Abstract
Identification of fungi and the International Code of Nomenclature underpinning this process, rests strongly on the characterisation of morphological structures. Yet, the value of these characters to define species in many groups has become questionable or even superfluous. This has emerged as DNA-based techniques have increasingly revealed cryptic species and species complexes. This problem is vividly illustrated in the present study where 105 isolates of the Botryosphaeriales were recovered from both healthy and diseased woody tissues of native Acacia spp. in Namibia and South Africa. Thirteen phylogenetically distinct groups were identified based on Internal Transcribed Spacer (ITS) rDNA PCR-RFLP and translation elongation factor 1-α (TEF1-α) sequence data, two loci that are known to be reliable markers to distinguish species in the Botryosphaeriales. Four of these groups could be linked reliably to sequence data for formerly described species, including Botryosphaeria dothidea, Dothiorella dulcispinae, Lasiodiplodia pseudotheobromae and Spencermartinsia viticola. Nine groups, however, could not be linked to any other species known from culture and for which sequence data are available. These groups are, therefore, described as Aplosporella africana, A. papillata, Botryosphaeria auasmontanum, Dothiorella capri-amissi, Do. oblonga, Lasiodiplodia pyriformis, Spencermartinsia rosulata, Sphaeropsis variabilis and an undescribed Neofusicoccum sp. The species described here could not be reliably compared with the thousands of taxa described in these genera from other hosts and regions, where only morphological data are available. Such comparison would be possible only if all previously described taxa are epitypified, which is not a viable objective for the two families, Botryosphaeriaceae and Aplosporellaceae, in the Botryosphaeriales identified here. The extent of diversity of the Botryosphaeriales revealed in this and other recent studies is expected to reflect that of other undersampled regions and hosts, and illustrates the urgency to find more effective ways to describe species in this, and indeed other, groups of fungi.
Keywords: Botryosphaeriales, morphotaxa, phylogeny, taxonomy, tree health
INTRODUCTION
Technological advances in DNA sequencing and accompanying software to store, share and compare the emerging data, have revolutionised the processes underpinning the discovery and identification of living organisms, including the fungi (Crous & Groenewald 2005, Cai et al. 2011, Maddison et al. 2012). These advances have fundamentally changed the way that the identification of fungi is undertaken, and concurrently, there have also been far ranging changes relating to our understanding of fungal diversity. It is logical that such fundamental changes will lead to changes in the taxonomic rules, which are based on approaches developed a century or more ago. In some cases this has already happened, such as the recent adoption of the ‘One Fungus One Name’ approach (Taylor 2011, Wingfield et al. 2012, Hibbett & Taylor 2013). Yet, in other areas, particularly in the case of older taxonomic names for which no sequence data are available, the rules have not changed. Here, comparisons with type specimens and descriptions linked to older names, following the rules of the International Code of Nomenclature for algae, fungi and plants (ICN), remains predominantly reliant on morphological features.
It has been estimated that fungal taxa defined based on morphology alone, often encompass at least two, but often more cryptic taxa, when molecular data from multiple gene regions are considered (Taylor et al. 2000). This is most likely an underestimation for many fungal taxa. For example, the Neofusiccoccum parvum–N. ribis complex (once considered to be a single species) has been shown to include at least seven cryptic taxa (Sakalidis et al. 2011), and unpublished data suggest that additional species exist in this group. Most of these species cannot be distinguished using traditional morphological characters (Pavlic et al. 2009a, b, Sakalidis et al. 2011). These characters are thus also not useful to link current species in the Botryosphaeriales to herbarium material or previous descriptions, which often include only very rudimentary morphological information (also see Phillips et al. 2013 for a more extensive discussion on this topic). This shortcoming of a taxonomic approach that is reliant on morphology, relegates even a dominant and important group such as the Botryosphaeriales to a very uncertain position when seeking to understand origins, invasions, patterns of disease outbreak, ecology in native areas, host associations, and a suite of other critical questions. This study addresses an example of the problem and argues that surveys in new regions and including unexplored plants can only realistically include comparisons with species for which sequence data are available.
Fungi belonging to the Botryosphaeriales are common endophytes, but some are also opportunistic pathogens, causing diseases of many woody species (Slippers & Wingfield 2007). They have a wide distribution and host range and typical symptoms include die-back, cankers and, in severe cases, tree death. Yet, the ecological role and biogeographical patterns of this group of fungi is poorly understood in most ecosystems. This is surprising in light of the fact that these fungi have been known for almost 180 years, are dominant in many ecosystems, readily isolated from healthy and dead plant material and regularly observed sporulating on this material. The reason why the wealth of taxonomic and isolation data linked to most of these taxa cannot be mined to address these questions, arises from recent studies showing that identifications are not reliable using traditional morphological characters of asexual and sexual forms (e.g. Slippers et al. 2004a, b, Pavlic et al. 2009a, b, Sakalidis et al. 2011, Phillips et al. 2013). While many studies on these fungi in recent years professed to have applied a combination of molecular and morphological characters to identify the species, a closer examination shows that they mostly rely on sequence data to distinguish species. Based on the delineations arising from analyses of sequence data, morphological characterisation is added to comply with regulations of the ICN. These characters are rarely useful for distinction of subsequently discovered species, and it is unrealistic to believe that they will be sufficient for the large numbers of species yet to be discovered. Clearly an urgent solution is needed to characterise and name the large numbers of species currently being discovered through extended sampling and the application of modern molecular tools (Hibbett et al. 2011, Hibbett & Taylor 2013).
Acacia mellifera, also known as the blackthorn, is regarded as one of the most valuable native trees found on cattle and game farms in southern Africa, but in Namibia it is threatened by a serious die-back disease (Holz & Schreuder 1989, Venter & Venter 2002). Symptoms of this disease are typical of the die-back that is caused by members of the Botryosphaeriales that were previously accommodated in the genus Botryosphaeria or its asexual morphs, now classified in the Botryosphaeriaceae (Crous et al. 2006, Slippers & Wingfield 2007, Slippers et al. 2013).
Reports of Botryosphaeriales from Acacia species in Africa have been limited until recently. A review on phytopathogens in South Africa by Crous et al. (2000) indicated no records of Botryosphaeriales on native Acacia species. Elsewhere in Africa, Lasiodiplodia theobromae, was reported causing a root disease on Acacia nilotica, which is native to Kenya (Lenné 1992). Recent studies, however, suggest that much more diversity exists on native Acacia, discovering and describing five new species from A. karroo from only two sites in South Africa, including Diplodia allocellula, Dothiorella dulcispinae, Do. brevicollis, Do. pretoriensis and Tiarosporella urbis-rosarum, together with Botryosphaeria dothidea, Diplodia pseudoseriata, Neofusicoccum vitifusiforme and Spencermartinsia viticola (Jami et al. 2012, 2013, 2014). Two species of the Botryosphaeriaceae have also been reported from non-native A. mearnsii trees from South Africa, namely L. theobromae (Stephens & Goldschmidt 1938, Gibson 1975, Roux & Wingfield 1997) and B. dothidea (Roux & Wingfield 1997, Roux et al. 1997).
In an effort to understand the association of the Botryosphaeriales with native Acacia species in southern Africa, a study was undertaken to characterise these fungi on various Acacia species in Namibia and South Africa, with a special focus on A. mellifera. The Botryosphaeriales associated with these trees were initially grouped using morphological characterisation of the asexual morphs, PCR-RFLP groupings and comparisons of sequence data for the internal transcribed spacer (ITS) and translation elongation factor 1-α (TEF 1-α) loci. The study also provided an opportunity to evaluate the complications of undertaking such a study using traditional taxonomic tools and increasingly intangible taxonomic characters. Finally, possible solutions are suggested for dealing with such problems.
MATERIALS AND METHODS
Collection of samples and isolations
Botryosphaeriales isolates were obtained from Acacia species during two main collection periods. The first set of isolates was obtained from a preliminary survey conducted in 2005 in the Prieska area of South Africa. The area surveyed (Libertas farm) is situated on the south bank of the Orange River at the foot of the Doringberg, Northern Cape Province, South Africa. Subsequently, during a second collection period, samples were collected from Windhoek, Dordabis, Grootfontein and Rundu in Namibia and Ditholo Air Force Base, near Pretoria (Gauteng Province) in South Africa. Acacia trees sampled in the Prieska area included A. erioloba, A. karroo, A. mellifera and A. tortilis. A total of 89 Acacia trees were sampled in the second collection period, of which 69 were from Namibia and 20 from Pretoria. Trees from Namibia included A. hebeclade (n = 5), A. karroo (n = 19) and A. mellifera (n = 45), while only A. mellifera (n = 20) was sampled in Pretoria. Both healthy branch tips and diseased plant material were collected. Healthy branch tips were used for isolations of the Botryosphaeriales existing as endophytes as described by Pavlic et al. (2004). Diseased plant material included symptoms such as lesions on branches, black pith in the branches, cankers, tip die-back, streaked lesions and a brownish black discolouration in the upper tap roots of dying trees. The plant material was surface disinfested with 76 % ethanol (v/v) and small pieces (± 5 mm2) of symptomatic tissue were plated onto 2 % MEA (20 g/L Biolab Malt Extract; 15 g/L Biolab Agar; Biolab, Midrand, South Africa).
Symptomatic tissue was also placed in moist chambers comprised of plastic bags containing wet paper towels. The plates and moist chambers were incubated at room temperature. Isolations were done from structures produced on plant tissue in moist chambers and cultures were purified from the plates. Cultures resembling the Botryosphaeriales were identified and initially grouped based on their morphological characteristics when grown in pure culture.
DNA isolation and PCR
DNA was extracted from cultures using a phenol:chloroform DNA extraction method modified from Raeder & Broda (1985). Modifications were similar to those of Barnes et al. (2001) except that the mycelium of 7-d-old cultures was scraped from the medium and transferred to Eppendorf tubes (1.5 mL) and extraction buffer was added. The mycelium was manually ground with a glass rod and incubated at 60 °C for 60 min in a heating block. Centrifugation was done at 10 000 rpm. DNA pellets were resuspended in 50 μL sterile SABAX water. RNAse (5 mg/mL) was added to DNA samples and incubated overnight at 37 °C to degrade residual RNA.
