Abstract
Experimental studies suggest that the β-blocker propranolol stimulates bone formation but little work has investigated its effect on fracture healing. In this study, we examined if a low dose of propranolol, previously shown to be preventive against bone loss in rats, improves bone repair. Female Wistar rats were injected with saline or propranolol (0.1 mg/kg/day) (n = 20/group), 5 days a week for 8 weeks. Three weeks after the beginning of treatment, all rats underwent a mid-diaphyseal transverse osteotomy in the left femur. Radiographic analysis of ostetomy healing was performed 2 and 5 weeks after osteotomy. Rats were sacrificed at 5 weeks and femora collected for measurements of fracture strength by torsional testing, callus volume, and mineral content by micro-CT analysis and histology of fracture callus. Eighty nine percent of osteotomies achieved apparent radiological union by 5 weeks in both groups. Propranolol treatment did not significantly alter the torsional strength of the fractured femur compared with controls. The volume and mineralization of fracture callus at 5 weeks were not significantly different in both groups. Histology showed that endochondral ossification was not affected by propranolol. Altogether, our results demonstrate that propranolol using the regimen described does not significantly improve or inhibit rat osteotomy healing and mechanical strength.
Keywords: propranolol, bone architecture, fracture healing, micro-CT, mechanical testing
Bone and periosteum are richly innervated by sympathetic and sensory nerves processes running in the vicinity to bone marrow and bone cells1–4 and neural regulation of bone metabolism has been extensively demonstrated in vitro and in vivo involving these different components of the nervous system.5–9 More interest was recently generated regarding the role of the sympathetic nervous system (SNS) in bone, due to the major discovery of a leptin-dependent central control pathway of bone remodeling involving the SNS.10 Bone cells have indeed functional receptors for several neuromediators, including noradrenergic receptors (AR).11–13 Osteoblasts and osteoclasts constitutively express the beta-2 adrenergic receptor (β2AR), which appears to be the main adrenergic receptor in bone cells, although β1, α1B, and α2BAR could also play a role in bone cell function.8,14,15 Multiple in vitro and in vivo studies have shown that β2AR activation inhibits bone formation while osteoclastogenesis and bone resorption are increased.8,14,16 In accordance, βAR antagonists (β-blockers) increase bone formation rate and can significantly rescue the bone loss induced by ovariectomy (OVX).10,13,17–19 Their effects on bone metabolism seem however dose-dependent, low doses being more protective against bone loss.17,18
The demonstration that β-blockers can increase bone mass in rodent experiments has led to the assumption that these drugs may play an important role in preventing bone loss and improving fracture healing in humans, in addition to treat cardiovascular disease. Several clinical retrospective studies have examined the use of β-blockers as potential therapeutic options for the bone loss observed in osteoporosis and associated fragility fractures but their results were conflicting, indicating the need for more randomized controlled trials to confirm the beneficial effect of β-blockers on bone mass and fracture risk.19–21 While animal studies have shown that complete peripheral nerve transection impairs fracture healing22,23 and that sensory innervation contributes to fracture healing,5,24 the role of the SNS and β-blockers on normal and fragility fracture healing has been poorly studied. Vascularization is essential for fracture healing and the SNS could be involved in bone formation during fracture repair, either directly through β2AR expressed on bone cells or indirectly through modulation of bone blood flow.25 The increase in autonomic innervation after fracture suggests indeed that this component of the nervous system plays a role in fracture repair.26 The only study performed in rats showed a positive effect of 19 days propranolol treatment on the union of 5 mm bone defects packed with demineralized bone matrix powder, suggesting that β-blockers could be beneficial to fracture healing.27
The aim of this study was to explore the hypothesis than 8 weeks injections of propranolol at a low dose of 0.1 mg/kg/day would induce beneficial changes in callus formation and strength during fracture healing in a 0.5 mm mid-diaphyseal transverse osteotomy model in the rat femur.
