Abstract
The fungal genus Curvularia includes numerous plant pathogens and some emerging opportunistic pathogens of humans. In a previous study we used morphology and sequences of the nuclear ribosomal internal transcribed spacer region (ITS) and the glyceraldehyde-3-phosphate dehydrogenase (gpd) gene to identify species within a set of 99 clinical Curvularia isolates from the USA. Seventy-two isolates could be identified while the remaining 27 isolates belonged in three unclassified clades that were tentatively labelled Curvularia sp. I, II and III. In the present study, we further assess the taxonomic placement of these isolates using sequences of ITS, gpd, the large subunit rDNA, and the second largest subunit of RNA polymerase II. DNA sequence comparisons with a set of 87 isolates representing 33 Curvularia spp. and members of the closely-related genera Bipolaris and Exserohilum revealed that Curvularia sp. I, II and III represent novel lineages in Curvularia. These lineages are morphologically different from the currently accepted species. In the phylogenetic tree, Curvularia sp. I and sp. III were each split into two distinct lineages. Morphology and phylogeny supported the proposal of five new species, to be named C. americana, C. chlamydospora, C. hominis, C. muehlenbeckiae and C. pseudolunata. The concatenated 4-locus phylogeny revealed the existence of six clades in Curvularia, which are associated with particular morphological features. They were named after representative species, namely americana, eragrostidis, hominis, lunata, spicifera and trifolii.
Keywords: Bipolaris, Curvularia, gpd, ITS, LSU, phylogeny, RPB2, systematics
INTRODUCTION
Curvularia, typified by C. lunata, is a species-rich genus, which includes numerous grass pathogens and saprobes occurring on plant material, dung and soil (Faurel & Schotter 1965, Sivanesan 1987, Jiang & Zhang 2007). At least eight species of this genus have been reported from opportunistic diseases in humans ranging from mild skin and nail infections to severe invasive disease, depending on route of infection and immune status of the host (Kamalam et al. 1992, Ismail et al. 1993, Lopes & Jobim 1998, Ebright et al. 1999, de Hoog et al. 2000). Morphologically, Curvularia is characterised by the production of sympodial conidiophores with tretic, terminal and intercalary conidiogenous cells and elongate, transversely septate conidia with a dark basal scar. Conidia are often curved at an asymmetrically swollen intermediate cell, but species with straight conidia also have been described (Sivanesan 1987). Authors such as Ellis (1971, 1976), de Hoog et al. (2000) and Revankar & Sutton (2010) have described the conidia as truly septate or ‘euseptate’, i.e. composed of a single wall with septa that are formed as inward extensions of that wall (Luttrell 1963). A similar genus is Bipolaris, type species B. maydis, which traditionally has been distinguished from Curvularia by producing conidia which lack an asymmetrically swollen intermediate cell and are ‘distoseptate’ (Domsch et al. 2007, Revankar & Sutton 2010), i.e. they have a common outer wall enclosing more or less spherical cells, each of which is surrounded by an individual wall (Luttrell 1963). The separation of the two genera has been a matter of controversy and many authors have stated that Curvularia species also have distoseptate conidia (Alcorn 1983a, Sivanesan 1987, Seifert et al. 2011).
Sexual stages of Bipolaris and Curvularia were traditionally placed in Cochliobolus. Typically, they feature thick-walled, ostiolate ascomata with pseudoparaphyses, and bitunicate asci that give rise to filiform, multiseptate ascospores (Sivanesan 1987, Zhang et al. 2012). The ascospores often appear more or less helically coiled within the ascus. A similar genus, Pseudocochliobolus, was segregated from Cochliobolus to accommodate species producing ascomata on columnar stromata, with ascospores appearing linearly parallel or loosely coiled within the asci. The asexual stages of Pseudocochliobolus species were Curvularia and Bipolaris species with short, rather straight conidia (Tsuda et al. 1977, Tsuda & Ueyama 1981). Most authors have not accepted Pseudocochliobolus as a separate genus because the degree of coiling of the ascospores can vary greatly within a species. Also, the addition of a second genus with Curvularia and Bipolaris asexual stages would introduce unnecessary complexity into the taxonomy of this group of fungi instead of clarifying it (Alcorn 1983a, 1988, Sivanesan 1987).
Cochliobolus, Pseudocochliobolus and their Bipolaris and Curvularia asexual morphs were previously considered to be related either to the Dothideales (Eriksson 1981) or to the Pleosporales (Barr 1979, Sivanesan 1984). Molecular data confirmed their placement in the latter order and more precisely in its largest family, Pleosporaceae, along with other important genera of plant pathogens and clinically-relevant fungi such as Alternaria and Exserohilum (Olivier et al. 2000, Zhang et al. 2009, 2012). Berbee et al. (1999) performed a phylogenetic study to assess the evolutionary relationships of Cochliobolus, Pseudocochliobolus, Curvularia and Bipolaris. Their phylogenetic trees, based on the internal transcribed spacer (ITS) region of the rDNA and the glyceraldehyde-3-phosphate dehydrogenase (gpd) gene, revealed that isolates were distributed mainly in two clades which were named ‘Cochliobolus groups 1 and 2’. Group 1 exclusively encompassed species with Bipolaris asexual morphs, including the type species, B. maydis, agent of southern corn leaf blight, as well as other economically-relevant phytopathogenic species. The sexual morph of B. maydis is Cochliobolus heterostrophus, type species of Cochliobolus (Sivanesan 1987). Group 2 included mostly plant pathogens and saprobes with Bipolaris and Curvularia asexual morphs, including the type species of the latter genus, C. lunata and all species of Pseudocochliobolus.