Primers ITS1 and ITS4 (White et al. 1990) were used to amplify the 3’ end of the 18S (small subunit) rRNA gene, ITS1, the complete 5.8S rRNA gene, ITS2 and the 5’ end of the 28S (large subunit) rRNA gene. The 3’ end of the second exon to the 5’ end of the last exon and two variable introns of the TEF1-α gene were also amplified with the primers EF1F and EF2R (Jacobs et al. 2004). To clarify the generic position of a species related to Barriopsis, Phaeobotryon and Sphaeropsis, the β-tubulin gene region was amplified for sequencing using primers Bt2a and Bt2b (Glass & Donaldson 1995) for a selected group of isolates.
PCR reaction mixtures contained 5–10 ng of genomic DNA, 0.2 mM dNTP, 0.2 mM of each primer, 1.5 mM buffer (10 mM Tris-HCL, 1.5 mM MgCl2, 50 mM KCL), 2.5 U Taq DNA polymerase and was adjusted to a final reaction volume of 25 μL with sterile distilled water. Parameters used for the PCR reactions included a 2 min step at 95 °C, followed by 10 cycles of 20 s at 94 °C, 40 s at 55 °C and 45 s at 72 °C. The 10 cycles were then repeated for another 30 cycles with a 5 s increase per cycle for the elongation step, and then a final elongation for 10 min at 72 °C. Amplification of the TEF1-α locus was problematic for some isolates for which the annealing temperatures were adjusted up to 68 °C and the MgCl2 concentrations increased up to 1.6 mM. PCR products were separated by electrophoresis using 2 % agarose gels, stained with ethidium bromide and visualized under UV illumination. Size estimates were made using a 100 bp size marker.
PCR-RFLP
A PCR-RFLP test was used on the ITS amplicons to confirm the initial groupings of isolates based on culture morphology of all the isolates from Namibia. Profiles were obtained using the enzyme HhaI (Fermentas International Inc., Canada), which recognizes the same sequence as CfoI, which has been used before to digest the ITS amplicons (Slippers et al. 2007). Each PCR-RFLP reaction consisted of 20 μL PCR product, 2 μL 10× Buffer Tango and 1 μL enzyme (10 U/μL) (Fermentas International Inc., Canada). The final reaction volume was adjusted to 40 μL with sterile distilled water. The reaction was incubated at 37 °C for 16 h in a heating block and the enzyme was inactivated at 80 °C for 20 min. The products were subjected to electrophoresis using a 3 % agarose gel at 60 V for 90 min, stained with ethidium bromide and visualized under UV illumination. Size estimates were made using a 100 bp size marker.
Sequencing and phylogenetic analyses
Thirteen isolates from the preliminary survey in the Prieska areas, and three representative isolates of each PCR-RFLP group (where possible), were used in sequence comparisons (Table 1). The PCR products were purified using 6 % Sephadex columns (1.33 g in 20 mL sterile water) (Sigma, Steinheim, Germany). The same primers were used as in the initial PCR reactions and both strands of the amplicons were sequenced. Reactions were performed using an ABI PRISM 3100 Autosequencer (Applied BioSystems, Foster City, California, USA) and sequences were analysed using Sequence Navigator v. 1.0.1 (Applied BioSystems, Foster City, California, USA).
Table 1.
Isolates of the Botryosphaeriales from this study, as well as previously identified reference isolates linked to some of them, that were used for phylogenetic and/or taxonomic studies.
| Identity1 | Culture no.2, 3 | Host | Locality | Collectors | GenBank accession numbers |
|
|---|---|---|---|---|---|---|
| ITS | TEF1-α | |||||
| Aplosporella africana | CMW 25424, CBS 121777 | Acacia mellifera | Dordabis, Namibia | F.J.J. van der Walt & J. Roux | EU101315 | EU101360 |
| CMW 25425, CBS 121778 | A. mellifera | Grootfontein, Namibia | F.J.J. van der Walt & J. Roux | EU101316 | EU101361 | |
| CMW 25426, CBS 121779 | A. mellifera | Dordabis, Namibia | F.J.J. van der Walt & J. Roux | EU101317 | EU101362 | |
| A. papillata | CMW 25427, CBS 121780 | A. tortillas | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101328 | EU101373 |
| CMW 25428, CBS 121781 | A. erioloba | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101329 | EU101374 | |
| CMW 25429, CBS 121782 | A. erioloba | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101330 | EU101375 | |
| Botryosphaeria auasmontanum | CMW 25413, CBS 121769 | A. mellifera | Windhoek, Namibia | F.J.J van der Walt & J. Roux | EU101303 | EU101348 |
| B. dothidea | CMW 8000 | Prunus sp. | Crocifisso, Switzerland | B. Slippers | AY236949 | AY236898 |
| CMW 25410 | A. mellifera | Dordabis, Namibia | F.J.J. van der Walt & J. Roux | EU101304 | EU101349 | |
| CMW 25411 | A. karroo | Windhoek, Namibia | F.J.J. van der Walt & J. Roux | EU101305 | EU101350 | |
| Dothiorella capri-amissi | CMW 25404, CBS 121878 | A. erioloba | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101324 | EU101369 |
| CMW 25405 | A. erioloba | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101325 | EU101370 | |
| CMW 25403, CBS 121763 | A. erioloba | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101323 | EU101368 | |
| Do. dulcispinae | CBS 130413 | A. karroo | Pretoria, South Africa | F. Jami & M. Gryzenhout | JQ239400 | JQ239387 |
| CBS 130414 | A. karroo | Pretoria, South Africa | F. Jami & M. Gryzenhout | JQ239401 | JQ239388 | |
| CBS 130415 | A. karroo | Pretoria, South Africa | F. Jami & M. Gryzenhout | JQ239402 | JQ239389 | |
| CMW 25406, CBS 121764 | A. mellifera | Rundu, Namibia | F.J.J. van der Walt & J. Roux | EU101299 | EU101344 | |
| Do. oblonga | CMW 25407, CBS 121765 | A. mellifera | Pretoria, South Africa | F.J.J. van der Walt & R.N. Heath | EU101300 | EU101345 |
| CMW 25408, CBS 121766 | A. mellifera | Pretoria, South Africa | F.J.J. van der Walt & R.N. Heath | EU101301 | EU101346 | |
| Lasiodiplodia pseudotheobromae | CMW 25417 | A. mellifera | Rundu, Namibia | F.J.J. van der Walt & J. Roux | EU101310 | EU101355 |
| CMW 25418 | A. mellifera | Rundu, Namibia | F.J.J. van der Walt & J. Roux | EU101311 | EU101356 | |
| L. pyriformis | CMW 25414, CBS 121770 | A. mellifera | Dordabis, Namibia | F.J.J. van der Walt & J. Roux | EU101307 | EU101352 |
| CMW 25415, CBS 121771 | A. mellifera | Dordabis, Namibia | F.J.J. van der Walt & J. Roux | EU101308 | EU101353 | |
| Neofusicoccum sp. | CMW 25409, CBS 121767 | A. mellifera | Windhoek, Namibia | F.J.J. van der Walt & J. Roux | EU101302 | EU101347 |
| Spencermartinsia rosulata | CBS 121760 | A. karroo | Windhoek, Namibia | F.J.J. van der Walt & J. Roux | EU101290 | EU101335 |
| CMW 25392, CBS 121761 | A. mellifera | Pretoria, South Africa | F.J.J. van der Walt & R.N. Heath | EU101293 | EU101338 | |
| CMW 25394 | A. karroo | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101318 | EU101363 | |
| CMW 25395, CBS 121762 | A. mellifera | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101319 | EU101364 | |
| CMW 25396 | A. mellifera | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101320 | EU101365 | |
| CMW 25397 | A. tortillis | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101321 | EU101366 | |
| CMW 25398 | A. tortillis | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101322 | EU101367 | |
| S. viticola | CBS 117009 | Vitis vinifera cv. Garnatxa Negra | Vimbodí, Spain | J. Luque & R. Mateu | AY905554 | AY905559 |
| CMW 25399 | A. mellifera | Pretoria, South Africa | F.J.J. van der Walt & R.N. Heath | EU101295 | EU101340 | |
| CMW 25400 | A. mellifera | Pretoria, South Africa | F.J.J. van der Walt & R.N. Heath | EU101296 | EU101341 | |
| CBS 117008 | V. vinifera cv. Xarel·lo | Sant Sadurní d’Anoia, Spain | J. Luque & J. Reyes | AY905557 | AY905560 | |
| Sphaeropsis variabilis | CMW 25420 | A. hebeclade | Windhoek, Namibia | F.J.J. van der Walt & J. Roux | EU101313 | EU101358 |
| CMW 25421, CBS 121775 | A. karroo | Windhoek, Namibia | F.J.J. van der Walt & J. Roux | EU101314 | EU101359 | |
| CMW 25422, CBS 121776 | A. mellifera | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101326 | EU101371 | |
| CMW 25423 | A. mellifera | Northern Cape, South Africa | F.J.J. van der Walt & G.J. Marais | EU101327 | EU101372 | |
| Guignardia sp. | MUCC 684 | Agonis flexuosa | Western Australia, Yalgorup | R. Adair & T. Burgess | EU675682 | EU686573 |
| MUCC 685 | Ag. flexuosa | Western Australia, Yalgorup | R. Adair & T. Burgess | EU675681 | EU686572 | |
1 Names in bold signify ex-type cultures, or from samples that have been linked morphologically to the type material.
2 CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CMW: CMW- FABI, University of Pretoria, South Africa; MUCC: Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie prefecture, Japan.
3 Isolates for which sequences were determined in this study are indicated in bold.
Sequences were compared to those of other Botryosphaeriales in GenBank using BLAST and related sequences were downloaded. Sequences were aligned with MAFFT (Katoh et al. 2002) v. 5.8 (http://mafft.cbrc.jp/alignment/server/) and manually adjusted. Phylogenetic analyses of the sequence data for Maximum Parsimony (MP) and Maximum Likelihood (ML) were made using PAUP v. 4.0b10 (Swofford 2002). For ML analyses, the best nucleotide substitution models for each dataset were found separately with Modeltest v. 3.7 (Posada & Buckley 2004). MP genealogies for single genes were constructed with the heuristic search option (100 random taxa additions, tree bisection and reconstruction or TBR in PAUP). The uninformative characters were removed from the analyses, gaps were treated as fifth character and all characters were unordered and of equal weight. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the tree(s) obtained was evaluated by 1 000 bootstrap replications. Congruence between the different datasets was tested using the Partition Homogeneity Test (PHT) in PAUP (Farris et al. 1995), with the uninformative characters removed before analysis. Other measures such as tree length (TL), homoplasy index (CI), rescaled consistency index (RC) and the retention index (RI) (Hillis & Huelsenbeck 1992) were recorded.
