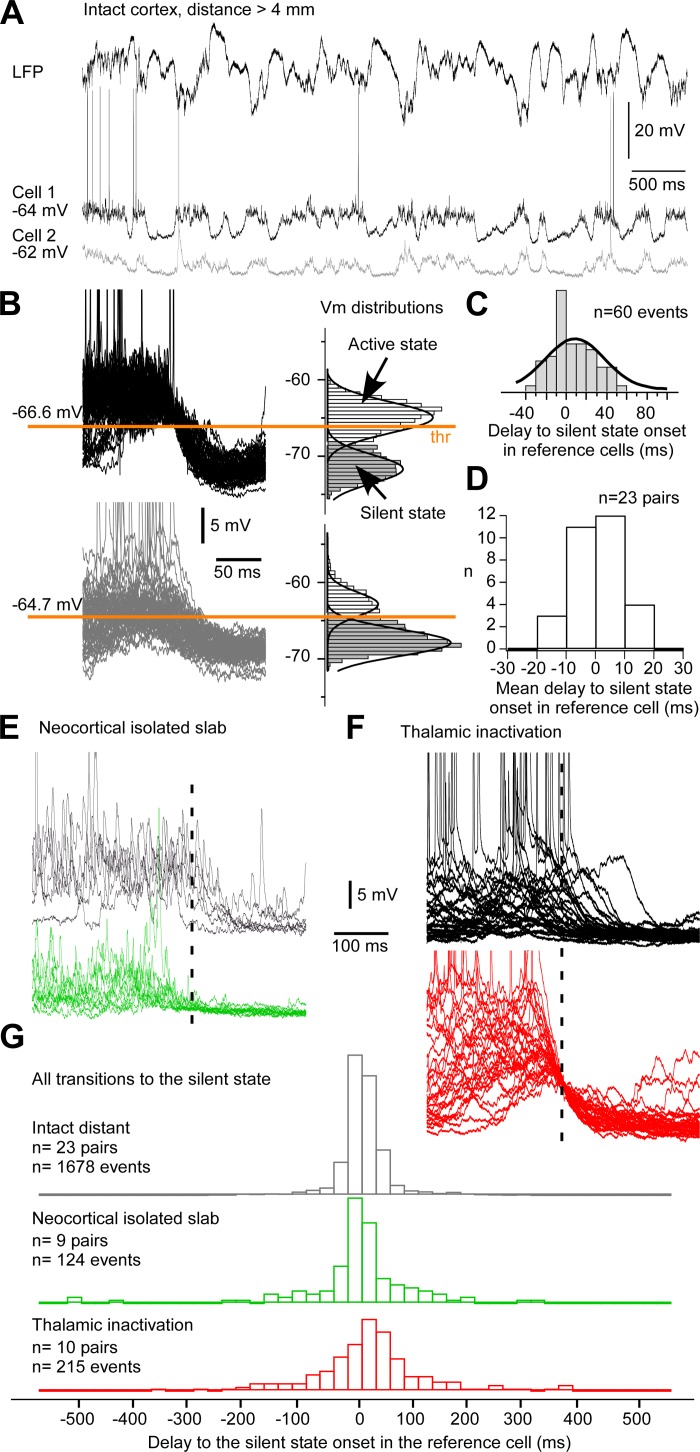
Synchrony of silent-state onset at the cellular level. A: example dual intracellular recording from the suprasylvian gyrus with a lateral distance of 4 mm. The LFP was obtained simultaneously in the same region. B: several transitions to the silent state in both cells (left) are shown with their corresponding membrane potential (Vm) distribution. The onset is defined when Vm crosses the active state threshold (thr). C: frequency histograms of the delays of the silent state onset in the gray cell (cell 2) to the onset in the black cell (cell 1). The thick black line is the Gaussian fitting of the distribution. D: histograms of mean delays in each pair. Note that the distribution is centered on zero. E: examples of silent-state onsets for 2 neurons at a distance >4 mm in the isolated neocortical slab. F: examples of superimposed transitions to the silent state for a pair of neurons at a distance <1.5 mm in the region affected by a lateral posterior nucleus inactivation. G: frequency histograms of the delays of the silent-state onset for all investigated conditions. Dashed lines in E and F indicate the crossing of the Vm threshold in cell 2.
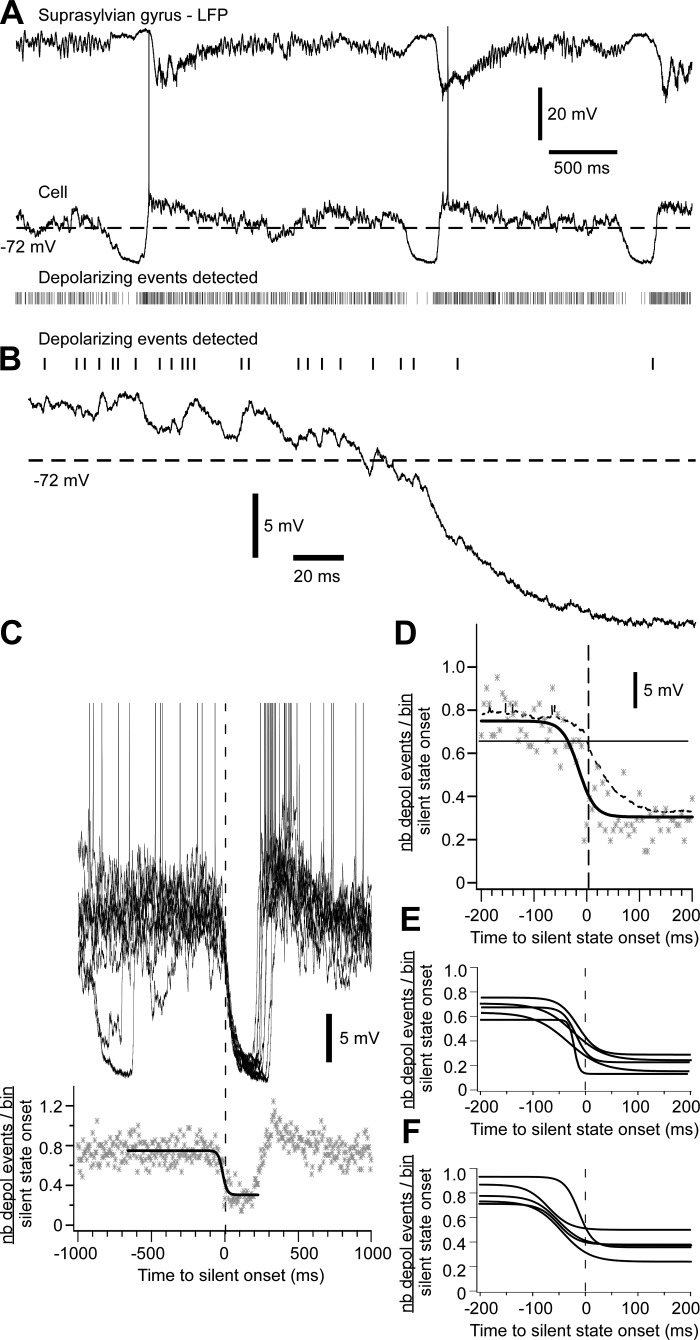
To evaluate the rate of synaptic inputs reaching the soma of cortical neurons, we extracted depolarizing events from intracellular recordings that had low electronic noise. Traces were filtered from 0 to 400 Hz to remove high-frequency noise and were derived over time to obtain the dV/dt of Vm. An event was selected as a peak in the dV/dt trace crossing a threshold (0.25–0.4 mV/ms depending on the cell). The number of depolarizing events was calculated in bins of 5 ms for a window of ±1,000 ms around the onset of the silent state. Data were normalized on the number of transitions to the silent state and fitted with a sigmoidal function ±200 ms around the onset of the silent state to describe the dynamic of synaptic inputs.
