Abstract
Aim
Strain elastography is a method for recording tissue hardness. Strain in different areas may be compared using strain ratio (SR). The aims of this study were to validate a previously proposed SR cut-off value of 1.25 for differentiating adenocarcinomas from adenomas and to compare the performance of endorectal ultrasonography (ERUS), strain elastography and MRI in the same patients.
Method
A prospective evaluation of 120 consecutive patients with rectal neoplasia, using a predetermined elastography strain ratio cut-off value, was performed to differentiate adenomas from adenocarcinomas. ERUS and MRI were performed according to standard routine at Haukeland University Hospital, defining T0 as adenomas and T1–T4 as adenocarcinomas. Subsequent histopathology was used as the reference standard.
Results
Histopathological evaluation revealed 21 adenomas and 99 adenocarcinomas. Sensitivity, specificity and accuracy (with 95% CI) were as follows: ERUS: 0.96 (0.90–0.99), 0.62 (0.40–0.80) and 0.90 (0.83–0.94); elastography SR: 0.96 (0.90–0.99), 0.86 (0.66–0.96) and 0.94 (0.88–0.97); and MRI: 0.99 (0.94–1.00), 0.07 (0.00–0.31) and 0.87 (0.80–0.93).
Conclusion
This study confirms that the elastography SR assessment accurately differentiates sessile adenomas from adenocarcinomas. SR assessment has a superior ability to differentiate adenomas and adenocarcinomas when compared with ERUS and MRI. MRI examination seems unable to recognize adenomas and should be interpreted with care when early-stage rectal neoplasia is suspected.
Keywords: Rectum, strain elastography, endorectal ultrasound, MRI, adenoma, adenocarcinoma
Introduction
Individualized treatment of rectal neoplasia is reliant upon the accuracy of the pretreatment assessment. Adenomas can be safely treated with local resection, reducing procedure-related morbidity and mortality. Screening programmes promote adenoma detection and therefore accurate staging is increasingly important 1,2.
MRI for rectal neoplasia does not adequately discriminate adenomas from adenocarcinomas 3. Biopsy sampling errors are also known to understage disease 4,5. Consequently, preoperative assessment may not adequately inform treatment selection. Endorectal ultrasonography (ERUS) is commonly regarded as the most accurate staging modality for rectal adenomas and early rectal cancer. High accuracies for ERUS T-staging have been shown by meta-analyses 6,7, but differentiation of adenoma from carcinoma is difficult 8–12. Up to 50% of early carcinomas may be falsely classified as adenomas preoperatively 13–15.
Strain elastography is a novel method for visualization of tissue stiffness based on the tissue's resistance to deformation (strain) 16,17. Low strain is found in stiff tissue, indicative of malignancy. A colour map representing tissue strain is superimposed on a B-mode image. As the elastography algorithm is based on the information provided by ERUS B-mode images, the method aims to improve ERUS assessment, rather than to replace it. Clinical application for the differentiation of benign and malignant tumours has been validated in several organs, including the breast 18, pancreas 19, liver 20, prostate 21 and thyroid gland 22. Studies on elastography evaluation of rectal tumours are scarce 9,23, but we have previously demonstrated the feasibility of performing elastography strain ratio (SR) examination in a standard outpatient setting. An SR cut-off value of 1.25 for the differentiation of rectal adenomas and adenocarcinomas was also proposed based on receiver–operating characteristics (ROC) curve analysis 9. Consequently, the primary aim of this study was to perform a prospective validation of the proposed SR cut-off value in a diverse group of rectal tumours encountered in clinical practice. A secondary aim was to compare the performance of elastography SR assessment with ERUS and MRI evaluations for the differentiation between adenomas and adenocarcinomas.
Method
Patients
One-hundred and twenty consecutive patients referred, from 1 November 2009 to 1 April 2011, to Haukeland University Hospital for evaluation and staging of suspected rectal tumours were included. There were 67 male and 53 female patients [median age 66 (range: 25–88) years%.
