Abstract
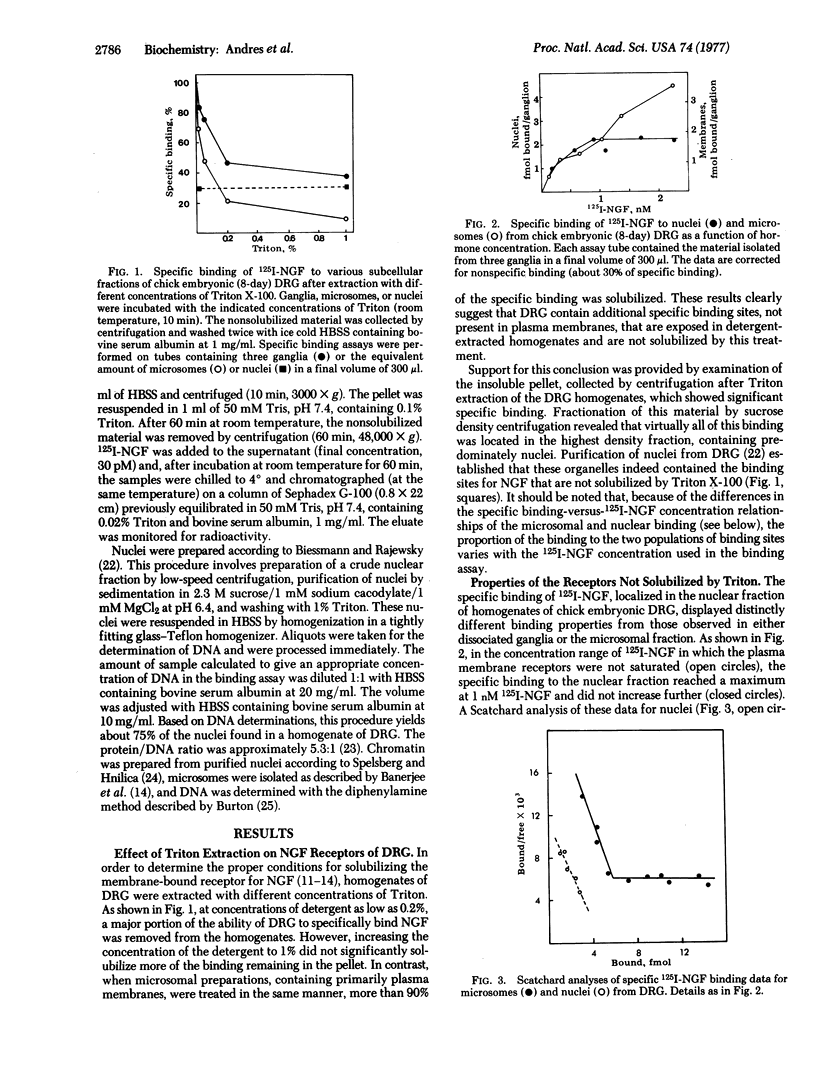
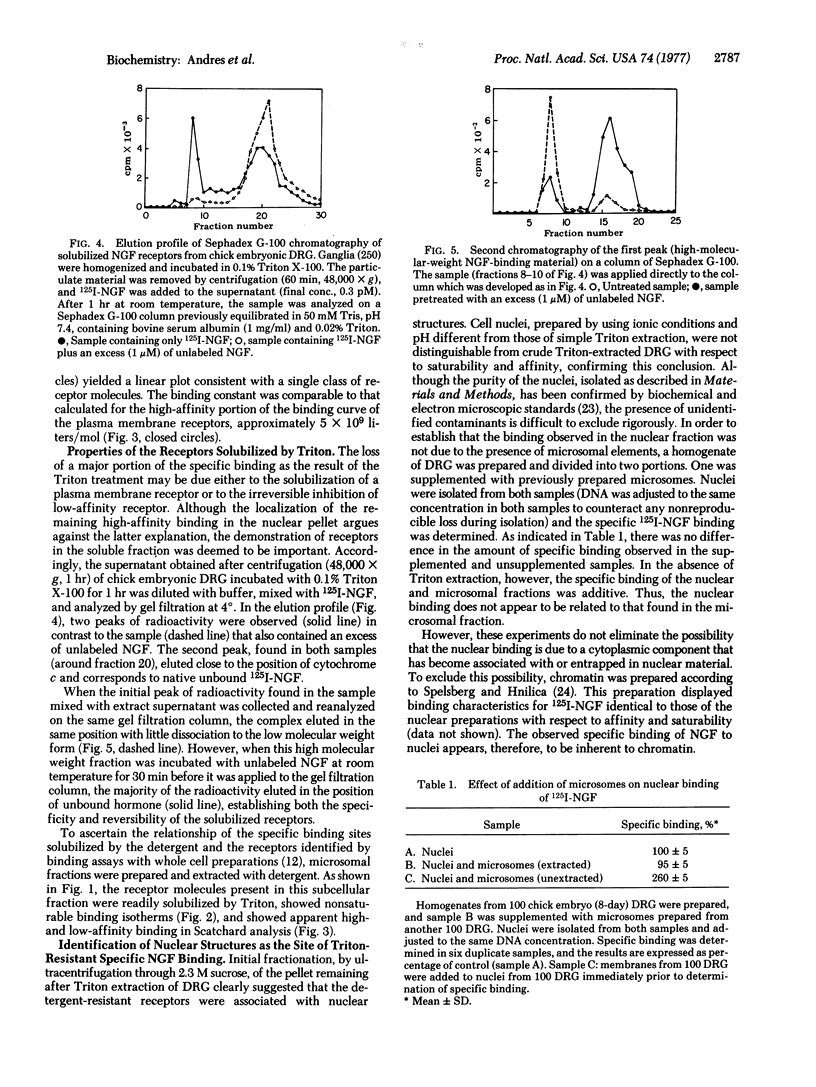
Two classes of receptors for 125I-labeled nerve growth factor in chick embryonic dorsal root neurons have been observed. One type is associated with the plasma membrane (or microsomal fraction) and can be completely solubilized by Triton X-100. These receptors display the nonsaturable binding isotherms and curvilinear Scatchard plots previously reported for nerve growth factor receptors in whole cells. The second class of binding sites is located in the nucleus, firmly bound to chromatin. These receptors are not solubilized by detergent, show saturable binding, and yield linear Scatchard plots of the type associated with a single class of binding sites of high affinity. The presence of the two receptor types suggests a bimodal mechanism of action for nerve growth factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELETTI P. U., GANDINI-ATTARDI D., TOSCHI G., SALVI M. L., LEVI-MONTALCINI R. METABOLIC ASPECTS OF THE EFFECT OF NERVE GROWTH FACTOR ON SYMPATHETIC AND SENSORY GANGLIA: PROTEIN AND RIBONUCLEIC ACID SYNTHESIS. Biochim Biophys Acta. 1965 Jan 11;95:111–120. doi: 10.1016/0005-2787(65)90216-9. [DOI] [PubMed] [Google Scholar]
- Angeletti P. U., Levi-Montalcini R., Caramia F. Ultrastructural changes in sympathetic neurons of newborn and adult mice treated with nerve growth factor. J Ultrastruct Res. 1971 Jul;36(1):24–36. doi: 10.1016/s0022-5320(71)80086-2. [DOI] [PubMed] [Google Scholar]
- Austoker J., Cox D., Mathias A. P. Fractionation of nuclei from brain by zonal centrifugation and a study of the ribonucleic acid polymerase activity in the various classes of nuclei. Biochem J. 1972 Oct;129(5):1139–1155. doi: 10.1042/bj1291139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. P., Snyder S. H., Cuatrecasas P., Greene L. A. Binding of nerve growth factor receptor in sympathetic ganglia. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2519–2523. doi: 10.1073/pnas.70.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Rajewsky M. F. Nuclear protein patterns in developing and adult brain and in ethylnitrosourea-induced neuroectodermal tumours of the rat. J Neurochem. 1975 Feb;24(2):387–393. doi: 10.1111/j.1471-4159.1975.tb11892.x. [DOI] [PubMed] [Google Scholar]
- Bjerre B., Björklund A., Stenevi U. Stimulation of growth of new axonal sprouts from lesioned monoamine neurones in adult rat brain by nerve growth factor. Brain Res. 1973 Sep 28;60(1):161–176. doi: 10.1016/0006-8993(73)90855-x. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V. The nerve growth factor. Amino acid composition and physicochemical properties. Eur J Biochem. 1970 Jul;15(1):127–131. doi: 10.1111/j.1432-1033.1970.