Abstract
Semantic priming is affected by the degree of association and how readily a word is imagined. In the association effect, activity in the perisylvian structures including the bilateral inferior frontal gyrus, the left middle temporal gyrus, and the supramarginal gyrus was correlated. However, little is known about the brain regions related to the effect of imagery word under the preconscious condition. Forty word pairs for high (HA)‐, low (LA)‐, and nonassociation (NA), nonword (NW) conditions were presented. Each 40 association word pairs (HA and LA) included 20 high (HI) and 20 low (LI) imagery prime stimuli, using a visually presented lexical decision task. A trial consisted of 30 ms prime, 30 ms mask, 500 ms probe, and 2–8 s stimulus onset asynchrony. Brain activation was measured using functional magnetic resonance imaging during word discrimination. Behavioral data indicated that the shortest response time (RT) was given for HA words, followed by LA and NA, and NW showed the longest RT (P < 0.01). RT was faster in HI than LI within HA, but not LA conditions (P < 0.01). Functional neuroimaging showed that differential brain regions for high imagery (HI) and low imagery (LI) words within low prime‐target word association were observed in the left precuneus, left posterior cingulate gyrus, and right cuneal cortex. The present findings demonstrate that the effect of the degree of imagery on semantic priming occurs during the early stage of language processing, indicating an “automatic imagery priming effect.” Our paradigm may be useful to explore semantic deficit related to imagery in various psychiatric disorders. Hum Brain Mapp 35:4795–4804, 2014. © 2014 The Authors. Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: imagery, association, semantics, precuneus, implicit processing
INTRODUCTION
The recognition and processing of word meanings is a central part of our everyday life and is especially important for learning, reasoning, and remembering. Impairment of these functions is related to brain disorders such as Alzheimer's, semantic dementia, schizophrenia, and autism [e.g., Laisney et al., 2011; Perri et al., 2011; Shen et al., 2012; Vistoli et al., 2011]. Previous studies reported that abnormalities in the semantic network were found in patients with schizophrenia, but not in controls [Jeong and Kubicki, 2010; Jeong et al., 2009]. Research into the semantic priming effect, focusing on the associative relationship between words, has contributed significantly to our understanding of the underlying neural mechanisms of semantic representations and word processing. This semantic priming effect is known to be influenced by word association and imagery. While brain areas related to word association have been well documented, those linked to word imagery have yet to be identified.
The semantic priming effect refers to the promoting effect observed in response to a target word when it is preceded by a semantically related word, compared with a semantically unrelated word [Meyer and Schvaneveldt, 1971]. Lexical activation tends to vary as a function of the degree of association between two words [Nelson et al., 1993]. Previous studies using functional magnetic resonance imaging (fMRI) have found that the activity in the anterior medial temporal cortex and left inferior frontal gyrus was increased as the association between word pairs decreased, and the activity in the left supramarginal gyrus at the temporal‐parietal junction was larger during the presentation of related word‐pairs [Fletcher et al., 2000; Rossell et al., 2003; Wagner et al., 2001]. When presenting related, unrelated, and nonword (NW) prime stimuli using an auditory lexical decision task, the left superior temporal gyrus, left and right middle temporal gyrus, and right caudate were less activated in related word conditions, compared to unrelated conditions [Rissman et al., 2003]. Based on these findings, Wible et al. [2006] further differentiated the degree of semantic relatedness and the connectivity between word pairs by comparing high connectivity word pairs, low connectivity word pairs, unrelated word pairs, and NW pairs using a semantic priming lexical decision task. The authors reported that the fastest reaction time was observed in high connectivity words pairs, followed by low connectivity and then by unrelated word pairs. The decrease in activity in the lateral superior and middle temporal regions was also observed as the semantic priming of word pairs increased (related high connectivity, related low connectivity, and unrelated). However, this study used spoken words in a conscious condition [the stimulus onset asynchrony (SOA): 750 ms]. It was anticipated that it would be worthwhile to investigate whether similar results would be observed when using a visually presented lexical decision task under preconscious conditions. This study thus seeks to extend the results of Wible et al. [2006] to a visually presented lexical decision task in a short‐SOA condition (30 ms).
Imagery of a word might yield a promoting effect on semantic priming, compared with nonimagery conditions. This is referred to as the imagery effect [Nittono et al., 2002; Paivio, 1991]. Indirect evidence on word imagery of the word can be found in studies using concrete versus abstract words. According to the concreteness effect, concrete words are more easily and quickly remembered and recognized than abstract words due to the difference in image ability between concrete and abstract words [Kroll and Merves, 1986]. For example, pencil is easier to visualize than happiness. Evidence on neural activity related to mental imagery includes brain areas involving the sensory‐motor processing cortex [Borst and Kosslyn, 2008; Kaas et al., 2010; Klein et al., 2004; Kosslyn et al., 2000; Simmons et al., 2007; Slotnick et al., 2005]. This observation has been explained by a dual coding model in which words are processed by information stored in both a linguistic semantic system and a nonverbal imagistic semantic system [Paivio, 1986]. According to this model, abstract words depend solely on information from the linguistic semantic system, whereas concrete words are influenced by both linguistic and imagistic semantic systems. Concrete words thus have processing advantages over abstract words since they have access to information from multiple systems [Paivio, 1991; Paivio and Sadoski, 2011]. Neuroimaging evidence supporting this model has shown that concrete words evoke brain activity in both hemispheres, whereas abstract words are engaged in linguistic related areas of the left hemisphere; the right hemisphere is more responsive to concrete than abstract words, whereas the left hemisphere displays activity in response to both concrete and abstract words [Chiarello et al., 1987; Coslett and Monsul, 1994; Nittono et al., 2002]. Binder et al. [2005], for example, found that the left inferior frontal regions were activated by abstract words whereas bilateral brain regions such as the angular gyrus and the dorsal prefrontal cortex were more likely to be activated by concrete words. A recent meta‐analysis on neuroimaging evidence of abstract and concrete words [Wang et al., 2010] indicated that some studies, however, failed to provide evidence that the processing of concrete words always involves the right hemisphere [Fiebach and Friederici, 2003; Noppeney and Price, 2004] and abstract words are exclusively engaged in the left hemisphere [Jessen et al., 2000; Kiehl et al., 1999]. The findings thus far, therefore, are inconclusive.