Histopathology evaluation of the resected specimens revealed 21 adenomas and 99 adenocarcinomas. Sixty-one of the 99 patients with biopsy-proven adenocarcinoma received neoadjuvant radiotherapy. ERUS and strain elastography were not performed (or were technically not feasible) in five adenocarcinomas, and were consequently not assessed according to ERUS and strain elastography protocols. MRI was not performed in seven adenomas and five adenocarcinomas. Only assessed tumours were included in the analysis. Consequently, 94 adenocarcinomas and 21 adenomas were assessed by ERUS and elastography SR measurements, and 94 adenocarcinomas and 14 adenomas were assessed by MRI. Only sessile (nonpedunculated) rectal adenomas or adenocarcinomas with a distal border ≤ 15 cm above the anal verge, as verified by rigid rectosigmoidoscopy, were included. Patients with previous rectal surgery or pelvic radiation therapy were excluded. Informed consent was mandatory.
The ERUS and strain elastography examiner was blinded to the results of endoscopy, biopsy and MRI. A brief rigid rectoscopy examination was performed before ERUS and strain elastography to verify a sufficient effect of rectal enema and to identify the localization of the rectal tumour. A second rectoscopy examination was performed after the elastography examination to describe tumour morphology and obtain biopsies if necessary.
ERUS and endorectal strain elastography
All examinations were performed by the same operator (JERW). The patients underwent a same-session standardized clinical examination, ERUS and strain elastography, as described previously 9. ERUS T-stage was assessed according to the TNM-classification system and conclusions were recorded before strain elastography evaluation. In five patients the ERUS and strain elastography examinations could not be performed according to protocol because of artefacts and/or painful examination. The ERUS evaluation was used in the multidisciplinary team assessment and treatment decision. Endorectal elastography SR measurements were considered experimental and were not part of the clinical decision-making process.
We used a standard ultrasonography scanner equipped with software for elastography (Hitachi EUB-8500, software version: V16-04A; Hitachi Medical Corporation, Kashiva, Japan). Both endorectal ultrasound imaging and elastography were performed with a single rigid 360° transrectal ultrasound probe (Hitachi EUP-R54AW-19) with a micro convex array probe (5–10 MHz). Briefly, the elastography method displays a colour-coded strain map, which is superimposed on the B-mode image in real time (Fig.1). The semiquantification of tissue hardness is enabled using a quasi-static autocorrelation real-time elastography method, as previously described 9,24–26. The strain of insonified tissues is calculated from the frame-to-frame movement of tissue echoes under relatively slow compression and decompression cycles. A water-filled balloon connected to a syringe and covering the ultrasound probe was used to create strain by rhythmic inflation/deflation. The investigator applied pulsatile pressure to the area of interest until a reproducible elastogram was obtained.
Figure 1.
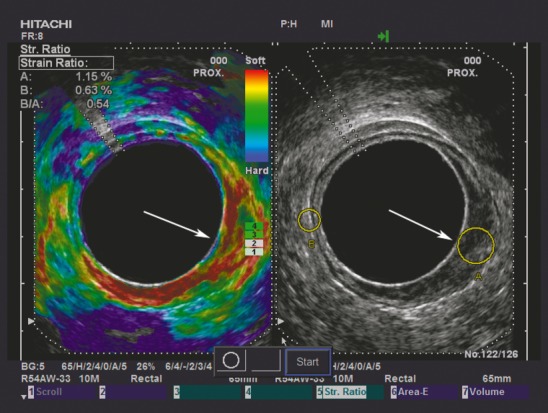
Split-screen image shows a B-mode image with strain ratio (SR) regions of interest on the right and an elastogram on the left. The tumour is situated from 2 to 7 o'clock (white arrow). The tumour appears softer (more red) than the same-depth reference tissue on the elastogram, and the SR (B/A) with SR measurement indicative of an adenoma (SR = 0.54) is displayed in the upper left-hand corner. When evaluated ultrasonographically it is difficult to determine whether the tumour is an early adenocarcinoma or an adenoma, as the hypoechoic mucosal layer is not clearly distinguished from the hyperechoic submocosal layer in the tumour region. The resection specimen was histopathologically confirmed as adenoma.
An elastogram was defined as representative if both a reproducible colour distribution and a constant B-mode image were present throughout a series of ≥ 80 consecutive frames. SR measurement was subsequently performed on five representative frames, and a mean SR was computed for statistical analysis. Tumour and reference area were selected on the B-mode image and not on the elastogram, to avoid strain field bias and focus on the sono-anatomy provided by the B-mode image. The selected areas for the SR measurements were circular and of approximately equal size and distance from the probe 25, representing tumour tissue (A) and reference tissue (B). Only tissue presenting as tumour on the ERUS B-mode image was selected for the tumour sample area, and tissue recognized as normal rectal wall and perirectal tissue, devoid of visible vessels or lymph nodes, was selected for the reference sample area (Figs.1 and 2).
