Abstract
The spheroids of 3-dimensional culture and Rho-associated kinase (ROCK) inhibitor Y-27632 have shown many advantages for the promotion of cellular viability and proliferation. The objective of this study was to investigate the effect of Y-27632 on the growth and injectability of bovine corneal endothelial cells (B-CECs) maintained in vitro as spheroid cultures. Immunofluorescence staining showed that Y-27632 did not alter the cell type specificity of B-CECs, but it significantly enhanced B-CEC spherical viability and proliferation by a Live/Dead assay, 5-ethynyl-2′-deoxyuridine (EdU) labeling assay, and Cell Counting Kit-8 (CCK-8) assay. The uniform B-CEC spheroids could easily form in multiwall agarose micromolds and had a higher stemness potential than single B-CECs. Injectable B-CEC spheroids were able to form monolayer growth, and polygonal B-CECs completely covered culture plates or Descemet's membrane of decellularized corneas under inverted microscopy and scanning electron microscopy (SEM). B-CEC spheroids were generated from agarose microwells on day 1 and then adherent culture with Y-27632 for day 5. However, small B-CEC spheroids still existed on culture plates or decellularized corneas when B-CEC spheroids were cultured in the same condition except for absence of Y-27632. Our findings that CEC spheroids with Y-27632 are injectable in vitro have important implications for the favorable treatment of CEC deficiency.
Introduction
Corneal endothelial cells (CECs) form a homogeneous single layer of flat hexagonal cells that attaches to the Descemet's membrane of the cornea. CECs are essential for maintaining corneal transparency, which is dependent upon the barrier and pump functions of CECs (Bi et al., 2013). However, the density of human CECs often decreases with increased age or in various diseases, such as intraocular surgery, glaucoma, and endothelium decompensation (Bourne et al., 2003). Endothelial keratoplasty may provide a significantly better visual outcome. Yet some complications, such as graft dislocation and primary graft failure, can occur. At the same time, the shortage of good-quality donors for endothelial keratoplasty also becomes the major limiting factor in the world. The future of endothelial keratoplasty may well entail the use of cultured endothelial cells (Anshu et al., 2012). However, the number of CECs with proliferative activity and ability is relatively low. Much variation in morphology and function exists after several cell passages (Gao et al., 2011; Jäckel et al., 2011; Peh et al., 2011). Therefore, there is the need for a stable and effective culture system to maximize cell proliferation and maintain the physiological function of CECs.
Y-27632 is a protein kinase inhibitor selective for Rho-associated kinase (ROCK). It involves various cellular functions that include actin cytoskeleton organization, cell adhesion, cell motility, and anti-apoptosis (Takahara et al., 2003). Zhang and co-workers (2011) reported that Y-27632 increased cloning efficiency of murine prostate basal epithelial cells. The increased cloning efficiency was due to the suppression of the dissociation-induced RhoA/ROCK activation-mediated apoptosis of prostate stem cells (Zhang et al., 2011). In our previous study, we successfully cultured bovine (B) CECs in vitro and immunofluorescence staining showed Na+/K+-ATPase, ZO-1, and AQP1 protein expression in cultivated B-CECs. Y-27632 significantly enhanced the adhesion and migration of B-CECs. B-CECs treated with Y-27632 exhibited more vigorous growth and a more spread morphology. Y-27632 was a potentially powerful reagent that was able to enhance the proliferation of cultured B-CECs (Li et al., 2013). In this study, we further investigated the effect of Y-27632 on the growth and injectability of B-CECs maintained in vitro as spheroid cultures. The goal of this study was to understand if Y-27632 could also be an appropriate reagent for cultivated B-CEC spheroids and their injectability in vitro. Our study will have helpful implications for future CEC engraftment in vivo.
Materials and methods
Ethics statement
The bovine eyes were obtained at a local slaughterhouse (Shipai, Guangzhou, China). The corneas were checked by slit lamp examination to be free of defects.
Materials
Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA, and Live/Dead assay kit were purchased from Invitrogen (Grand Island, NY, USA). The Cell Counting Kit-8 (CCK-8) was from Dojindo (Kyushu, Japan). AlexaFluor 488-labeled goat anti-rabbit immunoglobulin G (IgG) secondary antibody was from Beyotime (Jiangsu, China). Rabbit polyclonal anti-AQP1 antibody, rabbit polyclonal anti-ZO-1 antibody, and rabbit polyclonal anti-Na+/K+-ATPase antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Y-27632 was obtained from Sigma-Aldrich (St. Louis, MO, USA). The 5-ethynyl-2′-deoxyuridine (EdU) labeling/detection kit was purchased from Ribobio (Guangzhou, China). The 81-well rubber micromolds were purchased from MicroTissues Inc. (Providence, RI, USA).
The isolation of B-CECs and preparation of decellularized cornea
B-CECs were cultivated as described previously (Li et al., 2013) with some modifications. Briefly, the corneal explants were washed three times with ice-cold phosphate-buffered saline (PBS) containing 2% penicillin-streptomycin and 50 μg/mL gentamicin. The Descemet's membrane was stripped from the posterior surface of the corneal tissue with sterile surgical forceps under a dissecting microscope. The strips were incubated in trypsin at 37°C for 8–10 min. The cells were then centrifuged (300×g, 5 min) and placed on a gelatin-coated six-well dish containing low-glucose (LG) DMEM supplemented with 10% FBS and 1% penicillin-streptomycin in a humidified incubator at 37°C containing 5% CO2. Fresh culture medium was replenished every 2 days. When B-CECs were grown to form a confluent monolayer (5–7 days after plating), subsequent subcultures were propagated in the same medium. The first- and third-passage cells were used as indicated for all experiments
The preparation of decellularized cornea was as follows: Fresh bovine eyes were obtained, and the corneas were excised, rinsed with saline containing antibiotic solution (prepared with 100 U/mL penicillin G sodium and 100 mg/mL streptomycin sulfate), and dissected under sterile conditions. Bovine posterior stromal lamella (1 mm thick) including Descemet's membrane were treated with 0.5% Triton X-100 and 20 mM NH4OH mixture for 5–10 min. After rinsing with PBS three times, the lamellae were frozen at −80°C for 3 days and then preserved in 100% glycerol at 4°C. Prior to use, the dehydrated bovine stroma lamellae were rehydrated in PBS. The stroma lamellae were cut into pieces and sterilized under ultraviolet light for 30 min.