Furthermore, prior studies have exclusively focused on investigation of the imagery effect in the context of concrete versus abstract words. Although concrete words tend to have highly imaginable characteristics, a concrete word itself does not represent an imaginable word. Furthermore, clear evidence supporting a dual coding model where concrete words consist of both linguistic and imagistic system is still indecisive. In an attempt to overcome this limitation, two studies directly compared high imagery (HI) and low imagery (LI) words using event related potentials (ERPs) [Klaver et al., 2005; Nittono et al., 2002]. However, these two studies still did not consider the other factors affecting imagery of a word. There is evidence that the imagery of a word and the association of the word are closely connected with each other. This was supported by a study showing that participants presented with HI words produced more associative words within a limited time and were faster to report the first associative word than those presented with LI words [de Groot, 1989]. This study, for the first time, aimed to investigate brain activity evoked by word imagery by employing a factorial design in which the effect of word imagery is crossed with the effect of word association [HI/high association (HA), HI/low association (LA), LI/HI, and LI/LA], using a visually presented lexical decision task under a preconscious (automatic) condition (30 ms prime). Replicating and adapting the design (high related word association, low related word association, unrelated word association, NW association) of Wible et al. [2006], this study aimed to investigate (1) brain regions related to the degree of word imagery and (2) brain regions related to the degree of word association, using visually presented lexical decision task under the preconscious condition (30 ms). More specifically, based on previous work on visual imagery [Addis et al., 2007; Choi et al., 2006; Ishai et al., 2000; Mellet et al., 2000; Sharot et al., 2007], it was predicted that: (1) an imagery word priming effect would be detected in bilateral verbal and visual imagery related networks (e.g., precuneus, posterior cingulate cortex) and (2) a semantic priming effect related to word association would be observed in the left linguistic sematic regions (e.g., left inferior frontal regions). Regarding behavioral measures, it was hypothesized that: (3) a differential imagery priming effect would be detected depending on the degree of imagery word; a faster reaction time and higher accuracy would be observed in HI word pairs, compared with LI word pairs, and (4) a differential semantic priming effect would be observed depending on the degree of word association; the fastest reaction time and the highest accuracy would be detected in HA word pairs, followed by LA word pairs and then by non‐associated word pairs.
METHODS
Participants
Fourteen healthy participants (age = 30.5 ± 6.6 years; male:female = 7:7) participated in this study. Participants were recruited through a hospital message board, from Eulji University Hospital, Daejeon, Korea. All participants were interviewed, by a psychiatrist, using DSM‐IV criteria based on the Structured Clinical Interview nonpatient Edition (SCID) [First et al., 1998]. No participants had an Axis‐I psychiatric disorder. The study was approved by the Eulji University Hospital's Institutional Review Board. All participants were given written informed consent prior to participation in the study.
Stimuli
The word stimuli used in this study were chosen from those presented in a study by Park [2004] study. Park [2004] reported 665 Korean nouns, ranked in terms of word association and word imagery. In this study, word association frequencies were obtained by multiple‐response free association; word imagery values were obtained using a 7‐point Likert scale. For this study, the HA condition included 40 word pairs that scored higher than 50 in word association frequency whereas the LA condition included 40 word pairs that scored under 20 in word association frequency. The forty HA word pairs were divided into two groups, 20 HAHI and 20 HALI, based on word imagery values. The same criteria were applied to the selection of 20 LAHI and 20 LALI word pairs. Eight word pairs that did not meet the criteria for HA and LA word pairs were randomly chosen. Forty word pairs were used for the nonassociation (NA) word pair condition. The other 40 word pairs were made up of pronounceable NWs created by changing either an initial consonant or vowel or a consonant placed under a vowel of the original word. Word frequencies were matched across the conditions based on the criteria obtained by Younsei University [1998]. The range of syllables across the conditions was one to three. The number of syllables was matched across the conditions.
The Semantic Priming Paradigm and Apparatus
Forty word pairs were presented for each condition (HA, LA, NA, and NW). Each of the 40 high and low association word pairs (HA and LA) included 20 HI and 20 LI prime stimuli. For the preconscious priming condition, a trial consisted of 30 ms prime, 30 ms mask, 500 ms probe, and 2–8 s SOA (Fig. 1). A lexical decision task of 7 min 12 s was presented on the computer screen using E‐prime 1.0 software. Participants were asked to judge whether the target was a real word or not by clicking a button. Brain activation was measured using functional MRI during word discrimination by participants. Both behavioral and functional neuroimaging data were analyzed to explore the effects of degree of word association and the effects of imagery.