Figure 2.
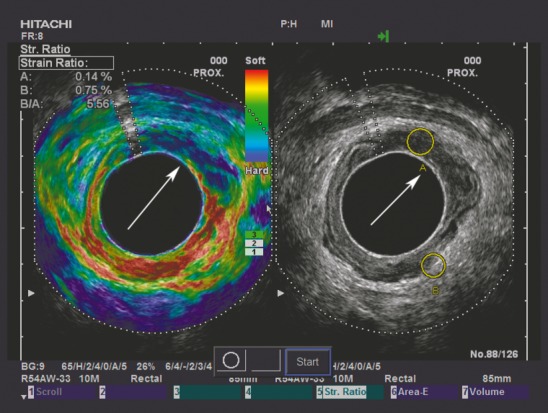
To contrast the adenoma in Fig.1, Fig. 2 demonstrates an adenocarcinoma situated from 11 to 3 o'clock, as indicated by the white arrow. The tumour appears harder (more blue) than the same-depth reference tissue on the elastogram, and a strain indicative of an adenocarcinoma (SR = 5.56) is displayed in the upper left-hand corner.
MRI evaluation
Rectal MRI was performed on 1.5-T Siemens Symphony or Siemens Symphony Vision, both running Syngo MR B17 (Siemens, Erlangen, Germany) using a phased-array, 12-channel body coil placed on the pelvis. In concordance with the examination protocol at Haukeland University Hospital, 100–150 ml of ultrasound gel (Eco supergel; Ceracarta, Forlì, Italy) was administered endorectally before imaging 3, in addition to intravenous administration of 20 mg of butylscopolamine bromide (Buscopan; Boehringer Ingelheim, Ingelhim am Rhein, Germany). The MRI protocol consisted of sagittal, coronal and axial T2-weighted turbo spin echo (TSE), high-resolution T2-weighted TSE and axial T1-weighted spin-echo sequences. High-resolution T2-weighted series were angled perpendicular to the long axis of the rectal lesion. The slice thickness in the high-resolution T2 series was 3 mm, with 3-mm spacing indicating no gap between slices 27.
The MRI reports used in the clinical decision process were revisited by a single experienced radiologist (CR) for evaluation of T-stages based on the original MRI report used in the preoperative work-up. Six patients did not undergo MRI examination before surgery, and eight MRI evaluations were inconclusive.
Histopathological evaluation
Transanal endoscopic microsurgery (TEM) specimens were pinned on a plate, fixed, serially sectioned at intervals of 2–3 mm and completely embedded. Rectal resection specimens were sliced at intervals of 3–4 mm and representative sections were selected for microscopy. Tissue sections were stained with haematoxylin-eosin. All tumours receiving neoadjuvant radiation therapy were verified by biopsy as adenocarcinomas before radiation therapy.
Statistical analysis
An SR cut-off defining malignancy as an SR of ≥ 1.25 was derived from pilot work in a discovery set of tumours 9. The test validity parameters sensitivity, specificity, accuracy, negative predictive value and positive predictive value were calculated for elastography SR evaluation, ERUS evaluation and MRI evaluation.
Histopathology of the resection specimens was used as the reference standard
One-way ANOVA was used to test for differences in SR values between pT-stages. The Blyth–Still–Casellas procedure was chosen to calculate the 95% CIs of test validity parameters 28,29. The spss version 21.0 (SPSS, Chicago, Illinois, USA), statxact 9.0 (Cytel Software Corporation, Cambridge, Massachusetts, USA) and Excel (Microsoft Office Excel 2003, Bellevue, Washington, USA) were used for data analysis.
Ethics
All patients received oral and written information, according to the Helsinki Declaration, before signing the consent form. The study was approved by the Regional Committee for Medical and Health Research Ethics of Western Norway.
Results
ERUS evaluation
The sensitivity, specificity and accuracy of ERUS evaluation were 0.96, 0.62 and 0.90, respectively (Table1). ERUS correctly identified 76% of the adenomas and 92% of the adenocarcinomas, with corresponding rates of false positives (24%) and false negatives (8%) when all histologically verified adenocarcinomas were included in the analysis.