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Drezner M. K., Lebovitz H. E. Inhibition of chondromucoprotein synthesis: an extraneuronal effect of nerve growth factor. J Pharmacol Exp Ther. 1975 Mar;192(3):630–634. [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Interaction of nerve growth factor with surface membranes: biological competence of insolubilized nerve growth factor. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2931–2935. doi: 10.1073/pnas.70.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Pulliam M. W., Szutowicz A., Bradshaw R. A. Properties and specificity of binding sites for 125I-nerve growth factor in embryonic heart and brain. J Biol Chem. 1974 Sep 25;249(18):5918–5923. [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Szutowicz A., Pulliam M. W., Bradshaw R. A. Specific binding sites for 125I-nerve growth factor in peripheral tissues and brain. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1096–1103. doi: 10.1016/0006-291x(74)90809-2. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Hogue-Angeletti R. A., Sherman R., Bradshaw R. A. Topography of mouse 2.5S nerve growth factor. Reactivity of tyrosine and tryptophan. Biochemistry. 1973 Aug 14;12(17):3281–3293. doi: 10.1021/bi00741a021. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta-nerve growth factor receptor in development. J Cell Biol. 1975 Oct;67(1):118–125. doi: 10.1083/jcb.67.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry. 1975 Aug 26;14(17):3845–3851. doi: 10.1021/bi00688a017. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Merrell R., Pulliam M. W., Randono L., Boyd L. F., Bradshaw R. A., Glaser L. Temporal changes in tectal cell surface specificity induced by nerve growth factor. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4270–4274. doi: 10.1073/pnas.72.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norr S. C., Varon S. Dynamic, temperature-sensitive association of 125-i-nerve growth factor in vitro with ganglionic and non-ganglionic cells from embryonic chick. Neurobiology. 1975 May;5(2):101–118. [PubMed] [Google Scholar]
- Paravicini U., Stoeckel K., Thoenen H. Biological importance of retrograde axonal transport of nerve growth factor in adrenergic neurons. Brain Res. 1975 Feb 7;84(2):279–291. doi: 10.1016/0006-8993(75)90982-8. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Guroff G., Schwab M., Thoenen H. The significance of retrograde axonal transport for the accumulation of systemically administered nerve growth factor (NGF) in the rat superior cervical ganglion. Brain Res. 1976 Jun 11;109(2):271–284. doi: 10.1016/0006-8993(76)90530-8. [DOI] [PubMed] [Google Scholar]
- Stöckel K., Paravicini U., Thoenen H. Specificity of the retrograde axonal transport of nerve growth factor. Brain Res. 1974 Aug 23;76(3):413–421. doi: 10.1016/0006-8993(74)90818-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]