With KCl-KAc simultaneous recordings, we identified synchronous inhibitory activities. These activities were characterized by a hyperpolarization in the neuron recorded with KAc and a depolarization in the neuron recorded with KCl. To detect these events, we used a window of ±100 ms with a sliding step of 10 ms. To estimate the onset and offset of detected events, we used the difference of the z-score transformation of both intracellular traces. A z-score trace from analyzed segments of several minutes was obtained by subtracting the Vm by the mean Vm of the cell (to set the mean Vm at zero) and then dividing it by the standard deviation of the Vm. The z-score difference was calculated by subtracting the z-score of the KCl trace by the z-score of the KAc trace. A hyperpolarization in the KAc trace and a depolarization in the KCl trace would give a positive difference. The threshold for the onset/offset was defined as 1 SD of the z-score difference trace. We built frequency histograms of the delays of onset of these events to the onset of the silent state. We also estimated a window of disfacilitation that was the difference of the time of silent-state onset and the time of long-duration inhibitory event offset. We tested whether the proportion of these events (number of events per silent-state onset) was significantly higher than expected at chance level with an exact binomial proportion test. This is a test based on the binomial distribution B(n, p), where n is the sample size and p is the probability of observation (5%). It verifies whether the number of events (X) is statistically different than expected by chance (K = n × p). The proportion of events was significant when Pr(X > K) ≤ 0.025 (α = 0.05 in a bilateral test).
RESULTS
Level of synchrony of the transition to the silent state.
Under ketamine-xylazine anesthesia, silent states occur nearly simultaneously in all recorded sites of the suprasylvian gyrus (Fig. 1, A–D). To assess the level of synchrony among these sites, we first defined the onset of the silent state as the time at half-amplitude of the sigmoidal fitting of the transition (Fig. 1B). For each site, we calculated the median delay to the onset of the silent state averaged from all sites (Fig. 1C). The median delays of all sites from all experiments (38 sites, 5 experiments) were centered on zero (Fig. 1D). The variance was used as a measure of synchrony of the silent-state onset. In the intact cortex, we found that the variance was 277.14 ms2. Starting from this baseline value, we next evaluated the effect of removing inputs to the cortex on the silent-state onset.
Fig. 1.
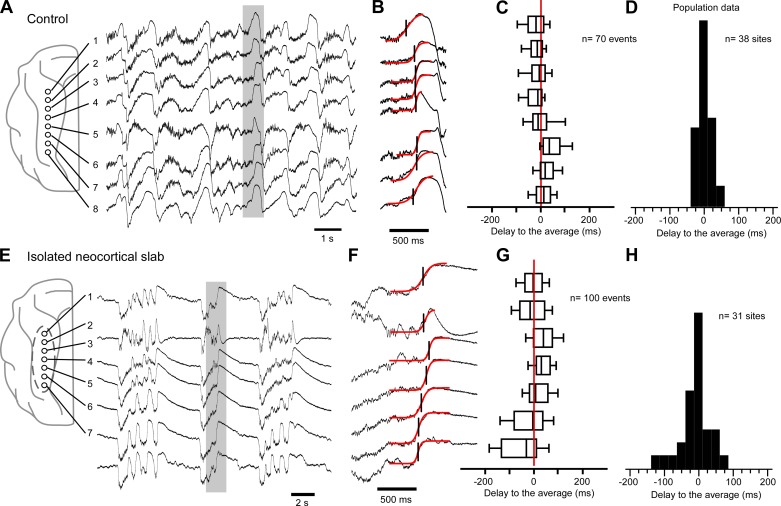
Removing inputs to the neocortex decreases the synchrony of silent-state onset. A: multisite local field potential (LFP) recordings in the intact suprasylvian gyrus reveal a synchronous slow oscillation in the 8 cortical sites. Schema at left indicates the location of recording electrodes. Traces in the gray box are enlarged in B to show the simultaneous occurrence of the silent state among sites. Transitions are fitted with a sigmoid curve (red), and the onset of the silent state is defined as the time corresponding to the half-amplitude of the curve. C: boxplots of the delays to the averaged silent-state onset for each site. The left and right boundaries of the box are the first and third quartiles, respectively, and the central line is the median. Horizontal lines contain percentiles 10 to 90. D: frequency histogram of the median delay to the averaged silent state for all sites (n = 38) from all experiments (n = 5). E: multisite LFP recordings in a neocortical slab. Schema at left indicates the location of the recording electrodes, and the dotted line delimitates the slab. Traces in the gray box are enlarged in F. G: boxplot of the delays to the averaged silent-state onset for each site. H: frequency histograms of the median delays to the averaged silent state for all sites (n = 31) from all experiments (n = 6).
We tested the effect of a complete deafferentation of the neocortex on the delays of the silent-state onset at different sites in an isolated neocortical slab (Fig. 1, E–H). The variance (3,652.78 ms2, n = 31 sites in 6 experiments) was significantly larger than in the intact cortex (F = 13.177, P < 0.05, F-test). These results show that long-range inputs are necessary to explain the level of synchrony of the silent-state onset within an intact cortical region.
We extended our investigation of the effects of external inputs to the neocortex on the synchrony of the silent-state onset at the cellular level. We first recorded simultaneously the intracellular activity of two neurons located at a distance of 4–12 mm in the intact cortex (Fig. 2A). We extracted the timing of the transition to the silent state for each neuron based on the crossing of a Vm threshold and calculated the delay in the pair (see example in Fig. 2, B and C). For all pairs recorded (n = 23; Fig. 2D), the mean delays were normally distributed around zero (D'Agostino and Pearson omnibus normality test, P = 0.6072) and had a low variance (61.07 ms2). Furthermore, the mean delay of the silent-state onset in pairs of neurons did not depend on the distance between neurons (P = 0.136, linear regression). The low temporal variability and the independence on the distance of the mean delay of the silent-state onset suggest the presence of synchronizing mechanisms.
After complete deafferentation, neocortical slab neurons recorded at distance of ∼4 mm (n = 9 pairs; Fig. 2, E and G) showed a more variable onset of the silent state than in the intact cortex (n = 124 silent states in the slab, n = 1,678 silent states in the intact cortex; F = 5.9, P < 0.05, F-test). After partial deafferentation [thalamic inactivation with QX-314, the same database as in our previous study (Lemieux et al. 2014)], the variances for neurons recorded in the affected region were similar to those recorded in the slab (F = 1.01, P > 0.05, F-test). These results suggest that long-range afferents to a neocortical region are necessary for the synchrony of the silent-state onset. As one of the main sources of afferents to the neocortex, thalamocortical cells might contribute to this fine-scale synchronization.