Table 1.
Validity parameters for evaluating elastography strain ratio, endorectal ultrasonography (ERUS) and MRI differentiation of adenomas and adenocarcinomas.
| Test validity parameter | ERUS (n = 115) | Elastography (n = 115) | MRI (n = 108) |
|---|---|---|---|
| Sensitivity | 0.96 (0.90–0.99) | 0.96 (0.90–0.99) | 0.99 (0.94–1.00) |
| Specificity | 0.62 (0.40–0.80) | 0.86 (0.66–0.96) | 0.07 (0.00–0.31) |
| Accuracy | 0.90 (0.83–0.94) | 0.94 (0.88–0.97) | 0.87 (0.80–0.93) |
| PPV | 0.92 (0.85–0.96) | 0.97 (0.91–0.99) | 0.88 (0.80–0.93) |
| NPV | 0.76 (0.51–0.92) | 0.82 (0.61–0.94) | 0.50 (0.03–0.97) |
Values are given as mean (95% CI), and 95% CIs were calculated using the Blyth–Still–Casella procedure.
NPV, negative predictive value; PPV, positive predictive value.
Endorectal elastography evaluation
There was a significant difference in elastography SR between adenomas and adenocarcinomas (P < 0.001, one-way ANOVA), with a mean SR of 1.03 (95% CI: 0.85–1.21) for adenomas and a mean SR of 5.53 (95% CI: 4.27–6.79) for adenocarcinomas. As demonstrated in Fig.3, adenocarcinomas treated with surgery alone did not differ significantly in mean SR from those deemed eligible for neoadjuvant radiotherapy (P = 0.38, one-way ANOVA).
Figure 3.
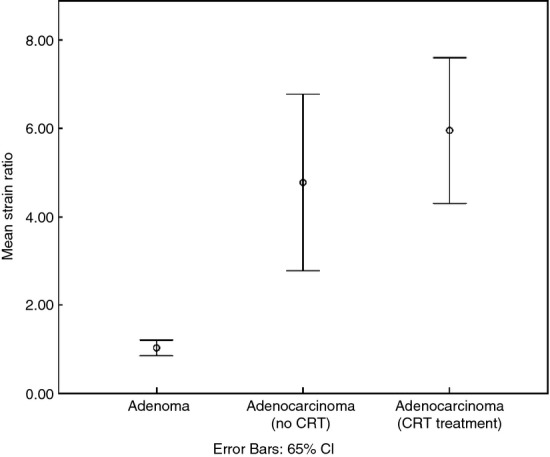
Error bar chart displaying the mean strain ratio (SR) of adenomas (n = 21) and adenocarcinomas that did (n = 61) or did not (n = 38) subsequently receive neoadjuvant radiotherapy, respectively. Adenomas were significantly different from both groups of adenocarcinomas (P < 0.001, one-way ANOVA). The mean SR of adenocarcinomas allocated to neoadjuvant treatment was not significantly different from that of adenocarcinomas treated with surgery alone (P = 0.38, one-way ANOVA). CRT, chemoradiotherapy.
The predefined elastography SR cut-off level of 1.25 9 yielded sensitivity, specificity and accuracy of 0.96, 0.86 and 0.94, respectively (Table1). Elastography evaluation correctly identified 82% of the adenomas and 97% of the adenocarcinomas, with corresponding rates of false positives (18%) and false negatives (3%) when all histologically verified adenocarcinomas were included in the analysis.
MRI evaluation
MRI yielded sensitivity, specificity and accuracy of 0.99, 0.07 and 0.87, respectively, in separating adenomas from adenocarcinomas (Table1). MRI correctly identified one of 14 adenomas, overstaging four as mrT1, four as mrT2, four as mrT3 and one as mrT4. A comparison of MRI with ERUS, strain elastography and histopathology is demonstrated in Fig.4(a–d).
Figure 4.