Generation of B-CEC spheroids in agarose micromolds
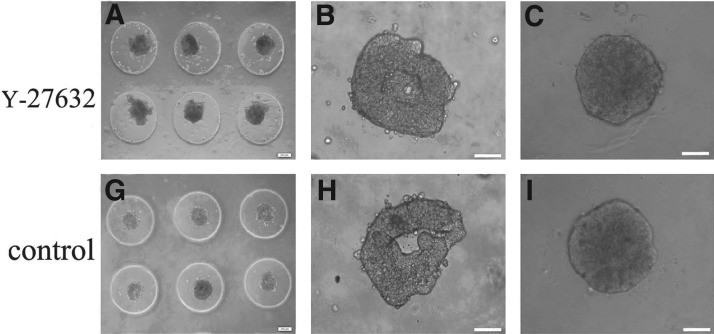
The rubber micromolds (Fig. 1I) were sterilized with absolute ethanol and allowed to dry in a sterile environment. Agarose powder was autoclaved, cooled, and mixed with LG- DMEM. The solution was microwaved to boiling until the agarose was completely dissolved. When the solution was still warm and liquid, 500 μL of 2% agarose were pipetted into each mold (Fig. 1II). After the agarose gelled, the micromold was carefully flexed to remove the agarose dish (Fig. 1III). The agarose dish was transferred to a 12-well tissue culture plate. Approximately 81,000 cells, (1000±cells per microwell) were carefully layered into the microwells of the agarose dish (Fig. 1IV). The tissue culture plate was placed in the incubator, and the medium surrounding the agarose dish was exchanged as needed. The cells aggregated and self-assembled, with one spheroid forming in one well of microwells. B-CEC spheroids were harvested by flushing spheroids out of the microwells with a pipette and placing them into adherent culture. The growth of B-CEC spheroids was observed under an inverted microscope.
FIG. 1.
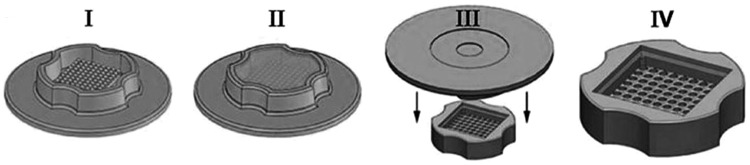
Schematic of agarose micromold casting system. A flexible, reusable rubber micromold with protrusions approximately 800 μm in diameter (I) was filled with liquid agarose (II). After cooling, the mold was flexed (III) to generate a multiwell agarose culture dish (IV).
To examine the gene and phenotype expression of B-CEC spheroids, the experiment was divided into six groups as follows: B-CECs cultured with Y-27632 on day 5 as group A and those without Y-27632 as group B. B-CEC spheroids generated from agarose microwells on day 2 and then adherent culture with Y-27632 on culture plates for day 3 as group C, and those without Y-27632 as group D. B-CEC spheroids generated from agarose microwells on day 2 and then adherent culture with Y-27632 on decellularized bovine corneas for day 3 were group E, and those without Y-27632 were group F.
Immunofluorescence assay
Immunofluorescence assay was used to examine the protein expression of AQP1, ZO-1, Na+/K+-ATPase, and nestin in B-CECs. Cells were treated with 10 μM Y-27632 before the immunofluorescence assay was performed. Cells not treated with Y-27632 were the control. Briefly, after fixation in 4% paraformaldehyde for 30 min at room temperature, cells were washed three times with PBS and incubated with PBS containing 5% FBS for 30 min at room temperature. The cells were incubated with rabbit polyclonal anti-APQ1 antibody (1:200), rabbit polyclonal anti Na+/K+-ATPase antibody (1:200), rabbit polyclonal anti ZO-1 antibody (1:200), and mouse monoclonal anti-nestin antibody (1:200) overnight at 4°C, and then with the secondary antibodies for 60 min before staining with 4′,6-diamidino-2-phenylindole (DAPI). Samples were then visualized and photographed under fluorescence microscope.
Cell viability assay
The viability of B-CEC spheroids treated with or without 10 μM Y-27632 on day 2 was evaluated using the Live/Dead assay in accordance with manufacturer's protocol. The kit includes green fluorescence of calcein acetoxymethyl ester (Calcein AM) stain for live cells and red fluorescence of ethidium homodimer III (EthD-III) stain for dead and damaged cells. Each well of 48-well culture plates included two B-CEC spheroids incubated with Live/Dead working solution (4 μM EthD-III and 2 μM Calcein AM in PBS) for 30 min at 37°C in the dark. This was followed by three wash steps in PBS. Samples were then visualized and photographed under a fluorescence microscope. The percentages of dead cells were calculated from three random fields in three wells using ImageJ, and the data were processed with SPSS. A t-test was used for comparing the mean of the groups.
EdU labeling assay
B-CEC spheroids treated with or without 10 μM Y-27632 on day 2 were plated in triplicate in 48-well plates and incubated overnight. According to the manual of the EdU labeling/detection kit, 50 μM EdU labeling medium was added to the cell culture to allow incubation for 2 h at 37°C under 5% CO2. Cultured B-CEC spheroids were fixed with 4% paraformaldehyde (pH 7.4) for 30 min and incubated with glycine for 5 min. After a wash with PBS, staining with anti-EdU working solution was performed at room temperature for 30 min. Following a wash with 0.5% Triton X-100 in PBS, the cells were incubated with 5 μg/mL Hoechst 33342 dye at room temperature for 30 min. Samples were then visualized and photographed under a fluorescence microscope. The percentages of EdU-positive cells were calculated from five random fields in three wells.
