Abstract
Eumycetoma is a chronic fungal infection characterised by large subcutaneous masses and the presence of sinuses discharging coloured grains. The causative agents of black-grain eumycetoma mostly belong to the orders Sordariales and Pleosporales. The aim of the present study was to clarify the phylogeny and taxonomy of pleosporalean agents, viz. Madurella grisea, Medicopsis romeroi (syn.: Pyrenochaeta romeroi), Nigrograna mackinnonii (syn. Pyrenochaeta mackinnonii), Leptosphaeria senegalensis, L. tompkinsii, and Pseudochaetosphaeronema larense. A phylogenetic analysis based on five loci was performed: the Internal Transcribed Spacer (ITS), large (LSU) and small (SSU) subunit ribosomal RNA, the second largest RNA polymerase subunit (RPB2), and translation elongation factor 1-alpha (TEF1) gene. In addition, the morphological and physiological characteristics were determined. Three species were well-resolved at the family and genus level. Madurella grisea, L. senegalensis, and L. tompkinsii were found to belong to the family Trematospheriaceae and are reclassified as Trematosphaeria grisea comb. nov., Falciformispora senegalensis comb. nov., and F. tompkinsii comb. nov. Medicopsis romeroi and Pseudochaetosphaeronema larense were phylogenetically distant and both names are accepted. The genus Nigrograna is reduced to synonymy of Biatriospora and therefore N. mackinnonii is reclassified as B. mackinnonii comb. Nov. Mycetoma agents in Pleosporales were phylogenetically quite diverse despite their morphological similarity in the formation of pycnidia, except for the ascosporulating genus Falciformispora (formerly in Leptosphaeria). Most of the species diagnosed from human mycetoma were found to be related to waterborne or marine fungi, suggesting an association of the virulence factors with oligotrophism or halotolerance.
Keywords: Madurella, mycetoma, Pleosporales, taxonomy, Trematosphaeriaceae
INTRODUCTION
Mycetoma is a chronic progressive, destructive inflammatory disease that initially infects subcutaneous tissues and extends slowly to eventually invade the bone. It is mainly characterised by multiple sinuses discharging white or coloured grains at the site of infection (McGinnis 1996, Fahal & Suliman 1994). As both bacteria and fungi are able to cause mycetoma, this disease is classified into two types, actinomycetoma and eumycetoma, which are of bacterial and of fungal nature, respectively (Chalmers & Archibald 1918). Both types have similar clinical presentations, with some differences in virulence and colour of the discharged grains (Fahal & Suliman 1994). Blackgrain mycetoma is caused by fungi only, but the etiological agents belong to different orders (mainly to Sordariales and Pleosporales) (de Hoog et al. 2004). The most frequent agents belong to the genus Madurella, which exclusively comprises human-pathogens.
Madurella was first described by Brumpt (1905) to accommodate Streptothrix mycetoma, which was later renamed as Madurella mycetomatis (Kwon-Chung & Bennett 1992). The genus was defined as exclusively producing melanised hyphae without sporulation, and hence prior to the use of molecular methods species were difficult to distinguish (de Hoog et al. 2000). During the 20th century several species were introduced to the genus by colony characteristics, but most of these proved to be synonyms of M. mycetomatis. Madurella pseudomycetomatis, M. tropicana, and M. fahalii were recently recognised as different species of Madurella on the basis of DNA sequence data (Yan et al. 2010, de Hoog et al. 2012).
For many decades, Madurella grisea (Mackinnon et al. 1949) was treated as the sister species of M. mycetomatis; it is distinguished by grey cultures without soluble pigments being exuded into the medium. In contrast, cultures of M. mycetomatis are blackish brown and produce a dark brown halo of diffusible melanin-like compounds. Physiological differences are found in assimilation of carbon sources and in thermotolerance: M. grisea assimilates sucrose but not lactose, and is mostly unable to grow at 37 °C, whereas M. mycetomatis assimilates lactose but not sucrose, and grows optimally at 37 °C (de Hoog et al. 2000). Madurella mycetomatis occurs at rather high frequency in arid climate zones of East Africa and India, whereas for M. grisea only occasional records from South America and India are available (Mackinnon et al. 1949, McGinnis 1996, de Hoog et al. 2007). A recent phylogenetic analysis showed the two species to belong to different orders: M. mycetomatis (as well as the related M. pseudomycetomatis, M. tropicana, and M. fahalii) belong to the Sordariales, whereas M. grisea is a member of the Pleosporales (de Hoog et al. 2004). The taxonomic position of M. mycetomatis within the Sordariales has been studied extensively (de Hoog et al. 2004, van de Sande 2012). However, the phylogeny and biology of M. grisea remains to be investigated.
Besides Madurella species, the order Pleosporales contains several other agents of black-grain mycetoma: Leptosphaeria senegalensis, L. tompkinsii, Medicopsis romeroi (syn. Pyrenochaeta romeroi), Nigrograna mackinnonii (syn. Pyrenochaeta mackinnonii), and Pseudochaetosphaeronema larense, as well as some undescribed species (Meis et al. 2000, de Hoog et al. 2004). The aim of the present study is to clarify the phylogeny and taxonomy of pleosporalean agents of mycetoma, with accent on Madurella grisea.
MATERIALS AND METHODS
Strains analysed
Fungal strains included in this study were obtained from the CBS-KNAW (Fungal Biodiversity Centre) reference collection (www.cbs.knaw.nl), supplemented with fresh isolates recovered from patients with mycetoma. When available, type strains of species concerned were included in the analyses. Information on source and origin of the analysed strains is provided in Table 1.
Table 1.
Source, origin, and GenBank accession No. for the species included in this study. CBS (CBS-KNAW Fungal Biodiversity Centre), dH: (G.S. de Hoog working collection). Accession numbers in bold were retrieved from GenBank.
| GenBank accessions |
||||||||
|---|---|---|---|---|---|---|---|---|
| Name | Culture no. | Source | Origin | ITS | LSU | SSU | RPB2 | TEF1 |
| Biatriospora mackinnonii | CBS 674.75 (T) | Mycetoma | Venezuela | KF015654 | KF015612 | GQ387613 | KF015703 | KF407986 |
| CBS 110022 | Mycetoma | Mexico | KF015653 | KF015609 | GQ387552 | KF015704 | KF407985 | |
| Falciformispora senegalensis | CBS 196.79 (T) | Mycetoma | Senegal | KF015673 | KF015631 | KF015636 | KF015717 | KF015687 |
| CBS 197.79 | Human | Senegal | KF015677 | KF015626 | KF015633 | KF015712 | KF015690 | |
| CBS 198.79 | Mycetoma | Senegal | KF015675 | KF015627 | KF015634 | KF015713 | KF015688 | |
| CBS 199.79 | Human | Senegal | KF015672 | KF015630 | KF015638 | KF015716 | KF015689 | |
| CBS 132257 | Mycetoma | Sudan | KF015676 | KF015629 | KF015635 | KF015714 | KF015692 | |
| CBS 132272 | Mycetoma | Sudan | KF015674 | KF015628 | KF015637 | KF015715 | KF015691 | |
| Falciformispora tompkinsii | CBS 200.79 | Man | Senegal | KF015670 | KF015625 | KF015639 | KF015719 | KF015685 |