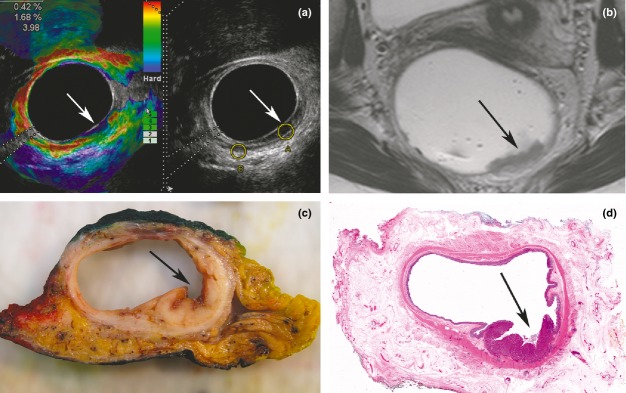
(a–d) Illustration of a rectal tumour situated 6–8 cm above the anal verge, from 3 to 6 o'clock (arrows). (a) Split-screen image with an elastogram on the left, demonstrating a strain ratio (SR) measurement of 3.98 in the upper left-hand corner, indicative of an adenocarcinoma. The B-mode image on the right of Fig. 4(a), in which a hypoechoic layer represents mucosa, seems to respect the hyperechoic submucosa layer in the tumour region.
Discussion
Endorectal elastography assessment of rectal tumours is a novel method yet to be properly introduced to clinical practice. We have previously shown that it is feasible within the constraints of a standard outpatient setting. The current study is the first prospective study to use a predefined SR cut-off value 9 to distinguish rectal adenomas and adenocarcinomas. This SR assessment of 120 consecutive patients with rectal tumours confirms a significant elastography SR difference between rectal adenomas and adenocarcinomas (P < 0.001), and the chosen SR cut-off value demonstrates an even higher accuracy (0.96; 95% CI: 0.90–0.99) than previously demonstrated. For evaluation of clinical relevance, strain elastography was compared with ERUS and MRI (Table1 and Fig.4). When comparing elastography SR evaluation with ERUS evaluation alone, the sensitivity was the same for both modalities; however, specificity, accuracy, and negative- and positive-predictive values were higher for elastography evaluation. The predictive values suggest that adding elastography to the standard ERUS examination could decrease the number of false-negative adenocarcinomas from 24% to 18% and the number of false-positive adenomas from 8% to 3%. This suggests that strain using elastography assessment as an add-on to ERUS assessment may improve the selection of tumours for local resection. A study including several low-volume institutions/examiners reporting to the UK TEMS database demonstrated poor ERUS accuracies in selecting rectal tumours for local resection 8. Although not directly addressed in our study, one might argue that the binary nature of an SR cut-off to differentiate adenomas and adenocarcinomas is probably easier to interpret by examiners with a low to medium volume of ERUS examinations. Consequently, elastography evaluation could simplify and improve selection of patients for local treatment in an even higher percentage of cases than in our study.
In hospitals not performing ERUS staging, more value in clinical decision making might be attributed to MRI examinations as MRI probably would represent the only imaging modality for local staging purposes. In this context, our finding that MRI evaluation is unable to differentiate adenomas from adenocarcinomas demonstrates a potential risk for overstaging and consequently overtreatment. These results are not contradictory to those previously published, as only verified malignant tumours tend to be included 7,30–32, and focus is frequently targeted at the ability of preoperative MRI to predict the need for neoadjuvant radiotherapy. In our study, only two tumours would have been selected for local resection based on MRI evaluation alone: one adenoma and one locally advanced adenocarcinoma (not suited for local resection). Implementation of standardized protocols for acquisition and interpretation of MRI images may potentially improve the assessment of rectal adenomas and early rectal cancer, as demonstrated by the MERCURY research project 33. Assessment of factors influencing MRI tumour evaluation was, however, beyond the scope of this study. Consequently, our findings reflect the actual contribution to clinical decision making of standard MRI in a routine clinical setting.
In clinical practice one would not look at ERUS, strain elastography or MRI in isolation, but rather as a combined approach supplementing the clinical evaluation and biopsy result. However, the primary aim of this study was to evaluate a novel method that has not been validated previously for the assessment of rectal tumours. Consequently, we argue that there was a need to compare each of the methods in isolation. Figure4 demonstrates the conflicting information with which one might be presented in such a multimodal approach. This underlines the need to understand the strengths and weaknesses of each method. Consequently, future studies should aim to assess the impact of strain elastography evaluation on treatment decisions in clinical practice.