CCK-8 assay
A CCK-8 was employed to identify the effect of Y-27632 on the proliferation of B-CECs. Briefly, B-CEC spheroids treated with or without 10 μM Y-27632 on day 2 were dissociated into single cells by a 5-min trypsin treatment. Then B-CECs cultured in 96-well plates at 1×104 cells/well and incubated at 37°C for 24 h. Subsequently, B-CECs were treated with or without Y-27632, in the presence of 10% FBS for a further 48 h. After 10 μL CCK-8 dyes were added to each well, cells were incubated at 37°C for 2 h. The absorbance at 450 nm was determined using a multimode reader. Six parallel experiments in each sample were used to assess the cell proliferation. The data were processed with SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL, USA). A t-test was used for comparing the mean of the groups.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA from B-CECs or B-CEC spheroids was isolated using a Tissue RNA Miniprep Kit, and the resulting RNA samples were quantified by measuring the optical density (OD) at 260 nm. OD 260/280 ratios for all RNA samples were between 1.8 and 2.1. Total RNA (1 μg) was reverse transcribed in a 10-μL reaction mixture containing 2 μL of 5× Reverse Transcriptase (RT) Buffer, 0.5 μL RT Enzyme Mix, 0.5 μL Primer Mix, and 6 μL nuclease-free water at 42°C for 1 h. One-tenth of the RT product was used for subsequent PCR, with the final concentration of PCR reaction being 1× Buffer, 0.2 mM deoxynucleotides (dNTPs), and 1.25 U Blend Taq® in a total volume of 50 μL, using the primers shown in Table 1. The PCR mixture was first denatured at 94°C for 2 min then amplified for 30 cycles [94°C, 30 sec; Tm (melting temperature) 58°C, 30 sec; 72°C, 1 min] using an authorized thermal cycler (Eppendorf, Hamburg, Germany). After amplification, 5 μL of each PCR product and 1 μL of 6× loading buffer were mixed and electrophoresed on a 1.5% agarose gel in 0.5× Tris-boric acid-EDTA containing 0.5 μg/mL ethidium bromide. Gels were photographed and scanned.
Table 1.
List of Primers
| Primers | Sequences (5′ to 3′) | Size (bp) | |
|---|---|---|---|
| β-actin | Sense | CTCTTCCAGCCTTCCTTCCT | 178 |
| β-actin | Antisense | GGGCAGTGATCTCTTTCTGC | |
| Na+/K+-ATPase | Sense | TGAGCCGAGGCTTAACAACT | 247 |
| Na+/K+-ATPase | Antisense | ATGACGACAGCAGACAGCAC | |
| Nestin | Sense | AGGACCCTCCTAGAGGCTGA | 169 |
| Nestin | Antisense | GTGAGGAGAGGGGAGTAGGG |
Injectability of B-CEC spheroids oton culture plates
To test whether B-CEC spheroids could maintain the structure and cell viability after injection for transplantation, an in vitro simulation experiment of injectability of B-CEC spheroids with or without Y-27632 onto culture plates was performed. One B-CEC spheroid was injected into the well of six-well culture plates with or without Y-27632 through the tip of a 200-mL pipette. Cellular morphology and growth after injection was observed under inverted microscopy each day. The adherent growing areas from the B-CEC spheroid periphery were measured using the ImageJ program on days 1, 2, and 3, respectively. Three parallel experiments in each group were used.
Injectability of B-CEC spheroids on decellularized cornea
To test whether B-CEC spheroids with or without Y-27632 on a biomimetic microenvironment could grow well, an in vitro simulation experiment of injectability of B-CEC spheroids onto decellularized Descemet's membrane of bovine corneal stroma was performed. One B-CEC spheroid was injected onto a thin piece of decellularized cornea in the wells of six-well culture plates with or without Y-27632 through the tip of a 200-mL pipette. Viable cell staining of Calcein AM was used for better observation of B-CEC spheroids on decellularized cornea under a fluorescence microscope. The adherent growing areas from the B-CEC spheroid periphery stained by Calcein AM were measured by the ImageJ program on days 2 and 3, respectively. Three parallel experiments in each group were used.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to observe the ultrastructure of the surface of the growing morphology of B-CEC spheroid on the decellularized cornea. Samples were fixed in 2.5% glutaraldehyde, washed three times for 30 min each time in 0.1 M PBS, and then postfixed in 1% osmium tetroxide for 30 min. Samples were washed three times again in PBS before passing through a graded series of alcohol (50%, 70%, 80%, 90%, and 100%). After three 5-min changes of 100% ethanol, the samples were then transferred to isoamyl acetate for 30 min, critical point dried, coated with gold, and mounted for viewing in the ULTRA 55 SEM (Zeiss, Germany).
Statistical analysis
The values were expressed as means±standard deviation (SD) from three to six samples. Statistical analyses were carried out using Student's t-test and a one-way analysis of variance (SPSS, Inc., Chicago, IL, USA). Results of *p<0.05 were considered statistically significant.
Results
Morphological characteristics and phenotype expression of B-CECs
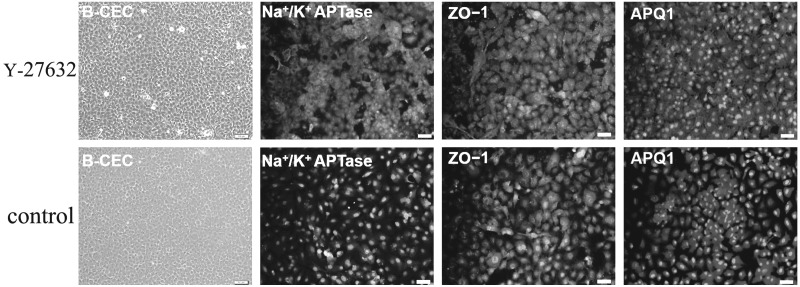
B-CECs were successfully isolated from Descemet's membrane of bovine corneas after trypsin-EDTA digestion. In the primary culture, the B-CEC monolayer reached confluence within 5–7 days. The typical hexagonal morphology was exhibited in confluent B-CECs from primary culture to passage 2. Immunofluorescence staining showed Na+/K+-ATPase, ZO-1, and AQP1 protein expression in cobblestone-shaped B-CECs at passage 2 on day 5. At the same time, confluent B-CECs treated with Y-27632 for 5 days also positively expressed AQP1, ZO-1, and Na+/K+ ATPase (Fig. 2). The result indicated that Y-27632 did not alter the cell-type specificity of confluent B-CECS. B-CECs treated with Y-27632 exhibited more vigorous growth and a more spread morphology than those without Y-27632.
FIG. 2.
The morphological characteristics and phenotype expression in confluent B-CECs. The typical hexagonal cobblestone shape was exhibited in confluent B-CECs. Immunofluorescence staining showed that the protein expression of Na+/K+-ATPase, ZO1, and AQP1 was positive not only in cultivated B-CECs (passage 2) on day 5 (upper), but also in B-CECs treated with 10 μM Y-27632 (down). Nuclei were stained with DAPI. Scale bars, 100 μm.