| CBS 201.79 | Man | Senegal | KF015671 | KF015624 | KF015640 | KF015718 | KF015686 | |
| Medicopsis romeroi | CBS 252.60 (T) | Mycetoma | Venezuela | KF366446 | EU754207 | EU754108 | KF015708 | KF015678 |
| CBS 132878 | Mycetoma | India | KF015658 | KF015622 | KF015648 | KF015709 | KF015682 | |
| CBS 122784 | Plant | KF366447 | EU754208 | EU754109 | KF015707 | KF015679 | ||
| CBS 123975 | Phaeohyphomycosis | India | KF015657 | KF015623 | KF015650 | KF015710 | KF015681 | |
| CBS 128765 | Subcutaneous cyst | Kuwait | KF015659 | KF015621 | KF015649 | KF015711 | KF015680 | |
| Pseudochaetosphaeronema larense | CBS 640.73 (T) | Mycetoma | Venezuela | KF015656 | KF015611 | KF015652 | KF015706 | KF015684 |
| CBS 639.94 | Man | Venezuela | KF015655 | KF015610 | KF015651 | KF015705 | KF015683 | |
| Roussoella sp. | CBS 128203 | Mycetoma | India | KF322117 | KF366448 | KF366450 | KF366453 | KF407988 |
| Roussoella sp. | CBS 868.95 | Mycetoma | Aruba | KF322118 | KF366449 | KF366451 | KF366452 | KF407987 |
| Trematosphaeria grisea | CBS 332.50 (AUT) | Mycetoma | Chili | KF015662 | KF015614 | KF015641 | KF015720 | KF015698 |
| CBS 246.66 | Submandibular abscess | India | KF015661 | KF015615 | KF015646 | KF366454 | KF015697 | |
| CBS 120271 | Tap water | The Netherlands | KF015660 | KF015613 | KF015647 | KF015726 | KF015699 | |
| CBS 135982 | Pastry gel | The Netherlands | KF015663 | KF015616 | KF015645 | KF015721 | KF015693 | |
| CBS 135984 | Water | The Netherlands | KF015666 | KF015618 | KF015632 | KF015724 | KF015694 | |
| CBS 136543 | Water | The Netherlands | KF015665 | KF015619 | KF015642 | KF015723 | KF015696 | |
| CBS 135985 | Water | The Netherlands | KF015667 | KF015620 | KF015643 | KF015722 | KF015700 | |
| CBS 135983 | Water | The Netherlands | KF015664 | KF015617 | KF015644 | KF015725 | KF015695 | |
| CBS 136537 | Water | The Netherlands | KF366444 | |||||
| V-0206-72 | Biopsy, human | The Netherlands | KF366445 | |||||
| Trematosphaeria pertusa | CBS 122371 | Plant | France | KF015669 | FJ201992 | FJ201993 | GU371801 | KF015702 |
| CBS 122368 | Plant | France | KF015668 | FJ201990 | FJ201991 | FJ795476 | KF015701 | |
Morphology and physiology
Morphology and growth characteristics of the species were studied on malt extract agar (MEA, Oxoid, UK) and oatmeal agar (OA, home-made at CBS, The Netherlands, http://www.cbs.knaw.nl/index.php/food-mycology/101-mycological-media-for-food-and-indoor-fungi). Culture plates were incubated at 30 °C for at least 4 wk, with a maximum of 8 wk and colony colours were described using the colour chart of Kornerup & Wanscher (1967). Cultures were firstly examined under a Nikon C-DSD 230 dissecting microscope. Microscopic mounts were made in lactic acid and examined using a light microscope (Nikon Eclipse 80i). Pictures were taken with a digital camera (Nikon, digital sight, DS-5M) and adjusted in Adobe Photoshop CS3. Cardinal growth temperatures were determined by incubating MEA plates in the dark at temperatures ranging from 15 to 40 °C at 3 °C intervals and including 10 and 37 °C.
Carbohydrate assimilation profiles were determined using ID32 C (BioMérieux, Marcy l’Étoile, France). For a review of carbohydrates and abbreviations see Table 2. Inocula were homogenised by ultrasonic treatment of fungal material in API C medium at maximum power (Soniprep, Beun de Ronde, Abcoude, The Netherlands). Final inocula were adjusted to obtain a transmission of 68–72 % at 660 nm (Novaspec II, Pharmacia Biotech, Cambridge, UK) and used according to the manufacturer’s instructions. Strips were incubated at 25 °C in a moist chamber and examined every 3 d for 9 d. Ability to produce urease was determined on 2 % urea agar plates (urea base, Oxoid, UK) incubated for one week. Laccase production was tested using ABTS agar medium containing 0.03 % ABTS (2, 2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt) (Kaluskar et al. 1999). ABTS plates were incubated at room temperature for 1 wk; Cryptococcus neoformans CBS 7229 was used as positive control. Salt tolerance was determined using nitrogen base medium (Difco) with sodium chloride at concentrations of 0, 2.5, 5, 10, 15, and 20 %.
Table 2.
Carbohydrate assimilation profile, and urease, laccase, and salt tolerance test results for the analysed species. – = Negative, + = positive, W = weak.
| Carbohydrate/Test | T. grisea | F. senegalensis | F. tompkinsii | Roussoella sp. | M. romeroi | B. mackinnonii | Pseud. larense |
|---|---|---|---|---|---|---|---|
| GAL (D-galactose) | + | + | + | + | + | + | + |
| ACT (actidione) | + | + | + | + | + | – | – |
| SAC (D-saccharose) | + | + | + | + | + | + | + |
| NAG (N-acetyl-glucosamine) | + | + | + | + | + | + | + |
| LAT (DL-lactate) | – | – | – | – | – | – | – |
| ARA (L-arabinose) | – | + | – | + | – | +/– | – |
| CEL (D-cellobiose) | + | + | + | + | + | + | + |
| RAF (D-raffinose) | + | + | + | + | + | + | + |
| MAL (D-maltose) | + | + | + | + | + | + | + |
| TRE (D-trehalose) | + | + | + | + | + | + | + |
| 2KG (potassium 2-keto-gluconate) | + | – | + | + | + | + | + |
| MDG (methyl-D-glucopyranoside) | + | + | + | + | – | + | + |
| MaN (D-mannitol) | + | +/– | + | + | + | + | + |
| LAC (D-lactose) | – | + | – | +/– | – | – | – |
| INO (inositol) | + | +/– | – | + | + | + | + |
| SOR (D-sorbitol) | + | +/– | – | + | + | + | + |
| XYL (D-xylose) | + | + | + | + | + | + | – |
| RIB (D-ribose) | – | – | – | – | – | +/– | –/+ |
| GLY (glycerol) | – | – | – | – | – | – | + |
| RHA (L-rhamnose) | + | + | + | + | + | + | + |
| PLE (palatinose) | + | + | + | + | + | + | + |
| ERY (erythritol) | +/– | – | + | + | + | + | + |
| MEL (D-melibiose) | + | – | +/– | + | +/– | + | + |
| GRT (sodium glucuronate) | – | – | – | – | – | +/– | – |
| MLZ (D-melezitose) | + | + | + | + | + | + | + |
| GNT (potassium gluconate) | – | – | – | – | – | – | – |
| LVT (levulinate) | +/– | – | – | – | – | + | – |
| GLU (D-glucose) | + | + | + | + | + | + | + |
| SBE (L-sorbose) | + | + | W | + | + | – | – |
| GLN (glucosamine) | – | – | – | –/W | – | – | – |
| ESC (esculin) | + | + | + | – | W | + | + |
| Salt tolerance test (NACL) | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | 10–20 % |
| Laccase test | + | + | –/W | + | + | + | + |
| Urease test | + | + | + | + | + | + | + |
DNA extraction
Genomic DNA was isolated from fungal mass grown on the surface of MEA plates. About 10 mm2 of material were added to a screw-capped tube containing 490 μL CTAB-buffer (2 % cetyltrimethyl ammonium bromide, 100 mM Tris-HCl, 20 mM EDTA, 1.4 M NaCl) and 6–10 acid-washed glass beads. Ten microliters proteinase K were added to the mixture and vortexed with a MoBio vortex for a few min. Tubes were incubated at 60 °C for 60 min. After incubation the tubes were again vortexed and 500 μL of chloroform : isoamylalcohol (24 : 1) were added followed by shaking for 2 min. Tubes were spun at 14 000 r.p.m. in a microfuge for 10 min and the upper layer was collected in new sterile tubes with 0.55 volume ice-cold iso-propanol and spun again. Finally, the pellets were washed with 70 % ethanol, air-dried and re-suspended in 100 μL TE buffer.