Figure 1.
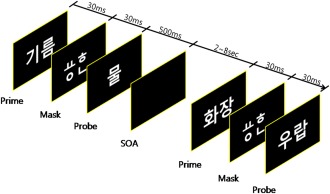
The semantic priming paradigm: event related design. Participants were presented by various conditions of prime‐target word pairs in the very short SOA (30 ms). Prime words were followed by HIHA target word (prime: judge, target: prosecutor), LIHA target word (prime: affection, target; love), HILA target word (prime: saucer, target: glass), LILA target word (prime: joke, target: smile), NA (prime: class, target: earth), and NW (prime: product, target: banul). Participants were asked to judge whether the target was a real word or not by clicking the button. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Behavioral Data
A two‐way ANOVA was performed to explore the main effect of association and imagery and the interaction between association and imagery on both response time (RT) and accuracy (Table 1). To compare the effect of the degree of association between two words (prime‐probe) on the response, one‐way analyses of variance (ANOVA) were performed. They were used to compare both RT and accuracy between the four association‐related conditions (HA vs. LA vs. NA vs. NW). The analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Table 1.
RT and accuracy during word discrimination task
| Measures | Association | Imagery | |||||
|---|---|---|---|---|---|---|---|
| AI | LI | HI | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| RT (msec) | NW | 704.51 | 87.76 | ||||
| NA | 599.66 | 89.67 | |||||
| LA | 591.17 | 72.55 | 593.34 | 74.59 | 589.01 | 71.59 | |
| HA | 556.82 | 68.08 | 568.19 | 71.80 | 545.43 | 66.53 | |
| Accuracy (No. of stimuli) | n = 40 | n = 20 | n = 20 | ||||
| NW | 35.43 | 3.44 | |||||
| NA | 38.71 | 1.68 | |||||
| LA | 39.21 | 0.70 | 19.50 | 0.52 | 19.71 | 0.47 | |
| HA | 39.64 | 0.50 | 19.86 | 0.36 | 19.79 | 0.43 | |
| Correct response only | |||||||
| RT (ms) | NW | 730.78 | 103.00 | ||||
| NA | 618.79 | 121.14 | |||||
| LA | 587.27 | 73.55 | 586.55 | 78.86 | 587.98 | 70.93 | |
| HA | 556.27 | 68.40 | 568.10 | 71.87 | 544.44 | 67.04 | |
Notes: AI: all imagery; HA: high association condition; HI: high imagery condition; LA: low association condition; LI: low imagery condition; NA: nonassociation condition; NW: nonword condition; RT: response time; SD: standard deviation.
MRI Data Acquisition
All participants underwent MRI procedures on a 3.0‐T whole body MRI Echospeed system (ISOL, Korea). Prior to functional acquisitions, a high‐resolution structural MRI examination [TE = 5.7 ms, TR = 10 ms, field of view (FOV) = 220 mm, matrix size = 256 × 256, slice thickness = 1.5 mm, MPRAGE sagittal slices] was performed for each patient to identify potential brain abnormalities. We measured a total of 220 EPI scans of the blood oxygen level‐dependent responses (TE 35 ms, TR 2 s, FOV = 192 × 220 mm, flip angle = 70°, 5.5 mm thick, 0 mm gap, 64 × 64 matrix, and 24 axial slices) and also acquired in‐plane T1‐weighted anatomical data (TE = 16 ms, TR = 2,800 ms, FOV = 192 × 220 mm, matrix size = 256 × 256, flip angle = 60°, slice thickness =5.5 mm, 0 mm gap, and 24 axial slices) for each participant.
Data Processing and Analysis
Functional magnetic resonance imaging
FMRI data were processed and analyzed with the FMRI Expert Analysis Tool (FEAT) of FSL software (http://www.fmrib.ox.ac.uk/fsl/feat5/index.html). The first four scans were discarded. The remaining 216 images were spatially realigned using rigid‐body. Next, a brain mask from the first volume of FMR data was created to eliminate signals outside of the brain in each subject. FMR images were filtered for 50 s with a high pass filter. To remove various noises including structured noise, and scanner‐related or physiological artifacts, an independent component analysis was performed using MELODIC of FSL software (http://www.fmrib.ox.ac.uk/fsl/melodic/index.html). Obvious noise components were removed by a researcher (M.J.K.). To reduce noise without reducing valid activation, spatial smoothing was performed using 5 mm FWHM. Prewhitening, for removal of serial correlations, was performed to maximize the validity of the statistics.