Disfacilitation and inhibition prior to the onset of the silent state.
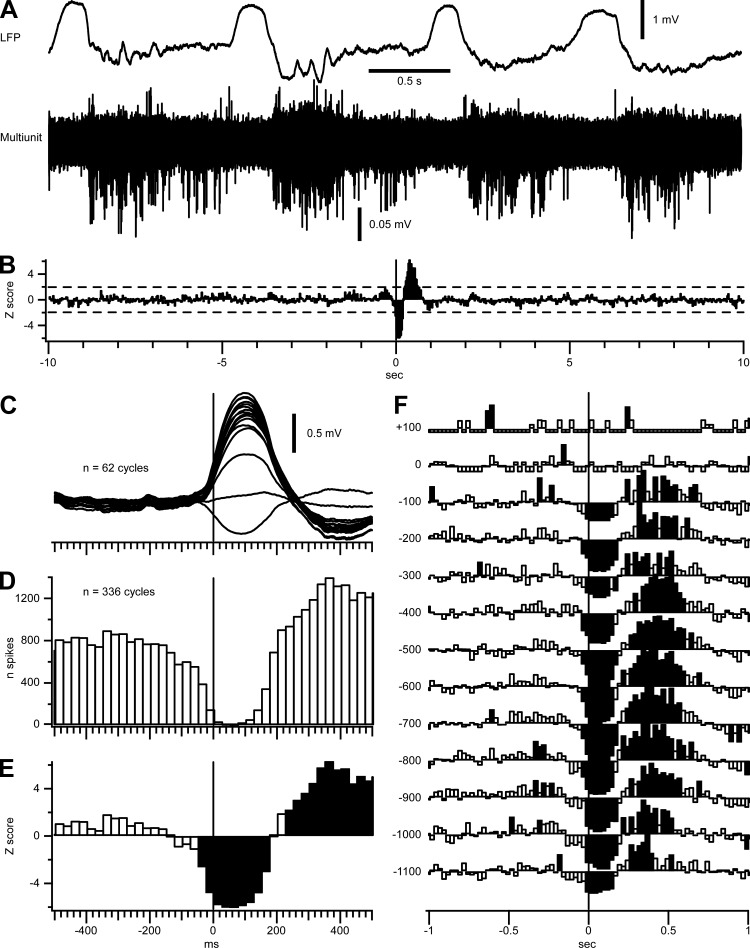
We hypothesized that a reduced synaptic drive could initiate the transition to the silent state. To test this idea, we estimated the rate of synaptic inputs on neurons by extracting depolarizing events of the Vm in intracellular recordings (Fig. 3, A and B). For 5 cells recorded under ketamine-xylazine, we extracted an average of 109–175 events/s. We next calculated the number of these depolarizing events in bins of 5 ms around the silent-state onset and fitted the transition with a sigmoid function (Fig. 3, C and D). Compared with the variation of the Vm at the transition to the silent state, the sigmoidal fitting of the number of depolarizing events started to decrease before the hyperpolarization of the neuron (Fig. 3, D–F). The dynamic varied from cell to cell but always preceded the variation of the Vm, both under anesthesia (Fig. 3E) and during natural sleep (Fig. 3F). These results supported our hypothesis that the transition to the silent state was initiated by a period of reduced synaptic drive. To further investigate this finding, we estimated the overall neuronal firing in the transition to silent states during slow-wave sleep in five separate experiments (Fig. 4). An overlay of cortical MUA recordings from 16 channels (Fig. 4A) illustrates a tendency to decreased populational firing rates before the onset of silent state. A perievent histogram of the overall firing referenced to the onset of LFP silent state (Fig. 4, C and D) further highlights this observation. Z-score transform shows that the firing is significantly decreased (P < 0.05) 50 ms before the onset of silent state (Fig. 4, B and E). A separate analysis at different cortical depths indicates that all the recordings below the reversal potential of LFP showed a significant decrease in the neuronal firing 25–50 ms before the onset of silent state (Fig. 4F). Because of the very sparse firing in upper cortical layers (Barth and Poulet 2012; Chauvette et al. 2010), we did not observe a significant reduction of firing in the transition to silent states in superficial channels.
Fig. 3.
Disfacilitation preceding the silent-state onset. A: example of LFP and intracellular recordings in the suprasylvian gyrus. Raster indicates depolarizing events detected in the intracellular trace. The dotted line is the Vm threshold used to define the onset of the silent state. B: examples of depolarizing events detected. C: 10 superimposed traces aligned on the onset of the silent state are shown (top). Graph (bottom) shows the number of depolarizing events per bin of 5 ms normalized to one silent-state onset (n = 41). Data are fitted with a sigmoidal curve (thick black line). Data are expanded ±200 ms around the silent-state onset in D. Note that the sigmoidal fitting of the number of depolarizing events precedes variation of the Vm. Sigmoidal fitting of 5 cells recorded under ketamine-xylazine anesthesia (E) and during natural sleep (F) are shown.
Fig. 4.
Decrease in populational cortical firing before the onset of silent states. A: deep LFP recorded during natural sleep of a cat (top) and superimposition of 16 filtered (0.5–10 kHz) LFP recordings for multiunit activities (bottom). Recordings were performed using a linear 16-channel silicon probe with recording sites separated by 100 μm; the probe was inserted perpendicularly to the cortical surface. Note the decreased firing toward the end of active states. B: z-score of multiunit firing triggered by silent-state onsets (n = 336). Mean and SD used for the z-score were calculated ±10 s from the silent-state onset. The dotted line represents z = ±1.96 (α = 0.05). C: wave-triggered averages of the LFPs recorded from a 16-channel silicon probe during one sleep episode. The deepest recording was used to detect the onset of silent states. D: perievent histogram of cortical firing calculated from 336 cycles from 5 sleep episodes (bins = 25 ms). E: close-up of B around the silent-state onset. F: z-score of the cortical depth profile of the significant variation of firing around silent-state onsets. Y-axis represents the cortical depth; depth 0 represents the depth at which the slow wave reversed and was used to align the recording depth from the 5 sleep episodes. Only depths with 5 recordings were used to calculate the depth profile z-score. For E and F, significant values are shown as filled bins; note the significant decreased firing starting 50 ms before onset of silent state.