DNA amplification and sequencing
DNA amplification was performed for the Internal Transcribed Spacer region (ITS), small ribosomal subunit (SSU), and (partial) large ribosomal subunit (LSU), RNA polymerase second largest subunit (RPB2), and translation elongation factor 1-alpha (TEF1) loci. Primers used for amplification and partial sequencing of LSU, SSU, RPB2, and TEF1 were LRoR and LR7 (Vilgalys & Hester 1990), NS1 and NS24 (Gargas & Taylor 1992, White et al. 1990), fRPB2-5F and fRPB2-7cR (Liu et al. 1999), and EF-983F and EF-2218R (http://www.aftol.org/pdfs/EF1primer.pdf), respectively. Additional primers that were used for sequencing SSU included NS2, NS3, NS6, and NS7, and rDNA ITS was amplified and sequenced using primers ITS1 and ITS4 (White et al. 1990). All PCR reactions were performed in a MyCycler™ Thermal Cycler (Bio-Rad, Munich, Germany) with 25 μL reaction mixture containing 10 ng of template DNA, 0.1 mM each dNTP, 1.0 U Taq polymerase, and 2.5 μL reaction buffer (0.1 M Tris-HCl, 0.5 M KCl, 15 mM MgCl2, 0.1 % gelatin, and 1 % Triton X-100). Amplification conditions for all fragments were taken from Feng et al. (in press) except for RPB2. Amplification conditions for RPB2 included pre-denaturation at 95 °C for 3 min followed by 5 cycles of denaturation at 95 °C for 45 s and annealing at 58 °C for 45 s (reduced to 56 °C in another 5 cycles) and elongation at 72 °C for 2 min followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 52 °C for 45 s, and elongation at 72 °C for 2 min, with a final post-elongation step at 72 °C for 8 min. PCR products were used for sequencing using Big Dye terminator cycle sequencing RR mix protocol (ABI PRISM v. 3.1, Applied Biosystems) with cycling conditions of 95 °C for 1 min, 30 cycles of 95 °C for 10 s, 50 °C for 5 s and 60 °C for 4 min. Final products were purified using Sephadex G-50 fine (GE Healthcare, Uppsala, Sweden) and analysed on an ABI 3730xl DNA Analyzer (Applied Biosystems).
Alignment and phylogenetic analysis
Consensus sequences for each locus were obtained and edited using SeqMan in the Lasergene software (DNAStar, WI, USA). Sequences retrieved from GenBank for Pleosporales species were mainly from studies of Schoch et al. (2009), Zhang et al. (2009), and Lutzoni et al. (2004). Sequences derived from this study were deposited in GenBank; accession numbers are listed in Table 1. Sequences of ribosomal genes were aligned with MUSCLE using the EMBL-EBI web server (http://www.ebi.ac.uk/Tools/msa/muscle/). Introns were found in some sequences of SSU and they were removed from the alignment. Protein-coding gene sequences were aligned after removing of introns using RevTrans 1.4 Server (Wernersson & Pedersen 2003). Alignments were constructed for each gene separately and adjusted manually in BioEdit v. 7.1.3 software (Hall 1999). Missing data for partial or complete sequences for some taxa were coded as ‘missing’ but still can be used in the final matrix according to Wiens (2006) and Wiens & Moen (2008). A combined alignment was constructed for partial sequences of LSU, SSU, TEF1, and RPB2, alignments were concatenated using DataConvert 1.0 (https://www.bigelow.org/research/srs/david_a_mcclellan/david_mcclellan_laboratory/dataconvert/).
Phylogenetic analyses were performed using Bayesian and maximum likelihood analyses. Bayesian command files were prepared using Mesquite v. 2.75 (Maddison & Maddison 2011). The combined dataset was partitioned per locus and the analysis was done in MrBayes v. 3.1.2 implemented in the Cipres web server (http://www.phylo.org/). Two parallel runs with four Markov chain Monte Carlo (MCMC) simulations for each run were set for 20 000 000 generations and the result was checked using Tracer v. 1.5 (Rambaut & Drummond 2007) for effective sample size (ESS). The run was then extended for another 10 000 000 generations with a sample frequency of 1 000 per each generation. Maximum likelihood analysis was done using RAxML v. 7.2.8 in the Cipres web server. All trees were constructed with outgroup method and edited in Mega v. 5.05 (Tamura et al. 2011). ITS sequences were aligned with MUSCLE and adjusted in BioEdit v. 7.1.3 (Hall 1999). Phylogenetic analysis for ITS was performed with Bayesian and maximum likelihood analysis as described before except with numbers of generations for MrBayes set at 10 000 000.
RESULTS
Phylogenetic analysis
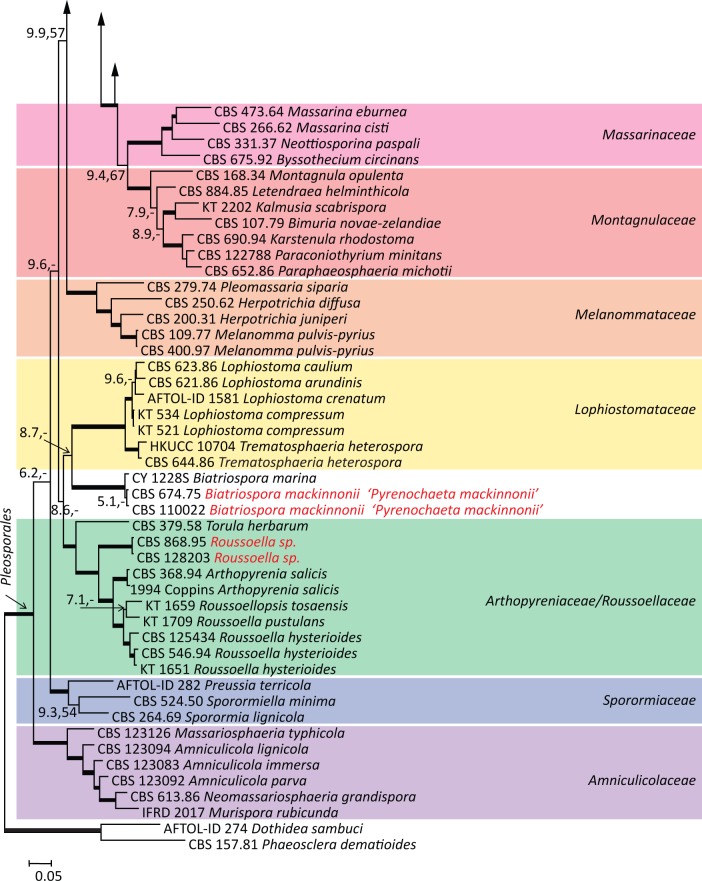
The combined dataset of SSU, LSU, RPB2, and TEF1 consisted of 101 taxa belonging to 13 families of Pleosporales. A total of 3 085 alignment characters were used of which 894 were derived from SSU, 706 from LSU, 711 from RPB2, and 774 from TEF1. Trees of the combined genes were rooted with Dothidea sambuci and Phaeosclera dematioides. The Bayesian and Maximum likelihood trees were congruent at both familial and intra-familial levels with few differences in the internal node support (Fig. 1). A 50 % majority consensus tree resulting from Bayesian analysis is presented in Fig. 1 in which the main clades represent the families of Pleosporales determined according to Zhang et al. (2009). An ITS alignment was constructed for 48 isolates belonging to 6 species. The total alignment length was 531 characters of which 335 were constant. The ITS tree was rooted with Medicopsis romeroi as outgroup.
Fig. 1.
Phylogram of combined dataset (LSU, SSU, RPB2, TEF1) obtained by Bayesian analysis and maximum likelihood (values of ≥ 0.70 for Bayesian probability and ≥ 70 % for maximum likelihood shown in bold branches). Species causing mycetoma shown in red, with obsolete names between inverted commas. Dothidea sambuci and Phaeosclera dematioides were used as outgroup.