Effects of Y-27632 on the formation and viability of B-CEC spheroids
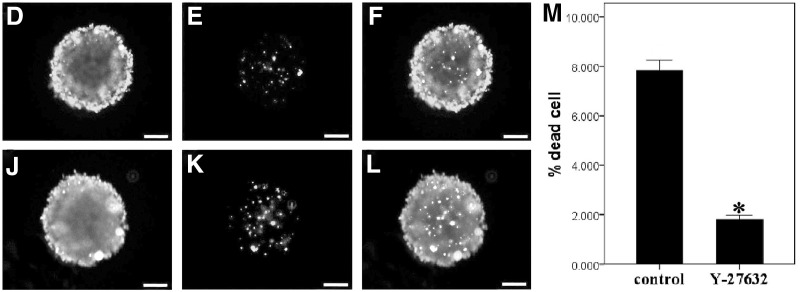
To determine whether the treatment with ROCK inhibitor would affect the generation and viability of B-CEC spheroids, B-CECs were placed in spheroid-forming culture molds in the presence or absence of 10 μM Y-27632. Primary B-CECs were seeded in multiwall agarose micromolds. Roughly preliminary spheroids (Fig. 3A, G) and hollow aggregates (Fig. 3B, H) formed on day 1 after seeding when they were treated with or without Y-27632. The 200-μm-size spherical aggregates with or without Y-27632 appeared in most of the micromolds on day 2 (Fig. 3C, I). The results revealed that Y-27632 had no apparent effect on B-CEC spheroid formation. The cell viability assay (Live/Dead assay) demonstrated that the vast majority of cells in B-CEC spheroids supplemented with or without Y-27632 were viable cells, which showed an intense green fluorescence in the live cytoplasm from the Calcein AM stain (Fig. 3D, J). However, B-CEC spheroids treated with Y-27632 (Fig. 3E) revealed less dead cells than those without Y-27632 (Fig. 3K), which showed red fluorescence in dead cell nuclei from the EthD-III stain. Dead cells were located mainly in the center of B-CEC spheroids (Fig. 3F, L). The percentages of dead cells of B-CEC spheroids in the Y-27632 group and control group were 1.80±0.16% and 7.82±0.37%, respectively (Fig. 3 M). There were significant differences between these two groups in percentages of dead cells (*p<0.05). The results showed that Y-27632 could decrease cell death inside the B-CEC spheroids.
FIG. 3.
The formation and viability of B-CEC spheroids treated with or without Y-27632. Rough preliminary B-CECs spheroids (A, G) and hollow aggregates (B, H) formed by 1 day after seeding in agarose micromolds. The uniform B-CECs spheroids with 200-μm sizes appeared on day 2 (C, I). A cell viability assay showed an intense green fluorescence in the live cytoplasm from Calcein AM stain (D, J) and red dot fluorescence in the dead cell nucleus from EthD-III stain (E, K). Dead cells were located mainly in the center of B-CEC spheroids (F, L). (M) The histogram compares the percentages of dead cells in B-CEC spheroids with or without Y-27632. (*) p<0.05 was considered statistically significant. Scale bars, 100 μm.
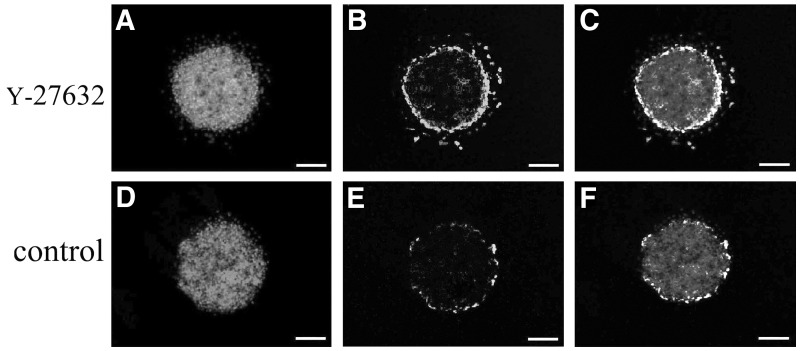
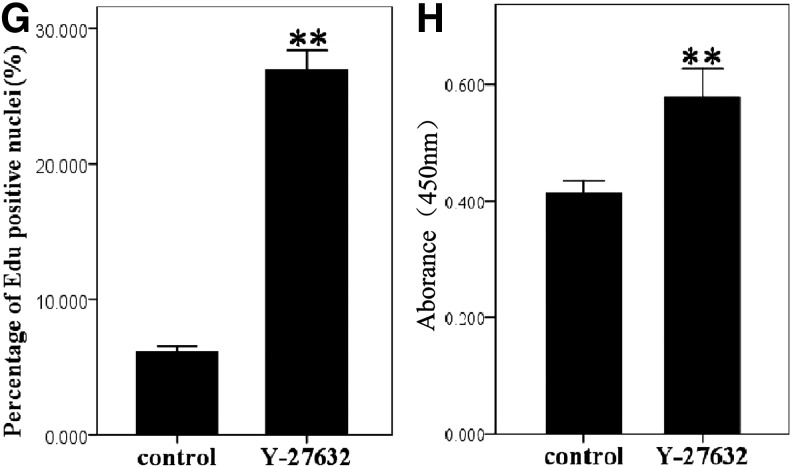
The EdU labeling assay demonstrated that the fluorescence from EdU-labeled cells was located mainly in the B-CEC spheroid periphery. B-CEC spheroids treated with Y-27632 (Fig. 4A–C) displayed more EdU-positive cells than those without Y-27632 (Fig. 4D–F) imaged under a fluorescence microscope and determined with ImageJ analysis software. The percentage of EdU-positive nuclei of B-CEC spheroids in the Y-27632 group and control group were 26.89±0.22% and 6.12±0.39%, respectively (Fig. 4G). There were significant differences between these two groups in percentages of EdU-positive nuclei (*p<0.05). The CCK-8 assay revealed that Y-27632 significantly promoted the proliferation of B-CEC spheroids (Fig. 4H). The results from EdU labeling and CCK-8 assays showed that Y-27632 significantly enhanced proliferating cells in B-CEC spheroids.
FIG. 4.
The proliferation of B-CEC spheroids treated with or without Y-27632. (A–F) EdU labeling assay in B-CEC spheroids treated with or without Y-27632. (G) The histogram compares the percentages of EdU-positive nuclei in B-CEC spheroids with or without Y-27632. (H) A CCK-8 assay showed that 10 μM Y-27632 increased the proliferation of B-CECs. (*) p<0.05 and (**) p<0.01 were considered statistically significant. Scale bars, 100 μm.
