Abstract
In most natural environments, the large majority of mammals harbour parasitic helminths that often live as adults within the intestine for prolonged periods (1–2 years) 1. Although these organisms have been eradicated to a large extent within westernized human populations, those living within rural areas of developing countries continue to suffer from high infection rates. Indeed, recent estimates indicate that approximately 2·5 billion people worldwide, mainly children, currently suffer from infection with intestinal helminths (also known as geohelminths and soil-transmitted helminths) 2. Paradoxically, the eradication of helminths is thought to contribute to the increased incidence of autoimmune diseases and allergy observed in developed countries. In this review, we will summarize our current understanding of host–helminth interactions at the mucosal surface that result in parasite expulsion or permit the establishment of chronic infections with luminal dwelling adult worms. We will also provide insight into the adaptive immune mechanisms that provide immune protection against re-infection with helminth larvae, a process that is likely to be key to the future development of successful vaccination strategies. Lastly, the contribution of helminths to immune modulation and particularly to the treatment of allergy and inflammatory bowel disease will be discussed.
Keywords: geohelminth, immune-expulsion, intestinal helminth, mucosal immunity, nematode, type 2 immunity
Introduction
Although they rarely kill, helminths often cause chronic infections and impact on human health through effects on nutrition leading to growth retardation, vitamin deficiencies and poor cognitive function 3,4. Hookworm infection is a major cause of iron-deficiency anaemia in endemic areas 5. While evidence is still limited to support important effects of intestinal helminths on intestinal function and immune responsiveness in humans, there is strong evidence for such effects in natural and experimental infections in animals including impaired immune responses to vaccines, increased susceptibility to other infectious diseases and a reduction in disease severity in experimental models of allergic and autoimmune disorders 6–12. Children living in poor regions of the rural tropics have a particularly high risk of infections with intestinal helminths and may harbour high parasite burdens with one or more of the major parasites, namely hookworm (Anyclostoma duodenale and Necator americanus), roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura) and threadworm (Strongyloides stercoralis). The disability adjusted life years (DALYs) lost each year as a result of intestinal helminth infections is approximately 39 million and is greater than that estimated for malaria and approaching that attributed to tuberculosis 1.
In contrast to other pathogens, intestinal helminths, with the exception of threadworms, are not able to replicate within their mammalian hosts. Thus, worm burdens tend to increase slowly over time as a result of constant exposure to infection in a faecally contaminated environment and tend to reach a peak during childhood after which infection intensities and prevalence may decline 1. Exceptions are hookworm and S. stercoralis for which peak prevalence tends to occur in adults. The convex age-prevalence and age-intensity profiles for intestinal helminths have been suggested to indicate the acquisition of age-dependent protective immunity 13. Such protective immunity may be targeted primarily at larval stages of intestinal helminths. Larvae that survive to mature into adults may survive for periods of years, and it has been suggested that the long-term survival of adults in the intestinal tract can be explained by the modulation of host immune responses. The exact mechanisms by which such modulation occurs remain poorly understood.
Immune Mechanisms Leading to Expulsion or Chronicity of Adult Worms
The majority of human helminths establish chronic infections in their host by subverting the host immune response, an effect that is also beneficial to the host because it minimizes the long-term harmful effects of inflammatory responses directed against the parasites. A better understanding of this complex relationship has been the subject of great interest, and much of the past research in the area of helminth immunobiology has focused on host–parasite relationships following primary infection. Much of our knowledge regarding host immunity has been necessarily derived from experimental murine models. Of these, three widely used models include Trichuris muris,Nippostrongylus brasiliensis and Heligmosomoides polygyrus bakeri (see Box 1 for detailed information on similarities and differences between these species). Trichuris muris is a murine pathogen closely related to T. trichuria (whipworm), the causative agent of human trichuriasis. Trichuris muris has been exploited in laboratory model systems for over 60 years to determine many of the immunological mechanisms associated with resistance and susceptibility. Nippostrongylus brasiliensis and H. p. bakeri belong to the Strongylida order, which includes the human hookworm parasites A. duodenale and N. americanus 14. While N. brasiliensis provides a suitable model for the lifecycle of human hookworm, infecting its host through the skin and migrating to the lung prior to entering the intestinal lumen, this parasite fails to persist and is instead expelled from immune competent animals within several weeks of infection. By contrast, primary infections with H. p. bakeri can persist for many months in susceptible strains of mice and thus represent a useful model for chronic intestinal helminthiases.
Box 1
Model Murine Intestinal Helminths
Numerous rodent parasites are routinely used to investigate the immune parameters of intestinal helminth infection. Although all of these helminths reside within the intestinal lumen in their adult form, many important differences exist in terms of their life cycle and chronicity. Indeed, as a result of their unique life cycles, and the likely abundant genetic differences, it is very likely that many features of the host immune response are species-specific and care needs be taken not to make generalized conclusions based on the findings of experiments using individual species. Details of the lifecycles for the three main murine nematode helminths discussed in this review are given below:
Heligmosomoides polygyrus bakeri: is a trichostrongylid nematode naturally infecting small rodents. It is ingested by the host and penetrates the submucosa of the small intestine as an L3 stage. Here is matures to an L4 stage then exists the mucosa to enter the intestinal lumen as an adult. Adults' anchor' themselves by coiling around intestinal villi and become sexually mature resulting in the production of eggs that are secreted through the faeces. Excreted eggs hatch within the soil where they develop over several weeks to the infective L3 stage and the life cycle continues. Adult worms form chronic infections in susceptible strains of mice and posses potent immune modulatory potential.
Nippostrongylus brasiliensis: is natural parasite of rats that can be adapted to use in murine experiments. Infective larvae enter their host through the skin, then enter the vasculature to be carried to the heart and lungs. Larvae exit the vasculature through small capillaries within the lung and develop into the L4 stage. They then penetrate the alveoli, are coughed up and swallowed, and migrate to the small intestine where they develop into sexually mature adults. Adults produce eggs, which are passed out in faeces, but are typically expelled by the host within a few weeks in a process that is dependent on type 2 immunity. As for H. p. bakeri excreted eggs hatch within the soil where they develop over several weeks to the infective L3 stage allowing the lifecycle to continue.
Trichuris muris: the life cycle of T. muris is entirely enteric, with orally ingested embryonated eggs hatching in the distal small intestine, releasing L1 larvae that migrate to the caecum and embed in the intestinal mucosa 55. Following four moults to adulthood, male and female worms copulate leading to the production of thousands of eggs per day, which are excreted in the faeces. Excreted embryonated eggs are not immediately infective, as they require approximately 3 weeks for larvae to develop. Infective eggs present in the environment are then ingested and the life cycle continues. Genetically, resistant strains of mice generate a strongly polarized type 2 immune response and reject larval stages of the parasite while susceptible strains exhibit a type 1-dominated response and develop chronic infections with adult worms.
Induction of type 2 immune responses
The majority of intestinal helminths elicit a strongly polarized type 2 immune response, with the exception of Trichuris spps that invoke a mixed type 2/type 1 response in many genetic backgrounds. Type 2 immunity is generally associated with protection against intestinal helminths and is characterized by a polarized cytokine response involving the secretion of interleukin (IL)-4, IL-13 and IL-5, B-cell isotype switching to IgG1 (mice), IgG4 (humans) and IgE, eosinophil and basophil haematopoiesis, and the expansion of alternatively activated macrophages, goblet cells and mast cells [reviewed in 15]. While type 2 cytokines were originally identified as being produced by T cells, an increasing number of studies have identified innate cell populations that contribute to their secretion. Of these, basophils 16 are a major source of IL-4 17,18 and a novel population of type 2 innate lymphoid cells (ILC2), which lacks T- or B-cell markers and is expanded by N. brasiliensis infection, represents an important early source of IL-13 and IL-5 19–22. Trichuris muris infection results in the expansion of a population of multipotent progenitor cells that produce IL-4 termed MPPtype 2 cells 23. These cells express several haematopoietic stem cell markers, but unlike ILC2, have the ability to differentiate into several cellular lineages including mast cells and macrophages 23. Expansion of ILC2 or MPPtype 2 cells following helminth infection represents one of the earliest events, and these cells likely shape the nature of the ensuing adaptive immune response. A better understanding of the development and function of these cells will undoubtedly be critical to our understanding of immune responses against intestinal helminths.