MATERIAL AND METHODS
Study Design
To examine the effect of propranolol on osteotomy healing, two different experiments were performed with the same protocol, one to enable destructive mechanical testing of fracture callus and the other one to measure callus volume and mineralization and perform morphological examinations. In both experiments, 20 female Wistar rats of approximately 250–300 g were divided randomly into two groups, one (n = 10) was injected with saline while the other (n = 10) was injected intraperitoneally with propranolol (0.1 mg/kg/day), 5 days a week for 8 weeks. Sample size was based on our previous experience and calculated so that 10 rats per group for each comparison are sufficient to establish significant differences between groups. Drinking water, along with food, was available ad libitum. Three weeks after the beginning of propranolol treatment all rats (n = 20) underwent a mid-diaphyseal transverse osteotomy (with a gap of 0.5 mm) in the left femur stabilized with an external fixator, as described previously.28,29 These procedures were performed under general anaesthesia with appropriate post-operative analgesia. Rats were kept 5 weeks after surgery and then euthanized under anaesthesia. Right and left femora were excised immediately after death at 5 weeks for mechanical testing in the first experiment and micro-CT analysis of fracture callus and histology in the second one. All animal experimentation procedures were in compliance with local ethical committee and Home Office approval and were performed under license in accordance with UK Animals (Scientific Procedures) Act 1986.
Radiographic Analysis
Both dorsal and ventral radiographs were taken at 2 weeks and 5 weeks under anaesthesia to assess the extent of in situ healing in terms of bridging of cortices across the osteotomy gap. The degree of union was scored on X-rays using a five point system, as previously described30; 0—no callus, 1—little to moderate callus, 2—profuse callus, 3—bridging callus, 4—mature callus with intrafragmentary bridging, 5—callus resorption after solid union.
Biomechanical Analysis
Femora were excised immediately after sacrifice at 5 weeks, individually stored in saline soaked gauze and frozen at −20°C. Immediately before testing, they were thawed and immersed in saline solution during the whole analysis. The torsional properties of osteotomized femora relative to those of intact contralateral ones were measured using the technique of Strömberg and Dalén31 using a Zwick/Roell (Slinfold, UK) testing machine DO-FB005 TN (Maximum test load 5000N). Torque was applied with a consistent angular velocity of 6° per second, with consistent direction, until failure. The torque and deflection angle were recorded using customized computer software. Graphical representation of torque versus angle permitted quantification of the maximum torque at failure (ultimate torsional strength, Nm) and the torsional stiffness (slope of the linear region of the torque versus angular displacement curve (Nm/deg)).
Micro-CT Analysis of Fracture Callus
Left femora (fractured side) were scanned at 14 µm resolution using micro-computed tomography (micro-CT, Skyscan 1172, Kontich, Belgium). A length of approximately 15 mm of the callus with the osteotomy in the centre was scanned and histomorphometric analysis in 2- and 3- dimensions (2D, 3D) performed by Skyscan software (v. 1.11.8.0), as described.29 Binarization of the reconstructed datasets involved two thresholds, one to delineate the low mineralized callus and the other one to define mineralized callus and cortical bone.
Histology
After micro-CT analysis, fracture calluses were decalcified and embedded in paraffin as described previously.29 Sagittal sections (5 µM) were cut in standardized plane using a microtome (HM360, Fisher Scientific Ltd, Loughborough, UK) and stained with Hematoxylin and Eosin (H&E) for basic morphology or Alcian blue and nuclear fast red for analysis of cartilage and bone.
Immunofluorescence
Sections of the osteotomy region were dewaxed, dehydrated, and incubated for 45 min at 37°C in pepsin (3 mg/ml in 0.02 M HCl) for antigen retrieval, then foetal calf serum (FCS) for 30 min to block non-specific binding. They were incubated with a primary rabbit antibody to β2AR (Thermo Scientific Pierce, Rockford, IL) at 1:1,000 in 20% FCS at 4°C overnight, washed and incubated with an anti-rabbit secondary antibody labelled with biotin (Dako, Carpinteria, CA) for 1 h at room temperature. Sections were washed, incubated with streptavidin-488 for 15 min, rinsed and mounted with a medium containing 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) for visualization of nucleic acids.