A general linear model was used for linear combination of the modeled response for HIHA, LIHA, HILA, LILA, NA, and NW conditions. Activation maps for several contrasts between conditions were constructed separately for each subject using a mixed effect analysis. The contrasts were letter vs. null event, and word vs. NW and for the main effect of association (association vs. nonassociation, HA vs. LA or NA, LA vs. NA), the main effect of imagery (HI vs. LI, HIHA vs. LIHA, HILA vs. LILA) and the interaction effect of association by imagery were considered. Each resulting Z statistic image in each subject was considered in the mixed effect analysis at the group level to show, which clusters of voxels above a Z value of 2.3 were activated at a significance level of P < 0.05. Activations identified at a spatial extent of at least 10 voxels. Before the group analysis, fMRI data were registered to the in‐plan T1‐weighted structural image with translation and to a high‐resolution structural image with linear transformation of 6 degrees‐of‐freedom and finally to standard space using a linear registration (FLIRT: FMRIB's Linear Image Registration Tool, http://www.fmrib.ox.ac.uk/fsl/flirt/index.html). The main template for the detection of the regions of interest was based on the Harvard Oxford Structural Atlas, which is provided by FSL. For example, as shown in Figure 3a, the results of the large cluster indicated three regions. We used the Harvard Oxford Cortical Structural Atlas to identify the names of the locations of the peak voxel coordinates of each of these regions. Regarding the results of Figure 3b,c, we checked each cluster's peak points (Table 2) in the Harvard Oxford Cortical Structural Atlas, and found that these peak points included the precuneus, cuneus, and posterior cingulate cortex, respectively.
Figure 3.
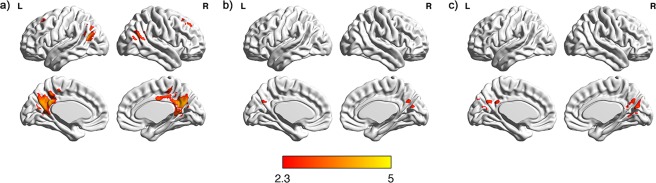
Brain regions modulated by semantic priming. (a) Brain regions activated by word stimuli (HA, LA, and NA), compared with NW stimuli, (b) brain regions activated by the negative interaction between word association and word imagery, (c) brain regions activated by HI word stimuli than LI word stimuli under LA condition.
Table 2.
Brain regions activated for the negative interaction between word association and word imagery (A) and brain regions activated for the contrast of HILA versus LILA conditions (B)
| Regions | Voxels | Peak voxel coordinate | Z‐Values | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| A | Precuneus, left | 295 | −2 | −66 | 28 | 3.08 |
| Precuneus, right | 4 | −68 | 30 | 2.97 | ||
| Cuneus, right | 2 | −74 | 20 | 2.94 | ||
| B | Cuneus, right | 1,098 | 2 | −74 | 20 | 3.14 |
| Precuneus, left | 0 | −56 | 28 | 3.08 | ||
| Posterior cingulate cortex, left | −6 | −46 | 30 | 3.08 | ||
RESULTS
Behavioral Data
In the two‐way ANOVA, the main effects of both association and imagery were significant (F = 28.21, P < 0.001 and F = 11.07, P = 0.01, respectively). The interaction between association and imagery was also significant (F = 4.93, P = 0.04: the higher the association and imagery the word pairs have, the faster the word discrimination task was). An imagery effect on RT was shown only within HA (t = −3.0, df = 14, and P = 0.01), not LA (t = −0.26, df = 14, and P = 0.80) word pairs (Fig. 2). Using the correct response data only, the main effects of association and imagery and their interaction were still significant (F = 38.63, P < 0.001; F = 5.00, P = 0.04; F = 5.66, P = 0.03, respectively). In terms of accuracy, there was no main effect of association (mean square = 0.64, F = 2.93, and P = 0.11), imagery (mean square = 0.07, F = 0.38, and P = 0.55), or their interaction (mean square = 0.29, F = 1.16, and P = 0.30).
Figure 2.
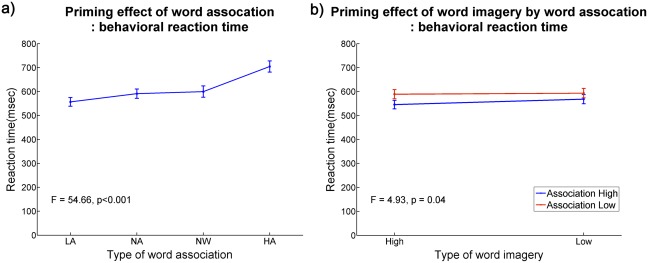
The mean reaction time with the standard error of the mean during lexical decision task for type of word association (left) and interaction of word association by word imagery (right). HA: high association between prime and target word; LA: low association between prime and target word; NA: no association between prime and target word; NW: nonword.
A one‐way ANOVA showed that the greater the association between two words, the faster the responses were on the word discrimination task (F = 54.66, P < 0.001). A post hoc contrast analysis showed that the RTs of HA and NW were the shortest and the longest, respectively (P < 0.001); however, the RTs between LA and NW did not differ significantly (P = 0.42; HA > LA = NA > NW; Fig. 2). Using the correct response data only, the post hoc analysis showed the responses to HA and NW were also the shortest and the longest, respectively (P < 0.001), but the RTs between LA and NA were not different (P = 0.26; HA > LA = NA > NW). The word discrimination for the associated word pairs was more accurate than that of non‐associated word pairs (F = 7.47, P = 0.01). The post hoc analysis showed that HA was more accurate than NA (P = 0.04) and NW (P < 0.001), LA than NW (P < 0.001), and NA than NW (P < 0.001). No difference in accuracy was seen between HA vs. LA (P = 0.11) or LA vs. NA (P = 0.25).