The mechanisms by which type 2 immunity is initiated in response to helminth infection remain unclear. Most host–pathogen interactions involve recognition of pathogenic molecular patterns by host pattern recognition receptors (PRR), yet the search for PRRs recognizing helminth products has yielded few results. Intestinal epithelial cells (IECs) have been identified as important in the initiation of type 2 immune responses following intestinal helminth infection 24. Mice with an IEC-specific defect in NF-κB activation (IkkbΔIEC mice) are susceptible to T. muris infection and produce decreased levels of IL-4, IL-5 and IL-13 and increased levels of IFN-γ leading to a failure to expel worms 24. IECs produce several cytokines that are required for the development of polarized type 2 immunity in response to T. muris including thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 24–28. IEC-intrinsic, NF-κB-dependent, production of TSLP is critical for licensing dendritic cells (DCs) to allow the development of adaptive CD4+ type 2 (Th2) cell responses 24,25,28. Mice deficient in the receptor for TSLP (TSLPR KO) are susceptible to T. muris infection 25, and antibody blockade of IL-12p40 or IFN-γ in either IkkbΔIEC mice or TSLPR KO mice following Trichuris infection renders these susceptible strains resistant 24,25,28. However, the development of type 2 immunity following infection with N. brasiliensis or H. p. bakeri does not require TSLPR 28. Thus, it is likely that TSLP is not required to directly promote protective immunity, but instead limits the development of nonprotective type 1 responses by suppressing the production of IL-12p40.
IL-25 and IL-33 (a member of the IL-1 family) have been shown to be produced by IEC rapidly following helminth infection. These cytokines play a crucial role in the regulation of type 2 cytokine production 26,27 and protective immunity against multiple helminth parasites including T. muris 27, N. brasilienis 29 and H. p. bakeri 22. IL-25 can also be produced by Th2 cells 30 and can act on a number of cell types including antigen-presenting cells 31, airway smooth muscle cells 32 and invariant natural killer T (iNKT) cells 33. Injection of recombinant IL-25 into naïve mice stimulates the production of type 2 cytokines by ILC2 19,29 or MPPtype 2 cells 23, induces IL-4 production by invariant iNKT cells 33 and facilitates the differentiation of Th2 cells 34. IL-25 has recently been shown to elicit both ILC2 and MPPtype 2 simultaneously, although these cell populations are distinct in their transcriptional profile, developmental programs and pluripotency 35. IL-33 is able to induce the secretion of type 2 cytokines (IL-4, IL-5 and IL-13) by Th2 cells 36–38, basophils and mast cells 39,40 and ILC2 29.
While ILC2 and MPPtype 2 cells are generally considered to arise directly as a result of IL-25 and IL-33 production, much controversy surrounds the issue of how Th2 cells are activated. DCs are the primary antigen-presenting cell (APC) of the immune system and are typically regarded as necessary for the activation of naïve CD4+ T cells. Yet, despite a clear increase in the frequency and number of DC in the tissues and mesenteric lymph nodes (mLN) following T. muris infection, DCs do not appear to be the primary APC required to promote Th2 differentiation. Mice that express MHC class II solely on DC are susceptible to infection with T. muris 41, indicating the existence of another APC population. Basophils, a rare granulocyte population (<0·5% of circulating cells), are often associated with helminth infection and can produce the Th2-polarizing cytokine IL-4 42. Recent studies highlighted this cell as playing a specific and critical role in antigen presentation during T. muris infection 41. Infection resulted in the transient appearance of these cells within the mLN where they functioned as APCs 41. Moreover, antibody depletion of basophils rendered normally resistant mice susceptible to T. muris infection. Interestingly, however, expulsion of adult worms following primary infection with N. brasiliensis 42,43 or secondary infection with H. p. bakeri 44 does not require basophils. Moreover, Th2 cell differentiation has been shown to be entirely dependent on CD11c+ DCs following N. brasiliensis or H. p. bakeri infection 45,46. Thus, depending on the context of the infection, DC or basophils promote Th2 cell differentiation by acting as APCs, with basophils additionally providing a source of IL-4 to boost type 2 immunity. Like DC and basophils, mast cells form a potent arm of the innate immune response and are capable of responding to the presence of pathogens. Of particular interest, mast cell degranulation following H. p. bakeri infection was recently reported to be required for the enhanced expression of IL-25, IL-33 and TSLP within the gut indicating that these cells play a role in the early response to helminth infection 47.
Immune mechanisms of worm expulsion
Resistance to infection with Trichuris spps in mice and pigs is associated with the activation of Th2 cells that produce the cytokines IL-4 and IL-13 48–51. As mentioned, the differentiation of Th2 cells is driven by binding of the type 2-associated cytokines IL-4 and IL-13 to IL-4Rα on the T-cell surface, leading to activation of signalling intermediates such as STAT6 and resulting in the activation of the master transcriptional activator, GATA3 52. Infection of humans also results in a Th2 cell-biased immune response, with elevated levels of IL-4 and IL-13 49,50, and immunoglobulin class-switching to IgG4 and IgA 53. Th2 cells and activation of STAT6 signalling pathways are also essential for the expulsion of N. brasiliensis and H. p. bakeri. Interestingly, transfer of ILC2 into wild type, but not RAG deficient mice (lacking T and B cells), promotes expulsion of N. brasiliensis 20,29, indicating that adaptive immune cells are necessary for the effector function of ILC2. Th2 cells represent a potent source of IL-25 and addition of exogenous IL-25 into RAG mice lacking Th2 cells could restore worm expulsion 29, indicating that the main role of Th2 cells in worm rejection may be to maintain the expansion of ILC2. However, other cells that are induced by IL-25 treatment may also promote ILC2 expansion. The ability of ILC2 to directly mediate worm expulsion likely results from the large quantity of IL-13 produced by these cells because addition of an exogenous source of the related cytokine, IL-4, in the form of immune complexes can also promote worm expulsion in H. p. bakeri- or N. brasiliensis-infected mice 54.
But what are the mechanisms by which IL-14/IL-13 mediate worm expulsion? These cytokines are well known to activate IL-4Rα-dependent responses in numerous intestinal cell types including epithelial cells (IEC), goblet cells, smooth muscle cells and macrophages. IECs are in a state of constant proliferation resulting in the regeneration of the intestinal epithelium. Trichuris muris larvae live embedded within IEC within the caecum 55, and increased epithelial cell proliferation and turnover can result in parasite expulsion. In susceptible strains of mice, increased epithelial proliferation is observed, but IEC turnover is limited, resulting in crypt elongation and a failure to expel the parasite 56. Surprisingly, the control of epithelial turnover during T. muris infection is controlled by the IFN-γ-dependent chemokine CXCL10. Antibody blockade of CXCL10 is sufficient to render susceptible mice, including immunodeficient mice, resistant to T. muris infection. Yet, signalling through IL-4Rα expressed on IEC is also critical for immunity to T. muris 57. Whether IL-4/IL-13 signalling in IEC promotes proliferation has not been examined. However, these cytokines promote intestinal permeability and increased fluid section, which are likely to contribute to the expulsion of luminal worms. Type 2 responses have also been shown to stimulate Paneth-cell growth and secretion of antibacterial products that may harm helminths 58,59.