Statistical Analysis
The results are presented as mean values ± SD. Statistical analysis was performed using a two-tailed Mann Whitney U test with GraphPad Prism software.
RESULTS
Beta-2 Adrenergic Receptor Was Highly Expressed in Fracture Callus
Detection of β2AR was carried out by immunocytochemistry on sections of the osteotomy region in the non-treated group (Fig. 1A). Figure 1B illustrates the area of section examined. Immunofluorescence for β2AR (Fig. 1C) is highly detected in the osteotomy gap and dissipated when moving further away from the osteotomy site. Increased magnification fluorescence images taken of the osteotomy site clearly showed that the fluorescence was more abundant in bone-forming osteoblasts rather than in hypertrophic chondrocytes. Our staining suggested that β2AR expression was mainly cytoplasmic.
Figure 1.

Expression of beta-2 adrenergic receptor in the osteotomy gap. (A) Representative image of Alcian blue-stained section of control fracture callus 5 weeks after fracture. The osteotomy gap (o), cortical bone (c) and bone marrow (bm) are indicated. (B) Higher magnification of the region shown in the box in section A and stained with H&E. Hypertophic chondrocytes (HC) and osteoblasts or osteoblast progenitors (Ob) are indicated by an arrow. (C) Image of osteotomy gap showing the presence of β2AR indicated by green fluorescence. (D) Same image of osteotomy gap showing the nuclei of cells. (E) Merge of C + D (Scale bars = 50 µm). (F) Negative control (no primary antibody).
Propranolol Had No Significant Effect on Body Weight and Radiographic Healing
Eight weeks propranolol treatment had no effect on body weight in both experiments (Fig. 2A). At 2 weeks, only 10% of osteotomies showed radiographic healing in one of the cortices. At 5 weeks, 89% of osteotomies achieved apparent radiological union in both groups (Fig. 2B).
Figure 2.

Effect of propranolol treatment on rat body weight and bone radiographic healing. (A) Body weight at start and end of propranolol treatment period in saline and propranolol-treated rats. (B) X-ray scoring results for fractured femora in saline and propranolol-treated rats 5 weeks after fracture. Bars represent mean ± SD of n = 8/9 rats/group.
Propranolol Treatment Did Not Significantly Improve the Mechanical Strength of the Fractured Femur
Torque-to-failure testing was performed 5 weeks after osteotomy, a time that gave sufficient healing to undergo testing. Propranolol treatment had no significant effect on the strength (Fig. 3A) and stiffness (Fig. 3C) of the fractured femur compared with controls. There was however a trend for increases in both strength and stiffness in the propranolol-treated group, close to significance in case of strength (Fig. 3A, 20%, P = 0.06). While intrinsic material properties of the intact femur were similarly not significantly modified by propranolol (Fig. 3B,D), the strength of the operated femur was 27% and 46% of the contralateral intact femur in saline- and propranolol-treated groups, respectively.
Figure 3.