Morphological characteristics
All isolates used for sequencing (Table 1) were induced to sporulate on sterilised pine needles that were placed on the surface of 2 % water agar (WA) at 25 °C under near UV light. Fungal structures were mounted on glass slides in lactic acid (75 %) and examined under a Zeiss Axioskop microscope and images were captured using a HRc Axiocam digital camera and Axiovision v. 3.1 software (Carl Zeiss Ltd., Germany). At least fifty measurements were made for each diagnostic character per species description. The minimum, maximum, standard deviation (SD), mean values and the length/width (l/w) ratios were calculated and are presented in this study as (min–) av. ± std. dev. (–max). Colours were identified based on the colour charts of Rayner (1970).
Growth characterisation
To determine the growth rate of species, two to three isolates (7-d-old) were selected to represent each taxon (Table 1). Agar discs (4 mm diam), overgrown with mycelium, were placed, mycelium side down, at the centres of 90 mm Petri dishes that contained 2 % MEA. Petri dishes were incubated in the dark at temperatures ranging from 5 °C to 35 °C at 5 °C intervals. The diameters of the colonies were measured after 2 d for fast growing cultures and 4 d for slower growing cultures. Average growth rates were calculated from five replicate plates for each isolate and temperature.
RESULTS
Collection of samples, isolations and preliminary grouping
Twenty-two cultures resembling members of the Botryosphaeriales were obtained from Acacia species in the Prieska area of the Northern Cape Province. Seventy-five isolates were collected from Acacia species in Namibia and eight from A. mellifera in Pretoria. Namibian isolates could be assigned to 12 groups based on culture morphology. Isolates from Pretoria were not included in these groups as they were obtained at a later stage.
PCR-RFLP
PCR produced amplicons of ~580 bp for the ITS gene region. PCR-RFLP profiles obtained from the HhaI digested ITS amplicons identified 11 PCR-RFLP groups from the Namibian isolates. These results corresponded well with the groups that were based on culture morphology, except in the case of one PCR-RFLP profile that represented two different culture morphology groups.
Sequencing and phylogenetic analyses
Following PCR-RFLP groupings, a total of 20 isolates representing the Namibian isolates were sequenced, as well as all eight isolates from Pretoria and all isolates from Prieska. BLAST searches and detailed DNA sequence comparisons were done on all sequences. Sequences from the Namibian, Pretoria and 13 of the Prieska isolates were then aligned with published sequences that were obtained from GenBank (Table 1), using MAFFT.
All datasets best fitted the GTR model with the ITS, TEF-1α, β-tubulin and combined datasets having the following parameters: for combined ITS and TEF-1α datasets G = 0.3510, I = 0.0 and for combined ITS, TEF-1α, β-tubulin datasets G = 0.267, I = 0.0. The analyses were performed in PAUP v. 4.0b10 and confidence levels were determined with 1 000 bootstrap replications. For the ITS and EF datasets (TreeBASE Accession No. 15092) were RI = 0.9229, RC = 0.5091, HI = 0.4483, TL = 2064, and the partition homogeneity test (PHT) on the datasets produced a P-value of 0.01. For the ITS, TEF-1α and β-tubulin datasets (TreeBASE Accession No. 15093) the tree statistics were RI = 0.9027, RC = 0.7607, HI = 0.1573, TL = 806.1.
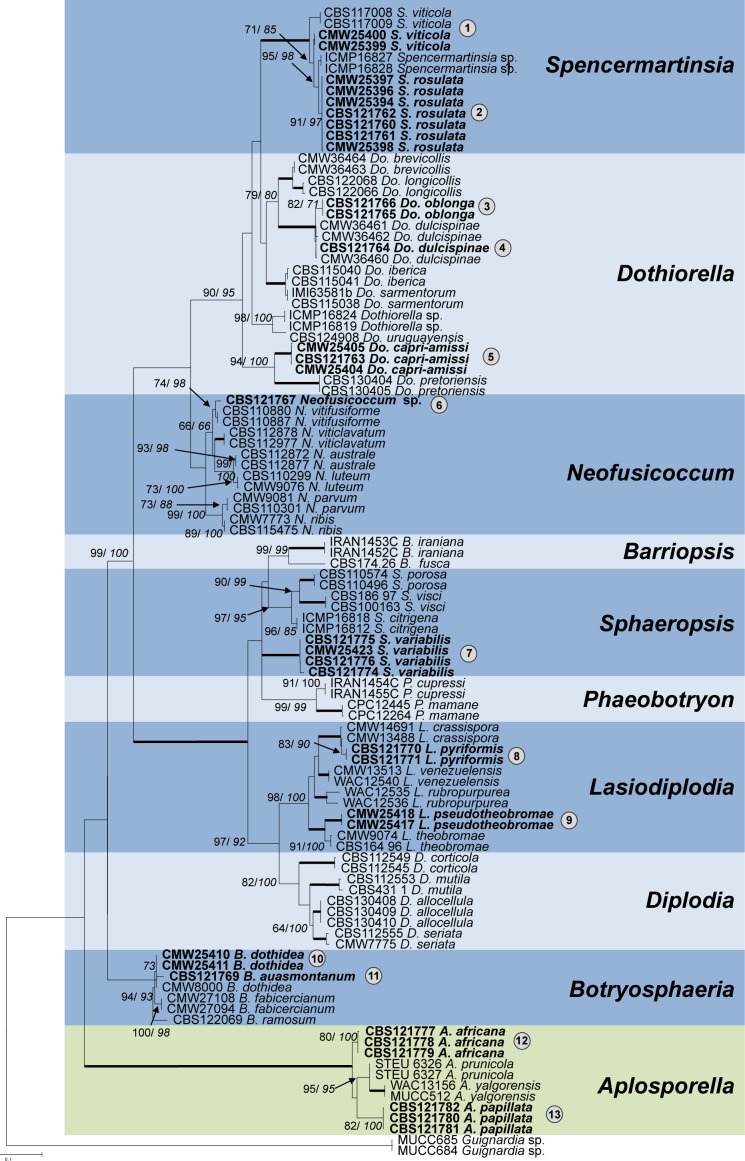
The phylogenetic analyses of the combined sequence data for the ITS and TEF1-α gene regions (Fig. 1) revealed 13 distinct taxa amongst the isolates collected in this study. The identified species included four previously described species, namely Do. dulcispinae, S. viticola, L. pseudotheobromae and B. dothidea (asexual morph = Fusicoccum aesculi) (Fig. 1). The remaining species did not match any species in GenBank, nor did their morphology (see below) match previous taxa described from these hosts or areas, and were considered to be undescribed taxa. These species included, two Aplosporella spp., a Botryosphaeria sp., two Dothiorella spp., a Lasiodiplodia sp., a Neofusicoccum sp., a Spencermartinsia sp. and a Sphaeropsis sp. In cases where the sister clades were not clearly distinguished, fixed polymorphisms across the loci were considered. Examples included a Spencermartinsia sp. (clade 2, Fig. 1, Table 2), a Botryosphaeria sp. (clade 11, Fig. 1, Table 3) and a Lasiodiplodia sp. (clade 8, Fig. 1, Table 4).
Fig. 1.
Maximum Likelihood tree of the combined dataset of ITS rDNA and TEF-1α loci sequences. Bootstrap values for ML and MP (italics) above 70 % are given at the nodes. Where both values were 100 %, the values are not shown but the branch is bolded. Isolates sequenced in this study are shown in bold. The species identified in the study are numbered 1–13, with these numbers bolded for species described in this study. The tree was rooted to Guignardia sp. (MUCC 684 and MUCC 685). Botryosphaeriaceae are shaded in blue and Aplosporellaceae in green.
Table 2.
Polymorphic nucleotides from sequence data of the ITS and TEF1-α to show the relationship between Spencermartinsia viticola and S. rosulata. Polymorphisms unique to S. rosulata are in bold type and shaded. Isolates from this study are indicated in bold.
| Identity | Culture no. | ITS |
EF1-α |
|||||
|---|---|---|---|---|---|---|---|---|
| 27 | 85 | 49 | 72 | 203 | 204 | 245 | ||
| S. viticola | CMW 25399 | C | T | C | C | C | A | G |
| CMW 25401 | C | T | C | C | C | A | G | |
| CBS 117006 | C | T | C | C | C | A | G | |
| CBS 117009 | C | T | C | C | C | A | G | |
| CBS 117008 | C | T | C | C | C | A | G | |
| S. rosulata | CBS 121760 | T | C | T | – | T | C | T |
| CBS 121761 | T | C | T | – | T | C | T | |
Table 3.
Sequence differences of the ITS and EF1-α gene regions to show the relationship between Botryosphaeria dothidea and B. auasmontanum. Differences unique to B. auasmontanum are in bold type and shaded. Isolates from this study are indicated in bold.
| Identity | Culture no. | ITS |
EF1-α |
|||||
|---|---|---|---|---|---|---|---|---|
| 56 | 57 | 91–106 | 110 | 133–135 | 214–230 | 236–239 | ||
| B. auasmontanum | CBS 121769 | T | G | – – – – – – – – – – – – – – – | G | – – – | – – – – – – – – – – – – – – – | – – – – |
| B. dothidea | CMW 8000 | – | – | GCCGCGGTTCTCCGCG | C | GGG | CTCCGCATCTGGATTTT | TTGT |
| CBS 116742 | – | – | GCCGCGGTTCTCCGCG | C | GGG | CTCCGCATCTGGATTTT | TTGT | |
| CBS 116743 | – | – | GCCGCGGTTCTCCGCG | C | GGG | CTCCGCATCTGGATTTT | TTGT | |
Table 4.
Polymorphic nucleotides (or alleles) from sequence data of the ITS and TEF1-α to show the relationship between Lasiodiplodia crassispora and the sibling species L. pyriformis. Polymorphisms unique to L. pyriformis are in bold type and shaded. Isolates from this study are indicated in bold.