IL-4/13 also stimulate goblet cells, and the goblet cell product resistin-like molecule (RELM) β plays an important role in the expulsion of N. brasiliensis and H. p. bakeri, presumably by interfering with their feeding upon host tissues 57. RELMβ is also highly induced during Trichuris infection and has been shown to bind to secretory structures on adult Trichuris worms 60. Surprisingly, however, RELMβ is dispensable for resistance to T. muris 61. Another major goblet cell product, mucin, has recently been identified as playing a role in parasite expulsion. The mucins Muc2 and Muc5ac are upregulated during worm expulsion and are required for clearance of T. muris and N. brasiliensis from the intestine 62,63. In contrast to the well-characterized intestinal mucin Muc2, Muc5ac is primarily expressed in the airways and is specifically induced in the intestine during helminth infection. In keeping with its persistence within the intestinal lumen, H. p. bakeri infection does not lead to increased Muc5ac expression 63,64. Additional goblet cell proteins including intelectin 60,65, chloride channel calcium-activated 3 59,66, pancreatic lipase-related protein 2 and pancreatic colipase 67 are upregulated during T. muris infection, but the roles of these proteins in parasite expulsion remain unknown.
IL-4 and IL-13 also induce increased contractility of intestinal longitudinal smooth muscle cells. However, deletion of the gene encoding the shared receptor subunit, IL-4Rα, specifically on smooth muscle cells only contributes partially to resistance against N. brasiliensis 68, indicating that this response plays a secondary or minor role in worm expulsion. Macrophages were recently identified as contributing to expulsion of N. brasiliensis 69, although their depletion with clodronate-loaded liposomes has no effect on the expulsion of T. muris 41. These cells contribute to smooth muscle hypercontractility following N. brasiliensis infection 69; however, their impact on host immunity and physiology may be much broader and should be investigated in more detail.
In addition to the canonical Th2 cell-associated cytokines IL-4 and IL-13, IL-9 has also been shown to be induced by T. muris infection and to play an important role in protective immunity 70–73. Transgenic over expression of IL-9 in mice resulted in rapid worm expulsion 73. In contrast, immunization with IL-9-ovalbumin complexes that led to a robust anti-IL-9 response 74, or treatment with anti-IL-9 antibodies 75, in normally resistant C57BL/6 mice rendered the animals susceptible to T. muris infection. Functionally, IL-9 can stimulate smooth muscle contractility 75, a potential mechanism of worm expulsion 76 and can potentiate IL-4-dependent immunoglobulin class-switching to IgE 73,77–79. IL-9 also promotes mucosal mastocytosis 73, a typical feature associated with type 2 immune responses and intestinal helminth infection [reviewed in 80]. Although mast cells are not required for expulsion of T. muris 81, they contribute to the development of type 2 immunity following H. p. bakeri infection 47 and are essential mediators of immune expulsion of Trichinella spiralis, a natural parasite of mice 82–84. Mucosal mast cells express a variety of effector molecules, including proteases that degrade tight junctions allowing the influx of fluids into the intestinal lumen, a process that likely contributes to their ability to promote worm expulsion 85.
Overall, the literature to date indicates that a variety of mechanisms contribute to the immune expulsion of adult helminths from the intestinal lumen, with the exact means of expulsion being highly dependent on the species present. A generalized summary of possible expulsion mechanisms is shown in Figure1.
Figure 1.
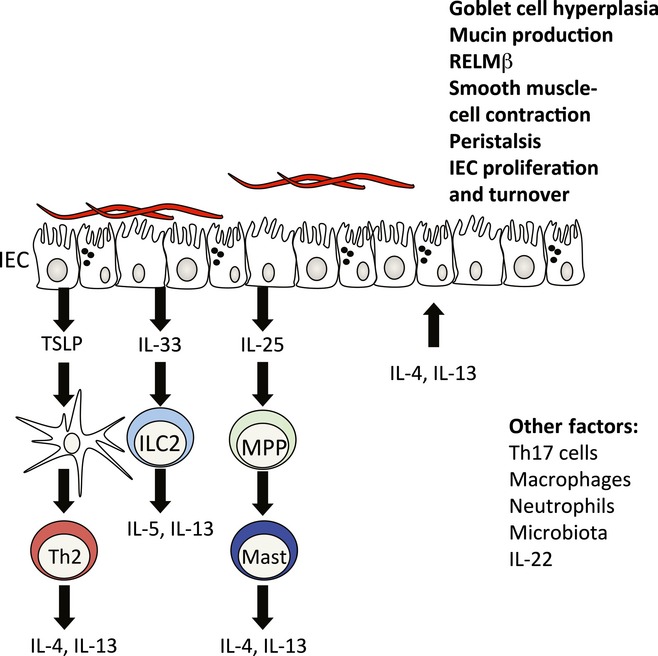
Mechanisms of expulsion of intestinal worms. Although the initial interaction between helminths and the host are poorly defined, infection results in the production of epithelial cell-derived cytokines such as thymic stromal lymphopoietin (TSLP), IL-33 and IL-25. Resistance to some helminth infections is independent of TSLP. Induction of TSLP regulates dendritic cell (DC) production of IL-12 and promotes basophilia (Baso), both leading to priming of type CD4+ T-cell responses (Th2). IL-33 is normally a nuclear protein that is released upon cellular damage. IL-33 is a potent activator of type 2 innate lymphoid cells (ILCs) that occurs early after helminth infection. IL-25 is induced in response to the microbiota and is increased following helminth infection. IL-25 induces a multipotent progenitor cell (MPP) that can give rise to other innate cell lineages. The result of these pathways is to promote a TH2 cell response and high levels of IL-4 and IL-13. These cytokines promote worm expulsion by inducing physiological changes in the intestinal epithelium. Some expulsion mechanisms include goblet cell hyperplasia and mucus secretion, increased proliferation and turnover and smooth muscle contractility and peristalsis. In addition, although they are not critical for resistance, other factors such as cells (neutrophils, macrophages and Th17 cells), cytokines (IL-22) and the microbiota are dynamically regulated during infection and most likely play a regulatory role in the development of protective immunity to helminth infection.
There are limited data on the mechanisms by which lumen-dwelling intestinal helminths are expelled in humans largely because of ethical and practical limitations to the investigation of the human intestinal tract. Histological studies of intestinal biopsy samples from individuals infected with intestinal helminth parasites generally show only mild alterations 86–88, indicating the close adaptation of these parasites to humans. In the case of trichuriasis, heavily infected children may develop colitis (inflammation of the large intestine) 89 or rarely a dysentery-like syndrome (Trichuris dysentery syndrome or TDS) 90. Children with TDS have greater numbers of mucosal IgE+ mast cells that show prominent degranulation by electron microscopy and high rates of spontaneous histamine release ex vivo 90, indicating that immediate hypersensitivity reactions per se may be ineffective in expelling T. trichiura adult worms. New data, derived from experimental infections of human volunteers with Necator americanus, have shed light on the mechanisms by which human hookworm may be expelled from the intestinal tract. Interestingly, most experimentally infected larvae reach the intestinal tract with little evidence of attrition during systemic migration 91,92. Experimentally infected individuals develop enteritis (inflammation of the small intestine) during primary infections 92, a clinical picture that is typical of human infections with the dog hookworm, Ancylostoma caninum 93. In the case of human infections with N. americanus, the mucosal inflammatory response appears to be restricted towards immature worms, characterized histologically by an intense eosinophilic inflammation associated with a shortened attachment time of the larvae and their progressive distal expulsion along the gut 92. The intensity of eosinophilic inflammation in biopsy samples was positively associated with the rate of larval expulsion 94. Immature worms are probably the primary target for expulsion mechanisms because, during repeat infections, mucosal histology is normal at sites adjacent to mature worms 92.