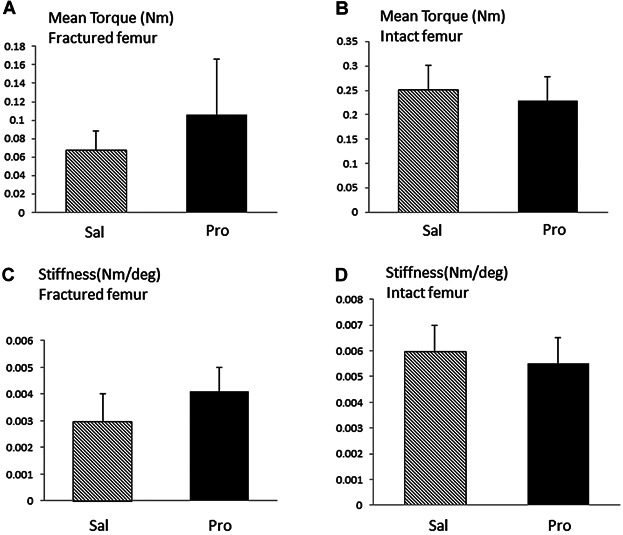
Effect of propranolol treatment on fracture callus mechanical properties. (A,B) Mean peak torque (Nm) in fractured (A) and intact (B) femur after 5 weeks of healing in saline and propranolol-treated groups. (C,D) Stiffness (Nm/deg) in fractured (C) and intact (D) femur after 5 weeks of healing in saline and propranolol-treated groups. Bars represent mean ± SD of n = 8/9 tested rats/group.
Propranolol Treatment Did Not Affect Fracture Callus Size or Mineralization
In both control and propranolol-treated groups, a large callus was still visible at the osteotomy site (Fig. 4A). Two binarization thresholds were applied to the micro-CT images of fracture calluses, one which took into account the low mineralized callus solely (Fig. 4Bi), the other one only taking into account cortical bone and highly mineralized callus (Fig. 4Bii). Propranolol treatment did not significantly affect either the volumes of low mineralized callus or that of highly mineralized callus and cortical bone (Fig. 4B).
Figure 4.

Effect of propranolol treatment on fracture callus size and mineralization. (A) Representative micro-CT images of control (i) and propranolol-treated (ii) fractured femora. (B) The volumes of low mineralized callus (i) and highly mineralized callus and bone (ii) are not significantly different in control and propranolol-treated groups. Bars represent mean ± SD of n = 8/9 rats/group. Examples of visual representations of the proportion of low mineralized callus highlighted in pink and high-mineralized callus and bone highlighted in yellow are shown below the graphs.
Propranolol Treatment Had No Effect On Endochondral Ossification
Figure 5Ai and Bi shows representative images of H&E- stained fracture calluses at 5 weeks in saline (A) and propranolol (B)-treated groups. In both groups, the periosteal callus still contained cartilage as well as small regions of primary trabecular-like bone. Higher magnifications of cartilage in the callus demonstrate hypertrophic chondrocytes in both propranolol-treated (Bii) and control group (Aii), suggesting that propranolol does not induce substantial advanced osteotomy healing.
Figure 5.

Effect of propranolol on endochondral ossification and osteotomy healing. Representative images of H&E stained longitudinal sections of fracture callus in control (A) and propranolol (B) -treated rats 5 weeks after fracture. The original cortical bone (c) and site of osteotomy (o) are evident. Higher magnifications of the boxes shown in Ai and Bi in the periosteal callus (pc) showed the presence of cartilage with hypertrophic chondrocytes (HC) in the propranolol- (Bii) and the control-treated groups (Aii) (Scale bars = 100 µm).
DISCUSSION
While many studies have investigated the role of propranolol on fracture risk, its crucial role in bone repair has not been extensively examined. In this study, we demonstrate that osteotomy healing and callus strength were not significantly improved by propranolol treatment at the dose used.