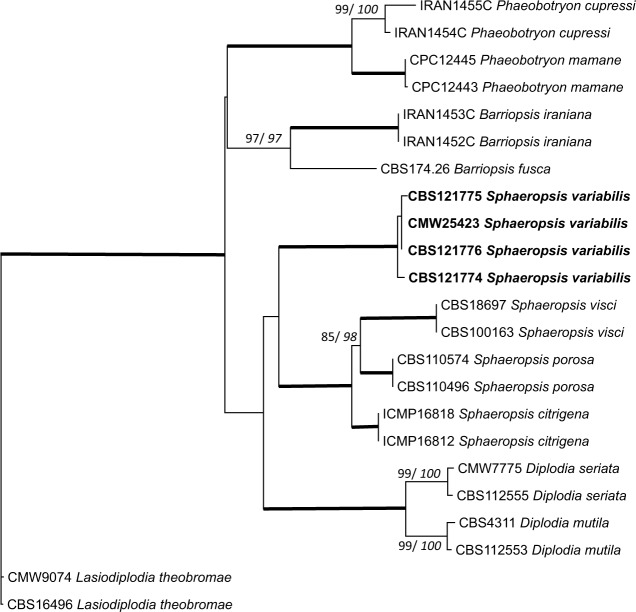
The grouping of the Sphaeropsis sp. was not clear based only on ITS and TEF-1α data. While ITS data grouped it with Sphaeropsis, TEF-1α data grouped it with Phaeobotryon. Additional β-tubulin sequence data, however, confirmed the grouping with Sphaeropsis (Fig. 2).
Fig. 2.
Maximum Likelihood tree of the combined dataset of ITS rDNA, TEF-1α and β-tubulin loci sequences for species closely related to Sphaeropsis variabilis. Bootstrap values for ML and MP (italics) above 70 % are given at the nodes. Where both values were 100 %, the values are not shown but the branch is bolded. Isolates sequenced in this study are shown in bold. The tree was rooted to Lasiodiplodia theobromae (CMW 9074 and CBS 16496).
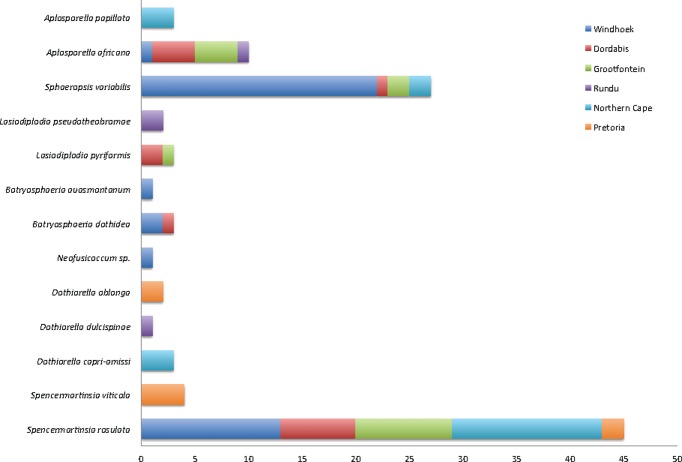
The geographic distribution of the Botryosphaeriales species identified in this study was mapped (Fig. 3). A number of species occurred at only one location. Others were found at many or even all of the locations considered in this study.
Fig. 3.
The distribution and numbers of isolates of Botryosphaeriales identified during this study from Acacia spp. in Namibia and South Africa.
Morphological characteristics
All the isolates used in the phylogenetic analyses were included in the morphological comparisons. Anamorph structures were produced on pine needles after 2–3 weeks. No teleomorph structures were observed and, therefore, descriptions are based only on the anamorphic states. The morphological characteristics of the isolates corresponded to groups emerging from the phylogenetic analyses and details are included in the descriptions of isolates. A Dichomera synasexual morph was identified in one of the B. dothidea cultures and its morphological characteristics are also described.
TAXONOMY
DNA sequence comparisons of the isolates from Acacia spp. revealed the presence of nine distinct taxa in the Botryosphaeriales that have not previously been identified. Eight of these are provided with names here. In addition to phylogenetic differences, the species on this host can be separated from each other based on distinct characteristics of the asexual morph. These characters are, however, not useful for distinction between many similar taxa from other regions and for which no sequence data are available.
Aplosporella africana F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518717; Fig. 4a
Fig. 4.
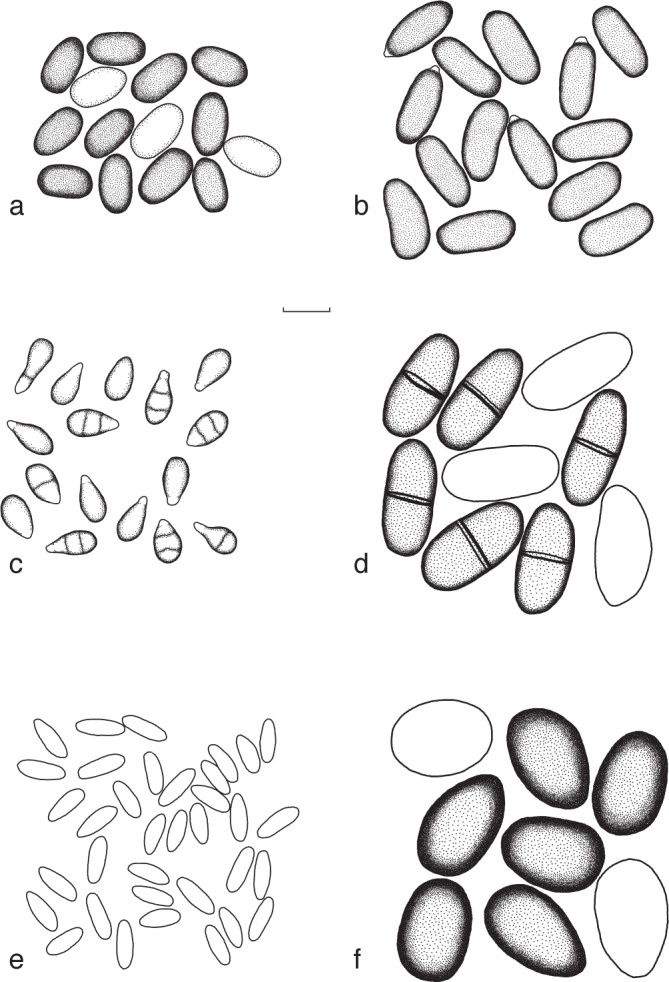
Conidia of newly described species of Botryosphaeriales, as well as species with newly described forms; a. Aplosporella africana; b. A. papillata; c. Dichomera state of Botryosphaeria dothidea; d. Dothiorella oblonga; e. B. auasmontanum; f. Lasiodiplodia pyriformis. — Scale bar = 10 μm.
Etymology. The name refers to the continent of Africa from which this genus and species was first described.
Sexual morph unknown. Conidiomata pycnidial, separate, covered in a small number of short hyphae, subglobose, up to 365 μm diam or occasionally pyriform, up to 465 μm in height, mostly superficial, occasionally semi-immersed or immersed. Paraphyses hyaline, filiform, aseptate, (15.5–)17–32.5(–38.5) × (1.2–)1.5–2.5 μm (av. 24.9 × 1.9 μm). Conidiogenous cells hyaline, holoblastic, cylindrical to ampuliform, proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations, (2.5–)3.5–6.5(–7.5) × 1.5–3.5(–4) μm (av. 5 × 2.5 μm). Conidia initially hyaline becoming honey coloured to brown, aseptate, ellipsoidal to broadly ellipsoidal, occasionally reniform, moderately thick-walled, granular content, smooth, occasionally slightly papillate apices, (10.5–)12.5–16(–19) × (5.5–)6.5–10(–13) μm (av. of 100 conidia: 14 × 8.5 μm, l/w ratio 1.7).
Culture characteristics — Mycelium blackish green-grey, occasionally blackish brown, effuse, with sparse mycelium. Thread-like growth on the edges of colonies. Reverse olivaceous black with thread-like growth at edges. Thickening of hyphae, chlamydospore-like, immersed in water agar. Temperatures for growth: min. 10 °C, growth rate of 18 mm/d at opt. 25 °C, max. 35 °C.
Specimens examined. NAMIBIA, Dordabis, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59640 – holotype, culture ex-type CMW 25424 = CBS 121777); Dordabis, from A. mellifera (PREM 59642 – paratype, culture ex-paratype CMW 25426 = CBS 121779); Grootfontein, from Acacia mellifera (PREM 59641 – paratype, culture ex-paratype CMW 25425 = CBS 121778).
Notes — Ten isolates were identified and described as A. africana. Nine of these were obtained from apparently healthy tissue and one was from wood with streaked discolouration. There are substantial morphological differences between A. africana and two related species, A. prunicola and A. yalgorensis (Damm et al. 2007, Taylor et al. 2009). Pycnidia and paraphyses of A. prunicola are much larger (up to 800 μm in length and 35–95 × 4–8 μm) than those of A. africana, while the conidia of A. yalgorensis (av. 19.9 × 10.7 μm) are larger than those of A. africana. Conidia of A. prunicola and A. yalgorensis are also hyaline at first, turning dark brown with age, which is different to those of A. africana that become honey to brown coloured. There are also no papillate apices on the conidia of A. prunicola or A. yalgorensis as are occasionally found in A. africana.
Aplosporella papillata F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518718; Fig. 4b
Etymology. Name refers to the papillation of the conidial apices.
Sexual morph unknown. Conidiomata pycnidial, globose or spherical to subglobose, separate, covered in small amounts of short hyphae, up to 895 μm diam, mostly semi-immersed or immersed and occasionally superficial. Paraphysis hyaline, filiform, (18–)23.5–36.5 × (1–)2–3 μm (av. 30.1× 2.2 μm). Conidiogenous cells hyaline, holoblastic, cylindrical to ampilliform, proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations, (5–)5.5–9.5(–11.5) × (1–)1.5–2.5(–3.5) μm (av. 7.4 × 2 μm). Conidia initially hyaline becoming blackish brown, ellipsoidal to broadly ellipsoidal, occasionally reniform, ovoid or constricted in the middle, moderately thick-walled, granular content, smooth, frequently with prominent pappillate apices (14.5–)16–18(–20) × (6–)6.5–9(–11.5) μm (av. of 50 conidia: 17 × 7.6, l/w ratio 2.2).