Functional Magnetic Resonance Imaging
Brain regions activated by the negative interaction between word association and word imagery were the bilateral precuneus and right cuneus (Table 2, Fig. 3). This suggests that the precuneus and cuneus are more likely to be activated in conditions of higher imagery but lower association. This interaction was further analyzed by the degree of word association. As a result, no differential brain area was found for HI and LI within the HA condition. However, within the LA condition, the right cuneal, left posterior cingulate cortex, and left precuneus were activated with HI words, compared with LI words (Table 2, Fig. 3).
When contrasting word (HA, LA, and NA) with NW stimuli, brain regions activated by word stimuli (all Ps < 0.01) included the left posterior cingulate gyrus, bilateral precuneus, bilateral superior lateral occipital cortex, left superior frontal gyrus, bilateral middle frontal gyrus, and right frontal poles (Table 3, Fig. 3). No region was identified when contrasting HA vs. LA or NA, LA vs. NA.
Table 3.
Brain regions activated for the contrast of Word (HA, LA, and NA) versus NW conditions
| Regions | Voxels | Peak voxel coordinate | Z‐Values | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Posterior cingulate cortex, left | 4724 | −2 | −48 | 30 | 4.48 |
| Precuneus, right | 2 | −66 | 28 | 4.20 | |
| Precuneus, left | −6 | −58 | 16 | 4.02 | |
| Superior lateral occipital cortex, left | 912 | −42 | −64 | 22 | 4.11 |
| Angular gyrus, left | −40 | −56 | 16 | 3.61 | |
| Superior lateral occipital cortex, right | 738 | 52 | −66 | 22 | 3.64 |
| Superior frontal gyrus, left | 355 | −22 | 22 | 44 | 3.67 |
| Middle frontal gyrus, left | −28 | 22 | 48 | 3.24 | |
| Frontal pole, right | 345 | 18 | 38 | 42 | 3.48 |
| Middle frontal gyrus, right | 26 | 26 | 42 | 3.29 | |
Notes: The coordinates of maximally activated voxels are given in MNI space. All activations identified at cluster‐level significance of P < 0.05 (corrected) for a spatial extent of at least 10 voxels.
DISCUSSION
The main aim of this study was to identify brain regions whose activation was based on the degree of word association and word imagery under an implicit (automatic) condition. The crucial finding was the activation of the left precuneus, left posterior cingulate gyrus, and right cuneus resulting from a difference in word imagery, but only under a LA condition. No brain region with activation depending on the degree of word association was detected under the preconscious condition.
Previous studies have found that the precuneus is involved in self‐consciousness [Kjaer et al., 2002; Lou et al., 2004; Vogt and Laureys, 2005], the recall of episodic memory [Lundstrom et al., 2003, 2005], and mental imagery related to visuospatial stimuli [Oshio et al., 2010]. Activation of the precuneus was also observed in both visual memory and visual imagery processing [Slotnick et al., 2012]. With the dorsolateral prefrontal cortex, the precuneus appeared to be activated upon presentation of visual three‐letter nouns under the supraliminal relative to subliminal condition, suggesting an awareness of visual verbal stimuli as one of the main functions of the precuneus [Kjaer et al., 2001]. Our findings extend previous studies by demonstrating that the activation of the precuneus is also promoted by the priming effect of HI words relative to LI words under the subliminal condition. The activation of the precuneus thus follows a function of the degree of word imagery in the early stage of verbal information processing. This is the first study to identify regional brain activity differentiating high and LI words in an implicit condition.
Our results differ from those of Kjaer et al. [2001]: the activation of the precuneus under subliminal conditions in our study might be due to uniqueness of the imagery effect of the word. For example, the present study contrasted HI with LI word pairs, whereas Kjaer et al. [2001], regardless of word imagery, repeatedly presented nouns with increasing duration (e.g., 28, 43, 57, … 1376 ms). Evidence that HI words are more easily processed and recalled compared with LI words has been presented, suggesting imagery effects of the words [de Groot, 1989; Nittono et al., 2002]. It is assumed that HI words, relative to LI words, facilitate activation of a stronger and denser semantic network. Accordingly, HI word pairs in this study might evoke enhanced precuneus activity, compared with LI words, under implicit processing conditions (beyond self‐awareness). This is further supported by previous findings that information processing of concrete words is faster and more efficient than that of abstract words in recognition, free and cued recall, paired associated learning, comprehension of a sentence, and a meaningfulness judgment task [Haberlandt and Graesser, 1985; Holmes and Langford, 1976; Paivio et al., 1994]. Similarly, West and Holcomb [2000] found that the RT to concrete words was faster than that for abstract words, which was further supported by evidence showing that the time difference between ERPs of concrete and abstract words was about 250–300 msec, with a larger activation of N400 for concrete words than abstract words. This is consistent with our finding that the priming effect of imagery words occurs outside of awareness.
The posterior cingulate cortex is known to be involved in episodic memory retrieval for standardized stimuli [Cabeza and Nyberg, 2000]. In particular, the left posterior cingulate, along with the left precuneus and cuneus, appeared to be more activated during cued recall of familiar people relative to unfamiliar people [Maddock et al., 2001]. The activation of the bilateral posterior cingulate cortex was also observed in awareness of emotional words, compared to neutral words [Maddock, 1999; Maddock and Buonocore, 1997; Maddock et al., 2003]. These prior studies suggest that the posterior cingulate cortex might be a common area for recall of episodic memory and perception of emotional words. Notably, imagery is a crucial component in episodic memory recall. Sharot et al. [2007] found that the posterior cingulate cortex plays a key role in imaging future events, and the left posterior cingulate, precuneus, and cuneus are involved in detailed elaboration of an imagined event [Addis et al., 2007]. Our data extend the role of the posterior cingulate gyrus to involve a priming effect related to HI words relative to LI words at an implicit level.