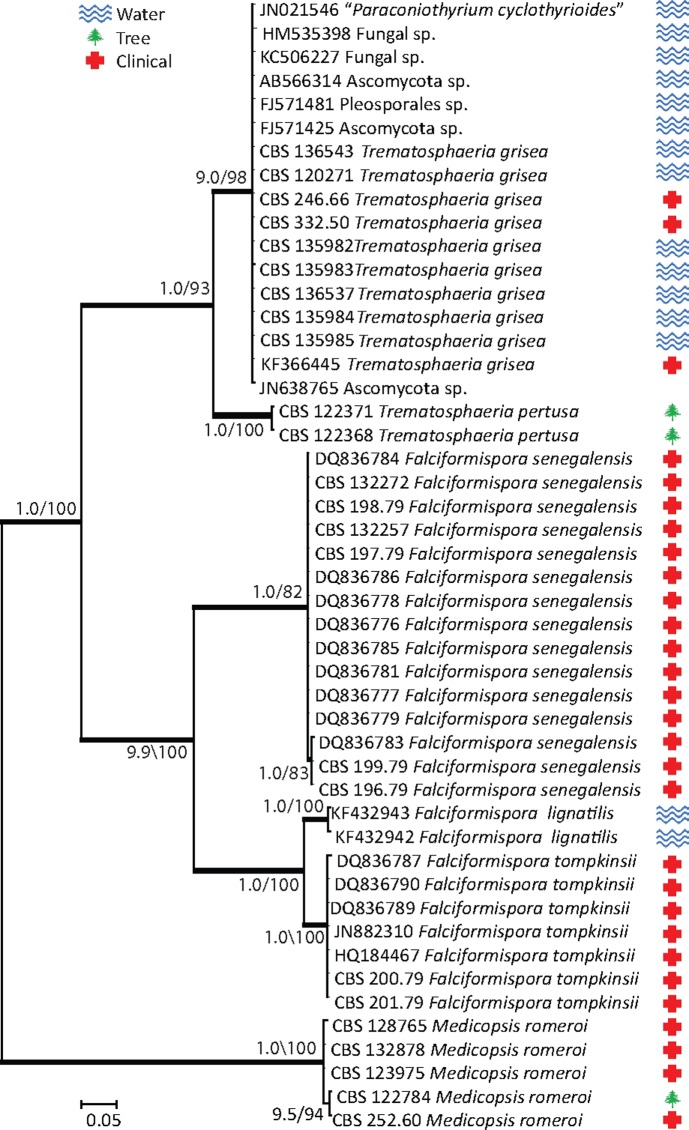
Based on the multi-gene phylogeny, pleosporalean mycetoma agents were found to belong to two families, viz. the Trematosphaeriaceae (Madurella grisea, Leptosphaeria senegalensis, and L. tompkinsii), and the Arthopyreniaceae / Roussoellaceae. Three additional agents of mycetoma were found to be unrelated to any known family. Within the Trematosphaeriaceae, Madurella grisea formed a well-supported clade with Trematosphaeria pertusa (Bayesian inference posterior probability (BII PP) / bootstrap values 1.00/100 %) (Fig. 1). This finding was confirmed with an ITS tree constructed for the family Trematosphaeriaceae (Fig. 2). In the ITS tree, additional ITS sequences were downloaded from GenBank and from strains in the CBS database which proved to be identical to the ITS sequence of M. grisea. These strains originally had been isolated from watery environments (Fig. 2, Table 1).
Fig. 2.
Phylogenetic tree resulting from Bayesian analysis and maximum likelihood for the ITS gene (values of ≥ 0.8 for Bayesian probability and ≥ 80 % for maximum likelihood are shown with bold branches). Medicopsis romeroi was used as outgroup.
Based on the multi-gene phylogeny, Leptosphaeria senegalensis and L. tompkinsii were found to cluster within the Trematosphaeriaceae, with Falciformispora lignatilis as nearest sibling species (Fig. 1). Leptosphaeria tompkinsii was closer to F. lignatilis than to its sister species L. senegalensis. This was noticed not only in the combined gene tree but also in the pairwise similarities between the three species at the individual loci (Fig. 2). Two sequences of ITS derived from Falciformispora lignatilis were included in the ITS tree (Fig. 2). The phylogeny of Falciformispora and the two Leptosphaeria species in the tree was congruent with the multi-gene tree.
Pseudochaetosphaeronema larense was found to be close to Massaria platani as a basal lineage to the Lentitheciaceae; however, this position was not well supported (Fig. 1). The relation to the genus Massaria also remained unclear since the genus is polyphyletic and Massaria platani is not the generic type species.
Nigrograna mackinnonii has recently been proposed as a new name for Pyrenochaeta mackinnonii, because it was found to be remote from the generic type species Pyrenochaeta nobilis (de Gruyter et al. 2010, 2013). In our multilocus analysis this species was found to be closely related to the monotypic genus Biatriospora, with B. marina as type species (Fig. 1). The two species could not unambiguously be separated using the five genes analysed.
Medicopsis romeroi (formerly Pyrenochaeta romeroi) was found in a basal lineage of Pleosporinae with no close relation to any pleosporalean family. The monotypic genus Medicopsis was recently erected to accommodate Pyrenochaeta romeroi (de Gruyter et al. 2013).
A non-sporulating eumycetoma agent represented by two strains; one strain morphologically identified by Meis et al. (2000) as Madurella mycetomatis, and a second isolate recovered from subcutaneous mycoses clustered in the pleosporalean family Arthopyreniaceae / Roussoellaceae (Fig. 1). This new species with its clinical data will be published at a later date.
Physiology
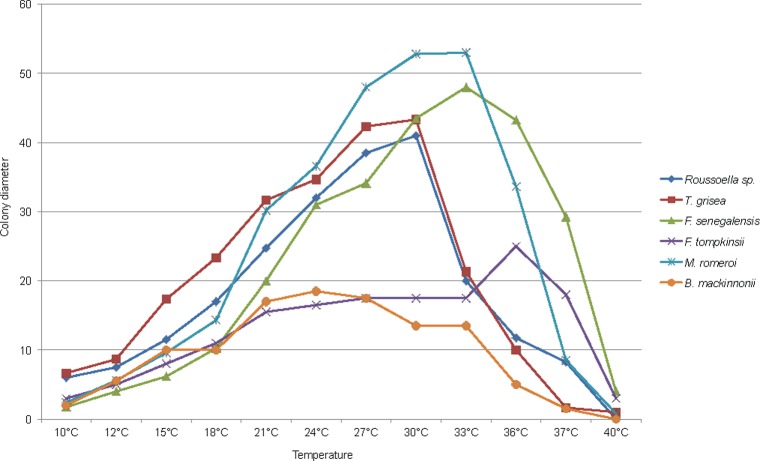
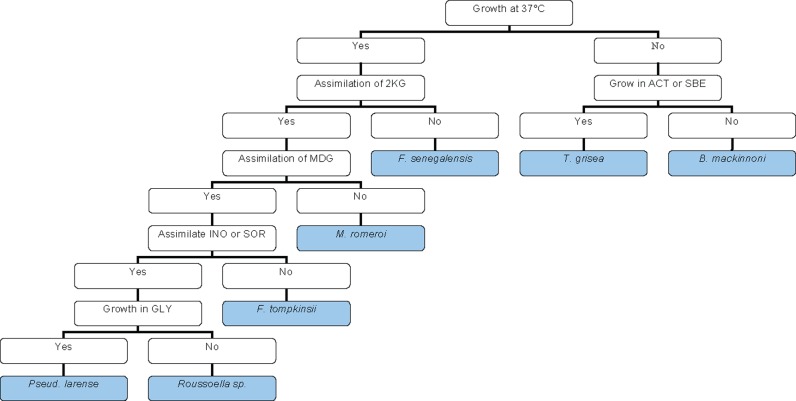
To determine possible diagnostic phenotypic or virulence markers, the black-grain eumycetoma species of the order of the Pleosporales were analysed for their physiological properties, i.e. cardinal growth temperatures and carbohydrate assimilation profiles. Growth profiles at different temperatures for the tested strains are shown in Fig. 3. Most of the species showed optimal growth at temperatures ranging from 27 to 36 °C, with the exception of N. mackinnonii, which had a lower optimum at 24 °C. The maximum growth temperature of all species tested was below 40 °C, but some individual strains of L. tompkinsii and L. senegalensis still were able to grow at this high temperature. Most strains were able to grow at 37 °C, with the exception of the clinical isolates of M. grisea (Fig. 3). However, strains of M. grisea still survived at 37 °C, because they could grow when re-incubated at 30 °C. Results of carbohydrate assimilation, urease, laccase, and salt tolerance tests are listed in Table 2. Madurella grisea, Leptosphaeria senegalensis, L. tompkinsii, Medicopsis romeroi, Nigrograna mackinnonii, Pseudochaetosphaeronema larense, and Roussoella sp. all were able to assimilate GAL, SAC, NAG, CEL, RAF, MAL, TRE, MAN, RHA, MLZ, PLE, and GLU, but not LAT, GNT, GRT, and GLN; no diagnostic difference was found between these carbohydrates. Leptosphaeria senegalensis was unique among the species tested in that it did not assimilate 2KG, ERY, and MEL, whereas the remaining species did. Furthermore, LAC was assimilated by this species, whereas the majority of the species was unable to use this compound. Leptosphaeria tompkinsii was the only species that did not assimilate INO and SOR. Medicopsis romeroiwas the only species not assimilating MDG, and Nigrograna mackinnoniiwas unique by its sensitivity to cycloheximide and ability to use XYL, unlike Pseudochaetosphaeronema larense, which did not assimilate XYL. Based on these physiological properties, an identification scheme was developed, which is presented in Fig. 4. It appeared that all species were able to produce laccase indicated by a green halo around the colony on ABTS medium (Fig. 8), except for one strain of L. tompkinsii. All species were found to be osmotolerant and were able to survive in concentrations of salt up to 20 %.
Fig. 3.
Average of colony diameters of studied species after 2 wk of incubation at various temperatures ranging from 15 to 36 °C, with 3 °C intervals, including 10 °C, 37 °C, and 40 °C.