Culture characteristics — Mycelium effuse, dark greenish olive with an orange-rufous concentric zone near the edge of the colony, occasionally with white mycelium. Reverse buffy brown with blackish brown concentric zones and thread-like growth in the centre. Occasionally with a thickening of the hyphae, chlamydospore-like, immersed in water agar, blackish brown. Temperatures for growth: min. 10 °C, growth rate of 17.4 mm/d at opt. 25 °C, max. 35 °C.
Specimens examined. SOUTH AFRICA, Northern Cape, Prieska, from Acacia tortillis, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2005, F.J.J. van der Walt & G.J. Marais (PREM 59643 – holotype, culture ex-type CMW 25427 = CBS 121780); Prieska, from A. erioloba (PREM 59644 – paratype, culture ex-paratype CMW 25428 = CBS 121781); Prieska, from A. erioloba (PREM 59645 – paratype, culture ex-paratype CMW 25429 = CBS 121782).
Notes — Aplosporella papillata is closely related to A. africana. Conidial sizes of the two species overlap, but there are differences in conidial colour and shape that distinguish the two species morphologically. Conidia of A. africana are hyaline at first, turning honey to brown with age, while those of A. papillata turn blackish brown with age. Furthermore, conidia of A. papillata may be constricted at the middle and more of the conidia apical papillae compared to those of A. africana, which also distinguishes it from A. prunicola and A. yalgorensis, apart from small size differences in conidia and paraphyses.
Botryosphaeria auasmontanum F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518721; Fig. 4e
Etymology. Name refers to the Auasberg Mountain surrounding Windhoek.
Sexual morph unknown. Conidiomata pycnidial, ovoid to ellipsoidal with a flat base, up to 410 μm in height, occasionally globose to subglobose, up to 235 μm diam, separate, covered in mycelium, superficial or semi-immersed. Conidiogenous cells hyaline, holoblastic, cylindrical, 5.5–8.5(–10.5) × (1.5–)2–2.5(–3) μm. Conidia ellipsoidal, rounded at the base and apex, hyaline, aseptate, (8–)8.5–11.5(–13) × (2.5–)3–4(–5) μm (av. of 28 conidia: 10.1 × 3.4 μm, l/w ratio 3).
Culture characteristics — Mycelium greyish olive to brownish olive, effuse. Reverse black to olive-brown. Temperatures for growth: min. 10 °C, growth rate of 9 mm/d at opt. 25 °C, max. 35 °C.
Specimen examined. NAMIBIA, Windhoek, Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59632 – holotype, culture ex-type CMW 25413 = CBS 121769).
Notes — Description is based on a single isolate from healthy tissue. Even though only one isolate was collected, obvious differences in morphology, host and geographical occurrence compared to those of known species strongly support the description of a unique species. There are clear morphological differences between B. dothidea (Slippers et al. 2004a), which is the species most closely related to B. auasmontanum. Conidiogenous cells and conidia are significantly smaller (conidiogenous cells and conidia of B. dothidea range from 6–20 × 2–5 and 17–22 × 4–5 μm, respectively). In addition, there was also significant sequence divergence compared to other species in the genus B. corticis and the closely related genus Cophinforma mamane (see Slippers et al. 2013, Phillips et al. 2013).
Botryosphaeria dothidea (Moug. ex Fr.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 212. 1863
= Fusicoccum aesculi Corda in Sturm, Deutschl. Fl., Abt. 3, 2: 111. 1829.
Specimens examined. NAMIBIA, Dordabis, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (CMW 25410); Windhoek, from A. karroo (CMW 25411); Windhoek, from A. karroo (CMW 25412 = CBS 121768).
Notes — There were no significant differences in conidial morphology for the isolates collected in this study compared to those in previous descriptions (Pennycook & Samuels 1985, Slippers et al. 2004a). Interestingly, dichomera-like conidia were observed in the cultures of some of these isolates, similar to what has previously been described in some isolates of B. dothidea and some Neofusicoccum spp. (Barber et al. 2005, Phillips et al. 2005, Crous et al. 2006, Inderbitzin et al. 2010). Hyaline conidia typical of F. aesculi and the dichomera-like conidia were observed in the same culture and in the same pycnidia (Fig. 4c). Buff and black masses of spores were visible on the pycnidia containing mostly both types of conidia.
Dothiorella capri-amissi F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518723; Fig. 5c
Fig. 5.
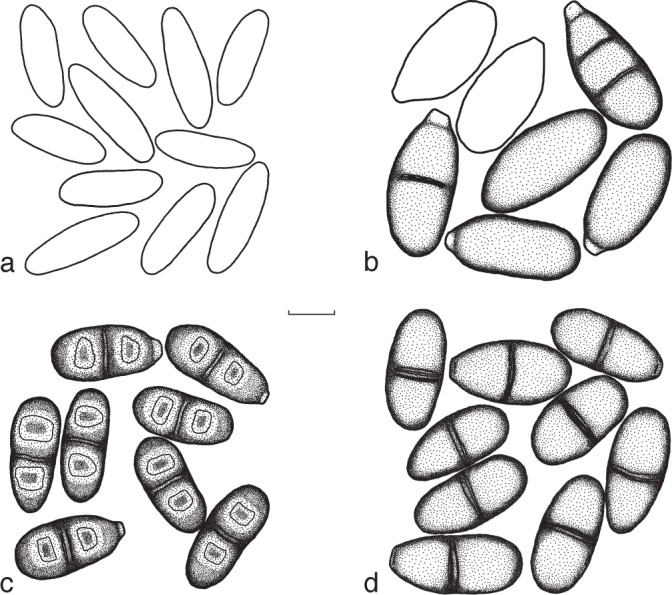
Conidia of newly described species of the Botryosphaeriaceae. a. Neofusicoccum sp.; b. Sphaeropsis variabilis; c. Dothiorella capria-missi; d. S. rosulata. — Scale bar = 10 μm.
Etymology. The name capri-amissi means ‘of the lost goat’, referring to the fact that Prieska, where this fungus was discovered, is a Khoisan word that means ‘place of the lost goat’.
Sexual morph unknown. Conidiomata pycnidial, separate or occasionally aggregated into botryose clusters, covered with mycelium, spherical to globose, up to 370 μm diam, superficial or immersed. Ostioles single, central, papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical to subcylindrical or broad lageniform, (4.5–)5–8.5(–10.5) × (2.5–)3–4.5(–5.5) μm (av. 7 × 3.8 μm), proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations. Conidia initially honey coloured to brown becoming blackish brown, 1-septate and rarely aseptate, mostly truncated at the base and constricted at the septum or with a thickening at the base of the septum and guttulate, moderately thick-walled, cylindrical to ovoid or broadly ellipsoidal, occasionally fusiform or reniform, (21.5–)23–26(–26.5) × (6.5–)8.5–10.5(–12) μm (av. of 50 conidia: 24.5 × 9.7 μm, l/w ratio 2.5).
Culture characteristics — Mycelium dark greyish brown to dark greyish olive with occasional smoke-grey tuft-like growth. Reverse dark olive to black and irregular edges. Thickening of the vegetative hyphae, chlamydospore-like, blackish brown, intercalary, terminal and dictyosporus and found superficial and immersed in water agar. Temperatures for growth: min. 10 °C, growth rate of 10.25 mm/d at opt. 25 °C, max. 30 °C.
Specimens examined. SOUTH AFRICA, Prieska, from Acacia erioloba, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2005, F.J.J. van der Walt & G.J. Marais (PREM 59626 – holotype, culture ex-type CMW 25404 = CBS 121878); Prieska, from A. erioloba (PREM 59625 – paratype, culture ex-paratype CMW 25403 = CBS 121763); Prieska, from A. erioloba (CMW 25405).
Dothiorella oblongaF.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518719; Fig. 4d
Etymology. Name refers to the oblong conidia.
Sexual morph unknown. Conidiomata pycnidial, separate, covered with short hyphae, ovoid, up to 840.5 μm in height or globose, up to 550 μm diam, occasionally aggregated into botryose clusters, superficial or immersed. Ostioles single, central, papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical to subcylindrical or broadly lageniform, (6–)8.5–11.5(–12.5) × (2.5–)4–4.5(–5.5) μm (av. 10 × 4.3 μm), proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations. Conidia honey coloured to brown, becoming blackish brown, aseptate or 1-septate, mostly truncate at the base and constricted at the septum or with a thickening at the base of the septum, moderately thick-walled, ovoid or oblong to ellipsoidal, (18.5–)23.5–27(–28) × (10–)11.5–13(–15) μm (av. of 100 conidia: 25.5 × 12.3 μm, l/w ratio 2.1).
Culture characteristics — Mycelium greyish olive or dark greyish olive, concentric zone at the edge with an effuse black centre. Reverse dark olive to blackish brown, thread-like growth in the centre of the colony. Occasional thickening of the hyphae, chlamydospore-like, blackish brown, intercalary, terminal and dictyosporus and found superficial and immersed in water agar. Temperatures for growth: min. 10 °C, growth rate of 18.5 mm/d at opt. 25 °C, max. 35 °C.
Specimens examined. NAMIBIA, Rundu, Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59627 – paratype, culture ex-paratype CMW 25406 = CBS 121764). – SOUTH AFRICA, Pretoria, Ditholo, A. mellifera, fruiting structures induced on needles of Pinus sp. on water agar, May 2006, F.J.J. van der Walt & R.N. Heath (PREM 59628 – holotype, culture ex-type CMW 25407 = CBS 121765); Ditholo, A. mellifera (PREM 59629 – paratype, culture ex-paratype CMW 25408 = CBS 121766).
Lasiodiplodia pseudotheobromaeA.J.L. Phillips, A. Alves & Crous, Fung. Diversity 28: 8. 2008
Specimens examined. NAMIBIA, Rundu, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (CMW 25417); Rundu, from A. mellifera (CMW 25418).
Notes — This species was described in a study considering cryptic species in L. theobromae (Alves et al. 2008). Two isolates were obtained from healthy Acacia tissue in the present study. There were no substantial differences between these isolates and those previously described except that the paraphyses in pycnidia from the present study were never longer than 45 μm compared to those of 58 μm described by Alves et al. (2008). In addition, they described a pink pigment in cultures grown at 35 °C, which was not seen in cultures from the present study. Furthermore, cultures of the previous study were able to grow at 10 °C, but those collected here were not able to grow at this temperature.
Lasiodiplodia pyriformisF.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. – MycoBank MB518722; Fig. 4f
Etymology. Name refers to the ovoid to pyriform conidia.