Mechanisms of chronicity
Treatment of mice with IL-4 complexes or exogenous recombinant IL-25 can promote worm expulsion in chronically H. p. bakeri-infected animals indicating that inadequate type 2 immunity is responsible for the initial failure of mice to expel these worms. This hypothesis is supported by the recent findings that H. p. bakeri-infected mice display little expansion of ILC2 95 and that transfer of IL-13-producing macrophages into mice harbouring a chronic H. p. bakeri infection can promote worm expulsion 96. Why host immunity is inadequate in expelling the worm is not clear, but it may be related the potent ability of this parasite to elicit regulatory T-cell expansion as depletion of these cells results in enhanced Th2 immunity 97. Interestingly, a recent study identified the existence of a TGF-β homologue within H. p. bakeri that functioned to promote conversion of naïve T cells to Foxp3+ regulatory T cells in vitro 98.
Genetics also plays a strong role in resistance or susceptibility to infection with intestinal helminths. For H. p. bakeri, rapid rejection of adult worms or the establishment of chronicity differs for various mouse strains with SJW and SJL mice expelling primary infection within 4–6 weeks, C57BL/6 and 129/J mice exhibiting an intermediate phenotype and CBA, C3H, SL and AJ mice exhibiting very little resistance to primary or challenge infections [reviewed in 99]. Infection of most strains of mice including BALB/c, C57BL/6, 129/J and C3H with T. muris results in worm expulsion between days 18 and 21. AKR/J mice are currently the only immunocompetent strain that fails to clear T. muris infection. A recent study has used F2 intercrosses between resistant BALB/c and susceptible AKR/J mice to identify quantitative trait loci (QTL) associated with chronic infection and inflammation 100. Seven QTL on seven chromosomes were identified (Tm1,Tm3,Tm4,Tm10,Tm11,Tm12 and Tm17). Consistent with a role in helminth immunity, the Tm1 and Tm17 loci have previously been identified in studies examining susceptibility to H. p. bakeri 101. Strikingly, one QTL, Tm3, completely overlaps with a region termed Cdcs1.1, previously identified in three unrelated spontaneous colitis models 102–104. Thus, genetic studies have shown that there appear to be genomic sites associated with both immunity to infection and regulation of intestinal inflammation.
The epidemiologic patterns of nonrandom clustering of infections and clustering at family and household levels have been interpreted as evidence for genetic susceptibility to ascariasis 105. Genome wide analyses for genes associated with susceptibility to ascariasis identified as a candidate gene, TNFSF13B, a regulator of B-cell activation and immunoglobulin secretion. Subsequent analyses have suggested additional possible genes associated with immune function 105, but these studies have shed little light so far on the mechanisms of susceptibility and establishment of chronic infections. Insights into the immunological mechanisms associated with the establishment of infections with mature adult hookworms have been provided by experimental infections of individuals with coeliac disease in remission 106. Comparisons of duodenal mucosal biopsies from such individuals compared with uninfected controls showed that the survival of mature adults in the small intestine during primary infections was associated with an increased protein and/or mRNA expression for Th2 (IL-4, IL-5, IL-9 and IL-13), regulatory (IL-10 and TGF-β) and mucosal healing (IL-22) cytokines 106. Comparison of pre- and post-infection biopsies, also indicated evidence for suppression of IL-23, a key cytokine involved in driving mucosal inflammation 106.
Protective Immunity against Re-Infection
For the majority of experimental helminths, repeated infection rounds of mice, of the appropriate genetic background, can elicit protective immunity if chemotherapy is administered between infection rounds to eliminate existing adult worms. Where observed, this protective response is normally targeted against the larval stages of the parasite. There is little evidence, however, that repeated rounds of chemotherapy, given to children living in endemic areas, contribute to the development of protective immunity against intestinal helminths.
Immune memory against intestinal helminths
Most experimental helminths can elicit the development of memory T cells, as evidenced by the more rapid production of IL-4 and IL-13 by CD4+ T cells following re-infection 107. In mice, long-term mucosal immunity against T. muris requires CD4+ Th2 cells and these cells persist even in the absence of chronic infection 107,108. Secondary infections are rapidly rejected (prior to day 12 post-infection), suggesting that infection-induced immunity is directed against larval stages. However, the mechanisms associated with this rapid immunity against re-infection with T. muris are unknown.
In humans, the magnitude of the Th2 cell response during T. trichiura infection is correlated with the probability of re-infection 49,50,109, indicating that protective immunity is mediated by mechanisms requiring type 2 cytokine production. Similarly in a hookworm endemic area of Papua New Guinea, resistance to re-infection following chemotherapy was associated with elevated production of IL-5 by PBMCs stimulated with parasite antigen 110.
For N. brasiliensis, clear evidence indicates that protective immunity against challenge infections occurs against larvae present in the lung 111. The development of immunity is dependent on Th2 cells, and many cell types increase in the lung following re-infection, including eosinophils and alternatively activated macrophages. However, again, the identity of the immune effectors that successfully target the helminth larvae has remained elusive. Antibodies are not necessary for immune protection following N. brasiliensis re-infection 112; however, passive transfer of serum from immune mice can confer some degree of resistance 113. More information is available for H. p. bakeri where protective immunity is targeted at larvae present within the intestinal submucosa. In this case, Th2 cells mediate the development of a granuloma around the larvae that is rich in eosinophils and alternatively activated macrophages, and protection is macrophage dependent 114. Antibodies also form an important arm of protective immunity in this model; however, the mechanisms by which these target larvae are not clear.
By contrast, a role for antibodies in protective immunity against intestinal helminths in humans is not established. Infected individuals including those with ascariasis have high circulating levels of specific antibodies of almost all isotypes and subclasses and antibody levels are generally positively associated with parasite burdens 115. However, levels of specific IgE against A. lumbricoides 116,117 and T. trichiura 118 and IgE reactive to larval antigens against hookworm 119 have been associated with resistance to infection with A. lumbricoides,T. trichiura and hookworm, respectively. Additional evidence for a possible protective role of IgE against intestinal helminth infections came from a placebo-controlled randomized trial of the use of anti-IgE therapy in asthma: in a region of Brazil where intestinal helminths are present, anti-IgE treatment was associated with a trend of increased risk of geohelminth infection, primarily with A. lumbricoides 120. It therefore seems reasonable to infer that specific IgE has a role to play in protective immunity either through the initiation of allergic-type responses to the parasites or in the amplification of other Th2-mediated mechanisms.
Vaccination
Vaccination relies on the development of immune memory with successfully vaccinated individuals raising a rapid and strong protective response following encounter with the true pathogen. Yet, despite the increased knowledge of the cellular and molecular requirements for protective immunity to intestinal helminths, there are currently no vaccines available against human species. In mice, subcutaneous vaccination with T. muris excretory/secretory (ES) products or adult worm homogenate in the presence of complete or incomplete Freund's adjuvant rendered susceptible mice resistant to infection and was associated with an increased Th2 cell response 121–126. In humans, an interesting vaccine target was identified as a 47 kDa antigen present in adult T. trichuria (43 kDa in T. muris) that is strongly recognized by human immune serum 127 and which can form pores in cells 128.
There has been considerable investment into the development of hookworm vaccines, and several promising candidates have been identified 129. A safety and immunogenicity study using Necator americanus Ancylostoma-secreted protein 2 (Na-ASP-2) caused generalized urticarial reactions in Brazilian adults previously infected with hookworm 130 and was associated with the presence of pre-existing specific IgE to this antigen. New vaccine candidates have targeted important parasite enzymes such as Na-GST-1 and Na-APR-1 that are required by the parasite for feeding on host blood 129. However, the need to induce Th2 responses for a vaccine to be useful for protection while avoiding such adverse allergic responses poses a major challenge for the development of vaccines against intestinal helminth parasites and it remains to be seen if a vaccine targeted against established adults in the intestine can be safe and effective.