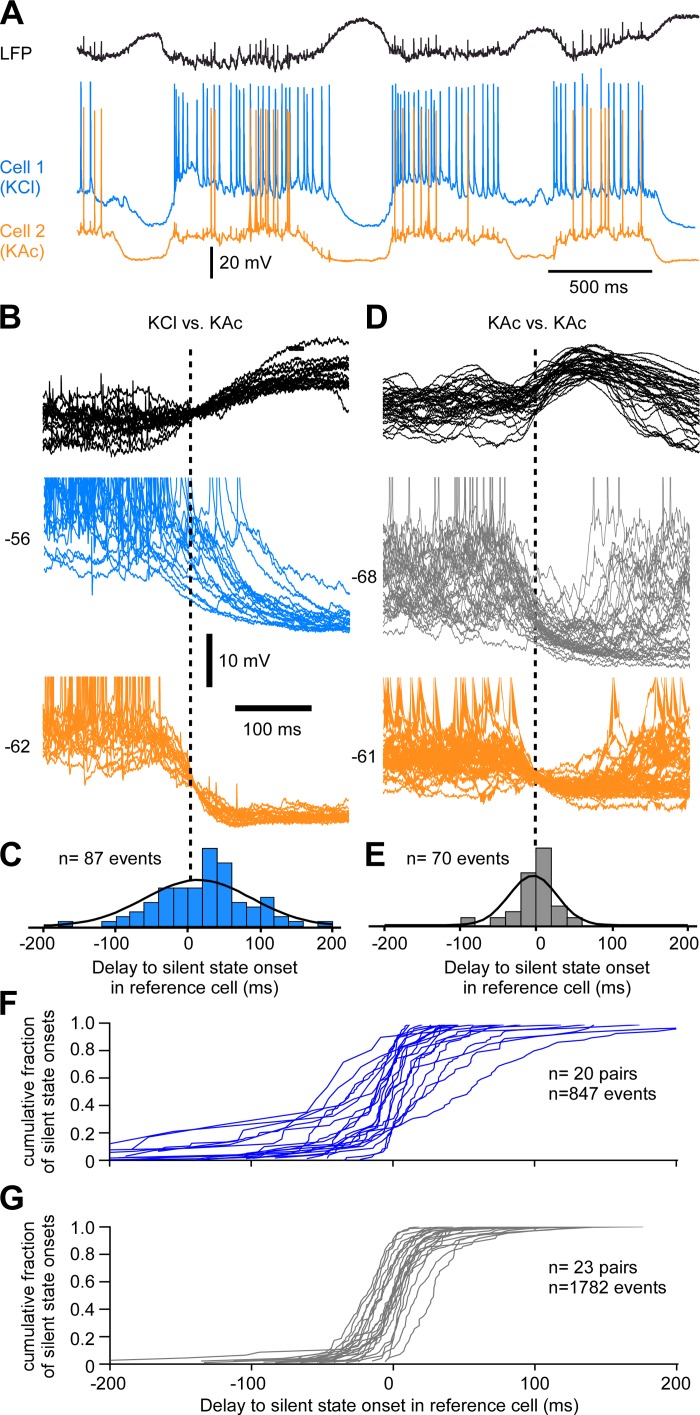
To investigate the role of chloride-mediated inhibition in the onset of the silent states, we performed dual intracellular recordings with one reference pipette filled with KAc and one test pipette filled with KCl (Figs. 5–7). Two neurons recorded at a distance <0.5 mm display similar fluctuations of the Vm, which suggests that they receive a similar amount of synaptic activities (Timofeev et al. 2004). Loading neurons with chloride reverses the hyperpolarizing effect of chloride-mediated inhibitory postsynaptic potential (IPSP) into a depolarizing one, and if active inhibition was involved directly at the transition to the silent state, we would expect to observe a longer delay in the KCl neuron compared with the KAc neuron. In the example shown in Fig. 5, A–C, the mean delay of the KCl cell to the silent-state onset of the reference cell was 14 ± 73.6 ms (n = 87 events). In comparison, the mean delay of in a pair of control cells (KAc-KAc) at a similar distance was −1.4 ± 31.3 ms (n = 70 events; Fig. 5, D and E). However, the cumulative density function of transitions to the silent states for each KCl-KAc recorded pair shows that such an example was rather the exception (Fig. 5F). The mean delay in most KCl neurons (n = 20 pairs) was still centered on zero, as was seen for control pairs (n = 23 pairs; Fig. 5G).
Fig. 5.
Chloride inhibition at the transition to the silent state. A: example of LFP and dual intracellular recordings of closely located (<0.5 mm) neurons. Cell 1 is recorded with a pipette filled with KCl (2 M) and cell 2 (reference cell) with a pipette filled with potassium acetate (KAc; 2 M). B: several superimposed examples of the transition to silent state triggered on the reference cell. Note the increased delay to silent-state onset in cell 1. C: frequency histogram of delays to the silent-state onset in cell 1 (KCl) compared with cell 2. D: example of 2 cells recorded with KAc closely located. E: frequency histogram of delays to the silent state. F: cumulative density functions for each KCl-KAc pair. G: cumulative density functions for each KAc-KAc pair. In B–E, the neuron indicated by orange color was used as reference cell and the silent-state onsets are indicated by a vertical dashed line.
Fig. 7.
Long-duration inhibition preceding the onset of the silent state during natural sleep. A: example of LFP, dual intracellular recordings, and associated z-score difference. Gray boxes indicate events of long-duration inhibition; black circles indicate the onset of silent states detected in the orange neuron. B: 2 examples from the same cells at a lower timescale. C: frequency histogram of the delays of onset of inhibitory events to the onset of the silent state. All data are from pairs with a proportion significantly higher than chance level. D: plot of the duration of the inhibitory event vs. the delay of onset of the event to the onset of the silent state. The slope of the linear regression is significant (P = 0.0107). E: frequency histograms of the window of disfacilitation.