Sexual morph unknown. Conidiomata pycnidial, superficial, semi-immersed to immersed, papillate, occasionally aggregated into botryose clusters, covered in mycelium, individual superficial pycnidia, globose, up to 695 μm diam. Paraphyses cylindrical, aseptate, hyaline, (27–)28.5–33.5 × 1.5–2 μm (av. 31.1 × 1.1 μm). Conidiogenous cells hyaline, holoblastic, cylindrical, (7–)9–16 × (2.5–)3–6.5 μ m (av. 12.3 × 4.7 μm). Conidia initially hyaline becoming sepia in colour, ovoid or pyriform to ellipsoidal or subglobose, thick-walled with granular content, rounded at apex and occasionally truncate at base, after 4 wk, faint longitudinal striations, aseptate, (19–)21.5–25(–28) × (13.5–)15.5–19.5(–21.5) μm (av. of 100 conidia: 23.3 × 17.6 μm, l/w ratio 1.3).
Culture characteristics — Columns of aerial mycelium reaching the Petri dish lid, dark greyish olive with white to smoke-grey tufts. Reverse dark olive to black, thread-like growth in the middle of the colony. Temperatures for growth: min. 15 °C, growth rate of 38.5 mm/d at opt. 30 °C, max. 35 °C.
Specimens examined. NAMIBIA, Dordabis, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59633 – holotype, culture ex-type CMW 25414 = CBS 121770); Dordabis, from A. mellifera (PREM 59634 – paratype, culture ex-paratype CMW 25415 = CBS 121771); Grootfontein, from A. mellifera (CMW 25416).
Notes — This description is based on three isolates from the leading edges of lesions on branches. Differences between L. pyriformis and those species of Lasiodiplodia described by Burgess et al. (2006) include conidia and paraphyses. Conidia in L. pyriformis are significantly smaller than those of all described species and they also have very faint longitudinal striations, which is unlike those of other species. The paraphyses in L. pyriformis were also significantly smaller and the growth rates of cultures were substantially more rapid than has been found for other species of Lasiodiplodia (Burgess et al. 2006).
Neofusicoccum sp. — Fig. 5a
Sexual morph unknown. Conidiomata aggregated into botryose clusters, covered in mycelium, immersed or semi-immersed, occasionally superficial, up to 955 μm diam. Conidiogenous cells hyaline, holoblastic, cylindrical, (6–)7.5–11(–11.5) × 2–3.5 μm (av. 9.2 × 2.6 μm). Conidia ellipsoidal to fusiform, hyaline, smooth, aseptate, (14.5–)17–21(–22.5) × (4.5–)5–6(–6.5) μm (av. of 100 conidia: 19.3 × 5.6 μm, l/w ratio 3.4).
Culture characteristics — White cottony mycelium in centre of colony and effuse dark olive at the edge of the colony. Reverse, concentric zones, olive-buff. Temperatures for growth: min. 10 °C, growth rate of 15 mm/d at opt. 25 °C, max. 35 °C.
Specimen examined. NAMIBIA, Windhoek, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (CMW 25409).
Notes — Only one isolate of this fungus was obtained, originating from healthy tissue. There were no significant morphological differences compared to the closely related species, N. vitifusiforme (van Niekerk et al. 2004) and Dichomera eucalypti (Barber et al. 2005).
Sphaeropsis variabilis F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518720; Fig. 5b
Etymology. Name refers to the variability in the shape of the conidia in this fungus.
Sexual morph unknown. Conidiomata pycnidial, globose to subglobose, covered in short hyphae, superficial, immersed or semi-immersed, up to 390 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical to ampuliform, (5.5–)6–11(–16.5) × 2.5–5.5(–7.5) μm (av. 7.3 × 3.4 μm), proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations. Conidia honey coloured to black brown, aseptate or 1–3-septate, mostly truncate at the base, smooth, moderately thick-walled, variable in shape ranging from cylindrical to ovoid or occasionally reniform or allantoid, occasionally guttulate, (24–)26.5–33.5(–37) × (8–)10.5–14(–17) μm (av. of 140 conidia: 30 × 12.3 μm, l/w ratio 2.4).
Culture characteristics — Mycelium olivaceous to olive-buff or white to smoke-grey, effuse. Reverse dark olive to buffy olive. Occasional thickening of the hyphae, chlamydospore-like, blackish brown, intercalary, terminal and found superficial and immersed in water agar. Cultures sometimes had what resembles a ‘clear zone’ or effuse to very little growth at the centres of the colony with white tuft-like mycelial growth and dark olive-grey to brownish olive mycelial growth surrounding the ‘clear zone’, with small white tufts around the pycnidia. Temperatures for growth: min. 10 °C, growth rate of 19 mm/d at opt. 25 °C, max. 35 °C.
Specimens examined. NAMIBIA, Windhoek, from Acacia karroo, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59637 – holotype, culture ex-type CMW 25419 = CBS 121774); Windhoek, from A. karroo (PREM 59638 – paratype, culture ex-paratype CMW 25421 = CBS 121775). – SOUTH AFRICA, Northern Cape, Prieska, from A. mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2005, F.J.J. van der Walt & G.J. Marais (PREM 59639, culture CMW 25422 = CBS 121776); Prieska, from A. mellifera (CMW 25423).
Notes — The generic placement of S. variabilis amongst Pheaobotryon, Barriopsis and Sphaeropsis was supported only after the addition of β-tubulin data. This placement also makes sense morphologically. Sphaeropsis variabilis does not have conidia with both ends rounded as in Phaeobotryon, or longitudinal striations as in Bariopsis. Rather, the obtuse conidia with truncate bases are more typical of Sphaeropsis.
Spencermartinsia rosulata F.J.J. van der Walt, Slippers & G.J. Marais, sp. nov. — MycoBank MB518724; Fig. 5d
Etymology. Name refers to the prominent rosette-like growth pattern of the fungus in culture.
Sexual morph unknown. Conidiomata pycnidial, abundant, superficial, immersed or semi-immersed, separate or aggregated into botryose clusters. Individual pycnidia pyriform to ovoid, up to 785 μm in height and covered with sparse mycelium, but also globose, up to 580 μm diam, totally covered in mycelium. Ostioles single, central, papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical to subcylindrical, (4–)5–12(–17) × (2.5–)3.5–5 μm (av. 8 × 4.5 μm), proliferating at the same level to form periclinical thickenings or rarely proliferating percurrently to form one or two annellations. Conidia honey coloured to brown becoming blackish brown, 1-septate, to a lesser extent aseptate, mostly guttulate, ovoid to subcylindrical or ellipsoidal, either with a rounded apex and base or a rounded apex and truncate base, occasionally constricted at the septum, moderately thick-walled, smooth, (19–)21–24.2(–26.7) × (8–)10–10.5(–13) μm (av. of 157 conidia: 22.6 × 10.4 μm, l/w ratio 2.2).
Culture characteristics — Greyish olive aerial mycelium, cottony and in a rosette form with lobed areas at edges of colony, can also be effuse. Occasionally with vinaceous grey cottony areas. Reverse olivaceous black to olive-buff or olivaceous, lobed with circular growth in the middle of the colony. Abundant thickenings of the hyphae, chlamydospore-like, blackish brown: intercalary, terminal and dictyosporus and found superficial and immersed in water agar. Temperatures for growth: min 10 °C, growth rate of 19 mm/d at opt. 25 °C, max. 35 °C.
Specimens examined. NAMIBIA, Windhoek, from Acacia karroo, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2006, F.J.J. van der Walt & J. Roux (PREM 59622 – holotype, culture ex-type CMW 25389 = CBS 121760); Grootfontein, from A. mellifera (CMW 25390); Dordabis, from A. mellifera (CMW 25391). – SOUTH AFRICA, Pretoria, Ditholo, from A. mellifera, fruiting structures induced on needles of Pinus sp. on water agar, May 2006, F.J.J. van der Walt & R.N. Heath (PREM 59623 – paratype, culture ex-paratype CMW 25392 = CBS 121761); Ditholo, from A. mellifera (CMW 25393); Prieska, from A. mellifera, fruiting structures induced on needles of Pinus sp. on water agar, Feb. 2005, F.J.J. van der Walt & G.J. Marais (PREM 59624, culture CMW 25395 = CBS 121762); Prieska, from A. karroo (CMW 25394); Prieska, from A. mellifera (CMW 25396, culture); Prieska, from A. tortillis (CMW 25397); Prieska, from A. tortillis (CMW 25398).
Notes — The DNA sequence data for this species is identical to that of an isolate identified as a Spencermartinsia sp. (= Diplodia medicaginis) in MycoBank. Unfortunately, this is not a type isolate and the identification is uncertain. Phillips et al. (2008) have already linked this specimen to Spencermartinsia, but declined to name it. The original description of D. medicaginis, from France, differs from S. rosulata, in that it has larger conidia and lack constrictions at the septa. While this might represent intra-specific variation, there is no logical way to connect these taxa, and the epithet is, therefore, not used.
Spencermartinsia viticola (A.J.L. Phillips & J. Luque) A.J.L. Phillips, A. Alves & Crous, Persoonia 21: 51. 2008
Specimens examined. SOUTH AFRICA, Pretoria, Ditholo, from Acacia mellifera, fruiting structures induced on needles of Pinus sp. on water agar, May 2006, F.J.J. van der Walt & R.N. Heath (CMW 25399); Ditholo, from A. mellifera (CMW 253400); Ditholo, from A. mellifera (CMW 25401); Ditholo, from A. mellifera (CMW 25402).
Notes — Spencermartinsia viticola (= Dothiorella viticola) was described from Vitis vinifera (Luque et al. 2005, Phillips et al. 2008). The morphology of the isolates collected in this study was very similar to that described by Luque et al. (2005). Isolates from the present study showed distinctive and abundant thickenings of the hyphae, chlamydospore-like structures that were intercalary, terminal and dictyosporus and found superficial and immersed in water agar. Conidiomata limited, separate, mostly globose to ovoid, covered in mycelium, superficial, up to 460 μm diam (av. 325.8 μm), individual superficial pycnidia up to 371 μm in height. Conidiogenous cells (7–)9.5–16 × 2–4.5(–6.5) μm (av. 12.6 × 3.4 μm). Conidia aseptate or 1-septate, mostly truncate at the base and constricted at the septum, ovoid to subcylindrical, (16.5–)18–21(–23.5) × 8.5–10.5(–12.5) μm (av. of 63 conidia: 19.6 × 9.9 μm, l/w ratio 2).