Fig. 4.
Identification scheme of eumycetoma causative agents based on physiological properties of studied species.
Fig. 8.
Trematosphaeria grisea (a–c. CBS 332.50, d–g. CBS 120271). Colonies after 2 wk of incubation on: a. MEA; b. OA c. ABTS media; d, e. pycnidia; f. conidia; g. conidiophores. — Scale bars = 10 μm.
Taxonomy
Biatriospora mackinnonii (Borelli) S.A. Ahmed, W.W.J. van de Sande, A. Fahal, de Hoog, comb. nov. — MycoBank MB805083
Basionym. Pyrenochaeta mackinnonii Borelli, Castellania 4: 230. 1976. MB322132.
≡ Nigrograna mackinnonii (Borelli) Gruyter, Verkley & Crous, Stud. Mycol. 75: 31. 2012. MB564795.
Colonies on MEA restricted, grey, velvety, becoming dark grey to black with age. Colonies on OA felty, greyish green to olivaceous. Description taken from de Gruyter et al. (2013): “Pycnidia solitary on the surface or submerged in agar, globose to subglobose or pyriform, with papillate ostioles. Conidiogenous cells hyaline, phialidic, discrete. Conidia subhyaline, brown in mass, aseptate, ellipsoidal”. Sexual morph unknown.
Cardinal temperatures — Min. below 10 °C, opt. 24 °C, max. 37 °C (Fig. 3).
Specimens examined. VENEZUELA, human mycetoma, CBS H-21354 holotype, culture ex-type CBS 674.75. Additional strain listed in Table 1.
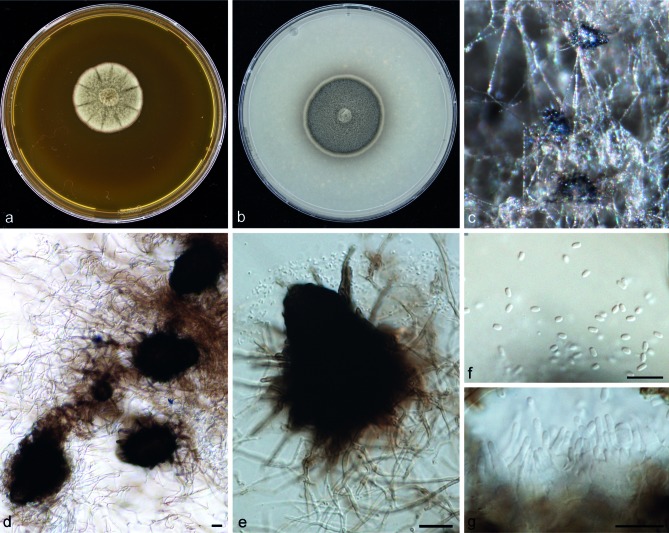
Falciformispora senegalensis (Segretain, Baylet, Darasse & Camain) S.A. Ahmed, W.W.J. van de Sande, A. Fahal & de Hoog,comb. nov. — MycoBank MB804853; Fig. 5
Fig. 5.
Falciformispora senegalensis (CBS 196.79). Colonies after 2 wk of incubation on: a. MEA; b. OA; c. cleistothecia; d, e. asci; f. ascospore; g. ascospore surrounded by thin sheath. — Scale bars = 10 μm.
Basionym. Leptosphaeria senegalensis Segretain, Baylet, Darasse & Camain, Compt. Rend. Acad. Sci. 248: 3732. 1959. MB299640.
Colonies on MEA radially folded, marble white, pale greyish green at some parts, velvety, with brown exudate produced on the colony surface; reverse dark brown to black, with dark brown pigment diffused into the agar. Colonies on OA dark greyish green, felty, radially folded with reddish brown pigment exuded below and around the colony. Asexual morph sporulation absent. Mature ascomata observed after 8 wk incubation as olivaceous, greyish green dots at the surface of the agar medium, 100–116 μm diam, black, solitary, spherical to subspherical or ovoidal, thick-walled. Pseudoparaphyses hyaline, septate. Asci clavate, rounded at the apex, bitunicate, containing 8 ascospores. Ascospores 30–32 × 9–10 μm ellipsoidal, 4-septate with constrictions at the septa, second cell from the top is the largest, leading to widening of the thin sheath surrounding the ascospore (Fig. 5). The species did not assimilate potassium 2-keto-gluconate (2KG), erythritol (ERY), and D-melibiose (MEL).
Cardinal temperatures — Min. below 10 °C, opt. 33 °C, max. above 40 °C (Fig. 3).
Specimens examined. SENEGAL, Dakar, C. de Bièvre, human mycetoma with black grains, CBS H-21349 holotype, culture ex-type CBS 196.79 = IHEM 3278 = IHEM 3814 = IP 614.60. Additional strains listed in Table 1.
Falciformispora tompkinsii (El-Ani) S.A. Ahmed, W.W.J. van de Sande, A. Fahal & de Hoog,comb. nov. — MycoBank MB804855
Basionym. Leptosphaeria tompkinsii El-Ani, Mycologia 58: 409. 1966. MB333276.
Colonies on MEA restricted, flat, initially marble white, becoming greyish green with age. Reverse dark brown to black at the centre and brown pigment seen on agar. Colonies on OA grey, with a halo of a pale red pigment diffusing into agar. Asexual morph sporulation absent. Sexual morph description according to El-Ani (1966): “Perithecia are non-ostiolate, scattered, innate or superficial, globose or subglobose, black, covered with brown flexuous hyphae, frequently flattened at the base measuring 214–535 μm. Asci are octosporic, clavate to cylinderical, bitunicate, rounded at the tip and tapered at the base 80–115 × 20–25 μm. The ascospores are hyaline, fusoid, straight or curved, 4–8-septate, predominantly 6- and 7-septate, constricted at the septa, 32–45 × 8.8–11 μm”. Unable to assimilate inositol (INO) and D-sorbitol (SOR).
Cardinal temperatures — Min. below 10 °C, opt. 36 °C, max. above 40 °C (Fig. 3).
Specimens examined. SENEGAL, Dakar, C. de Bièvre, human, CBS H-21350 holotype, culture ex-type CBS 200.79. Additional strain listed in Table 1.
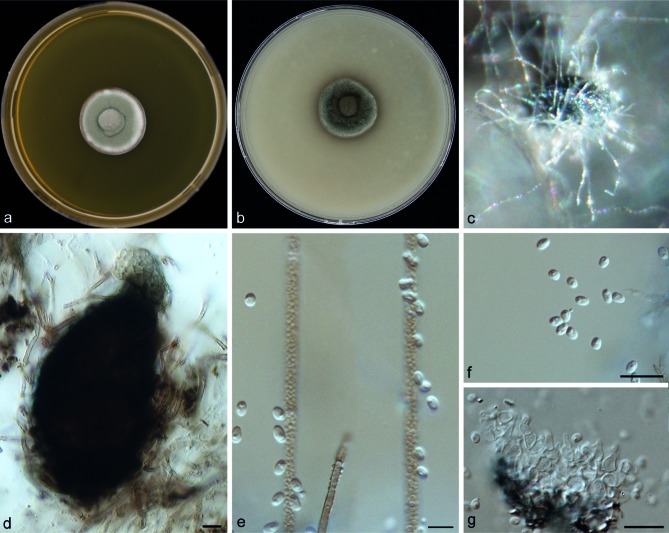
Medicopsis romeroi (Borelli) Gruyter, Verkley & Crous, Stud. Mycol. 75: 28. 2012. — MycoBank MB564792; Fig. 6
Fig. 6.
Medicopsis romeroi (CBS 252.60). Colonies after 2 wk of incubation on: a. MEA; b. OA; c–e. pycnidia; f. conidia; g. conidiophores. — Scale bars = 10 μm.
≡ Pyrenochaeta romeroi Borelli, Dermatol. Venez. 1: 326. 1959. MB338075.
Colonies on MEA greyish green, velvety, becoming dark grey and floccose with age; reverse dark grey to black. Colonies on OA flat, dark grey to olivaceous or black. Hyphae broad, septate, branched, dark brown, verruculose. Pycnidia observed after 4 wk incubation as small grey black dots on the surface of agar plates, 105–127 × 70–96 μm, black, ostiolate, pyriform, solitary with short dark brown to black setae. Conidia 2.0–2.8 × 1.2–1.5 μm, hyaline, unicellular, cylindrical to ellipsoidal. Conidiophores seen after crushing the pycnidia, phialidic, smooth-walled, hyaline, ampulliform (Fig. 6). Sexual morph unknown. Differential diagnosis from other pleosporalean mycetoma agents: inability to assimilate methyl-α-D-glucopyranoside (MDG).