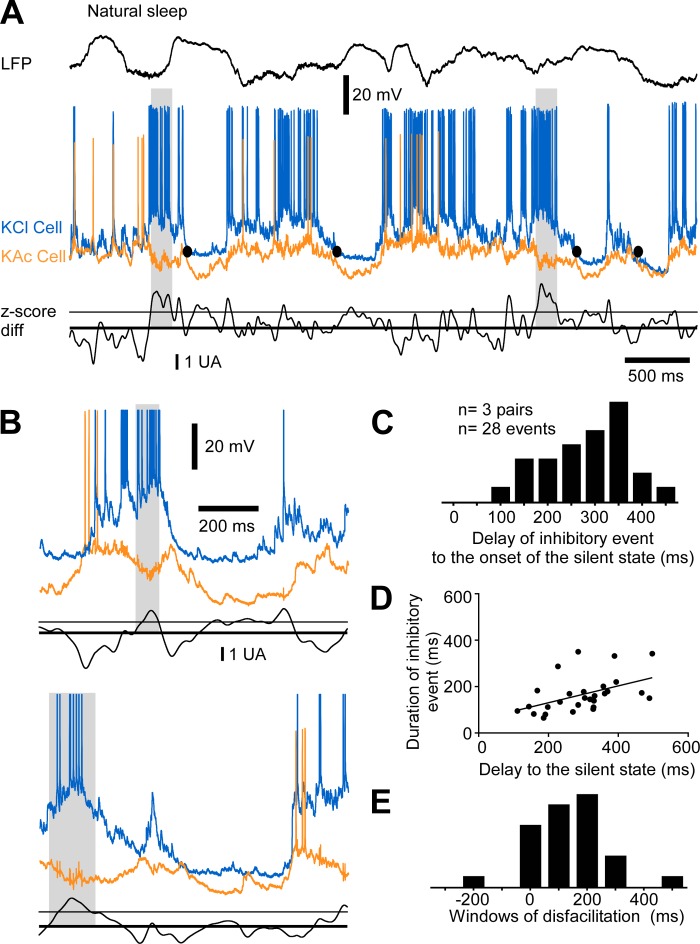
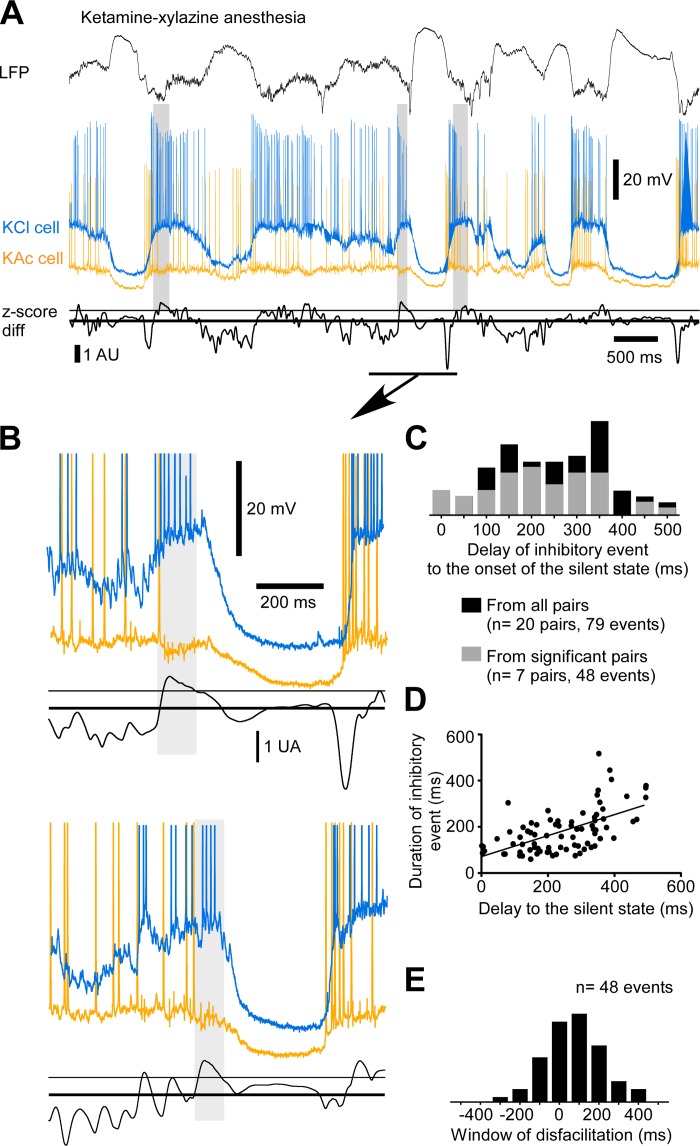
Because we found disfacilitation preceding the onset of the silent state by 10–100 ms, we investigated whether there was an increase in inhibition for this time period. We found that long-duration inhibitory activities often preceded the transition to the silent state (Figs. 6 and 7), and they were occasionally observed at the beginning of the active state (Fig. 6A). This phenomenon was characterized by a hyperpolarization of 3.11 ± 2.87 mV in the neuron recorded with the KAc pipette and by a depolarization of 5.40 ± 4.83 mV in the neuron recorded with the KCl pipette (n = 79 inhibitory events from 20 pairs of cells). The onset of these events was 227 ± 122 ms before the onset of the silent state (n = 79 events; Fig. 6C) and lasted on average 167 ± 78 ms. Events occurring earlier were generally longer in duration (P < 0.0001, linear regression; Fig. 6D). We estimated the delay between the end of inhibitory events and the onset of silent states (window of disfacilitation) and found there was a period of 71.2 ± 144.85 ms after the end of the identified inhibitory events and before the transition to the silent state (Fig. 6E). This time window matched the time course of reduction in depolarizing events at the end of the active states (Fig. 3).
Fig. 6.
Long-duration inhibition preceding the onset of the silent state under ketamine-xylazine anesthesia. A: example of LFP, dual intracellular recordings, and associated z-score differences. Z-score differences are presented in arbitrary units (AU). The thick line indicates a null difference and the thin line the standard deviation of the difference (threshold for inhibitory event onset). Gray boxes indicate events of long-duration inhibition. The epoch underlined is enlarged in B, top; another example from the same pair of cells is shown in B, bottom. C: frequency histogram of the delays of onset of inhibitory events to the onset of the silent state. Black bars are data from all pairs, and gray bars are data for pairs with a proportion significantly higher than chance level. D: plot of the duration of the inhibitory event vs. the delay of onset of the event to the onset of the silent state. The slope of the linear regression is significant (P < 0.0001). E: frequency histograms of the window of disfacilitation.
These long-duration inhibitory events were detected in almost every pair (18 of 20) and in 2.6–23.0% of the silent-state transitions. We evaluated that the occurrence of these inhibitory events was above chance level (P < 0.05, exact binomial proportion test) in 35% of the pairs (7 of 20). In these 35%, such inhibitory events would occur in 14.4 ± 4.1% of the transitions to the silent state. If that proportion holds at the population level, we would expect to observe this inhibitory activity in 3.6% (0.35 × 10.3%, the lowest approximation) to 6.5% of the cortical neurons (0.35 × 18.5%, the highest approximation). These results suggest that under ketamine-xylazine anesthesia, inhibiting 3.6–6.5% of neurons would be sufficient to terminate the active state and initiate a transition to the silent state.
We extended our investigation of these long-duration inhibitory events in relation to the onset of the silent state during natural sleep (n = 6 pairs of neurons) and found they also occurred before the silent-state onset (Fig. 7, A and B). The hyperpolarization in the KAc neuron was 4.06 ± 3.05 mV, and the depolarization in the KCl neuron was 10.19 ± 7.64 mV (n = 28 events from 6 pairs of neurons). The long-duration inhibition occurred 296 ± 102 ms before silent-state onset (n = 28 events; Fig. 7C) and lasted 165 ± 78 ms. As seen under anesthesia, events starting earlier lasted longer (P = 0.0107, linear regression; Fig. 7D). The window of disfacilitation was 140 ± 138 ms (Fig. 7E). This period was two times longer than that seen under anesthesia (Fig. 6E), but the time course of synaptic depression was also longer during natural sleep (Fig. 3F). These events were detected in half of the pairs (3 of 6) and seen in 19–26.8% of the silent-state onset. With these numbers, we can estimate that at every silent-state onset, 8–13.4% (0.5 × 19% for the lowest approximation and 0.5 × 26.8% for the highest one) of the neurons received an inhibition of long duration and were withdrawn from the pool of active neurons contributing to recurrent activity.