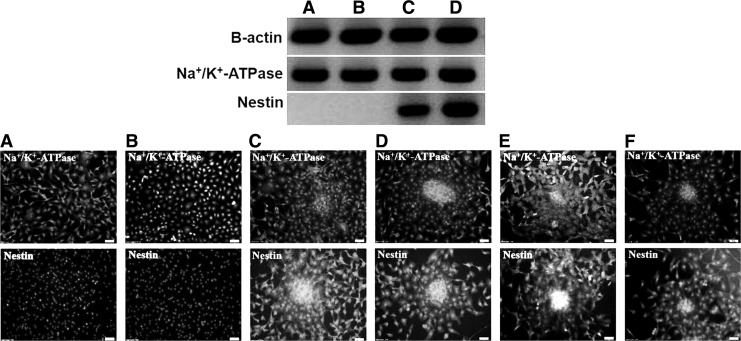
Effects of Y-27632 on the phenotype expression of B-CEC spheroids
RT-PCR analysis (Fig. 5, upper) and immunofluorescence staining (Fig. 5, lower) showed that the gene transcript and phenotype protein of Na+/K+-ATPase were positively expressed in all groups. Na+/K+-ATPase is a key B-CEC transmembrane protein, and the results demonstrated that both single B-CECs and B-CEC spheroids generated from agarose microwells on day 2 and then adherent culture for day 3 all expressed Na+/K+-ATPase. Y-27632 did not alter such CEC physiological characteristics. Furthermore, RT-PCR analysis (Fig. 5, upper) and immunofluorescence staining (Fig. 5, lower) also showed that the gene transcript and phenotype protein of nestin were negatively expressed in all dissociated single B-CECs, no matter whether treated with Y-27632 (Fig. 5A) or without Y-27632 (Fig. 5B). However, all B-CEC spheroids, no matter with or without Y-27632 on the culture plates (Fig. 5C, D) or decellularized bovine corneas (Fig. 5E, F), positively expressed nestin by RT-PCR analysis and immunofluorescence staining. Nestin is an immature cell marker, and the results revealed that the B-CEC spheroids with or without Y-27632 had a higher stemness potential than single B-CECs.
FIG. 5.
Phenotype expression and RT-PCR analysis of B-CEC spheroids treated with or without Y-27632. RT-PCR analysis (upper) and immunofluorescence staining (lower) showed that the gene transcript and phenotype protein of Na+/K+-ATPase were positively expressed in all groups. The gene transcript and phenotype protein of nestin were negatively expressed in all single B-CECs, no matter with Y-27632 (A, group A) or without Y-27632 (B, group B). All B-CEC spheroids, no matter with or without Y-27632 on culture plates (C, group C; D, group D) or decellularized bovine corneas (E, group E; F, group F), positively expressed nestin. Nuclei were stained with DAPI. Scale bars, 100 μm.
Injectability of B-CEC spheroids on culture plates
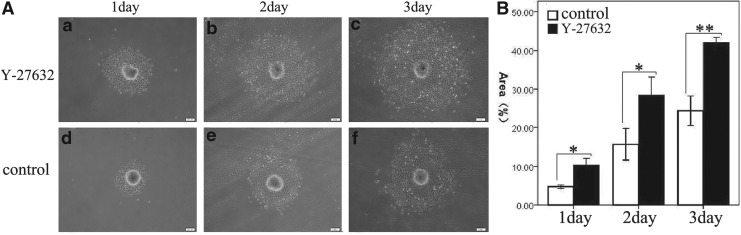
To observe B-CEC spheroids changes and determine whether B-CEC spheroids would remain viable after in vivo transplantation, an in vitro simulation experiment of injectability of B-CEC spheroids on culture plates was performed. B-CEC spheroids cultured on day 2 were collected and injected into conventional six-well culture plates by use of a micropipette. After passing through the micropipette tip, the spheroids first maintained an aggregated morphology. Soon, these spheroids displayed adherent growth and there were cells eventually repopulated as a confluent monolayer from spheroidal peripheral areas. With time, the adherent areas gradually became larger (Fig. 6A). The adherent area percentages of B-CEC spheroids in the Y-27632 group were 10.37±0.46%, 28.46±1.34%, and 41.98±0.41%, respectively on days 1, 2, and 3, whereas those in control group on culture plates were 4.73±0.13%, 15.65±1.17%, and 24.40±1.12%, respectively, on days 1, 2, and 3 (Fig. 6B). There were significant differences between these two groups in adherent area percentages on days 1 (*p<0.05), 2 (*p<0.05), and day 3 (**p<0.01). The results demonstrated that B-CEC spheroids in Y-27632 group showed better status and greater adherent areas of cell growth on the culture plates than controls.
FIG. 6.
Injectability of B-CEC spheroids on culture plates. (A) With time, the adherent areas gradually became larger and increased by the in vitro simulation experiment of injectability of B-CEC spheroids on culture plates. Y-27632 treatment obviously enhanced the adherent areas of B-CEC spheroids on days 1, 2, and 3, respectively. (B) The adherent area percentages of B-CEC spheroids in Y-27632 group were obviously larger than those in control group on days 1, 2, and 3. Three parallel experiments in each group were used and (*) p<0.05 and (**) p<0.01 were considered statistically significant. Scale bars, 200 μm.
Injectability of B-CEC spheroids on decellularized corneas
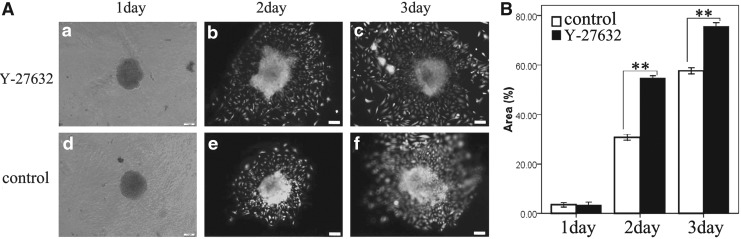
To observe the adherent growth of B-CEC spheroids on a natural microenvironment, the injectability of B-CEC spheroids on Descemet's membrane of decellularized cornea was performed. B-CEC spheroids cultured on day 2 were collected and injected onto decellularized bovine corneal stroma. The adherent areas from the B-CEC spheroid periphery were also observed to gradually become larger and increased with time by viable cell staining of Calcein AM under a fluorescence microscope (Fig. 7A). The adherent area percentages of B-CEC spheroids in the Y-27632 group and the control group on decellularized bovine corneal stroma were 54.70±0.31% and 30.72±0.35%, 75.58±0.46%, and 58.06±0.31%, respectively, by live cells staining on days 2 and 3 (Fig. 7B). There were significant differences between these two groups in adherent area percentages on day 2 (**p<0.01) and day 3 (**p<0.01). The results suggested that Y-27632 also had a positive regulatory effect on the injectability of B-CEC spheroids on Descemet's membrane of the decellularized cornea in vitro simulation experiment. B-CEC spheroids were able to maintain the structural integrity and cellular viability after being injected onto biomimetic decellularized cornea. Y-27632 could promote the migration and proliferation of B-CEC spheroids and improve cell viability on a natural microenvironment.