Impact on Inflammatory Diseases
Evidence of heavy helminth burdens can be found in the mummified remains of early hominids reflecting our long co-evolution with these pathogens 131. Although helminth infections still infect almost one-third of the human population, the introduction of municipal sanitation has largely resulted in their eradication from developed countries. The relatively recent absence of intestinal helminths within developed societies has been hypothesized to be associated with a possible increased incidence of immune-mediated inflammatory diseases, including allergies, autoimmunity and inflammatory bowel disease (IBD) [reviewed in 132]. In support of this concept, helminths were recently described to represent the main selective force for the selection of human genes associated with autoimmunity and allergy 133.
Immunomodulation by intestinal helminths
There is growing interest in the potential effects of intestinal helminths in modulating inflammatory diseases of the mucosa. Modulatory effects of intestinal helminths have been reported for inflammation in the intestine and in the lungs, but the evidence for a clinically relevant role is still much stronger in experimental murine models than in humans.
Asthma
Temporal trends of increased asthma prevalence over recent decades have been attributed to changes in the living environment that includes declining exposures to infectious diseases and microbial products 134,135. Helminth parasites have attracted considerable interest as a potential exposure that might modify allergic inflammation and asthma. Murine models have shown clearly that intestinal helminth infections can modulate airway inflammation 7,136. The intestinal helminth H. p. bakeri has been shown to suppress allergen-induced airway eosinophilia 9,136 and bronchial hyper-reactivity 9 induced by sensitization with ovalbumin (OVA) 9,136 or Dermatophagoides pteronyssinus allergen p 1 (Der p 1) 136. Suppression was transferable to uninfected animals by splenocytes 9 or mesenteric lymph node cells 136 and has been associated with regulatory T cells 136 and regulatory B cells 137. Nippostrongylus brasiliensis can induce alternatively activated macrophages during primary infections 138, and infections are associated with a protracted suppression of airway hyper-reactivity and inflammation to D. pteronyssinus 139. Findings from human studies have been less clear. In cross-sectional studies, hookworm infection has been associated with a reduced prevalence of wheeze or asthma 140, but A. lumbricoides and T. trichiura, or markers of infection, have been associated with an increased risk 140–143. So far, no well-controlled trial has shown an effect of periodic antihelminthic treatment on the prevalence of asthma 144,145. Randomized therapeutic studies using small infective doses with hookworm larvae have not shown clear clinical benefits of infection on symptoms of asthma 146 or allergic rhinitis 146. Similarly, a randomized controlled trial of the efficacy of the pig whipworm, T. suis, in the treatment of allergic rhinitis showed no demonstrable benefit 147. The ability to show a clinical benefit in asthma likely requires very marked effects on inflammation in the lungs, and it seems unlikely that helminth treatment, at doses and infection periods that are free of significant adverse effects, will be a useful therapeutic approach. More useful, perhaps, will be the isolation of helminth products that have specific effects in distinct inflammatory pathways.
Inflammatory bowel disease
Inflammatory bowel diseases that include Crohn's diseases and ulcerative colitis are associated with impairments in epithelial function and dysregulated immune responses to commensal bacteria in the intestinal tract 148. Increases in the prevalence of IBD over recent years have been attributed to improved hygiene and the disappearance of intestinal helminth infections 149. Experimental murine models have shown that intestinal helminths can reduce inflammation in chemical-induced colitis 8,130, and the Th2 response induced by H. p. bakeri infection can attenuate experimental gastritis caused by Helicobacter pylori infection 150. The mechanisms by which helminths may protect against colitis include the induction of a Th2 cytokine environment in the mucosa 151,152, downregulation of Th1 96 and Th17 mucosal responses 153, upregulation of IL-10 and TGF-β by colonic regulatory T cells 152,154, the induction of regulatory DCs 155 or alternatively activated macrophages in the intestinal mucosa 156, and by regulatory effects on mucosal innate immune responses 157–159.
The usefulness of therapeutic infections with intestinal helminths has been evaluated in patients with IBD. Treatment with T. suis ova was associated with clinical improvement in a randomized controlled trial of patients with ulcerative colitis 160. Two other studies have reported temporary improvements in symptoms of patients with ulcerative colitis 161 and Crohn's disease 160,161 following T. suis therapy, but these studies are difficult to evaluate because no comparison groups were included. The use of N. americanus infections for the treatment of IBD has been evaluated for Crohn's 162 disease and for coeliac disease 163: in the case of Crohn's disease, a small open trial provided some evidence for reductions in disease activity 162, but for coeliac disease, a small randomized controlled trial showed no demonstrable clinical benefit of hookworm infection 163, although there was evidence for reductions in Th1 and Th17 immune responses in the duodenal mucosa of hookworm-treated subjects 132. Therefore, as for asthma, the potential for the clinical use of helminth therapy for the treatment of IBD remains doubtful, and adequately powered, properly controlled, randomized trails will be required to fully evaluate the therapeutic usefulness of such a strategy.
Interactions with the intestinal microbiota
As intestinal helminths and commensal bacteria inhabit the same environmental niche, it is likely that these organisms interact with, and impact on, each other. Intestinal helminths are well known to alter intestinal physiology, permeability, mucous secretion and the production of antimicrobial peptides – all of which may impact on bacterial survival and spatial organization. Yet, despite rapid advances in our understanding of host–intestinal bacteria interactions, the impact of helminths on the relationship has remained largely unexplored. That such interactions do take place was highlighted in a recent report 164, indicating that bacterial interactions are essential for the hatching of embronyated T. muris eggs. Such interactions are likely to also be required in the intestine as active T. muris infection was not observed in mice infected with embronyated eggs and additionally treated with broad-spectrum antibiotics to diminish the numbers of intestinal bacteria. It also appears that the presence of helminths can alter the nature and complexity of intestinal bacterial communities as H. p. bakeri infection of mice was observed to result in an increase in abundance of Lactobacillaceae family members at 14 days post-infection 165. However, whether this bacterial dysbioses resulted from helminth infection per se or from the introduction of bacteria associated with the faecal-hatched larvae was not established. Nevertheless, interactions of helminths with bacterial communities may occur raising the possibility that such interactions contribute to immune modulation and to the general well-being of the host. Increasing evidence suggests that alterations to intestinal bacterial communities (dysbioses) are associated with chronic inflammatory diseases including obesity, IBD, diabetes and allergy 166. Thus, the exact nature of helminth–bacterial interactions – and the contribution of this to host immunity and disease – will be an important topic for future studies in both man and mice.
Concluding Comments and Perspectives
The large majority of intestinal helminths elicit polarized type 2 immune responses, and type 2 cytokine production is essential for effective immune expulsion of adult worms or protection against re-infection. The distinction between innate and adaptive immunity has become increasingly blurred, and type 2 immunity following helminth infection is likely to involve important contributions first from innate immune cells and later from T cells that act to both amplify type 2 cytokine secretion and to sustain the activity of innate immune cells. Yet, despite the protective role of type 2 immune responses in animal infections, most human intestinal helminths remain chronically within their host for years and children tend to suffer increasing worms burdens acquired through constant re-infection. These findings indicate that immunity is often inadequate against these macro-parasites. Evidence in mouse models indicates that the immune system can effectively target helminths, but that the response must be both strong and rapid. Helminths efficiently evade host immunity through a number of mechanisms including but not limited to (i) promoting a strong regulatory T-cell response that diminishes type 2 immunity, (ii) immune deviation towards type 1 cytokine production and (iii) production of molecules with immune dampening properties.