To examine the effect of propranolol on bone repair, we used a model of mid-diaphyseal transverse osteotomy in rats, routinely employed in our lab.28,29 The osteotomy was stabilized by external fixation, which provided greater control of the mechanical environment and thus a better assessment of the effect of propranolol treatment alone. Rats were injected with a low dose of propranolol, previously used to prevent bone loss in OVX rats.17 Propranolol effects on the skeleton were dose-dependent in rodent models, low doses of 0.1 and 1 mg/kg being beneficial for preserving bone mass in OVX rats while higher doses having mainly no effect on bone architecture,17,32–34 although propranolol treatment at 20 mg/kg was shown to reduce the unloading-induced bone loss in mice.35 Different delivery modes of propranolol were also used,10,28,32,33,35 and most studies agree that doses of propranolol ranging from 0.1 to 5 mg/kg (intraperitoneal injections) are efficient to prevent cancellous bone loss after estrogen deficiency and disuse. However, the mechanisms of action are not always concordant as some studies suggest predominant effects on bone resorption while others show that propranolol increases osteoblast number and bone formation rate.20 Most studies have examined the skeletal effect of propranolol in models of bone loss. In our study, rats were not OVX or immobilized. The low propranolol dose that we used did not affect body weight, in accordance with previous studies in rodents.32,33,35
Both intramembranous and endochondral ossification are taking place during bone regeneration.36 We evaluated the effect of propranolol 5 weeks after fracture, a time at which complete periosteal bridging had occurred in the model used but where cartilage persisted in the fracture callus, suggesting that endochondral ossification was still taking place. Our present histology data corroborate this and do not indicate that the rate of chondrocyte maturation in the periosteal fracture callus was different in the propranolol-treated group compared to controls. We also showed no effect of propranolol on callus size and density of mineral, although there was a trend for a decrease in low mineralized callus in the propranolol group, suggesting that mineralization and therefore speed of healing could be slightly more rapid in this group. While our power calculation has shown that 10 rats/group was sufficient to show significant effects in this osteotomy model, we cannot exclude that propranolol may have a significant positive effect on mineralization with the use of larger groups. However, a more rapid mineralization of the cartilaginous callus in the propranolol-treated group would indicate that propranolol affects mechanical qualities of the fracture callus and our present data show no significant effects. Mechanical integrity was evaluated by torsional testing, a widely used method since it most closely mimics physiologic loading in vivo and accommodates the callus asymmetry.37 Callus torsional strength was not significantly enhanced by propranolol after 5 weeks treatment, despite small increases in callus stiffness and strength in the propranolol-treated group which could correlate with the little increase in mineral content within the external callus. Correlations between fractured and intact femurs show that, 5 weeks after osteotomy, propranolol- treated group had reached 46% of the strength of the contralateral intact femur against 27% for the saline group, confirming the tendency for enhanced strength of the fractured bones in the propranolol group, although this result was not statistically significant.
One single publication reported the effect of propranolol on bone repair in a rat femoral defect model of 5 mm packed with demineralized bone matrix. Propranolol treatment for 19 consecutive days, at similar range of doses to our study, significantly increased callus formation by 33% as well as mineral apposition rate measured after 12 weeks by densitometry and histology, respectively.27 In contrast, preliminary work by Aspenberg's group38 and Bostrom et al.39 indicated no effect of propranolol on bone healing of osteotomized mice. In this latest report, propranolol was added in the drinking water at low (4 mg/kg) and high (20 mg/kg) doses for 3 weeks and no significant difference between treatments was demonstrated. While propranolol was started at time of osteotomy in previous studies, we administered propranolol 3 weeks before osteotomy to mimic fractures in patients on pre-existing propranolol. We cannot exclude that greater effects could be present by delaying the administration of propranolol until the bone anabolic response after osteotomy or by performing the analysis at later time points. The weakness of all these studies, ours included, is also that the efficacy of propranolol treatment was not evaluated in terms of expected pharmacological action, as the effect of propranolol was not assessed on blood flow parameters. Fracture healing like any wound healing is a well-coordinated process that requires cell migration and proliferation at the wound. Interestingly, β2AR activation decreased cell migration and proliferation, delaying wound healing, while in contrast blockade of β2AR by antagonists promoted wound repair, partly by increasing angiogenesis.40 Fracture healing requires revascularization and nerves, which are frequently associated with blood vessels and may also affect fracture healing indirectly via their effects on angiogenesis and blood flow. Propranolol is an unspecific β-blocker but most effects on bone of βAR signalling seem to be mediated by β2AR expressed by bone cells,18,19 although other βAR subtypes may contribute to the regulation of bone mass by having different or even opposite effects on bone.14,15 We found high expression of β2AR in the fracture callus, supporting a role for adrenergic nerves in the process of bone healing. The SNS plays a very important role in osteoporosis and other osteoporotic conditions such as spinal cord injury and reflex sympathetic dystrophy syndrome,21 which are important clinical issues contributing to a high number of fractures each year in the UK. The possible role of beta-blockers in the maintenance of bone mass and improvement of fracture healing in those patients may therefore depend on the development and use of highly selective beta-blockers.