FIG. 7.
Injectability of B-CEC spheroid on decellularized corneas. (A) With time, the adherent areas by viable cell staining of Calcein AM gradually became larger and increased by the in vitro simulation experiment of injectability of B-CEC spheroids on decellularized cornea. Y-27632 treatment obviously enhanced the adherent areas of B-CEC spheroids on days 2 and 3, respectively. (B) The adherent area percentages of B-CEC spheroids in the Y-27632 group were obviously larger than those in control group on days 2 and 3. Three parallel experiments in each group were used and (**) p<0.01 was considered statistically significant. Scale bars, 100 μm.
Monolayer growth of injectable B-CEC spheroids
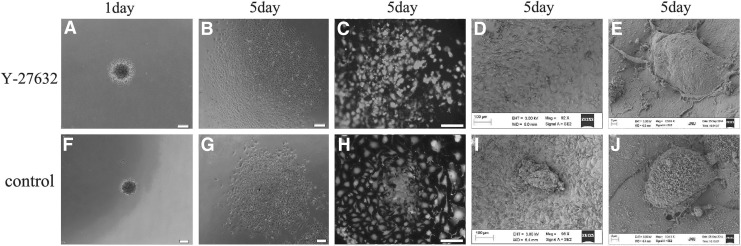
To obtain the monolayer growth of B-CEC spheroids after the in vitro simulation experiment of injectability, we decreased the period of B-CEC spheroid formation and increased the observation time. When B-CEC spheroids generated from agarose microwells on day 1 and then adherent culture with Y-27632 for day 5, B-CEC spheroids disappeared and monolayer B-CECs covered completely either on culture plates (Fig. 8A, B) or Descemet's membrane of decellularized corneas (Fig. 8C, D) as observed under an inverted microscope and SEM. However, small B-CEC spheroids still existed either on culture plates (Fig. 8F, G) or on decellularized corneas (Fig. 8H, I) when B-CEC spheroids were generated from agarose microwells on day 1 and then adherent culture without Y-27632 for day 5. High-magnification views of SEM revealed a flattened polygonal appearance of monolayer B-CECs with surface microvilli in the groups when B-CEC spheroids generated from agarose microwells on day 1 and then adherent culture for day 5 on Descemet's membrane of decellularized corneas with (Fig. 8E) or without Y-27632 (Fig. 8J).
FIG. 8.
Monolayer growth of injectable B-CEC spheroids. B-CEC spheroids generated from agarose microwells on day 1 and then adherent culture with Y-27632 for day 5. B-CEC spheroids disappeared and monolayer B-CECs covered either culture plates (A, B) or decellularized corneas (C, D), as seen under inverted microscopy and SEM, in the Y-27632 group. Small B-CEC spheroids still existed either on culture plates (F, G) or on decellularized corneas (H, I) in control group. High-magnification images of SEM revealed a polygonal appearance and surface microvilli of B-CECs from B-CEC spheroids on decellularized corneas with (E) or without Y-27632 (J). Scale bars, 100 μm.
Discussion
Corneal endothelial dysfunction associated with vision loss is a major indication for corneal transplant surgery (Darlington et al., 2006). Endothelial keratoplasty provides significantly good visual outcomes. But the lack of healthy donor corneas has limited the extensive corneal transplant surgery. In recent years, tissue-engineered cornea (TEC) has provided a new feasible way to solve this problem. For example, TEC composed of human amniotic epithelial cells and decellularized porcine cornea could be used to repair severe rabbit corneal injury. The transplanted TEC was transparent and completely inoculated into the host cornea (Luo et al., 2013). Bioengineering of corneas is being developed to meet a shortage of donor corneas, which moves toward the use of materials derived from native sources including decellularized cornea (Griffith and Harkin, 2014). The recently discovered ability of CECs to proliferate in vitro has opened the possibility of regenerating the corneal endothelium through bioengineering. Corneal endothelial tissue bioengineering using cultured CECs can be used for treating corneal endothelial defects (Sabater et al., 2013). Currently, research is aimed at identifying optimal conditions for the isolation and in vitro expansion of CECs (Numata et al., 2014; Zavala et al., 2013).
Recently, Okumura et al. showed that the transplantation of CECs in combination with Y-27632 successfully achieved the recovery of corneal transparency with a monolayer hexagonal cell phenotype at a high cell density in rabbit and primate models (Okumura et al., 2012). They also found that ROCK inhibitors Y-27632 and Y-39983 both stimulated the proliferation of both monkey CECs and human CECs. Y-39983 may be a more potent agent than Y-27632 for facilitating corneal endothelium wound healing (Okumura et al., 2014). ROCK is one of the main downstream effectors of the Ras-homologous (Rho) family of GTPases, which are involved in a number of cellular functions, including cell proliferation, apoptosis, invasion, and metastasis (Gallo et al., 2012).