Because there was a gap between the enhanced inhibitory drive (Fig. 6) and the reduced synaptic drive (Fig. 3) and populational firing (Fig. 4), we extended our analysis of the decrease in neocortical excitatory drive to intracellular recordings obtained from regular spiking (presumably pyramidal) neurons (Fig. 8). Figure 8, A and B, shows an example of a cell vigorously firing 100–200 ms before the onset of the silent state. The timing of that decrease matches the disfacilitation time window caused by the period of long-duration inhibitory activities (Fig. 6E). To quantify that phenomenon, we transformed histograms of cortical firing ±1 s to the silent state onset (Fig. 8C) into z-score histograms (Fig. 8D). We extracted two temporal values: the onset time of the sustained decrease in firing and the time when firing was significantly depressed compared with the average firing (P < 0.05, or z = −1.96). When firing was too low to achieve the significant value of −1.96, we selected the first time bins of lowest firing (usually bin 0). For our sample (n = 30 cells), we found that the decrease could start 50–200 ms before the onset of the silent state (Fig. 8E). These neurons were located either in the lower part of superficial layers or in the upper part of deep layers. These results also show that the firing of one-third of recorded neurons decreased concurrently with the onset of the silent state. There was a significant decrease in firing 50–100 ms before the transition from active to silent states in 5 of 30 neurons (Fig. 8F). These results show that a decrease in firing occurs in individual neurons in between the period of long-duration inhibitory activities and the period of reduced synaptic drive. They also suggest that a preferential distribution in the cortical column of these neurons withdrawn from the excitatory neuronal pool.
Laminar profile of stimulation efficacy to induce a silent state.
The finding that synchronous onset of the silent state within a neocortical region requires long-range afferents (Figs. 1 and 2) suggested that an excitatory input to the cortex could terminate the active state. We asked if an electrical stimulation could trigger a silent state and whether the efficacy was higher at different cortical depths. We stimulated the neocortical slab with a silicon probe from the pia mater to a depth of 2,250 μm (step of 150 μm). Before stimulation, we recorded the spontaneous activity (Fig. 9A, left part of traces). The amplitude of the LFP was maximal below 1 mm, and the polarity reversed between 750 and 600 μm as expected. An electrical stimulus delivered through a coaxial electrode located 1 mm away from the probe triggered a silent state that was generalized throughout the cortical column (Fig. 9A, right part of traces). Once the laminar position of the electrodes was assessed, we used the 16-channel silicon probe to stimulate and the coaxial electrode to record the LFP. We used two adjacent electrodes on the probe to stimulate and tested current intensity ranging from 45 to 150 μA at a step of 15 μA. Electrical stimulation was delivered after at least 400 ms since the active state onset. This procedure was repeated 10 times, and the efficacy was calculated as the number of successes divided by 10. A successful stimulation led to a silent state within tens of milliseconds. The mapping of efficacy was done for five experiments and data were averaged (Fig. 9B). The lowest efficacy to induce a silent state was found when stimuli were applied above 600 μm, and it was maximal for stimuli applied between 900 and 1,950 μm. These results show that the excitatory inputs (here, electrical stimuli) can trigger silent states and that the highest efficacy was for depths partially overlapping those containing neurons withdrawn from the excitatory pool but also extending in between the superficial and deep clusters (Fig. 8, E and F).
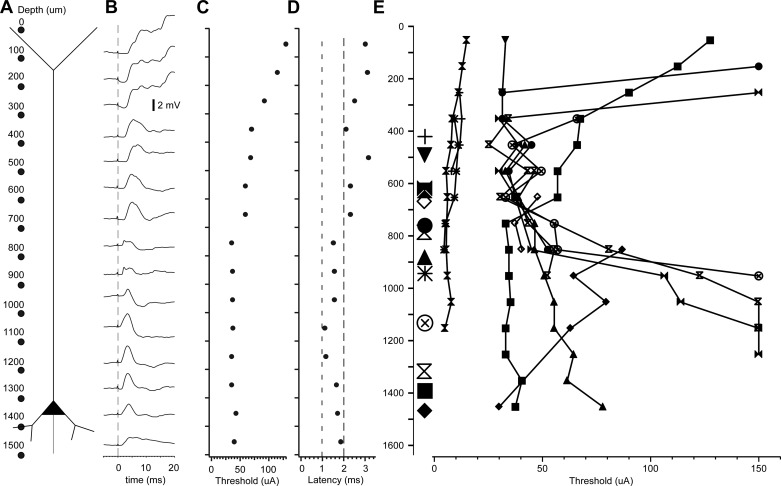
The laminar stimulation in the intact cortex of a neuron whose soma was located at a depth of 1,300–1,400 μm showed the same tendency of higher excitability at depths below 700 μm. The intracellular recording was obtained at a distance of 200 μm from the silicon probe, and stimuli were always delivered between electrodes at a regular distance of 100 μm (see schematic in Fig. 10A). The average of the postsynaptic response for each site of stimulation is shown in Fig. 9B. The lowest threshold of current intensity to obtain a response in the neuron was found when sites below 700 μm were stimulated (Fig. 10C). Furthermore, these deeper sites had a shorter latency (<2 ms) to evoke a synaptic response than more superficial sites (Fig. 10D). Using the same approach, we investigated 13 cortical neurons. The lowest thresholds were located at a depth of 400–900 μm (Fig. 10E), which correspond to layers 3 and 4 of cat suprasylvian gyrus (Hassler and Muhs-Clement 1964). Results on laminar profile of synaptic thresholds match the decrease in firing of individual neuron of the superficial cluster (Fig. 8) but differ from results of field potential findings in the isolated neocortical slab (see discussion).
Fig. 10.
Depth profile of neuronal response to electrical stimuli. A: schematic representation of the experimental approach. The 16 dots represent the 16 stimulating sites of the silicon probe at regular intervals of 100 μm. B: stimuli-triggered averages of responses obtained for every pair of stimulating site. The averages were obtained from at least 30 stimuli. C: stimulus intensity required to induce responses shown in B. D: latency of responses shown in B. E: threshold to elicit responses to laminar stimulation in 13 cortical neurons.
DISCUSSION
In this study, we found that under normal conditions, the transition from the active to the silent states of the slow oscillation occurs synchronously over a large distance (>10 mm) in the cat suprasylvian gyrus. The removal of long-range inputs to the neocortex decreases this synchrony. We identified at least three processes that precede the onset of the silent states: 1) a reduced density of excitatory synaptic events in a majority of investigated neurons concurrent with a decrease in firing by cortical neurons, 2) increased inhibitory activities in a subpopulation of neurons, and 3) an excitatory input that can initiate the transition. We propose that long-range afferents to a given cortical area activate a subset of inhibitory interneurons that hyperpolarize a subset of cortical neurons below the firing threshold. The lower firing of a subset of neurons reduces the excitatory drive on their targets and leads to a decrease in populational firing 50 ms before the silent-state onset. That triggers a chain reaction of reduced excitation underlying the synchronous onset of the silent states.
Balance of excitation and inhibition.