In conclusion, our data indicate that low dose of propranolol had no significant positive or negative effect on bone healing and callus strength in a rodent model of osteotomy. Although this dose has been previously proven to be effective on bone mass, our study is limited by the use of this single dose of propranolol without any measurement of its efficacy on bone flow parameters in our model. A single agent such as propranolol that could have beneficial effects on both the heart and bone could have huge significance in terms of public health. However, our study indicates that the commonest clinical setting would be simple fractures in patients on pre-existing propranolol for its cardiac benefit.
Acknowledgments
This study was supported by a Special Trustees Pump-priming Grant. We are grateful to Prof. David Marsh for his help in study conduct and Mark Harrison for his technical assistance with mechanical testing.
REFERENCES
- Serre CM, Farlay D, Delmas PD, et al. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- Hara-Irie F, Amizuka N, Ozawa H. Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone. 1996;18:29–39. doi: 10.1016/8756-3282(95)00425-4. [DOI] [PubMed] [Google Scholar]
- Hohmann EL, Elde RP, Rysavy JA, et al. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Ding Y, Arai M, Kondo H, et al. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone. 2010;46:1591–1596. doi: 10.1016/j.bone.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Cherruau M, Facchinetti P, Baroukh B, et al. Chemical sympathectomy impairs bone resorption in rats: a role for the sympathetic system on bone metabolism. Bone. 1999;25:545–551. doi: 10.1016/s8756-3282(99)00211-2. [DOI] [PubMed] [Google Scholar]
- Elefteriou F. Regulation of bone remodelling by central and peripheral system. Arch Biochem Biophys. 2008;473:231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togari A, Arai M. Pharmacological topics on bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. 2008;106:542–546. doi: 10.1254/jphs.fm0070227. [DOI] [PubMed] [Google Scholar]
- Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Chenu C, Serre CM, Raynal C, et al. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–299. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- Bliziotes M, Eshleman A, Burt-Pichat B, et al. Serotonin transporter and receptor expression and function in osteocytic MLO-Y4 cells. Bone. 2006;39:1313–1321. doi: 10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodelling and its implications for the treatment of osteoporosis. J Musculoskel Neuron Interact. 2008;8:94–104. [PubMed] [Google Scholar]
- Pierroz DD, Nonnet N, Bianchi EN, et al. Deletion of β-adrenergic receptor 1,2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012;27:1252–1262. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- Kajimura D, Hinoi E, Ferron M, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N, Laroche N, Vico L, et al. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther. 2006;318:1118–1127. doi: 10.1124/jpet.106.105437. [DOI] [PubMed] [Google Scholar]
- Arai M, Sato T, Takeuchi S, et al. Dose effects of butoxamine, a selective β2-adrenoreceptor antagonist, on bone metabolism in spontaneously hypertensive rat. Eur J Pharmacol. 2013;701:7–13. doi: 10.1016/j.ejphar.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Graham S, Hammond-Jones D, Gamie Z, et al. The effect of beta-blockers on bone metabolism as potential drugs under investigation for osteoporosis and fracture healing. Expert Opin Investig Drugs. 2008;17:1281–1299. doi: 10.1517/13543784.17.9.1281. [DOI] [PubMed] [Google Scholar]
- Reid IR. Effects of beta-blockers on fracture risk. J Musculoskelet Neuronal Interact. 2008;8:105–110. [PubMed] [Google Scholar]