In our previous study, we discovered that the ROCK inhibitor Y-27632 could promote the adherence and proliferation of bovine CECs and inhibit their apoptosis in vitro. B-CECs with fibroblastic-like appearance shape at about 60% confluence using Y-27632 for 24 h expressed Na+/K+-ATPase and AQP1 by immunofluorescence staining (Li et al., 2013). In this study, we further confirmed that B-CECs with a cobblestone shape at confluence using Y-27632 for 5 days brightly expressed Na+/K+-ATPase, ZO-1, and AQP1. The results indicated that this ROCK inhibitor apparently did not alter the cell-type specificity of B-CECs at different cell confluences. Moreover, we found that Y-27632 had no apparent effect on B-CEC spheroid formation. Yet ROCK inhibitor could decrease cell death inside B-CEC spheroids and enhance proliferating cells in the B-CEC spheroid periphery. The cellular spherical culture represents a bridge for linking cells in vitro to the tissues and organs in vivo. Spheroid culture may form distinct extracellular matrix (ECM) and establish new cell–matrix interactions to influence cell fate and improve cell activity (Garcion et al., 2004; Jensen et al., 1999; Jones et al., 1995; Song et al., 2002). Numerous studies confirmed that the aggregation state of the cells was more conducive to rebuilding and regeneration of body tissues. The exchange of biochemical and mechanical signals between cells among spherical culture was further strengthened (Abbott et al., 2003; Griffith and Swarz, 2006; Pampaloni et al., 2007).
There were many methods for generation and growth of spheroids, which included hanging drop culture, centrifugation pellet culture, and spinner or rotary dynamic culture systems (Lin and Chang, 2008). In this study, rubber micromolds for making agarose “culture dishes” were used. These micromolds allowed the simultaneous generation of hundreds of spheroids in one simple step. The microfabrication of multicellular spheroids with uniform defined size could form at the bottom of nonadhesive agarose microwells. Birenboim and co-workers reported that such agarose micromolds allowed simple and repeatable generation of aggregates of human embryonic stem cells (hESCs) that could be differentiated into neurons (Birenboim et al., 2013). We found that all B-CEC spheroids, no matter with or without Y-27632, on culture plates or decellularized bovine corneas, positively expressed the immature cell marker nestin, whereas single B-CECs cultured with or without Y-27632 negatively expressed nestin. This was consistent with the results of other researchers. It was reported that Y-27632 not only did not alter important morphological features of human CEC spheres, but also promoted their adhesion, nestin expression, and cell pump function (Bi et al., 2013).
In the 1990s, Reynolds and Weiss first cultured cells that exhibited stem cell properties as spheroids, called neurospheres, from the adult brain (Reynolds and Weiss, 1992). Thereafter, spheroid-forming assays have been widely used in stem cell isolation. Spheroid culture helps to maintain the stemness of cells. Additionally, aggregated sphere culture can induce non-stem cells to acquire stem cell properties (Pastrana et al., 2011; Su et al., 2013). Spheroids generated from human CECs were reported to contain precursors with high expression of the immature cell marker nestin. (Mimura et al., 2010; Yamagami et al., 2006; Yokoo et al., 2005).
Bayoussef and colleagues reported that contrary to single cells, aggregation promotes muscle cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. Cell aggregation could be beneficial as a parameter to test for with in vivo experimentation (Bayoussef et al., 2012). In vitro simulation experiments of injectability of spheroids could be used to determine whether spheroid microtissues remained viable after in vivo transplantation.
Huang and co-workers reported that cells from dermal papilla (DP) spheroids were able to maintain their structural integrity and cellular viability after in vitro simulation experiments of the injectability of DP spheroids on culture plates were performed (Huang et al., 2013). We confirmed similar results and further found that in vitro simulation experiments of injectability of B-CEC spheroids on biomimetic decellularized cornea also revealed high cellular viability. Y-27632 significantly enhanced proliferating cells in B-CEC spheroids. For the first time, we found that Y-27632 obviously promoted the injectability of B-CEC spheroids onto both conventional culture plates and natural decellularized corneas in vitro. Moreover, B-CEC spheroids could disappear, and monolayer B-CECs covered either culture plates or decellularized corneas as soon as B-CEC spheroids were generated from agarose microwells on day 1 and then adherent culture with Y-27632 for day 5. But small B-CEC spheroids still existed on culture plates or decellularized corneas when B-CEC spheroids were cultured in the same condition except for absence of ROCK inhibitor. The results demonstrated that Y-27632 was able to promote the monolayer growth of injectable B-CEC spheroids.
In conclusion, compared with conventional two-dimensional culture, B-CEC spheroids of three-dimensional culture had higher stemness potential in vitro. The ROCK inhibitor Y-27632 could enhance B-CEC spherical viability, proliferation, and migration. At the same time, Y-27632 did not alter important morphological features of B-CECs. CEC spheroids generated from agarose microwells could be used for scalable production of size-controllable injectable CEC spheroids. Such CEC spheroids were able to achieve monolayer growth. Injected B-CECs covered completely on Descemet's membrane of decellularized corneas after treatment with ROCK inhibitor, which will be beneficial to either CEC transplantation or pharmacological testing. Mimura and co-workers reported that CEC-derived sphere therapy was an effective treatment in a rabbit CEC deficiency model (Mimura et al., 2005). Our results demonstrated that the combination of agarose microwells and Y-27632 is a potentially powerful tool for the generation of spheroids containing precursors and their migration in vitro simulation experiment of injectability. We believe that such a combination will have important implications for future CEC therapies.