Investigations of our relationship with intestinal helminths can thus reveal fascinating information about immune regulation. A better understanding of how intestinal helminths regulate mucosal inflammatory responses may prove useful for the development of new treatments for chronic inflammatory diseases of the mucosa that are driven by over-exaggerated or inappropriate immune stimulation. By contrast, an improved understanding of protective immune mechanisms against intestinal helminths will be key to the development of vaccines against these important pathogens. Recent studies have also demonstrated the importance of rapid wound healing for host survival following helminth infection 167. Further studies investigating the impact of immune cells on this response are likely to reveal novel insights into this fundamental process. Lastly, we can no longer consider the host–helminth relationship in isolation. Helminths and bacteria live alongside one another within the intestine and both have been shown to have important impacts on the host immune system. Future studies should consider this ‘ménage à trois’ situation, particularly when investigating the immunomodulatory potential of helminths because bacterial dysbioses is linked to a variety of inflammatory disease.
Acknowledgments
PJC is supported by the Wellcome Trust (grant 088862/Z/09/Z). C.Z. is supported by the Canadian Institutes of Health Research, the Michael Smith Foundation for Health Research and Canada Foundation for Innovation. C.Z. is a CIHR New Investigator and an MSFHR Career Investigator. N.L.H. is supported by the Swiss Vaccine Research Institute and by a Swiss National Foundation (grant 310030-133104).
References
- 1.Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli AF. Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis. 2008;2:e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. WHO Information Series on School Health-Document 1. Strengthening Interventions to Reduce Helminth Infections: An Entry Point for the Development of Health Promoting Schools. Geneva: WHO; 1997. [Google Scholar]
- 4.WHO. Deworming for Health and Development: Report of the Third Partners for Parasite Control Meeting. Geneva: WHO; 2005. [Google Scholar]
- 5.Pawlowski Z. Schad G and Stott G. Geneva: WHO; 1991. [Google Scholar]
- 6.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF., Jr Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 7.Fallon PG. Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 8.Khan WI, Blennerhasset PA, Varghese AK, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV. Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 10.Su Z, Segura M. Stevenson MM. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect Immun. 2006;74:2138–2144. doi: 10.1128/IAI.74.4.2138-2144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban JF, Jr, Steenhard NR, Solano-Aguilar GI, et al. Infection with parasitic nematodes confounds vaccination efficacy. Vet Parasitol. 2007;148:14–20. doi: 10.1016/j.vetpar.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MS. Maizels RM. Regulatory T cells induced by parasites and the modulation of allergic responses. Chem Immunol Allergy. 2006;90:176–195. doi: 10.1159/000088892. [DOI] [PubMed] [Google Scholar]
- 13.Bundy D. Population ecology of intestinal helminth infections in human communities. Philos Trans R Soc Lond. 1988;321:405–420. doi: 10.1098/rstb.1988.0100. [DOI] [PubMed] [Google Scholar]
- 14.Gouy de Bellocq J, Ferte H, Depaquit J, Justine JL, Tillier A. Durette-Desset MC. Phylogeny of the Trichostrongylina (Nematoda) inferred from 28S rDNA sequences. Mol Phylogenet Evol. 2001;19:430–442. doi: 10.1006/mpev.2001.0925. [DOI] [PubMed] [Google Scholar]
- 15.Allen JE. Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 16.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima H, Gleich GJ. Kita H. Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J Immunol. 1996;156:4859–4866. [PubMed] [Google Scholar]
- 18.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–1027. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 19.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Muto T, Kawagoe T, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 25.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owyang AM, Zaph C, Wilson EH, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphreys NE, Xu D, Hepworth MR, Liew FY. Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 28.Massacand JC, Stettler RC, Meier R, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 31.Gratchev A, Kzhyshkowska J, Duperrier K, Utikal J, Velten FW. Goerdt S. The receptor for interleukin-17E is induced by Th2 cytokines in antigen-presenting cells. Scand J Immunol. 2004;60:233–237. doi: 10.1111/j.0300-9475.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 32.Lajoie-Kadoch S, Joubert P, Letuve S, et al. TNF-alpha and IFN-gamma inversely modulate expression of the IL-17E receptor in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1238–L1246. doi: 10.1152/ajplung.00301.2005. [DOI] [PubMed] [Google Scholar]
- 33.Terashima A, Watarai H, Inoue S, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CK, Li PW. Lam CW. Intracellular JNK, p38 MAPK and NF-kappaB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112:82–91. doi: 10.1016/j.imlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Saenz SA, Siracusa MC, Monticelli LA, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurowska-Stolarska M, Kewin P, Murphy G, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 37.Matsuba-Kitamura S, Yoshimoto T, Yasuda K, et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22:479–489. doi: 10.1093/intimm/dxq035. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Kondo Y, Yoshimoto T, Yasuda K, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 40.Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 41.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnmacht C. Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G. Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbst T, Esser J, Prati M, et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA. 2012;109:14954–14959. doi: 10.1073/pnas.1117584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KA, Harcus Y, Garbi N, Hammerling GJ, MacDonald AS. Maizels RM. Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c(+) cell depletion. Infect Immun. 2012;80:3481–3489. doi: 10.1128/IAI.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hepworth MR, Danilowicz-Luebert E, Rausch S, et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci USA. 2012;109:6644–6649. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kringel H, Iburg T, Dawson H, Aasted B. Roepstorff A. A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int J Parasitol. 2006;36:915–924. doi: 10.1016/j.ijpara.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Jackson JA, Turner JD, Rentoul L, et al. T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J Infect Dis. 2004;190:1804–1811. doi: 10.1086/425014. [DOI] [PubMed] [Google Scholar]
- 50.Jackson JA, Turner JD, Rentoul L, et al. Cytokine response profiles predict species-specific infection patterns in human GI nematodes. Int J Parasitol. 2004;34:1237–1244. doi: 10.1016/j.ijpara.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Cliffe LJ. Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 52.Murphy KM. Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 53.Needham CS, Lillywhite JE, Didier JM. Bundy DA. Serum isotype responses after treatment of human trichuriasis. Trans R Soc Trop Med Hyg. 1994;88:354–355. doi: 10.1016/0035-9203(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 54.Urban JF, Jr, Maliszewski CR, Madden KB, Katona IM. Finkelman FD. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol. 1995;154:4675–4684. [PubMed] [Google Scholar]
- 55.Tilney LG, Connelly PS, Guild GM, Vranich KA. Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J Exp Zool A Comp Exp Biol. 2005;303:927–945. doi: 10.1002/jez.a.214. [DOI] [PubMed] [Google Scholar]
- 56.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C. Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 57.Herbert DR, Yang JQ, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamal M, Dehlawi MS, Brunet LR. Wakelin D. Paneth and intermediate cell hyperplasia induced in mice by helminth infections. Parasitology. 2002;125:275–281. doi: 10.1017/s0031182002002068. [DOI] [PubMed] [Google Scholar]