Cardinal temperatures — Min. below 10 °C, opt. 30−33 °C, max. 40 °C (Fig. 3).
Specimens examined. VENEZUELA, from human mycetoma, D. Borelli, culture ex-type CBS 252.60 = ATCC 13735 = FMC 151 = UAMH 10841. Additional strains listed in Table 1.
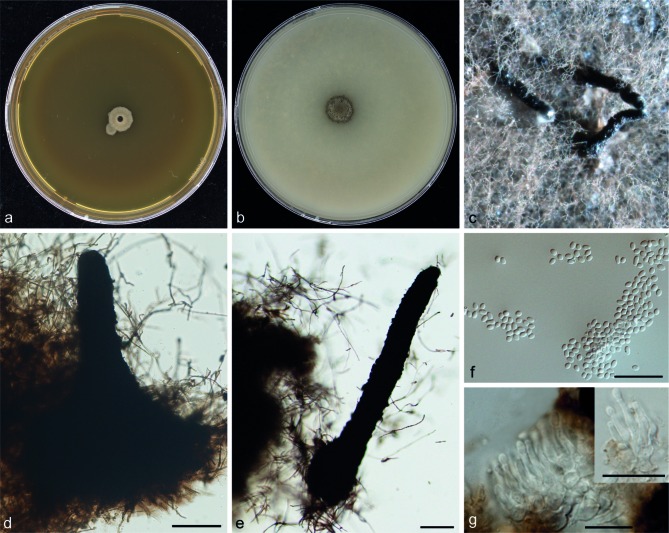
Pseudochaetosphaeronema larense (Borelli & R. Zamora) Punith., Nova Hedwigia 31: 127. 1979. — MycoBank MB321757; Fig. 7
Fig. 7.
Pseudochaetosphaeronema larense(CBS 640.73). Colonies after 2 wk of incubation on: a. MEA; b. OA; c–e. pycnidia; f. conidia; g. conidiophores. — Scale bars = 10 μm.
Colonies on MEA restricted, velvety, grey green; reverse dark grey to black. Colonies on OA woolly, dark grey to olivaceous. Pycnidia observed after 8 wk of incubation on OA, 245–248 × 177–231 μm black, solitary, obpyriform with long neck reaching 140 μm in length. Conidia 2.7–3.6 × 2.0–2.5 μm, hyaline, unicellular, subspherical to ellipsoidal. Conidiophores ampulliform hyaline, phialidic. No assimilation of xylose; sensitive to cyclohexamide. Sexual morph unknown.
Cardinal temperatures — Min. below 10 °C, opt. 24–30 °C, max. 40 °C (Fig. 3).
Specimens examined. VENEZUELA, human mycetoma, D. Borelli, culture ex-type CBS 640.73 = FMC 250. Additional strain listed in Table 1.
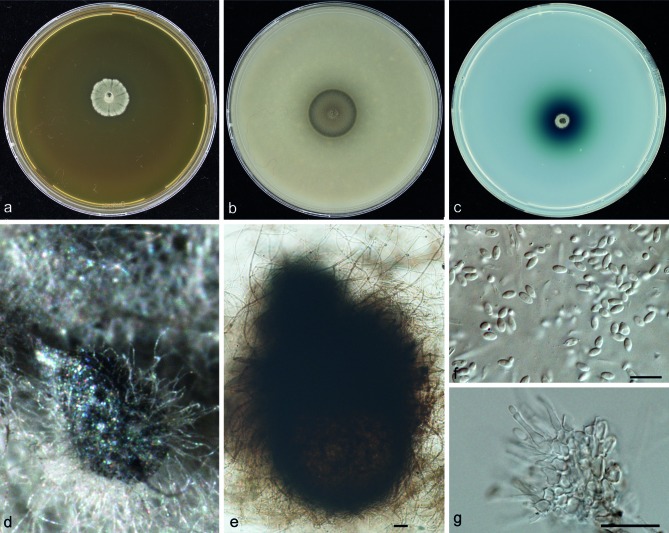
Trematosphaeria grisea (J.E. Mackinnon, Ferrada & Montemart) S.A. Ahmed, W.W.J. van de Sande, A. Fahal & de Hoog, comb. nov. — MycoBank MB804854; Fig. 8
Basionym. Madurella grisea J.E. Mackinnon, Ferrada & Montemart, Mycopathologia 4: 389. 1949. MB287885.
Colonies on MEA restricted, felty to floccose, greenish grey at the centre, becoming faint towards the margin; reverse dark brown to black. Colonies on OA flat, olivaceous to black (Fig. 8). Environmental isolates of T. grisea rapidly growing, with expanding, grey colonies. Hyphae branched, septate, hyaline or brown, thick-walled, verruculose. Pycnidia observed in environmental strains after 8 wk incubation as small black dots with white droplets on top, 128–190 × 96–115 μm, black, solitary or aggregated, subspherical to pyriform, ostiolate, setose. Conidia 4.0–5.4 × 2.0–2.4 μm, hyaline to pale brown, unicellular, clavate to ellipsoidal. Conidiophores hyaline, rostrate with very short, hardly detectable collarette. Sexual morph unknown.
Cardinal temperatures — Min. below 10 °C, opt. 27−30 °C, max. 40 °C (Fig. 3).
Specimens examined. CHILI, Mycetoma, J.E. MacKinnon, CBS-H 21348 holotype, culture ex-type CBS 332.50 = ATCC 10794 = IHM 927. Strain CBS 120271 isolated from tap water, The Netherlands, was used for morphological description of pycnidia and conidia, additional strains listed in Table 1.
Trematosphaeria pertusa (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 161. 1870. — MycoBank MB197662; Fig. 9
Fig. 9.
Trematosphaeria pertusa (CBS 122368). Colonies after 2 wk of incubation on: a. MEA; b. OA. c, d. pycnidia; e. verrucose hyphae and conidia; f. conidia; g. conidiophores. — Scale bars = 10 μm.
Basionym. Sphaeria pertusa Pers., Syn. Meth. Fung.: 83. 1801. MB405380.
Colonies on MEA velvety, dark greyish green with pale grey margin; reverse dark grey to black. Colonies on OA floccose, dark greyish green. Pycnidia 70–100 × 38–63 μm, black, solitary or in aggregates, ovoidal, ostiolate, with dark brown to black setae. Conidia 2.0–3.6 × 2.0–2.3 μm, hyaline to pale brown, unicellular, ellipsoidal. Conidiophores short, hyaline, obpyriform. Sexual morph: description according to Zhang et al. (2008): “Ascomata were 350–550 × 320–480 μm, solitary or in groups and subglobose. Asci were 100–145 × 15–17 μm, contain 8 ascospores 27.5–32.5 × 7.5–8.5 μm, verruculose with 1–3 septa, deeply constricted at the median septum, the upper cell often shorter and broader than the lower one”.
Cardinal temperatures — Min. below 10 °C, opt. 27 °C, max. 37 °C.
Specimens examined. FRANCE, 25 Apr. 2004, from Fraxinus excelsior, J. Fournier,Herbarium IFRD 2002, culture ex-type CBS 122368. Additional strain listed in Table 1.
DISCUSSION
A wide range of fungal species has been reported as causative agents of human eumycetoma. To date more than 30 species are listed, belonging to different genera throughout the Ascomycota, predominantly in the orders Sordariales and Pleosporales (de Hoog et al. 2004). In the present study, we analysed the molecular taxonomy of pleosporalean agents of mycetoma using five loci, to establish their higher phylogenetic affiliations as well as their specific taxonomy. Some of the species have exclusively been recovered from cases of human mycetoma and are not known from the environment. They are nevertheless judged to be environmental opportunists causing a general foreign body response in the host (de Hoog et al. 2000). A habitat involving living plants or plant debris is known for numerous species of genera such as Leptosphaeria and Pyrenochaeta, but for the clinical members of these genera the natural niche has yet to be discovered.