DISCUSSION
This study is one of the most detailed to date considering fungi in the Botryosphaeriales associated with Acacia species. Despite the fact that it still represents only a small number of sites and Acacia species that occur in the area considered, it nevertheless revealed a rich diversity of Botryosphaeriales. Given the lack of resolution provided by the available morphological characters to identify species in this family of fungi, most of this diversity cannot be reliably linked to taxa that were described from other plants and regions of the world and for which cultures and sequence data are not available. Thus, despite these shortcomings and in the interests of describing this diversity in a manner that allows it to be communicated and linked to information from future studies, the new species have been described.
In this study, as in numerous other recent studies, it was not possible to compare species in the Botryosphaeriales based only on morphology and without DNA sequence data (e.g. Slippers et al. 2004a, Crous et al. 2006, Pavlic et al. 2009a, Inderbitzin et al. 2010). Morphology clearly has an important role in mycology; enabling us to visualize the structures that underpin the biology of these organisms. But it is becoming increasingly recognised as a suite of weak characters, when comparing species occurring in different environments and on different hosts. Once the DNA-based identifications have been made, the linked morphology can prove useful to group further isolates from Acacia in these regions, but even in such cases, caution is advised. For example, the sampling for the study of Jami et al. (2012) was conducted subsequent to the present study, and again revealed a substantial number of new species that would most likely have been overlooked if only morphological characteristics had been considered. Fortunately, the cost of DNA sequencing continues to decrease, and the capacity to analyse molecular data continue to increase, making it feasible to determine identity using an ITS barcode (Schoch et al. 2012) for large numbers of isolates. It is likely that in the future, most identifications of fungi will be made in this way and increasingly in the absence of morphological characters.
The Dothiorella and Spencermartinsia species isolated in this study vividly illustrates the difficulties encountered with morphological taxonomy and the application of old names. The morphological concepts now applied to these genera have in the past been used for, and could have easily been confused with many of the more than 1 800 species in the genera Sphaeropsis and Diplodia (Index Fungorum; www.indexfungorum.org). Furthermore, more than 360 names are attached to Dothiorella alone, but the morphological concepts as they are currently used after redefining the phylogenetic position of the group (Phillips et al. 2005, 2008) have been applied to few of them. Work with these older names rapidly reveals that there are few options to link the available information and characters to taxonomic hypotheses that can currently be tested. For example, there appears to be little value in ecological distinctions based on host, with some species occurring on numerous hosts (see Sakalidis et al. 2013). Furthermore, morphological characteristics shared between cryptic sister taxa, distantly related species and even some genera (e.g. Dothiorella and Spencermartinsia, or Botryosphaeria and Neofusicoccum), can be virtually indistinguishable (e.g., Slippers et al. 2004a, Crous et al. 2006, Pavlic et al. 2009a). Using older names justified due to an overlap of only some of these characters is not logically defensible. When overlapping host, geography and morphology data are found, a case (albeit still very circumstantial) might then be made to use an older name. In such cases it is essential that an epitype is designated (e.g., Slippers et al. 2004a).
Here we report at least 13 Botryosphaeriales species from these hosts and environments collected in South Africa and Namibia. Of these, eight species are described for the first time as Aplosporella africana, A. papillata, Botryosphaeria auasmontanum, Dothiorella capri-amissi, Do. oblonga, Lasiodiplodia pyriformis, Spencermartinsia rosulata and Sphaeropsis variabilis. Previously described species include, B. dothidea, Do. dulcispinae, L. pseudotheobromae and S. viticola. One isolate of a possible new Neofusicoccum sp. was found, but it was not described due to the lack of sufficient evidence to clearly define it. This pattern of discovery of new species of the Botryosphaeriales in this study and that of Jami et al. (2012, 2013, 2014) is expected to continue as more regions are sampled.
Three of the previously described species identified in this study, B. dothidea, L. pseudotheobromae and S. viticola, all have wide host ranges and broad geographical distributions (Slippers et al. 2004b, van Niekerk et al. 2004, Luque et al. 2005, Alves et al. 2008, Begoude et al. 2010, 2011, Piškur et al. 2011). Slippers & Wingfield (2007) considered that some of the most damaging pathogens in the Botryosphaeriales have wide host and geographical distributions. In the present study, B. dothidea, L. pseudotheobromae and S. viticola were, however, all isolated from healthy material and in very low numbers. Nonetheless, these species should be considered in future pathology studies, not only due to their wide host and geographical distributions, but their apparent ability to move between hosts and previous evidence that they are pathogens (e.g. Begoude et al. 2010, 2011, Piškur et al. 2011).
The predominant fungi identified from A. mellifera, a tree on which widescale die-back has been reported (Holz & Schreuder 1989) were S. rosulata and S. variabilis. All the S. variabilis isolates were obtained from diseased material while approximately half of the S. rosulata isolates were from diseased tissue. Symptoms from which these fungi were isolated included black-discoloured pith in the branches and roots, branch and stem cankers, tip die-back and a brownish black discolouration in the upper taproots of the trees. Although their pathogenicity was not considered, it is possible that these fungi play a role in the decline of the trees and they deserve further study.
Ten of the Botryosphaeriales species were obtained only from healthy or from both healthy and diseased plant tissue. Most of these species were present in very low numbers. Slippers & Wingfield (2007) found that most of the genera in the Botryosphaeriales are known as endophytes, which in some cases dominate the endophyte community. The results of this study add credence to the notion that most species in the Botryosphaeriales are endophytic (e.g. see Dakin et al. 2010, Jami et al. 2013, Slippers et al. 2013). In light of their common occurrence and in some cases dominance, it is unfortunate that their ecological role as endophytes in nature is so poorly understood.
There was significant geographic structure to the Botryosphaeriales characterised in this study. A high level of diversity in the Botryosphaeriales was encountered at all sites sampled. The composition in terms of both species identity and dominance, however, differed between all sites. Some species had limited distribution, often occurring at only one site. These included A. papillata, B. auasmontanum, Do. capri-amissi, B. dothidea, Do. dulcispinae, Do. oblonga, L. pseudotheobromae, a Neofusicoccum sp. and S. viticola. The remainder of the species were found at multiple sites, including A. africana (3 sites), S. variabilis (4 sites), L. pyriformis (2 sites) and S. rosulata (5 sites). Whether the distribution of these species is dependent on biological interactions (e.g. host diversity) or non-biological environmental factors at the sites is not clear. To realise the full extent of the distribution and host specificity of these fungi, further studies will be required that include more intensive sampling at specific sites and a greater number of hosts per site. Such studies should also specifically test hypotheses linked to factors influencing distribution and diversification. These are critical ecological and evolutionary questions pertaining to the Botryosphaeriales, and its answer would substantially promote an understanding of their potential as pathogens following introduction into new areas and environmental changes. Whatever the underlying cause of diversification, the present and previous studies (e.g. Pavlic et al. 2008, Taylor et al. 2009, Abdollahzadeh et al. 2010, Inderbitzin et al. 2010, Perez et al. 2010, Mehl et al. 2011, Sakalidis et al. 2011, Jami et al. 2012, 2013, 2014) emphasise the conclusion that sampling of new areas is likely to continue to yield fairly large numbers of new species of Botryosphaeriales.
Spencermartinsia and Dothiorella, two closely related genera, were the most diverse and most common genera found on native Acacia spp. in southern Africa, in this and previous studies (Jami et al. 2012, 2013, 2014). This includes ten species in these genera, of which S. rosulata appears to be one of the most dominant species of the Botryosphaeriaceae on native Acacia spp. in southern Africa. This fungus was obtained from both healthy and diseased tissue from Namibia and it is the only species of the Botryosphaeriales that was found at all four sites. Spencermartinsia rosulata has not been found on any other hosts in the region or elsewhere, and this is despite extensive sampling. It is expected that many of these species are native to southern Africa and they represent an interesting group to consider with regards to ecology and evolution of Botryosphaeriales in the region.
The conidia of B. dothidea are known to become brown and septate in some cases, superficially resembling Dichomera conidia (Phillips et al. 2005). The shape of the dichomera-like conidia observed in one of the B. dothidea cultures in this study contradicts conclusions of Crous et al. (2006), who suggested that it is possible to distinguish between the genera Neofusicoccum and Botryosphaeria based on the morphology of their associated dichomera-like conidia. In that study, dichomera-like conidia of Botryosphaeria were considered to be fusiform to ellipsoid, while those associated with Neofusicoccum were described as globose to pyriform (Crous et al. 2006). Results of the present study, however, show that Dichomera conidia of Botryosphaeria can also be globose to pyriform in shape. The dichomera-like conidial morphology is thus a questionable characteristic by which to distinguish between Botryosphaeria and Neofusicoccum. This is also the first report of pleomorphism in B. dothidea in Africa.
Members of the Botryosphaeriales are clearly prominent and diverse on Acacia species in southern Africa. Studies of this kind emphasise the need to consider the factors underlying patterns related to the diversity of the Botryosphaeriales, which might include host, climate and geographic distribution. Furthermore, the relevance of these Botryosphaeriales species as pathogens and their potential threat to native hosts remains unclear and needs further consideration. In order to address such questions, there is an urgent need to resolve the problems arising from a taxonomy based on morphology in a group of fungi that is phylogenetic diverse yet morphological uniform. This problem is not restricted to the Botryosphaeriales despite the fact that more efficient tools for species identification have been available for 20 years (Taylor 2011) and are now commonly accessible and at a lower cost than traditional tools. The threats associated with global climate change, increasing numbers of invasions and the need to more efficiently preserve and utilise natural resources, make it imperative that we adopt the most efficient methods to accurately identify and describe species. At least for the Botryosphaeriales, species names without attached sequence data has very limited and dubious value. This could imply that older names in this order will have to be disregarded, unless viable options exist to epitypify them (see also Phillips et al. 2013 for discussion on this topic). We propose here that this process of epitypification must be vigorously pursued for the Botryosphaeriales when exploring new hosts and new environments. While we have continued to characterise the morphology of the species treated in this study, the value of this practice must be questioned for future studies. This is particularly true because it is unlikely to be used for the purpose for which it was originally intended, namely species identification.