The cuneus is most known for its involvement in basic visual information processing. A previous study found that bilateral cuneus is activated in task switching related to cognitive control [Dove et al., 2000]. Another study showed that isolated lateralized ERP activity was detected in the bilateral cuneus in the early stages of the visuo‐spatial cue‐target SOA (250–300ms) [McDonald and Green, 2008]. These findings suggest that the cuneus is linked to attentional‐control activity related to visual symbol stimuli. Based on the obtained results, we speculate that the cuneus plays a pivotal role in differentiating HI words from LI words in the early stages of cue‐target SOA (30 ms), a finding that has not previously been reported.
Our results showing that highly related prime‐target word pairs displayed a shorter latency and higher correct response than nonrelated prime‐target word pairs or NW pairs support the semantic priming effect. This was further supported by the findings of differential brain activities between prime‐target word pairs (HA, LA, and NA) and prime‐target NW pairs. According to spreading‐activation theories [Collins and Loftus, 1975; Neely, 1976], the activation of the prime spreads automatically into the semantically related nodes, and thereby a semantically related target can be processed more rapidly than an unrelated target. There is evidence that associative priming effects using a lexical decision task occur even at a very short SOA, suggesting an automatic priming effect [Dietrich and Thieos, 1992; Perea and Gotor, 1997; Sereno, 1991]. In this sense, these findings reflect automatic, implicit access to semantic priming. However, we failed to show brain activity dependent on the degree of the relatedness of prime‐target word associations (e.g., HA vs. LA or LA vs. NA). A previous study conducted by Wible et al. [2006], however, found differential brain activity depending on the degree of the relatedness of prime‐target word pairs, but under a long prime‐target SOA condition (750 ms). These results suggest that the semantic priming effect based on the degree of the relatedness of prime‐target words might not be generated under preconscious conditions (short prime‐target SOA; 30 ms). We speculate that the lack of a differential effect of the degree of the relatedness of word associations might be attributable to passive and shallow spread of activation into the semantically related nodes during the very short prime‐target SOA condition (30 ms), which also hinders brain activity from differentiating related and unrelated word pairs. As such, a semantic priming effect of the degree of the relatedness of word associations appears to occur at SOA conditions of more than 30 ms.
These findings have significant theoretical implications in terms of the context availability and the dual coding model. The context availability model [Bransford and McCarrell, 1974] hypothesizes that words are processed in a single semantic system whereas a dual coding model [Paivio, 1986] assumes that word processing depends on dual semantic systems including both a verbal and nonverbal system. Our findings that the differential effects of word imagery (HI vs. LI) were observed in only the LA condition, not the HA condition, suggest a main effect of word imagery. That is, there is a sole effect of word imagery when the semantic effect of word association was decreased under the preconscious condition. This result supports the dual coding model.
There are several limitations that should be noted. The present study did not match word imagery of unrelated word association, which precluded examining the differential effect of word imagery in terms of related words versus unrelated words. A future study including the imagery of unrelated word association might provide useful information on the facilitating imagery effect between related and unrelated word association. Another limitation of this study was the inclusion of only a very short SOA. This was because a previous study already conducted a similar design under long SOA conditions [Wible et al., 2006]. Nonetheless, it might be interesting to investigate whether the imagery effect of word association would be promoted under the conscious condition given that Wible et al. [2006] did not directly examine the imagery effect of prime‐target word associations. A future study including various ranges of prime‐target SOAs will provide useful information in investigating both automatic and controlled imagery and the semantic priming effect. Whereas previous studies have used concrete and abstract words to investigate the facilitating effect of word imagery, this study directly classified HI and LI words regardless of concreteness and abstractness. Future studies controlling for concreteness and abstractness of the word would offer a more rigorous design for investigating the word imagery effect.
Despite the aforementioned limitations, the present investigation provides a novel finding of identifying brain regions related to the imagery words. Our findings suggest that when we recognize a word, the imagery of the word can be activated and processed at a very early stage (30 ms SOA), and the right cuneal, left posterior cingulate gyrus, and left precuneus are involved in this function. Previous studies have shown that the aforementioned brain regions are related to self‐consciousness, episodic memories, imagery processing, and the recognition of visually presented words at a conscious level. These findings extend the previous research by examining the unique function of the right cuneal, left posterior cingulate gyrus, and left precuneus in recognizing word imagery at a preconscious level, indicating an “automatic imagery priming effect.” Given the evidence showing that people with high degrees of alexithymia displayed less activation of the posterior cingulate cortex while imagining past and future happy memories, compared with the control condition (seeing a cross symbol) [Mantani et al., 2005], and that the posterior cingulate cortex is reported to play a key role in imagining future events [Addis et al., 2007; Sharot et al., 2007], the brain areas detected in this study might be considered as targets for clinical intervention in depressed people. For example, it would be interesting to investigate whether Cognitive Bias Modification training targeting the posterior cingulate cortex using imagery words would be helpful for depressed people [Koster et al., 2009; Mathews and Mackintosh, 2000].