- 59.Steenwinckel V, Louahed J, Lemaire MM, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 60.Artis D, Wang ML, Keilbaugh SA, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair MG, Guild KJ, Du Y, et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasnain SZ, Wang H, Ghia JE, et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasnain SZ, Evans CM, Roy M, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasnain SZ, Thornton DJ. Grencis RK. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 2011;33:45–55. doi: 10.1111/j.1365-3024.2010.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datta R, deSchoolmeester ML, Hedeler C, Paton NW, Brass AM. Else KJ. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infect Immun. 2005;73:4025–4033. doi: 10.1128/IAI.73.7.4025-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabo-Attwood T, Ramos-Nino M, Bond J, et al. Gene expression profiles reveal increased mClca3 (Gob5) expression and mucin production in a murine model of asbestos-induced fibrogenesis. Am J Pathol. 2005;167:1243–1256. doi: 10.1016/S0002-9440(10)61212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eydoux C, Aloulou A, De Caro J, et al. Human pancreatic lipase-related protein 2: tissular localization along the digestive tract and quantification in pancreatic juice using a specific ELISA. Biochim Biophys Acta. 2006;1760:1497–1504. doi: 10.1016/j.bbagen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Horsnell WG, Cutler AJ, Hoving JC, et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 2007;3:e1. doi: 10.1371/journal.ppat.0030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao A, Urban JF, Jr, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. doi: 10.1053/j.gastro.2008.03.077. e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bancroft AJ, Else KJ. Grencis RK. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur J Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- 71.Else KJ, Entwistle GM. Grencis RK. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite Immunol. 1993;15:595–600. doi: 10.1111/pim.1993.15.10.595. [DOI] [PubMed] [Google Scholar]
- 72.Else KJ, Hultner L. Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 73.Faulkner H, Renauld JC, Van Snick J. Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun. 1998;66:3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard M, Grencis RK, Humphreys NE, Renauld JC. Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci USA. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan WI, Richard M, Akiho H, et al. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vallance BA, Blennerhassett PA. Collins SM. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol. 1997;272:G321–G327. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- 77.Dugas B, Renauld JC, Pene J, et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 78.Faulkner H, Humphreys N, Renauld JC, Van Snick J. Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 79.Petit-Frere C, Dugas B, Braquet P. Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- 80.Pennock JL. Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–140. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 81.Betts CJ. Else KJ. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 82.Alizadeh H. Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984;70:767–773. [PubMed] [Google Scholar]
- 83.Oku Y, Itayama H. Kamiya M. Expulsion of Trichinella spiralis from the intestine of W/Wv mice reconstituted with haematopoietic and lymphopoietic cells and origin of mucosal mast cells. Immunology. 1984;53:337–344. [PMC free article] [PubMed] [Google Scholar]
- 84.Wong GW, Foster PS, Yasuda S, et al. Biochemical and functional characterization of human transmembrane tryptase (TMT)/tryptase gamma. TMT is an exocytosed mast cell protease that induces airway hyperresponsiveness in vivo via an interleukin-13/interleukin-4 receptor alpha/signal transducer and activator of transcription (STAT) 6-dependent pathway. J Biol Chem. 2002;277:41906–41915. doi: 10.1074/jbc.M205868200. [DOI] [PubMed] [Google Scholar]
- 85.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR. Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia FT, Sessions JT, Strum WB, et al. Intestinal function and morphology in strongyloidiasis. Am J Trop Med Hyg. 1977;26:859–865. doi: 10.4269/ajtmh.1977.26.859. [DOI] [PubMed] [Google Scholar]
- 87.MacDonald TT, Choy MY, Spencer J, et al. Histopathology and immunohistochemistry of the caecum in children with the Trichuris dysentery syndrome. J Clin Pathol. 1991;44:194–199. doi: 10.1136/jcp.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tandon BN, Das BC, Saraya AK. Deo MG. Functional and structural studies of small bowel in ankylostomiasis. Br Med J. 1966;1:714–716. doi: 10.1136/bmj.1.5489.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bundy DA. Cooper ES. Trichuris and trichuriasis in humans. Adv Parasitol. 1989;28:107–173. doi: 10.1016/s0065-308x(08)60332-2. [DOI] [PubMed] [Google Scholar]
- 90.Cooper ES, Spencer J, Whyte-Alleng CA, et al. Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet. 1991;338:1104–1107. doi: 10.1016/0140-6736(91)91964-v. [DOI] [PubMed] [Google Scholar]
- 91.Croese J. Speare R. Intestinal allergy expels hookworms: seeing is believing. Trends Parasitol. 2006;22:547–550. doi: 10.1016/j.pt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Croese J, Wood MJ, Melrose W. Speare R. Allergy controls the population density of Necator americanus in the small intestine. Gastroenterology. 2006;131:402–409. doi: 10.1053/j.gastro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 93.Croese J. Hookworm-provoked IgE-mediated pathology: capricious damage or remarkable strategy? Parasitol Today. 1998;14:70–72. doi: 10.1016/s0169-4758(97)01166-6. [DOI] [PubMed] [Google Scholar]
- 94.Croese J, Gaze ST. Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int J Parasitol. 2013;43:275–282. doi: 10.1016/j.ijpara.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Hepworth MR, Maurer M. Hartmann S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes. 2012;3:476–481. doi: 10.4161/gmic.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Z, Grinchuk V, Urban JF, Jr, et al. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS ONE. 2013;8:e59441. doi: 10.1371/journal.pone.0059441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rausch S, Huehn J, Loddenkemper C, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 98.Grainger JR, Smith KA, Hewitson JP, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reynolds LA, Filbey KJ. Maizels RM. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol. 2012;34:829–846. doi: 10.1007/s00281-012-0347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levison SE, Fisher P, Hankinson J, et al. Genetic analysis of the Trichuris muris-induced model of colitis reveals QTL overlap and a novel gene cluster for establishing colonic inflammation. BMC genomics. 2013;14:127. doi: 10.1186/1471-2164-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behnke JM, Iraqi FA, Mugambi JM, et al. High resolution mapping of chromosomal regions controlling resistance to gastrointestinal nematode infections in an advanced intercross line of mice. Mamm Genome. 2006;17:584–597. doi: 10.1007/s00335-005-0174-0. [DOI] [PubMed] [Google Scholar]
- 102.Borm ME, He J, Kelsall B, Pena AS, Strober W. Bouma G. A major quantitative trait locus on mouse chromosome 3 is involved in disease susceptibility in different colitis models. Gastroenterology. 2005;128:74–85. doi: 10.1053/j.gastro.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 103.Farmer MA, Sundberg JP, Bristol IJ, et al. A major quantitative trait locus on chromosome 3 controls colitis severity in IL-10-deficient mice. Proc Natl Acad Sci USA. 2001;98:13820–13825. doi: 10.1073/pnas.241258698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ermann J, Garrett WS, Kuchroo J, et al. Severity of innate immune-mediated colitis is controlled by the cytokine deficiency-induced colitis susceptibility-1 (Cdcs1) locus. Proc Natl Acad Sci USA. 2011;108:7137–7141. doi: 10.1073/pnas.1104234108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams-Blangero S, Criscione CD, VandeBerg JL, et al. Host genetics and population structure effects on parasitic disease. Philos Trans R Soc Lond B Biol Sci. 2012;367:887–894. doi: 10.1098/rstb.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McSorley HJ, Gaze S, Daveson J, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE. 2011;6:e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P. Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee TD, Wakelin D. Grencis RK. Cellular mechanisms of immunity to the nematode Trichuris muris. Int J Parasitol. 1983;13:349–353. doi: 10.1016/s0020-7519(83)80039-3. [DOI] [PubMed] [Google Scholar]
- 109.Turner JD, Jackson JA, Faulkner H, et al. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197:1204–1212. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- 110.Quinnell RJ, Pritchard DI, Raiko A, Brown AP. Shaw MA. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J Infect Dis. 2004;190:430–438. doi: 10.1086/422256. [DOI] [PubMed] [Google Scholar]
- 111.Harvie M, Camberis M, Tang SC, Delahunt B, Paul W. Le Gros G. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun. 2010;78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Q, Kreider T, Bowdridge S, et al. B cells have distinct roles in host protection against different nematode parasites. J Immunol. 2010;184:5213–5223. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogilvie BM. Jones VE. Passive protection with cells or antiserum against Nippostrongylus brasiliensis in the rat. Parasitology. 1968;58:939–949. doi: 10.1017/s0031182000069705. [DOI] [PubMed] [Google Scholar]