Acknowledgments
We thank the Department of Science and Technology (DST) / National Research Foundation (NRF) Centre of Excellence in Tree and Health Biotechnology (CTHB), the National Research Foundation (NRF) and the THRIP initiative of the Department of Trade and Industry, South Africa for financial assistance. We are very grateful to Dr Percy M. Chimwamurombe and Mr Jean Damascene Uzabakiriho from the University of Namibia who invited us to survey diseased Acacia spp. in that country and who provided field assistance during this investigation. We also thank the farmers of Namibia and Flight Sergeant Mauritz on the Dithololo Training Area in Pretoria, as well as Mr Petrus Fourie from Libertas farm near Prieska, who allowed us to collect plant material. We further acknowledge Dr Ronald Heath and Dr Martin Coetzee who assisted us with the field work.
REFERENCES
- Abdollahzadeh J, Javadi A, Mohammadi Goltapeh E, Zare R, Phillips AJL. 2010. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A, Crous PW, Correia A, Phillips AJL. 2008. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28: 1–13. [Google Scholar]
- Barber PA, Burgess TJ, Hardy GEStJ, Slippers B, Keane PJ, Wingfield MJ. 2005. Botryosphaeria species from Eucalyptus in Australia are pleoanamorphic, producing Dichomera synanamorphs in culture. Mycological Research 109: 1347–1363. [DOI] [PubMed] [Google Scholar]
- Barnes I, Roux J, Coetzee MPA, Wingfield MJ. 2001. Characterization of Seiridium spp. associated with cypress canker based on β-tubulin and histone sequences. Plant Disease 85: 317–321. [DOI] [PubMed] [Google Scholar]
- Begoude BAD, Slippers B, Wingfield MJ, Roux J. 2010. Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycological Progress 9: 101–123. [Google Scholar]
- Begoude BAD, Slippers B, Wingfield MJ, Roux J. 2011. The pathogenic potential of endophytic Botryosphaeriaceous fungi on Terminalia species in Cameroon. Forest Pathology 41: 281–292. [Google Scholar]
- Burgess T, Barber PA, Mohali S, Pegg G, Beer W de, Wingfield MJ. 2006. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98: 423–435. [DOI] [PubMed] [Google Scholar]
- Cai L, Giraud T, Zhang N, Begerow D, Cai G, Shivas R. 2011. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Diversity 50: 121–133. [Google Scholar]
- Crous PW, Groenewald JZ. 2005. Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470. [Google Scholar]
- Crous PW, Phillips AJL, Baxter AP. 2000 Phytopathogenic fungi from South Africa: 73–75 Department of Plant Pathology Press, University of Stellenbosch, South Africa. [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin N, White D, Hardy GEStJ, Burgess TI. 2010. The opportunistic pathogen, Neofusicoccum australe, is responsible for crown dieback of peppermint (Agonis flexuosa) in Western Australia. Australasian Plant Pathology 39: 202–206. [Google Scholar]
- Damm U, Fourie PH, Crous PW. 2007. Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Diversity 27: 35–43. [Google Scholar]
- Farris JS, Kallersjo M, Kluge AG, Bult C. 1995. Constructing a significance test for incongruence. Systematic Biology 44: 570–572. [Google Scholar]
- Gibson IAS. 1975 The Leguminosae. In: Gibson IAS.(ed), Diseases of forest trees widely planted as exotics in the tropics and southern hemisphere. Part I. Important members of the Myrtaceae, Leguminosae, Verbenaceae and Meliaceae: 21–34 Commonwealth Mycological Institute, University of Oxford, England. [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Ohman A, Glotzer D, Nuhn M, Kirk P, Nilsson RH. 2011. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biology Reviews 25: 38–47. [Google Scholar]
- Hibbett DS, Taylor JW. 2013 Fungal systematics: is a new age of enlightenment at hand? Nature Reviews Microbiology 11: 129–133. [DOI] [PubMed] [Google Scholar]
- Hillis DM, Huelsenbeck JP. 1992. Signal, noise, and reliability in molecular phylogenetic analyses. Journal of Heredity 83: 189–195. [DOI] [PubMed] [Google Scholar]
- Holz G, Schreuder W. 1989. Dieback of blackthorn (Acacia mellifera subsp. detinens) in South West Africa. Agricola 7: 32–36. [Google Scholar]
- Inderbitzin P, Bostock RM, Trouillas FP, Michailides TJ. 2010. A six-locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 102: 1350–1368. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, et al. 2004. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycological Research 108: 411–418. [DOI] [PubMed] [Google Scholar]
- Jami F, Slippers B, Wingfield MJ, Gryzenhout M. 2012. Five new species of the Botryosphaeriaceae from Acacia karroo in South Africa. Cryptogamie Mycologie 33: 245–266. [Google Scholar]
- Jami F, Slippers B, Wingfield MJ, Gryzenhout M. 2013. Botryosphaeriaceae diversity greater in healthy than associated diseased Acacia karroo tree tissue. Australasian Plant Pathology 42: 421–430. [Google Scholar]
- Jami F, Slippers B, Wingfield MJ, Gryzenhout M. 2014. Botryosphaeriaceae species overlap on four unrelated, native South African hosts. Fungal Biology 118: 168–179. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenné JM. 1992 Diseases on multi purpose woody legumes in the tropics: A review. Nitrogen Fixing Tree Research Reports 10: 13–29. [Google Scholar]
- Luque J, Martos S, Phillips AJL. 2005. Botryosphaeria viticola sp. nov. on grapevines: a new species with a Dothiorella anamorph. Mycologia 97: 1111–1121. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Guralnick R, Hill A, Reysenbach A-L., McDade LA. 2012. Ramping up biodiversity discovery via online quantum contributions. Trends in Ecology and Evolution 27: 72–77. [DOI] [PubMed] [Google Scholar]
- Mehl JWM, Slippers B, Roux J, Wingfield MJ. 2011. Botryosphaeriaceae associated with Pterocarpus angolensis (kiaat) in South Africa. Mycologia 103: 534–553. [DOI] [PubMed] [Google Scholar]
- Niekerk JM van,, Crous PW, Groenewald JZ, Fourie PH, Halleen F. 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96: 781–798. [PubMed] [Google Scholar]
- Pavlic D, Barber PA, Hardy GEStJ, Slippers B, Wingfield MJ, Burgess TI. 2008. Seven new species of the Botryosphaeriaceae discovered on baobabs and other native trees in Western Australia. Mycologia 100: 851–866. [DOI] [PubMed] [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ. 2004. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology 50: 313–322. [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Wingfield MJ. 2009a. Multiple gene genealogies and phenotypic data reveal cryptic species of the Botryosphaeriaceae: A case study on the Neofusicoccum parvum/N. ribis complex. Molecular Phylogenetics and Evolution 51: 259–268. [DOI] [PubMed] [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Wingfield MJ. 2009b. Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum/N. ribis complex. Mycologia 101: 636–647. [DOI] [PubMed] [Google Scholar]
- Pennycook SR, Samuels GJ. 1985. Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (Kiwifruit) in New Zealand. Mycotaxon 24: 445–458. [Google Scholar]
- Perez CA, Wingfield MJ, Slippers B, Altier NA, Blanchette RA. 2010. Endophytic and canker-associated Botryosphaeriaceae occurring on non-native Eucalyptus and native Myrtaceae trees in Uruguay. Fungal Diversity 41: 53–69. [Google Scholar]
- Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, et al. 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. 2008. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Rumbos IC, Alves A, Correia A. 2005. Morphology and phylogeny of Botryosphaeria dothidea causing fruit rot of olives. Mycopathologia 159: 433–439. [DOI] [PubMed] [Google Scholar]
- Piškur B, Pavlic D, Slippers B, Ogris N, Maresi G, Wingfield MJ, Jurc D. 2011. Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. European Journal of Forest Research 130: 235–249. [Google Scholar]
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Raeder U, Broda P. 1985. Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1: 17–20. [Google Scholar]
- Rayner RW. 1970 A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, UK. [Google Scholar]
- Roux J, Wingfield MJ. 1997. Survey and virulence of fungi occurring on diseased Acacia mearnsii in South Africa. Forest Ecology and Management 99: 327–336. [Google Scholar]
- Roux J, Wingfield MJ, Morris MJ. 1997 Botryosphaeria dothidea as a pathogen of Acacia mearnsii in South Africa. South African Journal Science : xii. [Google Scholar]
- Sakalidis ML, Hardy GEStJ, Burgess TI. 2011. Use of the Genealogical Sorting Index (GSI) to delineate species boundaries in the Neofusicoccum parvum-Neofusicoccum ribis species complex. Molecular Phylogenetics and Evolution 60: 333–344. [DOI] [PubMed] [Google Scholar]
- Sakalidis ML, Slippers B, Wingfield BD, Hardy GEStJ, Burgess TI. 2013. The challenge of understanding the origin, pathways and extent of fungal invasions: global populations of the Neofusicoccum parvum – N. ribis species complex. Diversity and Distributions 19: 873–883. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Boissin E, Phillips A, Groenewald JZ, Lombard L, et al. 2013. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Studies in Mycology 76: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ. 2004a. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96: 83–101. [PubMed] [Google Scholar]
- Slippers B, Fourie G, Crous PW, Coutinho TA, Wingfield BD, et al. 2004b. Speciation and distribution of Botryosphaeria spp. on native and introduced Eucalyptus trees in Australia and South Africa. Studies in Mycology 50: 343–358. [Google Scholar]
- Slippers B, Smit WA, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ. 2007. Taxonomy, phylogeny and identification of Botryosphaeriaceae associated with pome and stone fruit trees in South Africa and other regions of the world. Plant Pathology 56: 128–139. [Google Scholar]
- Slippers B, Wingfield MJ. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biology Reviews 21: 75–89. [Google Scholar]
- Stephens RP, Goldschmidt WB. 1938. A preliminary report on aspects of wattle pathology. South African Forestry Journal 2: 32–42. [Google Scholar]
- Swofford DL. 2002 PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]
- Taylor JW. 2011. One fungus = One name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus 2: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Taylor K, Barber PA, Barber PA, Hardy GEStJ, Burgess TI. 2009. Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including the description of four new species. Mycological Research 113: 337–353. [DOI] [PubMed] [Google Scholar]
- Venter F, Venter J-A. 2002 Making the most of indigenous trees, 2nd ed Briza Publications; Pretoria, South Africa. [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. 1990 Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA. [Google Scholar]
- Wingfield MJ, Beer ZW de, Slippers B, Wingfield BD, Groenewald JZ, et al. 2012. One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]