ACKNOWLEDGMENTS
The authors thank T.M. Kim and H.J. Choi for taking care of subjects, and C.H. Lee and J.C. Yu for reviewing this manuscript providing feedback to the investigators throughout the project.
Conflict of Interest: None declared.
REFERENCES
- Addis DR, Wong AT, Schacter DL (2007): Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA (2005): Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci 17:905–917. [DOI] [PubMed] [Google Scholar]
- Borst G, Kosslyn SM (2008): Visual mental imagery and visual perception: Structural equivalence revealed by scanning processes. Mem Cognit 36:849–862. [DOI] [PubMed] [Google Scholar]
- Bransford JD, McCarrell NS (1974): A sketch of a cognitive approach to comprehension: Some thoughts about understanding what it means to comprehend In: Weimer W, Paleromo D, editors. Cognition and the symbolic processes. Hillsdale, New Jersey: Erlbaum; p 189–230. [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Senehi J, Nuding S (1987): Semantic priming with abstract and concrete words: Differential asymmetry may be postlexical. Brain Lang 31:43–60. [DOI] [PubMed] [Google Scholar]
- Choi JW, Jeong BS, Lee CH, Kim JW, Lee SW, Kim DK (2006): The neural correlates of the retrieval of previously acquired information during top‐down control. Psychiatry Investig 3:66–71. [Google Scholar]
- Collins AM, Loftus EF (1975): A spreading‐activation theory of semantic processing. Psychol Rev 82:407. [Google Scholar]
- Coslett HB, Monsul N (1994): Reading with the right hemisphere: Evidence from transcranial magnetic stimulation. Brain Lang 46:198–211. [DOI] [PubMed] [Google Scholar]
- de Groot AM (1989): Representational aspects of word imageability and word frequency as assessed through word association. J Exp Psychol Learn Mem Cogn 15:824. [Google Scholar]
- Dietrich D, Thieos J (1992): Priming outside of awareness and subsequent stimulus identification. Percept Mot Skills 75:483–493. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: an event‐related fMRI study. Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD (2003): Processing concrete words: fMRI evidence against a specific right‐hemisphere involvement. Neuropsychologia 42:62–70. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1998): Structured Clinical Interview for DSM‐IV Axis I Disorders, Non‐patient Edition. (SCID‐I/NP). New York: New York State Psychiatric Institue, Biometrics Research. [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ (2000): “Sculpting the response space”—an account of left prefrontal activation at encoding. Neuroimage 12:404–417. [DOI] [PubMed] [Google Scholar]
- Haberlandt KF, Graesser AC (1985): Component processes in text comprehension and some of their interactions. J Exp Psychol Gen 114:357. [Google Scholar]
- Holmes VM, Langford J (1976): Comprehension and recall of abstract and concrete sentences. J Verbal Learn Verbal Behav 15:559–566. [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV (2000): Distributed neural systems for the generation of visual images. Neuron 28:979–990. [DOI] [PubMed] [Google Scholar]
- Jeong B, Kubicki M (2010): Reduced task‐related suppression during semantic repetition priming in schizophrenia. Psychiatry Res 181:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong B, Wible CG, Hashimoto R, Kubicki M (2009): Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. Hum Brain Mapp 30:4138–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath DO, Klose U, Papassotiropoulos A, Grodd W (2000): The concreteness effect: Evidence for dual coding and context availability. Brain Lang 74:103–112. [DOI] [PubMed] [Google Scholar]
- Kaas A, Weigelt S, Roebroeck A, Kohler A, Muckli L (2010): Imagery of a moving object: The role of occipital cortex and human MT/V5+. Neuroimage 49:794–804. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD (1999): Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp 7:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC (2001): Precuneus‐prefrontal activity during awareness of visual verbal stimuli. Conscious Cogn 10:356–365. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC (2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 17:1080–1086. [PubMed] [Google Scholar]
- Klaver P, Fell J, Dietl T, Schur S, Schaller C, Elger CE, Fernandez G (2005): Word imageability affects the hippocampus in recognition memory. Hippocampus 15:704–712. [DOI] [PubMed] [Google Scholar]
- Klein I, Dubois J, Mangin J‐F, Kherif F, Flandin G, Poline J‐B, Denis M, Kosslyn SM, Le Bihan D (2004): Retinotopic organization of visual mental images as revealed by functional magnetic resonance imaging. Cogn Brain Res 22:26–31. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Costantini‐Ferrando MF, Alpert NM, Spiegel D (2000): Hypnotic visual illusion alters color processing in the brain. Am J Psychiatry 157:1279–1284. [DOI] [PubMed] [Google Scholar]
- Koster EH, Fox E, MacLeod C (2009): Introduction to the special section on cognitive bias modification in emotional disorders. J Abnorm Psychol 118:1–4. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Merves JS (1986): Lexical access for concrete and abstract words. J Exp Psychol Learn Mem Cogn 12:92. [Google Scholar]
- Laisney M, Giffard B, Belliard S, de la Sayette V, Desgranges B, Eustache F (2011): When the zebra loses its stripes: Semantic priming in early Alzheimer's disease and semantic dementia. Cortex 47:35–46. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH (2004): Parietal cortex and representation of the mental Self. Proc Natl Acad Sci USA 101:6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M (2003): Isolating the retrieval of imagined pictures during episodic memory: Activation of the left precuneus and left prefrontal cortex. Neuroimage 20:1934–1943. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM (2005): The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27:824–834. [DOI] [PubMed] [Google Scholar]