- 114.Anthony RM, Urban JF, Jr, Alem F, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Figueiredo CA, Amorim LD, Alcantara-Neves NM, et al. Environmental conditions, immunologic phenotypes, atopy, and asthma: new evidence of how the hygiene hypothesis operates in Latin America. J Allergy Clin Immunol. 2013;131:1064–1068. doi: 10.1016/j.jaci.2013.01.016. , 1068 e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McSharry C, Xia Y, Holland CV. Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67:484–489. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turner JD, Faulkner H, Kamgno J, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–996. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 118.Faulkner H, Turner J, Kamgno J, Pion SD, Boussinesq M. Bradley JE. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J Infect Dis. 2002;185:665–672. doi: 10.1086/339005. [DOI] [PubMed] [Google Scholar]
- 119.Bethony J, Loukas A, Smout M, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- 120.Cruz AA, Lima F, Sarinho E, et al. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clin Exp Allergy. 2007;37:197–207. doi: 10.1111/j.1365-2222.2007.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wakelin D. Selby GR. Functional antigens of Trichuris muris. The stimulation of immunity by vaccination of mice with somatic antigen preparations. Int J Parasitol. 1973;3:711–715. doi: 10.1016/0020-7519(73)90061-1. [DOI] [PubMed] [Google Scholar]
- 122.Jenkins SN. Behnke JM. Impairment of primary expulsion of Trichuris muris in mice concurrently infected with Nematospiroides dubius. Parasitology. 1977;75:71–78. doi: 10.1017/s0031182000048332. [DOI] [PubMed] [Google Scholar]
- 123.Jenkins SN. Wakelin D. Functional antigens of Trichuris muris released during in vitro maintenance: their immunogenicity and partial purification. Parasitology. 1983;86(Pt 1):73–82. doi: 10.1017/s0031182000057188. [DOI] [PubMed] [Google Scholar]
- 124.Else KJ. Wakelin D. Genetically-determined influences on the ability of poor responder mice to respond to immunization against Trichuris muris. Parasitology. 1990;100(Pt 3):479–489. doi: 10.1017/s0031182000078793. [DOI] [PubMed] [Google Scholar]
- 125.Robinson K, Bellaby T. Wakelin D. Efficacy of oral vaccination against the murine intestinal parasite Trichuris muris is dependent upon host genetics. Infect Immun. 1995;63:1762–1766. doi: 10.1128/iai.63.5.1762-1766.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dixon H, Little MC. Else KJ. Characterisation of the protective immune response following subcutaneous vaccination of susceptible mice against Trichuris muris. Int J Parasitol. 2010;40:683–693. doi: 10.1016/j.ijpara.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lillywhite JE, Cooper ES, Needham CS, Venugopal S, Bundy DA. Bianco AE. Identification and characterization of excreted/secreted products of Trichuris trichiura. Parasite Immunol. 1995;17:47–54. doi: 10.1111/j.1365-3024.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 128.Drake L, Korchev Y, Bashford L, et al. The major secreted product of the whipworm, Trichuris, is a pore-forming protein. Proc Biol Sci. 1994;257:255–261. doi: 10.1098/rspb.1994.0123. [DOI] [PubMed] [Google Scholar]
- 129.Hotez PJ, Diemert D, Bacon KM, et al. The human hookworm vaccine. Vaccine. 2013;31(Suppl. 2):B227–B232. doi: 10.1016/j.vaccine.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diemert DJ, Pinto AG, Freire J, et al. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130:169–176. doi: 10.1016/j.jaci.2012.04.027. e166. [DOI] [PubMed] [Google Scholar]
- 131.Araujo A, Reinhard KJ, Ferreira LF. Gardner SL. Parasites as probes for prehistoric human migrations? Trends Parasitol. 2008;24:112–115. doi: 10.1016/j.pt.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 132.McSorley HJ, Hewitson JP. Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 133.Fumagalli M, Pozzoli U, Cagliani R, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.von Mutius E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: farm lifestyles and the hygiene hypothesis. Clin Exp Immunol. 2010;160:130–135. doi: 10.1111/j.1365-2249.2010.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR. Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilson MS, Taylor MD, O'Gorman MT, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reece JJ, Siracusa MC. Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF., Jr Scott AL. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun. 2008;76:3511–3524. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leonardi-Bee J, Pritchard D. Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174:514–523. doi: 10.1164/rccm.200603-331OC. [DOI] [PubMed] [Google Scholar]
- 141.Moncayo AL, Vaca M, Oviedo G, et al. Effects of geohelminth infection and age on the associations between allergen-specific IgE, skin test reactivity and wheeze: a case-control study. Clin Exp Allergy. 2013;43:60–72. doi: 10.1111/cea.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alcantara-Neves NM, Badaro SJ, dos Santos MC, Pontes-de-Carvalho L. Barreto ML. The presence of serum anti-Ascaris lumbricoides IgE antibodies and of Trichuris trichiura infection are risk factors for wheezing and/or atopy in preschool-aged Brazilian children. Respir Res. 2010;11:114. doi: 10.1186/1465-9921-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cooper PJ, Chico ME, Vaca MG, et al. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: a cluster-randomised trial. Lancet. 2006;367:1598–1603. doi: 10.1016/S0140-6736(06)68697-2. [DOI] [PubMed] [Google Scholar]
- 145.Flohr C, Tuyen LN, Quinnell RJ, et al. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double-blind, placebo-controlled trial in Vietnam. Clin Exp Allergy. 2010;40:131–142. doi: 10.1111/j.1365-2222.2009.03346.x. [DOI] [PubMed] [Google Scholar]
- 146.Feary JR, Venn AJ, Mortimer K, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bager P, Arnved J, Ronborg S, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. e121–. [DOI] [PubMed] [Google Scholar]
- 148.Xavier RJ. Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 149.Elliott DE, Urban JJ, Argo CK. Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease? FASEB J. 2000;14:1848–1855. doi: 10.1096/fj.99-0885hyp. [DOI] [PubMed] [Google Scholar]
- 150.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 151.Chen CC, Louie S, McCormick B, Walker WA. Shi HN. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun. 2005;73:5468–5481. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Setiawan T, Metwali A, Blum AM, et al. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Elliott DE, Metwali A, Leung J, et al. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hang L, Blum AM, Setiawan T, Urban JP, Jr, Stoyanoff KM. Weinstock JV. Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis. J Immunol. 2013;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blum AM, Hang L, Setiawan T, et al. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J Immunol. 2012;189:2512–2520. doi: 10.4049/jimmunol.1102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weng M, Huntley D, Huang IF, et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen CC, Louie S, McCormick BA, Walker WA. Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ilic N, Colic M, Gruden-movsesijan A, Majstorovic I, Vasilev S. Sofronic-Milosavljevic L. Characterization of rat bone marrow dendritic cells initially primed by Trichinella spiralis antigens. Parasite Immunol. 2008;30:491–495. doi: 10.1111/j.1365-3024.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 159.Ince MN, Elliott DE, Setiawan T, et al. Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFbeta after lipopolysaccharide stimulation. J Immunol. 2006;176:726–729. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 160.Summers RW, Elliott DE, Urban JF, Jr, Thompson R. Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Summers RW, Elliott DE, Qadir K, Urban JF, Jr, Thompson R. Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 162.Croese J, O'Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut. 2006;55:136–137. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease–a randomised double-blinded placebo controlled trial. PLoS ONE. 2011;6:e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS. Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science. 2010;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Walk ST, Blum AM, Ewing SA, Weinstock JV. Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spor A, Koren O. Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 167.Chen F, Liu Z, Wu W, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]