The present study shows that agents of mycetoma are phylogenetically remote from the genera where they were originally classified, and thus entirely different habitats may be expected. A striking example is Nigrograna mackinnonii (= Pyrenochaeta mackinnonii), which we found to be molecularly indistinguishable from Biatriospora marina, a marine ascomycete consistently inhabiting tropical mangrove wood (Hyde & Borse 1986, Jones et al. 2006). Nigrograna was proposed to accommodate Pyrenochaeta mackinnonii. However, the high degree of sequence similarity demonstrated the single identity of the two species, N. mackinnonii and B. marina. Therefore, the genus Nigrograna proved to be redundant and Nigrograna mackinnonii might be the asexual morph of the ascosporulating species B. marina. Mangrove wood may be the natural habitat of the fungus; cases of mycetoma are extremely rare, so that human infection is unlikely to be the natural behaviour of the fungus. An unidentified Biatriospora species was isolated from a marine sponge in Brazil by Passarini et al. (2013). In addition to mycetomata, N. mackinnonii was also reported from phaeohyphomycosis in a renal transplant patient in Brazil (Santos et al. 2013). No morphological similarity was found between B. marina and N mackinnonii and the two strains of N. mackinnonii (B. marina) included in our analysis, originating from Mexico and Venezuela, did not show any sporulation even after 3 mo of incubation. de Gruyter et al. (2013) reported the formation of pycnidia and conidia by the ex-type strain of N. mackinnonii, CBS 674.75.
Most clinical isolates from mycetoma are non-sporulating or produce conidia or ascospores only after a very long incubation time. Applying phenotypic, morphological diagnostics has thus led to many misidentifications and also to misclassifications of the fungus within the fungal kingdom. Previous generic concepts based on sterile mycelia led to assignment of Madurella mycetomatis and M. grisea as sister and only species in the genus, whereas we now know that the DNA sequence differences are great enough that they are placed in different orders (de Hoog et al. 2004). More precisely, whereas Madurella mycetomatis, the most frequent agent of eumycetoma (McGinnis 1996) appeared to be closely related to dung-associated species of Chaetomium in the Sordariales (van de Sande 2012, de Hoog et al. 2013), we found M. grisea to be related to marine wood-inhabiting species of Trematosphaeria in the Pleosporales.
Madurella grisea, Leptosphaeria senegalensis, and L. tompkinsii were found to belong to the Trematosphaeriaceae, a small family of fungi in marine environments affiliated to the sub-order Massarineae (Zhang et al. 2012). This adds to N. mackinnonii above, where also a marine natural habitat was suggested. De Hoog et al. (2005) noted that mild halotolerance tends to be associated with opportunism and may thus be regarded as a potential virulence factor. The ecological similarity with Trematosphaeria is striking; possibly also here mild osmotolerance can be regarded as an ancestral virulence factor in these fungi.
The family Trematosphaeriaceae consists of three genera, Trematosphaeria, Halomassarina, and Falciformispora. These genera mainly comprise fungi inhabiting marine environments, e.g. mangrove wood (Suetrong et al. 2011). Madurella grisea was found closely related to Trematosphaeria pertusa, the type species of the genus Trematosphaeria. Both species produce a coelomycetous asexual morph forming well-characterised conidiomata (Fig. 8, 9), but T. pertusa, described from its natural habitat, produces a sexual morph with ascomata and ascospores (Zhang et al. 2008). In our study, we analysed two strains of T. pertusa, including the ex-type strain (CBS 122368). Both strains only displayed asexual sporulation, which was not described before (Fig. 9). Zhang et al. (2008) reported formation of hyphopodia-like structures in T. pertusa after 6 mo of incubation in water. To compare the morphology with other species of the genus Trematosphaeria, strains of T. heterospora, T. hydrela, and T. crassiseptata have been analysed, but they were found to be phylogenetically distant from T. pertusa and could not be included in the tree (data not shown) (Wang et al. 2007). Because of similarities in both phylogenetic position and morphology with T.pertusa, M. grisea is transferred to the genus Trematosphaeria and renamed Trematosphaeria grisea. Physiological properties established for T. grisea,i.e. assimilation of sucrose and galactose but not lactose are consistent with the original description of the species given by Mackinnon et al. (1949). The GenBank database contained several unnamed depositions of identical ITS sequences, which are now re-identified as T. grisea (Fig. 2). Interestingly, all sequences for which information on origins was available were derived from marine and marine-related environments (Fig. 2). In addition, several strains from the CBS were added which were mainly obtained from water or other environmental sources in The Netherlands (Table 1). In the present study, only 3 out of 10 strains genotypically identified as T. grisea and included in the ITS tree (Table 1, Fig 2) originated from a human infection (mycetoma, abscess, biopsy specimen). Thus T. grisea is an occasional opportunist. Six of the strains were recovered from water, and one from pastry gel. This suggests that T. grisea is likely to be an oligotrophic saprobe. Reported cases of mycetoma caused by T. grisea originated from South America and India. Unconfirmed reports were published from North and Central America, Africa, and Asia (Green & Adams 1964, Butz & Ajello 1970, Mahgoub 1973, Venugopal et al. 1990, Vilela et al. 2004). In most of these publications, identification was based on morphological or physiological properties and may have been misidentifications (Desnos-Ollivier et al. 2006). One of the strains of T. grisea examined by Mackinnon et al. (1949) in the original description of the species was re-identified with ITS sequences as Madurella tropicana (Sordariales, Chaetomiaceae). Recently, a case of mycetoma in an Indian patient in the UK was ascribed as T. grisea by Gulati et al. (2012). However, sequence comparison revealed that the strain was phylogenetically remote from T. grisea (data not shown).
Two Leptosphaeria agents of mycetoma appear in the literature, viz. L. senegalensis and L. tompkinsii. Both species were found to be phylogenetically remote from the Leptosphaeriaceae but instead clustered in the Trematosphaeriaceae close to Falciformispora, another monotypic genus in which F. lignatilis is associated with the mangrove environment (Hyde 1992). The position of L. tompkinsii and F. lignatilis is concordant with the morphology, both forming ascospores with 6–8 septa, whereas in L. senegalensis ascospores have 4 septa (El-Ani 1966, Hyde 1992). With F. lignatilis as the type species of the genus Falciformispora, both L. senegalensis and L. tompkinsii are to be transferred to this genus. All strains of F. senegalensis and F. tompkinsii included in this study originated from human infections, mostly mycetoma or other subcutaneous infections, and were geographically distributed in tropical areas of Sudan and Senegal. Segretain & Mariat (1968) reported the isolation of F. senegalensis from Acacia thorns in West and Central Sub-Saharan Africa. Falciformispora lignatilis has thus far only been recovered from mangrove wood and fresh water in North America and Thailand (Hyde 1992, Suetrong et al. 2009).
Of the five strains of Pyrenochaeta romeroi included in the present study, two were derived from mycetoma, two from phaeohyphomycosis or subcutaneous cysts, and one was recovered from plant material (Table 1). de Gruyter et al. (2010) excluded P. romeroi from the genus Pyrenochaeta, because it was proven to be phylogenetically distant from its type species P. nobilis. In another study, de Gruyter et al. (2013) proposed a new genus Medicopsis to accommodate P. romeroi. In our multi-gene phylogeny the as yet monotypic genus Medicopsis was found as a basal lineage to the Pleosporinae rather than as a member of Trematosphaeriaceae as reported by de Gruyter et al. (2013) based on rDNA LSU analyses. This might be due to the larger number of loci analysed, a more extended taxon sampling of species of Trematosphaeriaceae, and more strains of M. romeroi included.
Very few cases of eumycetoma have been reported with Pseudochaetospheronema larense as causative agent. Two strains isolated from Venezuela were analysed in the present study. The species formed a well-supported clade with strains identified as Massaria platani, and with Lentitheciaceae as the closest family, but at a significant phylogenetic distance. This was in accordance with the phylogenetic tree published by Schoch et al. (2009), while another tree published by Suetrong et al. (2009) showed that M. platani strains were basal lineage of Trematosphaeriaceae. In general, the phylogeny of many genera of Pleosporales is poorly resolved at the familial level mainly because this order is still under-sampled (Schoch et al. 2009).
Two sterile strains of a madurella-like species from human mycetoma were studied, one originating from the Caribbean and the other from India. Both strains appeared to represent a new pathogenic species in the family Arthopyreniaceae / Roussoellaceae (Ahmed et al. 2014).
The different species of mycetoma analysed in the present study share some physiological and virulence factors like growth at human body temperature, laccase production, and melanisation, in addition to halotolerance. The ecology and epidemiology of these species need to be further determined by analysing more strains from different regions.
Acknowledgments
We thank Satinee Na and E. Gareth Jones for providing sequences of Falciformispora strains. Katrien Lagrou is thanked for providing information on some clinical strains.