- Maddock RJ (1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22:310–316. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH (1997): Activation of left posterior cingulate gyrus by the auditory presentation of threat‐related words: An fMRI study. Psychiatry Res 75:1–14. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2001): Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S (2005): Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: A functional magnetic resonance imaging study. Biol Psychiatry 57:982–990. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B (2000): Induced emotional interpretation bias and anxiety. J Abnorm Psychol 109:602–615. [PubMed] [Google Scholar]
- McDonald JJ, Green JJ (2008): Isolating event‐related potential components associated with voluntary control of visuo‐spatial attention. Brain Res 1227:96–109. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio‐Mazoyer N, Bricogne S, Mazoyer B, Kosslyn SM, Denis M (2000): Functional anatomy of high‐resolution visual mental imagery. J Cogn Neurosci 12:98–109. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW (1971): Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval operations. J Exp Psychol 90:227–234. [DOI] [PubMed] [Google Scholar]
- Neely JH (1976): Semantic priming and retrieval from lexical memory: Evidence for facilitatory and inhibitory processes. Mem Cognit 4:648–654. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Bennett DJ, Gee NR, Schreiber TA, McKinney VM (1993): Implicit memory: Effects of network size and interconnectivity on cued recall. J Exp Psychol Learn Mem Cogn 19:747–764. [DOI] [PubMed] [Google Scholar]
- Nittono H, Suehiro M, Hori T (2002): Word imageability and N400 in an incidental memory paradigm. Int J Psychophysiol 44:219–229. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ (2004): Retrieval of abstract semantics. Neuroimage 22:164–170. [DOI] [PubMed] [Google Scholar]
- Oshio R, Tanaka S, Sadato N, Sokabe M, Hanakawa T, Honda M (2010): Differential effect of double‐pulse TMS applied to dorsal premotor cortex and precuneus during internal operation of visuospatial information. Neuroimage 49:1108–1115. [DOI] [PubMed] [Google Scholar]
- Paivio A (1986): Mental representations: A dual coding approach. Oxford University Press: New York. [Google Scholar]
- Paivio A (1991): Dual coding theory: Retrospect and current status. Can J Psychol 45:255. [Google Scholar]
- Paivio A, Sadoski M (2011): Lexicons, contexts, events, and images: commentary on Elman (2009) from the perspective of dual coding theory. Cogn Sci 35:198–209. [DOI] [PubMed] [Google Scholar]
- Paivio A, Walsh M, Bons T (1994): Concreteness effects on memory ‐ when and why. J Exp Psychol Learn Mem Cogn 20:1196–1204. [Google Scholar]
- Park T (2004): Investigation of Association Frequency and Imagery Value of Korean Words. The Korean Journal of Experimental Psychology 16:237–260. [Google Scholar]
- Perea M, Gotor A (1997): Associative and semantic priming effects occur at very short stimulus‐onset asynchronies in lexical decision and naming. Cognition 62:223–240. [DOI] [PubMed] [Google Scholar]
- Perri R, Zannino GD, Caltagirone C, Carlesimo GA (2011): Semantic priming for coordinate distant concepts in Alzheimer's disease patients. Neuropsychologia 49:839–847. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE (2003): An event‐related FMRI investigation of implicit semantic priming. J Cogn Neurosci 15:1160–1175. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC (2003): The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia 41:550–564. [DOI] [PubMed] [Google Scholar]
- Sereno JA (1991): Graphemic, associative, and syntactic priming effects at a brief stimulus onset asynchrony in lexical decision and naming. J Exp Psychol Learn Mem Cogn 17:459. [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA (2007): Neural mechanisms mediating optimism bias. Nature 450:102–105. [DOI] [PubMed] [Google Scholar]
- Shen MD, Shih P, Ottl B, Keehn B, Leyden KM, Gaffrey MS, Muller RA (2012): Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: Evidence from functional and effective connectivity. Neuroimage 62:1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW (2007): A common neural substrate for perceiving and knowing about color. Neuropsychologia 45:2802–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Thompson WL, Kosslyn SM (2005): Visual mental imagery induces retinotopically organized activation of early visual areas. Cereb Cortex 15:1570–1583. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Thompson WL, Kosslyn SM (2012): Visual memory and visual mental imagery recruit common control and sensory regions of the brain. Cogn Neurosci 3:14–20. [DOI] [PubMed] [Google Scholar]
- Vistoli D, Passerieux C, Houze B, Hardy‐Bayle MC, Brunet‐Gouet E (2011): Neural basis of semantic priming in schizophrenia during a lexical decision task: A magneto‐encephalography study. Schizophr Res 130:114–122. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S (2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare‐Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 31:329–338. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV (2010): Neural representation of abstract and concrete concepts: A meta‐analysis of neuroimaging studies. Hum Brain Mapp 31:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WC, Holcomb PJ (2000): Imaginal, semantic, and surface‐level processing of concrete and abstract words: An electrophysiological investigation. J Cogn Neurosci 12:1024–1037. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Mh N, Jolesz FA, McCarley RW, Nestor P (2006): Connectivity among semantic associates: An fMRI study of semantic priming. Brain Lang 97:294–305. [DOI] [PubMed] [Google Scholar]
- Younsei University (1998): Younsei Korean Language Dictionary. Seoul: Dusan Dong‐A. [Google Scholar]