Although the issue of selecting patients for neoadjuvant (chemo-) radiotherapy is solved, to a great extent, by advances in MRI evaluation, the assessment of treatment effect is still challenging. Evaluation of the radiation effect was beyond the scope of this study, but this issue is being addressed in an ongoing study. In the current study, the elastography SR measurements were not significantly different in adenocarcinomas eligible for primary resection and those in need of radiation therapy. However, a recent elastography study on the response of radiation therapy in rectal cancer has shown promising results 23.
Although a rigorous protocol for choosing tumour tissue and reference tissue was designed to minimize selection bias, all elastography examinations were performed by a single examiner immediately following the ERUS examination. As elastography SR assessment is based on a high-quality B-mode ERUS image, which is performed before the SR measurement by the same observer, it may be argued that crucial information, such as invasive tumour growth seen by ERUS, would influence the subsequent elastography SR evaluation in its favour, but not vice versa. Consequently, an observer bias is possible, but the elastography method is, by design, an add-on to B-mode ERUS. A set-up with two observers performing separate ERUS or combined ERUS and elastography evaluations would address this shortcoming, but was not logistically feasible. Because of technical limitations regarding the format of saved elastography video loops and images, a blinded SR re-examination was not possible, making a reliability evaluation of SR measurements unattainable. Further studies are being conducted to address some of these issues.
In conclusion, this study validates an endorectal elastography SR of 1.25 as an accurate cut-off for the discrimination of rectal adenomas and adenocarcinomas. Elastography evaluation adds precision to ERUS and MRI examinations. Our results suggest that MRI should be interpreted with care when adenomas or early adenocarcinomas are suspected. Future studies should be aimed at further clarification of the potential role of endorectal strain elastography staging of rectal tumours and at assessing the impact on clinical decision making.
Acknowledgments
The study was founded by Helse Vest Research Foundation, but they did not play any part in the development or approval of this manuscript. We would like to thank Geir Egil Eide for insightful statistical advice and Borghild Straume for valuable logistical assistance in the outpatient clinic.
Author contributions
Jo Erling Riise Waage: Conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the submitted manuscript. Sabine Leh: Preparation of protocol, acquisition of pathology data, data interpretation, critical revision of the article and final approval of the submitted manuscript. Cornelia Røsler: Preparation of MRI protocol, acquisition and interpretation of MRI data, critical revision of the article and final approval of the submitted manuscript. Simon P. Bach: Substantial contributions to conception and design, data interpretation, critical revision of the article and final approval of the submitted manuscript. Frank Pfeffer: Substantial contributions to design, data interpretation, critical revision of the article and final approval of the submitted manuscript. Roald Flesland Havre: Substantial contributions to conception and design, data interpretation, critical revision of the article and final approval of the submitted manuscript. Ingfrid Haldorsen: Preparation of MRI protocol, data interpretation, critical revision of the article and final approval of the submitted manuscript. Svein Ødegaard: Substantial contributions to conception and design, data interpretation, critical revision of the article and final approval of the submitted manuscript. Gunnar Baatrup: Substantial contributions to conception and design, data interpretation, critical revision of the article and final approval of the submitted manuscript.
References
- 1.Steele RJ, McClements P, Watling C, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576–81. doi: 10.1136/gutjnl-2011-300535. [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Kaur H, Choi H, You YN, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012;32:389–409. doi: 10.1148/rg.322115122. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer R, MacDonald A, Matthews R, Gunn J, Monson JR, Hartley JE. Brush cytology for the diagnosis of colorectal cancer. Dis Colon Rectum. 2009;52:598–601. doi: 10.1007/DCR.0b013e3181a0ad44. [DOI] [PubMed] [Google Scholar]
- 5.Colleypriest BJ, Marden PF, Linehan JD. What is the optimal number of biopsies to diagnose a tumor found during colonoscopy? J Clin Gastroenterol. 2009;43:1012–3. doi: 10.1097/MCG.0b013e31819fcd0a. [DOI] [PubMed] [Google Scholar]