- He J-Y, Jiang L-S, Dai L-Y. The roles of the sympathetic nervous system in osteoporotic diseases: a review of experimental and clinical studies. Ageing Res Rev. 2011;10:253–263. doi: 10.1016/j.arr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Aro H. Effect of nerve injury on fracture healing. Callus formation studied in the rat. Acta Othop Scand. 1985;56:233–237. doi: 10.3109/17453678508993002. [DOI] [PubMed] [Google Scholar]
- Madsen JE, Hukkanen M, Aune AK, et al. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res. 1998;351:230–240. [PubMed] [Google Scholar]
- Apel PJ, Crane D, Northam CN, et al. Effect of selective sensory denervation on fracture healing. J Bone Joint Surg. 2009;91:2886–2895. doi: 10.2106/JBJS.H.01878. [DOI] [PubMed] [Google Scholar]
- Moran CG, McGrory BJ, Roorda J, et al. Adrenergic control mechanisms of blood flow in a vascularised canine tibial allograft. J Orthop Res. 1993;11:429–437. doi: 10.1002/jor.1100110316. [DOI] [PubMed] [Google Scholar]
- Long H, Ahmed M, Ackermann P, et al. Neuropeptide Y innervation during fracture healing and remodelling. A study of angulated tibial fractures in the rat. Acta Orthop. 2010;81:639–646. doi: 10.3109/17453674.2010.504609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkowitz B, Boskey AL, Jane LM, et al. Effects of propranolol on bone metabolism in the rat. J Orthop Res. 1991;9:869–875. doi: 10.1002/jor.1100090613. [DOI] [PubMed] [Google Scholar]
- Harrison LJ, Cunningham JL, Stromberg L, et al. Controlled induction of a pseudoarthrosis: a study using a rodent model. J Orthop Trauma. 2003;17:11–21. doi: 10.1097/00005131-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Jeyabalan J, Viollet B, Smitham P, et al. The anti-diabetic drug metformin does not affect bone mass in vivo or fracture healing. Osteoporos Int. 2013;24:2659–2670. doi: 10.1007/s00198-013-2371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A, Zijerdi D, Zhang J, et al. BMP-14 deficiency inhibits long bone fracture healing: a biochemical, histologic, and radiographic assessment. J Orthop Trauma. 2005;19:629–634. doi: 10.1097/01.bot.0000177108.38461.9c. [DOI] [PubMed] [Google Scholar]
- Strömberg L, Dalén N. Experimental measurements of maximum torque capacity of long bones. Acta Ortop Scand. 1976;47:257–263. doi: 10.3109/17453677608991987. [DOI] [PubMed] [Google Scholar]
- Baek K, Bloomfield SA. Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J Bone Miner Res. 2009;24:792–799. doi: 10.1359/jbmr.081241. [DOI] [PubMed] [Google Scholar]
- Marenzana M, De Souza R, Chenu C. Blockade of β-adrenergic signalling does not influence the bone mechano-adaptive response in mice. Bone. 2007;41:206–215. doi: 10.1016/j.bone.2007.04.184. [DOI] [PubMed] [Google Scholar]
- Rodrigues WF, Madeira MFM, da Silva TA, et al. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br J Pharmacol. 2012;165:2140–2151. doi: 10.1111/j.1476-5381.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Nifuji A, Takeda S, et al. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem. 2005;280:30192–33200. doi: 10.1074/jbc.M504179200. [DOI] [PubMed] [Google Scholar]
- Marsell R, Heinhorn TA. The Biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenberg P. Drugs and fracture repair. Acta Orthop. 2005;76:741–748. doi: 10.1080/17453670510045318. [DOI] [PubMed] [Google Scholar]
- Bostrom MP, Yang X, Carson J, et al. Blockade of beta-adrenergic signalling does not influence fracture healing in a mouse model. J Bone Joint Surg. 2010;92-B:71. (Suppl 1) [Google Scholar]
- Pullar CE, Provost GS, O'Leary AP, et al. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol. 2012;132:2076–2084. doi: 10.1038/jid.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