Acknowledgments
We would like to thank Shuiliang Cao and Yunfeng Shi for examining cultures with scanning electron microscopy. This work was supported by the National Natural Science Foundation of China (81371689), collaborated grant for HK-Macao-TW of Ministry of Science and Technology (2012DFH30060), and the Natural Science Foundation of Guangdong Province (S2013010013391).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Abbott A. (2003). Cell culture: Biology's new dimension. Nature 424, 870–872 [DOI] [PubMed] [Google Scholar]
- Anshu A., Price M.O., Tan D.T., and Price F.W., Jr. (2012). Endothelial keratoplasty: A revolution in evolution. Surv. Ophthalmol. 57, 236–252 [DOI] [PubMed] [Google Scholar]
- Bayoussef Z., Dixon J.E., Stolnik S., and Shakesheff K.M. (2012). Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J. Tissue Eng. Regen. Med. 6, e61–e73 [DOI] [PubMed] [Google Scholar]
- Bi Y.L., Zhou Q., Du F., Wu M.F., Xu G.T., and Sui G.Q. (2013). Regulation of functional corneal endothelial cells isolated from sphere colonies by Rho-associated protein kinase inhibitor. Exp. Ther. Med. 5, 433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birenboim R., Markus A., and Goldstein RS. (2013). Simple generation of neurons from human embryonic stem cells using agarose multiwall dishes. J. Neurosci. Methods 214, 9–14 [DOI] [PubMed] [Google Scholar]
- Bourne W.M. (2003). Biology of the corneal endothelium in health and disease. Eye (Lond) 17, 912–918 [DOI] [PubMed] [Google Scholar]
- Darlington J.K., Adrean S.D., and Schwab I.R. (2006). Trends of penetrating keratoplasty in the United States from 1980 to 2004. Ophthalmology 113, 2171–2175 [DOI] [PubMed] [Google Scholar]
- Gallo R.M., Khan M.A., Shi J., Kapur R., Wei L., Bailey J.C., Liu J., and Brutkiewicz R.R. (2012). Regulation of the actin cytoskeleton by rho kinase controls antigen presentation by CD1d. J. Immunol. 189, 1689–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhou Q., Qu M., Yang L., Wang Y., and Shi W. (2011). In vitro culture of human fetal corneal endothelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 249, 663–669 [DOI] [PubMed] [Google Scholar]
- Garcion E., Halilagic A., and Faissner A. (2004). Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131, 3423–3432 [DOI] [PubMed] [Google Scholar]
- Griffith L.G., and Swartz M.A. (2006). Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211–224 [DOI] [PubMed] [Google Scholar]
- Griffith M., and Harkin D.G. (2014). Recent advances in the design of artificial corneas. Curr. Opin. Ophthalmol. 25, 240–247 [DOI] [PubMed] [Google Scholar]
- Huang Y.C., Chan C.C., Lin W.T., Chiu H.Y., Tsai R.Y., Tsai T.H., Chan J.Y., and Lin S.J. (2013). Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials 34, 442–451 [DOI] [PubMed] [Google Scholar]
- Jäckel T., Knels L., Valtink M., Funk R.H., and Engelmann K. (2011). Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br. J. Ophthalmol. 95, 123–130 [DOI] [PubMed] [Google Scholar]
- Jensen U.B., Lowell S., and Watt F.M. (1999). The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: A new view based on whole-mount labelling and lineage analysis. Development 126, 2409–2418 [DOI] [PubMed] [Google Scholar]
- Jones P.H., Harper S., and Watt F.M. (1995). Stem cell patterning and fate in human epidermis. Cell 80, 83–93 [DOI] [PubMed] [Google Scholar]
- Li S., Wang C., Dai Y., Yang Y., Pan H., Zhong J., and Chen J. (2013). The stimulatory effect of ROCK inhibitor on bovine corneal endothelial cells. Tissue Cell 45, 387–396 [DOI] [PubMed] [Google Scholar]
- Lin R.Z., and Chang H.Y. (2008). Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 3, 1172–1184 [DOI] [PubMed] [Google Scholar]
- Luo H., Lu Y., Wu T., Zhang M., Zhang Y., and Jin Y. (2013). Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 34, 6748–6759 [DOI] [PubMed] [Google Scholar]
- Mimura T., Yamagami S., Yokoo S., Yanagi Y., Usui T., Ono K., Araie M., and Amano S. (2005). Sphere therapy for corneal endothelium deficiency in a rabbit model. Invest. Ophthalmol. Vis. Sci. 46, 3128–3135 [DOI] [PubMed] [Google Scholar]
- Mimura T., Yamagami S., Yokoo S., Usui T, and Amano S. (2010). Selective isolation of young cells from human corneal endothelium by the sphere-forming assay. Tissue Eng. Part C Methods. 16, 803–812 [DOI] [PubMed] [Google Scholar]
- Numata R., Okumura N., Nakahara M., Ueno M., Kinoshita S., Kanematsu D., Kanemura Y., Sasai Y., and Koizumi N. (2014). Cultivation of corneal endothelial cells on a pericellular matrix prepared from human decidua-derived mesenchymal cells. PLoS One 9, e88169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N., Koizumi N., Ueno M., Sakamoto Y., Takahashi H., Tsuchiya H., Hamuro J., and Kinoshita S. (2012). ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 181, 268–277 [DOI] [PubMed] [Google Scholar]
- Okumura N., Nakano S., Kay E.P., Numata R., Ota A., Sowa Y., Sakai T., Ueno M., Kinoshita S., and Koizumi N. (2014). Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest. Ophthalmol. Vis. Sci. 55, 318–329 [DOI] [PubMed] [Google Scholar]
- Pampaloni F., Reynaud E.G., and Stelzer E.H. (2007). The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 [DOI] [PubMed] [Google Scholar]
- Pastrana E., Silva-Vargas V., and Doetsch F. (2011). Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8, 486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh G.S., Beuerman R.W., Colman A., Tan D.T., and Mehta J.S. (2011). Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 91, 811–819 [DOI] [PubMed] [Google Scholar]
- Reynolds B.A., and Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 [DOI] [PubMed] [Google Scholar]
- Sabater A.L., Guarnieri A., Espana E.M., Li W., Prósper F., and Moreno-Montañés J. (2013). Strategies of human corneal endothelial tissue regeneration. Regen. Med. 8, 183–195 [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C.H., Doan C., and Xie T. (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855e7. [DOI] [PubMed] [Google Scholar]
- Su G., Zhao Y., Wei J., Han J., Chen L., Xiao Z., Chen B., and Dai J. (2013). The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials 34, 3215–3222 [DOI] [PubMed] [Google Scholar]
- Takahara A., Sugiyama A., Satoh Y., Yoneyama M., and Hashimoto K. (2003). Cardiovascular effects of Y-27632, a selective Rho-associated kinase inhibitor, assessed in the halothane-anesthetized canine model. Eur. J. Pharmacol. 460, 51–57 [DOI] [PubMed] [Google Scholar]
- Yamagami S., Mimura T., Yokoo S., Takato T., and Amano S. (2006). Isolation of human corneal endothelial cell precursors and construction of cell sheets by precursors. Cornea 25, S90–S92 [DOI] [PubMed] [Google Scholar]
- Yokoo S., Yamagami S., Yanagi Y., Uchida S., Mimura T., Usui T., and Amano S. (2005). Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest. Ophthalmol. Vis. Sci. 46, 1626–1631 [DOI] [PubMed] [Google Scholar]
- Zavala J., López Jaime G.R., Rodríguez Barrientos C.A., and Valdez-Garcia J. (2013). Corneal endothelium: Developmental strategies for regeneration. Eye (Lond) 27, 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Valdez J.M., Zhang B., Wei L., Chang J., and Xin L. (2011). ROCK inhibitor Y-27632 suppresses dissociation-induced apoptosis of murine prostate stem/progenitor cells and increases their cloning efficiency. PLoS One 6, e18271. [DOI] [PMC free article] [PubMed] [Google Scholar]