REFERENCES
- Ahmed SA, Stevens DA, Sande WW van de. et al. 2014 Roussoella percutanea, a novel opportunistic pathogen causing subcutaneous mycoses. Medical Mycology 52: 689–698. [DOI] [PubMed] [Google Scholar]
- Brumpt E. 1905. Sur le mycétome à grains noirs, maladie produite par une Mucédinée dugenre Madurella n. g. Comptes Rendus des Scéances de la Société de Biologie et deses Filiales 58: 997–999. [Google Scholar]
- Butz WC, Ajello L. 1970 Black grain mycetoma: a case due to Madurella grisea. Archives of Dermatology 104: 197–201. [DOI] [PubMed] [Google Scholar]
- Chalmers AJ, Archibald RG. 1918 The classification of mycetomas. Journal of Tropical Medicine and Hygiene 21: 121–123. [Google Scholar]
- Desnos-Ollivier M, Bretagne S, Dromer F, et al. 2006 Molecular identification of black-grain mycetoma agents. Journal of Clinical Microbiology 44: 3517–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ani AS. 1966 A new species of Leptosphaeria, an etiologic agent of mycetoma. Mycologia 58: 406–411. [PubMed] [Google Scholar]
- Fahal AH, Suliman SH. 1994 Clinical presentation of mycetoma. Sudan Medical Journal 32: 46–66. [Google Scholar]
- Feng P, Lu Q, Najafzadeh MJ, et al. In press. Cyphellophora and its relatives in Phialophora: biodiversity and possible role in human infection. Fungal Diversity. doi 10.1007/s13225-012-0194-5. [Google Scholar]
- Gargas A, Taylor JW. 1992 Polymerase chain reaction (PCR) primers for amplifying and sequencing 18S rDNA from lichenized fungi. Mycologia 84: 589–592. [Google Scholar]
- Green WO, Jr, Adams TE. 1964. Mycetoma in the United States: A review and report of seven additional cases. American Journal of Clinical Pathology 42: 75–91. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JH, Aveskamp MM, et al. 2010 Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JH, Aveskamp MM, et al. 2013. Redisposition of phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati V, Bakare S, Tibrewal S, et al. 2012 A rare presentation of concurrent Scedosporium apiospermum and Madurella grisea umycetoma in an immunocompetent host. Case Reports in Pathology 2012: 154–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. 1999 BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hoog GS de, Adelmann D, Ahmed AOA, et al. 2004. Phylogeny and typification of Madurella mycetomatis, with a comparison of other agents of eumycetoma. Mycoses 47: 121–130. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Ahmed AO, McGinnis MR, et al. 2007 Fungi causing eumycotic mycetoma. In: Murray PR, Baron EJ, Jorgensen JH, et al. (eds), Manual of Clinical Microbiology:1918–1927 American Society for Microbiology Press, Washington, DC, USA. [Google Scholar]
- Hoog GS de, Ahmed SA, Najafzadeh MJ, et al. 2013 Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Neglected Tropical Diseases 7: e2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Diepeningen AD van, Mahgoub el-S, et al. 2012 New species of Madurella, causative agents of black-grain mycetoma. Journal of Clinical Microbiology 50: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J. et al. 2000 Atlas of Clinical Fungi, 2nd edition Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Hoog GS de, Zalar P, Gerrits van den Ende AHG, et al. 2005 Relation of halotolerance to human-pathogenicity in the fungal tree of life: an overview of ecology and evolution under stress. In: Gunde-Cimerman N, Oren A, Plemenitaš A. (eds), Adaptation to life at high salt concentrations in Archaea Bacteria, and Eukarya: 373–395 Springer, The Netherlands. [Google Scholar]
- Hyde KD. 1992. Intertidal mangrove fungi from the west coast of Mexico, including one new genus and two new species. Mycological Research 96: 25–30. [Google Scholar]
- Hyde KD, Borse BD. 1986. Marine fungi from Seychelles. V. Biatriospora marina gen. et sp. nov. from mangrove wood. Mycotaxon 26: 263–270. [Google Scholar]
- Jones EBG, Pilantanapak A, Chatmala I, et al. 2006 Thai marine fungal diversity. Songklanakarin Journal of Science and Technology 28: 687–708. [Google Scholar]
- Kaluskar VM, Kapadnis BP, Jaspers C, et al. 1999. Production of laccase by immobilized cells of Agaricus sp.: induction effect of xylan and lignin derivatives. Applied Biochemistry and Biotechnology 76: 161–170. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1967 Methuen handbook of colour. 2nd ed Methuen & Co Ltd, London. [Google Scholar]
- Kwon-Chung KJ, Bennett JE. 1992 Medical mycology. Lea & Febiger; Philadelphia. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA Polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Kauff F, Cox C, et al. 2004 Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- Mackinnon JE, Ferrada-Urzúa LV, Montemayor L. 1949 Madurella grisea n. sp. Mycopathologia 4: 384–393. [Google Scholar]
- Maddison WP, Maddison DR. 2011 Mesquite: a modular system for evolutionary analysis. Version 2.75 http://mesquiteproject.org. [Google Scholar]
- Mahgoub ES. 1973 Mycetomas caused by Curvularia lunata, Madurella grisea, Aspergillus nidulans, and Nocardia brasiliensis in Sudan. Sabouraudia 11: 179–182. [PubMed] [Google Scholar]
- McGinnis MR. 1996 Mycetoma. Dermatology Clinic 41: 97–104. [Google Scholar]
- Meis JF, Schouten RA, Verweij PE, et al. 2000 Atypical presentation of Madurella mycetomatis mycetoma in a renal transplant patient. Transplantation and Infectious Disease 2: 96–98. [DOI] [PubMed] [Google Scholar]
- Passarini MR, Santos C, Lima N, et al. 2013. Filamentous fungi from the Atlantic marine sponge Dragmacidon reticulatum. Archives of Microbiology 195: 99–111. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. 2007 Tracer v. 1.4. Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Sande WWJ van de. 2012. Phylogenetic analysis of the complete mitochondrial genome of Madurella mycetomatis confirms its taxonomic position within the order Sordariales. PLoS One 7: e38654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos DW, Padovan AC, Melo AS, et al. 2013. Molecular identification of melanised non-sporulating moulds: a useful tool for studying the epidemiology of phaeohyphomycosis. Mycopathologia 175: 445–454. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretain G, Mariat F. 1968 Recherches sur la présence d’agents de mycétomes dans le sol et sur les épineux du Sénégal et de la Mauritanie. Bulletin de la Societé de Pathologie Exotique 61: 194–202. [PubMed] [Google Scholar]
- Suetrong S, Hyde KD, Zhang Y, et al. 2011. Trematosphaeriaceae fam. nov. (Dothideomycetes, Ascomycota). Cryptogamie, Mycologie 32: 343–358. [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, et al. 2009. Molecular systematics of the marine Dothideomycetes. Studies in Mycology 64: 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal PV, Venugopal TV, Laing WN, et al. 1990 Black grain mycetoma caused by Madurella grisea in Saudi Arabia. International Journal of Dermatology 29: 434–435. [DOI] [PubMed] [Google Scholar]
- Vilela R, Duarte OM, Rosa CA, et al. 2004 A case of eumycetoma due to Madurella grisea in northern Brazil. Mycopathologia 158: 415–418. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990 Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HK, Aptroot A, Crous PW, et al. 2007 The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycological Research 111: 1268–1276. [DOI] [PubMed] [Google Scholar]
- Wernersson R, Pedersen AG. 2003 RevTrans – Constructing alignments of coding DNA from aligned amino acid sequences. Nucleic Acids Research 31: 3537–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990 Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR Protocols: a guide to methods and applications:315–322 Academic Press, New York, USA. [Google Scholar]
- Wiens JJ. 2006. Missing data and the design of phylogenetic analyses. Journal of Biomedical Informatics 39: 34–42. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Moen DS. 2008. Missing data and the accuracy of Bayesian phylogenetics. Journal of Systematics and Evolution 46: 307–314. [Google Scholar]
- Yan J, Deng J, Zhou CJ, et al. 2010. Phenotypic and molecular characterization of Madurella pseudomycetomatis sp. nov., a novel opportunistic fungus possibly causing black-grain mycetoma. Journal of Clinical Microbiology 48: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, et al. 2012. Pleosporales. Fungal Diversity 53: 1–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fournier J, Pointing SB, et al. 2008 Are Melanomma pulvis-pyrius and Trematosphaeria pertusa congeneric? Fungal Diversity 33: 47–60. [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, et al. 2009. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]