- 6.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–65. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 7.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging – a meta-analysis. Radiology. 2004;232:773–83. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 8.Ashraf S, Hompes R, Slater A, et al. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14:821–6. doi: 10.1111/j.1463-1318.2011.02830.x. [DOI] [PubMed] [Google Scholar]
- 9.Waage JE, Havre RF, Odegaard S, Leh S, Eide GE, Baatrup G. Endorectal elastography in the evaluation of rectal tumours. Colorectal Dis. 2011;13:1130–7. doi: 10.1111/j.1463-1318.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- 10.Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51:38–42. doi: 10.1007/s10350-007-9104-4. [DOI] [PubMed] [Google Scholar]
- 11.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR. Can endoscopic ultrasound predict early rectal cancers that can be resected endoscopically? A meta-analysis and systematic review. Dig Dis Sci. 2010;55:1221–9. doi: 10.1007/s10620-009-0862-9. [DOI] [PubMed] [Google Scholar]
- 12.Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 13.Baatrup G, Elbrond H, Hesselfeldt P, et al. Rectal adenocarcinoma and transanal endoscopic microsurgery. Diagnostic challenges, indications and short term results in 142 consecutive patients. Int J Colorectal Dis. 2007;22:1347–52. doi: 10.1007/s00384-007-0358-z. [DOI] [PubMed] [Google Scholar]
- 14.Worrell S, Horvath K, Blakemore T, Flum D. Endorectal ultrasound detection of focal carcinoma within rectal adenomas. Am J Surg. 2004;187:625–9. doi: 10.1016/j.amjsurg.2004.01.005. ; discussion 9. [DOI] [PubMed] [Google Scholar]
- 15.Bach SP, Hill J, Monson JR, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–90. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 17.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 18.Alhabshi SM, Rahmat K, Abdul HN, et al. Semi-quantitative and qualitative assessment of breast ultrasound elastography in differentiating between malignant and benign lesions. Ultrasound Med Biol. 2013;39:568–78. doi: 10.1016/j.ultrasmedbio.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Saftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 20.Onur MR, Poyraz AK, Ucak EE, Bozgeyik Z, Ozercan IH, Ogur E. Semiquantitative strain elastography of liver masses. J Ultrasound Med. 2012;31:1061–7. doi: 10.7863/jum.2012.31.7.1061. [DOI] [PubMed] [Google Scholar]
- 21.Nygard Y, Haukaas SA, Waage JE, et al. Combination of real-time elastography and urine prostate cancer gene 3 (PCA3) detects more than 97% of significant prostate cancers. Scand J Urol. 2013;47:211–6. doi: 10.3109/00365599.2012.727859. [DOI] [PubMed] [Google Scholar]
- 22.Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–11. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 23.Rafaelsen SR, Vagn-Hansen C, Sorensen T, Lindebjerg J, Ploen J, Jakobsen A. Ultrasound elastography in patients with rectal cancer treated with chemoradiation. Eur J Radiol. 2013;82:913–7. doi: 10.1016/j.ejrad.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Havre RF, Leh S, Gilja OH, et al. Strain assessment in surgically resected inflammatory and neoplastic bowel lesions. Ultraschall Med. 2014;35:149–58. doi: 10.1055/s-0032-1325535. [DOI] [PubMed] [Google Scholar]
- 25.Havre RF, Waage JR, Gilja OH, Odegaard S, Nesje LB. Real-Time Elastography: Strain Ratio Measurements Are Influenced by the Position of the Reference Area. Ultraschall Med. 2012;33:550–8. doi: 10.1055/s-0031-1273247. [DOI] [PubMed] [Google Scholar]
- 26.Havre RF, Elde E, Gilja OH, et al. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol. 2008;34:1638–50. doi: 10.1016/j.ultrasmedbio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Beets-Tan RG, Lambregts DM, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522–31. doi: 10.1007/s00330-013-2864-4. [DOI] [PubMed] [Google Scholar]
- 28.Casella G. Refining binominal confidence intervals. Can J Stat. 1986;14:113–29. [Google Scholar]
- 29.Still C. Binominal confidence intervals. J Am Stat Assoc. 1983;78:108–16. [Google Scholar]
- 30.Fernandez-Esparrach G, Ayuso-Colella JR, Sendino O, et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347–54. doi: 10.1016/j.gie.2011.03.1257. [DOI] [PubMed] [Google Scholar]
- 31.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–64. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 32.Maas M, Lambregts DM, Lahaye MJ, et al. T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging. 2012;37:475–81. doi: 10.1007/s00261-011-9770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown G, Daniels IR. Preoperative staging of rectal cancer: the MERCURY research project. Recent Results Cancer Res. 2005;165:58–74. doi: 10.1007/3-540-27449-9_8. [DOI] [PubMed] [Google Scholar]


