Abstract
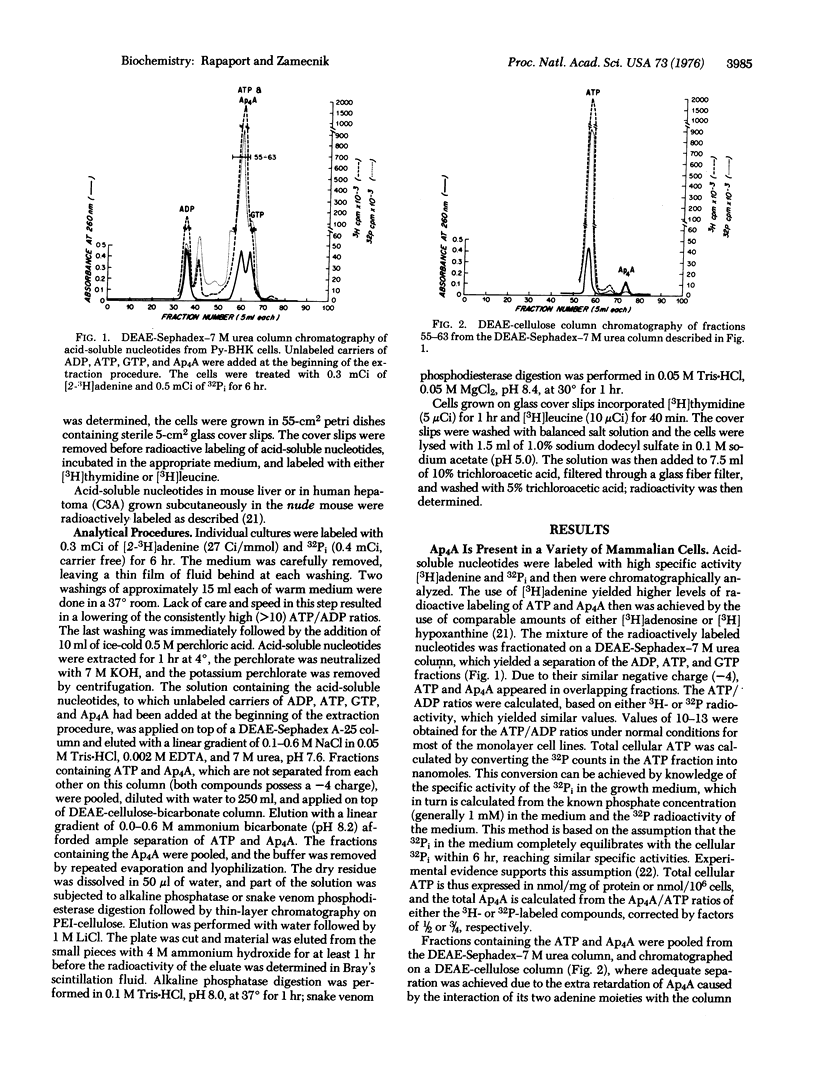
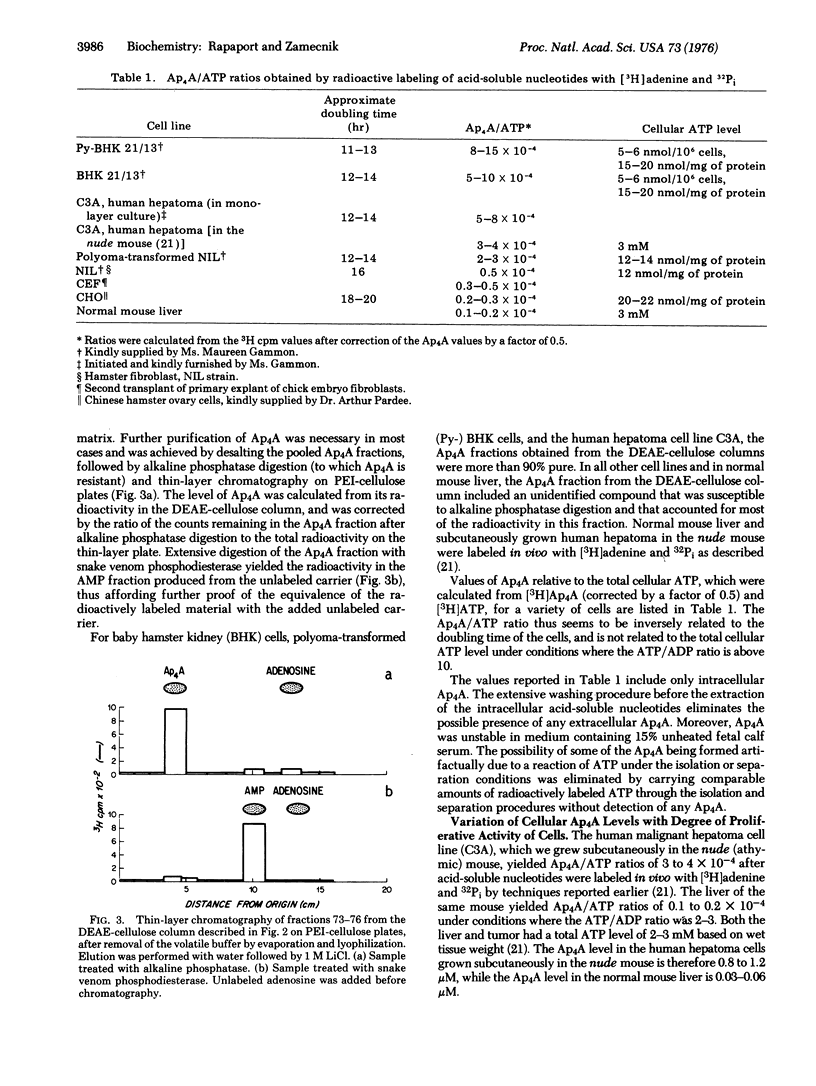
An accurate assay of diadenosine 5',5'''- P1,P4-tetraphosphate [A(5') pppp(5')A], which was shown to be formed in vitro in the backreaction of the amino acid activation step, has been developed in various cell lines in culture and in normal mouse liver or hepatoma in vivo. Use of radioactive labeling of acid-soluble nucleotides to high specific activity followed by chromatographic separation techniques yielded levels of Ap4A varying from 5 to 0.05 muM (from 30 pmol/mg of protein to 0.15 pmol), depending on the doubling time of the cell line or the proliferative state of the cells. The levels of Ap4A incells is inversely related to their doubling time, varying from 0.1 X 10(-4) of the cellular ATP levels in slowly growing cells to 20 X 10(-4) of the ATP levels of cells with rapid doubling times. The steady-state levels of ATP of different cell lines, although showing some fluctuations, are not related to the doubling time of the cells. Arrest of cellular proliferation by serum deprivation or amino acid starvation, which does not alter the cellular ATP levels more than 2-fold, does nevertheless cause a decrease of 30 to 50-fold in the Ap4A levels. Inhibition of protein synthesis by pactamycin or puromycin, or inhibition of DNA synthesis by hydroxyurea, leads to a more dramatic decrease of 50 to 100-fold in intracellular Ap4A levels. The metabolic lability of Ap4A is also demonstrated by its rapid depletion after decreases in the ATP/ADP ratio. The possibility of Ap4A being a metabolic "signal nucleotide" that is formed at the onset of protein synthesis and is active in positive growth regulation (positive pleiotypic activation) is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., ZAMECNIK P. C. The effect of puromycin on rabbit reticulocyte ribosomes. Biochim Biophys Acta. 1962 Jun 11;55:865–874. doi: 10.1016/0006-3002(62)90899-5. [DOI] [PubMed] [Google Scholar]