A recent study showed that activation of halorhodopsin-dependent chloride-permeable channels inhibiting 14% of pyramidal neurons from infragranular (but not supragranular) layers could induce a silent state (Beltramo et al. 2013), suggesting that removing only 14% of the intracortical excitatory drive was more than sufficient to cease the recurrent activity in the neocortex. Our results suggest that under ketamine-xylazine anesthesia, removing 3–6% of the neurons is sufficient to create a period of disfacilitation (reduced excitatory postsynaptic potential barrages), which ultimately ends with a silent state. This lower proportion of inhibited neurons is lower than under natural sleep (8–13%) and may be explained by the antagonizing action of ketamine on NMDA receptors (Anis et al. 1983; MacDonald et al. 1987), which already reduced the excitatory drive in the neocortical network.
Active states are maintained by a tight balance of excitatory and inhibitory conductances (Haider et al. 2006; Shu et al. 2003), although in the natural states of vigilance this balance is shifted toward inhibition (Haider et al. 2013; Rudolph et al. 2007). The inhibition controls the firing of regular-spiking neurons; inhibitory conductances are always larger than excitatory ones except before an action potential (Hasenstaub et al. 2005; Rudolph et al. 2007). Furthermore, GABAA conductances have been shown to be important to maintain activity within the network during the active state; the removal of this inhibition shortens the active state's duration and induces epileptiform burst (Mann et al. 2009; Sanchez-Vives et al. 2010). If inhibition controls the firing of neurons, a strong and synchronous recruitment of interneurons would have the consequence that inhibition would silence neurons and cause a disfacilitation resulting into a full silent state.
The higher efficacy of inhibition toward the end of the active state is supported by a higher frequency-dependent depression of excitatory inputs than inhibitory ones (Galarreta and Hestrin 1998). Furthermore, the electrical coupling specifically among fast-spiking neurons (Galarreta and Hestrin 1999) and the strong thalamocortical drive (a source of long-range afferents to the neocortex) on these inhibitory neurons combined with a strong intracortical excitation of inhibitory low-threshold spiking neurons (Gibson et al. 1999) might favor inhibition over excitation and thus terminate the active state.
The canonical cortical circuit is characterized by pyramidal neurons making strong connections to other pyramidal neurons and GABAergic interneurons. These interneurons innervate somatic, initial axon segmental, and dendritic compartments on pyramidal neurons and/or interneurons (Somogyi et al. 1998). GABAergic baskets cells target essentially the soma of neocortical pyramidal neurons (Tamás et al. 1997), where GABAergic synapses dominate (Freund et al. 1983; Gu et al. 1993). Because deep layer neurons fire more than those of superficial layers (Barth and Poulet 2012; Chauvette et al. 2010; Sakata and Harris 2009; Sanchez-Vives and McCormick 2000), layers V and VI are more likely to maintain the recurrent activity generating the active state. In other words, silencing these neurons directly at the soma would be the most efficient way to trigger a disfacilitation and silent state.
Results from laminar stimulations in the isolated neocortical slab reveal that stimulation of layer V exhibits the lowest threshold to trigger network silent states (Fig. 9). However, subthreshold neuronal responses in the intact cortex have lower threshold if the stimuli are applied around layers III and IV (Fig. 9). This discrepancy suggests a larger contribution of deeper layers in the isolated slab. A source of excitation of the superficial layer comes from intracortical long-range projecting neurons of the ipsilateral (Gilbert and Kelly 1975) and contralateral hemispheres (Jacobson and Trojanowski 1974). In other words, the lower level of synchrony in the isolated neocortical slab may be caused by the loss of both ipsilateral and commissural long-range intracortical connectivity. These findings are also in agreement with results showing that upper cortical layers stimuli can easily induce local responses but that deeper cortical layers stimuli can drive responses in the whole network (Beltramo et al. 2013).
Although inhibiting the soma is probably the most efficient way to induce a silent state, we cannot rule out the involvement of inhibition in other compartment, such as the one occurring at spines and dendritic shaft (Tamás et al. 1997). Also, we did not investigate the potential role of GABAB receptors in the termination of active states that was shown in layers II and III of the cingulated gyrus of rats in vitro (Mann et al. 2009).
Synchronization of the cortical inhibition.
The cortical synchrony of the silent-state onset decreased after the pharmacological inactivation of thalamocortical inputs (Fig. 2, F and G) similarly to the complete cortical deafferentation (neocortical isolated slab). This suggests that the thalamocortical inputs to the neocortex may synchronize inhibitory interneurons. There is indeed a high convergence of thalamocortical cells projections on a given suspected interneuron associated with a high divergence of a single thalamocortical cell on cortical interneurons in the neocortex (Swadlow 1995). It is very likely that long-range intracortical projections are implicated in the silent-state onset. Inhibition appears when intracortical stimulation intensity is increased and coincides with a higher firing by interneurons (Hirsch and Gilbert 1991). The emergent inhibition due to the supralinearity of neocortical horizontal connections recruiting inhibitory networks (Tucker and Katz 2003) could thus play a synchronizing role of inhibition during slow-wave sleep to promote the onset of the silent state.
Electrical stimulation.
Consistent with electrical stimulation in ferret slices of frontal and occipital cortex (Shu et al. 2003), we were able to shift the current state of an isolated neocortical slab with an electrical stimulation. As the active state progresses in time, it is likely that the excitation is decreased and an input to the cortex would favor inhibition over sustained excitation. The intracellular recordings of fast-spiking neurons in neocortical slices of ferrets showed that these interneurons increased their firing more in response to stimuli triggering a silent state than to those that induced an active state onset (Shu et al. 2003). A similar ability to induce a silent state (slow wave) was obtained in naturally sleeping rats (Vyazovskiy et al. 2009). It is of interest that transcranial magnetic stimulation in humans can noninvasively generate slow waves during the waking state (reviewed in Massimini et al. 2009). Although it is unknown whether electrical stimulations activated directly the synapses or the axons (or both), our data show that layer IV and V appears as a hotspot to induce a silent state.
Sleep architecture, cortex, and thalamus.
Our results point to an active contribution of thalamocortical neurons to the termination of cortical active states during slow-wave sleep. The thalamus is also known as a site of origin of spindle, at least fast spindles (Morison and Dempsey 1942; Steriade and Deschenes 1984; Timofeev and Chauvette 2013; Zheng et al. 2014). Thalamocortical neurons are hyperpolarized during slow-wave sleep and depolarized during rapid eye movement (REM) sleep (Hirsch et al. 1983) and likely waking state (Steriade et al. 1993a). On removal of cortical inputs, thalamocortical neurons are progressively hyperpolarizing and spindle activity is progressively replaced by delta clocklike bursting (Timofeev and Steriade 1996). At the beginning of sleep, the EEG activity is dominated by slow-delta rhythms, whereas toward the second half of sleep, it is dominated by spindles (Borbely et al. 1981). On that basis, we propose that thalamocortical neurons might be more hyperpolarized at the beginning of sleep and that their powerful bursting triggers synchronized onset of cortical silent states. Later in the night, the membrane potential of thalamocortical neurons becomes more depolarized and they contribute to spindle generation. Such changes of membrane potential of thalamocortical neurons could occur either as changes in the activities of neuromodulatory systems (Steriade and McCarley 2005) or as an upregulation of h-current (Luthi and McCormick 1999), which eventually leads to a reduction of T-current (Leresche et al. 2004) and low-threshold Ca2+ spikes. Therefore, sleep pressure expressed as enhanced slow-wave activity at the beginning of sleep or sleep deprivation might partially be mediated by the thalamus.
Functional implications.
The present study suggests that the inhibition by the activation of GABAA receptors (chloride mediated) in about 5% of cortical neurons under anesthesia and twice that number during natural slow-wave sleep is sufficient to induce a transition from the active to the silent states of the slow oscillation. The silent state is a key signature of slow-wave sleep, occupying ∼20% of the time (Chauvette et al. 2011) during slow oscillation. Silent states allow the replenishment of extracellular calcium (Massimini and Amzica 2001) or may simply give the cortical and thalamic neurons a metabolic break (Vyazovskiy and Harris 2013). The silent state also provides the ideal conditions for massive entries of calcium in neurons at the beginning of the next active states with a high synaptic efficacy (Crochet et al. 2005). This high calcium influx is essential to intracellular pathways involved in the long-term potentiation of synapses (Chauvette et al. 2012; Lamprecht and LeDoux 2004). A possible role of the silent state could be to pave the way to memory consolidation.
GRANTS
This work was supported by the Canadian Institutes of Health Research, National Institutes of Health, Natural Sciences and Engineering Research Council of Canada, and Fonds de la Recherche en Santé du Québec.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L. and I.T. conception and design of research; M.L., S.C., and I.T. performed experiments; M.L., S.C., and I.T. analyzed data; M.L. and I.T. interpreted results of experiments; M.L., S.C., and I.T. prepared figures; M.L. drafted manuscript; M.L., S.C., and I.T. edited and revised manuscript; M.L., S.C., and I.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Ftomov for technical support and J. Seigneur for logistic support.
REFERENCES
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79: 565–575, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci 35: 345–355, 2012. [DOI] [PubMed] [Google Scholar]
- Beltramo R, D'Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat Neurosci 16: 227–234, 2013. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51: 483–495, 1981. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex 20: 2660–2674, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron 75: 1105–1113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of slow oscillation during slow-wave sleep and anesthesia in cats. J Neurosci 31: 14998–15008, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Chauvette S, Skorheim S, Timofeev I, Bazhenov M. Interneuron-mediated inhibition synchronizes neuronal activity during slow oscillation. J Physiol 590: 3987–4010, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol 406: 443–468, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol 494: 251–264, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci 21: 1030–1044, 2005. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 13: 9–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RC, Leresche N, Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J Neurosci 33: 19599–19610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Smith AD, Somogyi P. Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axoaxonic cells and of presumed basket cells in synaptic contact with pyramidal neurons of the cat's visual cortex. J Comp Neurol 221: 263–278, 1983. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci 1: 587–594, 1998. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402: 72–75, 1999. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Kelly JP. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol 163: 81–105, 1975. [DOI] [PubMed] [Google Scholar]
- Gu Q, Perez-Velazquez JL, Angelides KJ, Cynader MS. Immunocytochemical study of GABAA receptors in the cat visual cortex. J Comp Neurol 333: 94–108, 1993. [DOI] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature 493: 97–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47: 423–435, 2005. [DOI] [PubMed] [Google Scholar]
- Hassler R, Muhs-Clement K. [Architectonic construction of the sensomotor and parietal cortex in the cat.] J Hirnforsch 6: 377–422, 1964. [PubMed] [Google Scholar]
- Hendry SH, Fuchs J, deBlas AL, Jones EG. Distribution and plasticity of immunocytochemically localized GABAA receptors in adult monkey visual cortex. J Neurosci 10: 2438–2450, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci 11: 1800–1809, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JC, Fourment A, Marc ME. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res 259: 308–312, 1983. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One 2: e276, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron 33: 947–958, 2002. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Trojanowski JQ. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res 74: 149–155, 1974. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci 5: 45–54, 2004. [DOI] [PubMed] [Google Scholar]
- Lemieux M, Chen JY, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci 34: 5689–5703, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche N, Hering J, Lambert RC. Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential. J Neurosci 24: 5592–5602, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. Modulation of a pacemaker current through Ca2+-induced stimulation of cAMP production. Nat Neurosci 2: 634–641, 1999. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58: 251–266, 1987. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci 29: 7513–7518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J Neurophysiol 85: 1346–1350, 2001. [DOI] [PubMed] [Google Scholar]
- Massimini M, Tononi G, Huber R. Slow waves, synaptic plasticity and information processing: insights from transcranial magnetic stimulation and high-density EEG experiments. Eur J Neurosci 29: 1761–1770, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison RS, Dempsey EW. A study of thalamo-cortical relations. Am J Physiol 135: 281–292, 1942. [Google Scholar]
- Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical up states. Proc Natl Acad Sci USA 105: 8428–8433, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci 27: 5280–5290, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64: 404–418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Mattia M, Compte A, Perez-Zabalza M, Winograd M, Descalzo VF, Reig R. Inhibitory modulation of cortical up states. J Neurophysiol 104: 1314–1324, 2010. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev 26: 113–135, 1998. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res Rev 8: 1–63, 1984. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum, 2005. [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993a. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillations and other sleep rhythms of electroencephalogram. J Neurosci 13: 3266–3283, 1993c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Dossi RC, Nuñez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamo-cortical neurons: Scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci 13: 3284–3299, 1993d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Influence of VPM afferents on putative inhibitory interneurons in S1 of the awake rabbit: evidence from cross-correlation, microstimulation, and latencies to peripheral sensory stimulation. J Neurophysiol 73: 1584–1599, 1995. [DOI] [PubMed] [Google Scholar]
- Tamás G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol 500: 715–738, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol 76: 4152–4168, 1996. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Chauvette S. The spindles: are they still thalamic? Sleep 36: 825–826, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Contreras D, Steriade M. Synaptic responsiveness of cortical and thalamic neurones during various phases of slow sleep oscillation in cat. J Physiol 494: 265–278, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci USA 98: 1924–1929, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Contribution of intrinsic neuronal factors in the generation of cortically driven electrographic seizures. J Neurophysiol 92: 1133–1143, 2004. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex 10: 1185–1199, 2000. [DOI] [PubMed] [Google Scholar]
- Tucker TR, Katz LC. Recruitment of local inhibitory networks by horizontal connections in layer 2/3 of ferret visual cortex. J Neurophysiol 89: 501–512, 2003. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave sleep. J Neurosci 26: 5665–5672, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci 14: 443–451, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol 101: 1921–1931, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng TW, O'Brien TJ, Kulikova SP, Reid CA, Morris MJ, Pinault D. Acute effect of carbamazepine on corticothalamic 5–9-Hz and thalamocortical spindle (10–16-Hz) oscillations in the rat. Eur J Neurosci 39: 788–799, 2014. [DOI] [PubMed] [Google Scholar]