- Abell C. W., Monahan T. M. The role of adenosine 3',5'-cyclic monophosphate in the regulation of mammalian cell division. J Cell Biol. 1973 Dec;59(3):549–558. doi: 10.1083/jcb.59.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Clarke G. D., Stoker M. G., Ludlow A., Thornton M. Requirement of serum for DNA synthesis in BHK 21 cells: effects of density, suspension and virus transformation. Nature. 1970 Aug 22;227(5260):798–801. doi: 10.1038/227798a0. [DOI] [PubMed] [Google Scholar]
- Colby C., Edlin G. Nucleotide pool levels in growing, inhibited, and transformed chick fibroblast cells. Biochemistry. 1970 Feb 17;9(4):917–920. doi: 10.1021/bi00806a029. [DOI] [PubMed] [Google Scholar]
- FINAMORE F. J., WARNER A. H. The occurrence of P1, P4-diguanosine 5'-tetraphosphate in brine shrimp eggs. J Biol Chem. 1963 Jan;238:344–348. [PubMed] [Google Scholar]
- Felicetti L., Colombo B., Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. II. The site of action of cycloheximide, streptovitacin A and pactamycin. Biochim Biophys Acta. 1966 Apr 18;119(1):120–129. [PubMed] [Google Scholar]
- HOAGLAND M. B. An enzymic mechanism for amino acid activation in animal tissues. Biochim Biophys Acta. 1955 Feb;16(2):288–289. doi: 10.1016/0006-3002(55)90218-3. [DOI] [PubMed] [Google Scholar]
- HOAGLAND M. B., KELLER E. B., ZAMECNIK P. C. Enzymatic carboxyl activation of amino acids. J Biol Chem. 1956 Jan;218(1):345–358. [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P., Hershko A., Kram R., Schacter L., Lust J., Tomkins G. M. The pleiotypic response in mammalian cells: search for an intracellular mediator. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1378–1384. doi: 10.1016/0006-291x(72)90865-0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Elliott D. C., Atkinson M. R. Nucleotide biosynthesis from preformed purines in mammalian cells: regulatory mechanisms and biological significance. Prog Nucleic Acid Res Mol Biol. 1970;10:87–119. doi: 10.1016/s0079-6603(08)60562-0. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Randerath K., Janeway C. M., Stephenson M. L., Zamecnik P. C. Isolation and characterization of dinucleoside tetra- and tri-phosphates formed in the presence of lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):98–105. doi: 10.1016/0006-291x(66)90416-5. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Svihovec S. K., Zamecnik P. C. Relationship of the first step in protein synthesis to ppGpp: formation of A(5')ppp(5')Gpp. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2653–2657. doi: 10.1073/pnas.72.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Incorporation of adenosine into ATP: formation of compartmentalized ATP. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3122–3125. doi: 10.1073/pnas.73.9.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss J. R., Moffatt J. G. Dismutation reactions of nucleoside polyphosphates. 3. The synthesis of alpha, omega-dinucleoside 5'-polyphosphates. J Org Chem. 1965 Oct;30(10):3381–3387. doi: 10.1021/jo01021a029. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M. The metabolic code. Science. 1975 Sep 5;189(4205):760–763. doi: 10.1126/science.169570. [DOI] [PubMed] [Google Scholar]
- Vallejo C. G., Lobaton C. D., Quintanilla M., Sillero A., Sillero M. A. Dinucleosidasetetraphosphatase in rat liver and Artemia salina. Biochim Biophys Acta. 1976 Jun 7;438(1):304–309. doi: 10.1016/0005-2744(76)90246-1. [DOI] [PubMed] [Google Scholar]
- Warner A. H., Huang F. L. Biosynthesis of the diguanosine nucleotides. II. Mechanism of action of GTP:GTP guanylyltransferase on nucleotide metabolism in brine shrimp embryos. Can J Biochem. 1974 Mar;52(3):241–251. doi: 10.1139/o74-037. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C. An historical account of protein synthesis, with current overtones--a personalized view. Cold Spring Harb Symp Quant Biol. 1969;34:1–16. doi: 10.1101/sqb.1969.034.01.005. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]