Manamgoda et al. (2012), with a wider sampling of species and based on the analysis of ITS, large subunit (LSU) rDNA, gpd and elongation factor 1-α (EF1-α) genes, applied the one fungus = one name concept (Hawksworth et al. 2011) to the Bipolaris-Curvularia, Cochliobolus-Pseudocochliobolus complex. Their phylogenies confirmed the existence of the same two main groups reported by Berbee et al. (1999). Based on those results, Cochliobolus and Pseudocochliobolus were synonymized with the more commonly used generic names Bipolaris and Curvularia, respectively, and the generic concept of the latter genus was expanded to accommodate some species with rather straight conidia formerly placed in Bipolaris but grouping in the Curvularia clade (Manamgoda et al. 2012). These included important agents of opportunistic infections in vertebrates, such as B. australiensis, B. hawaiiensis and B. spicifera (de Hoog et al. 2000). The last of these had been previously considered a Curvularia species by Boedijn (1933).
Curvularia spp. have been identified mostly based on morphology, but the names applied often do not correlate with DNA sequence-based identifications. Furthermore, the species most commonly reported from humans, C. lunata, appeared to be a species complex (Berbee et al. 1999, Yanagihara et al. 2010). Da Cunha et al. (2013) recently characterised a set of 99 clinical Curvularia strains from the USA using sequences of the ITS region and the gpd gene. They could identify 73.2 % of the isolates, including C. aeria, which was the most common species. The remaining isolates were distributed over three different lineages which did not correlate with any known species. In this study we used DNA sequence data of four nuclear loci to further assess the taxonomic position of these isolates.
MATERIALS AND METHODS
Fungal isolates
Twenty-seven clinical Curvularia isolates from the USA were studied (Table 1). These isolates were obtained from the Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, and represent the clades named Curvularia sp. I, II and III in the study by da Cunha et al. (2013). These isolates were compared with ex-type or reference strains of different Curvularia spp. from the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands.
Table 1.
Isolates included in the phylogenetic study, their origins, and GenBank accession no.
| Taxon | Isolate no.1 | Source | GenBank accession no.2 |
|||
|---|---|---|---|---|---|---|
| ITS | LSU | gpd | RPB2 | |||
| Bipolaris chloridis | CBS 242.77B | Chloris gayana, Australia | HF934928 | HF934869 | HG779083 | HF934830 |
| B. cynodontis | CBS 285.51 | Cynodon transvaalensis, Kenya | HF934929 | HF934874 | HG779081 | HF934831 |
| CBS 305.64 | Cynodon dactylon, USA | HF934930 | HF934883 | HG779082 | HF934832 | |
| B. maydis | CBS 130.26 | Unknown | HF934923 | HF934873 | HG779084 | HF934825 |
| CBS 136.29 | Zea mays, Japan | HF934926 | HF934879 | HG779086 | HF934828 | |
| CBS 307.64 | Zea mays, USA | HF934925 | HF934875 | HG779085 | HF93482 | |
| B. microlaenae | CBS 280.91T | Microlaena stipoides leaf, Australia | HF934933 | HF934877 | HG779092 | HF934835 |
| B. oryzae | CBS 157.50 | Oryza sativa grain, Indonesia | HF934931 | HF934870 | HG779090 | HF934833 |
| CBS 199.54 | Oryza sativa grain, New Guinea | HF934932 | HF934884 | HG779091 | HF934834 | |
| B. sorghicola | CBS 249.49 | Sorghum vulgare var. sudanense, Locality unknown | HF934927 | HF934868 | HG779087 | HF934829 |
| B. sorokiniana | CBS 140.31 | Substrate unknown, Japan | HF934935 | HF934876 | HG779088 | HF934837 |
| CBS 145.32 | Triticum durum, Locality unknown | HF934934 | HF934885 | HG779089 | HF934836 | |
| B. zeae | CBS 127716 | Unknown | HG778980 | HG779027 | HG779095 | HG779158 |
| B. zeicola | CBS 316.64 | Zea mays, USA | HF934938 | HF934871 | HG779093 | HF934840 |
| CBS 317.64 | Zea mays, USA | HF934939 | HF934878 | HG779094 | HF934841 | |
| Curvularia aeria | CBS 294.61T | Air, Brazil | HF934910 | HF934902 | HF565450 | HF934812 |
| C. affinis | CBS 154.34T | Manihot utilissima, Java | HG778981 | HG779028 | HG779126 | HG779159 |
| CBS 185.49 | Manihot utilissima, Java | HG778982 | HG779029 | HG779127 | HG779160 | |
| C. akaii | CBS 318.86 | Substrate unknown, Japan | HF934921 | HF934897 | HG779118 | HF934823 |
| CBS 127728 | Substrate unknown, Japan | HF934920 | HF934898 | HG779119 | HF934822 | |
| CBS 127730 | Substrate unknown, Japan | HF934922 | HF934899 | HG779120 | HF934824 | |
| C. australiensis | CBS 172.57 | Oryza sativa seed, Vietnam | HF934912 | HF934901 | HG779139 | HF934814 |
| C. brachyspora | CBS 186.50 | Soil, Java | HG778983 | HG779030 | HG779150 | HG779161 |
| C. carica-papayae | CBS 135941T | Carica papaya leaf, India | HG778984 | HG779031 | HG779146 | HG779162 |
| C. coicis | CBS 192.29T | Coix lacrima-jobi var. typica, Japan | HF934917 | HF934895 | HG779130 | HF934819 |
| C. cymbopogonis | CBS 419.78 | Yucca sp. leaf, Netherlands | HG778985 | HG779032 | HG779129 | HG779163 |
| C. ellisii | CBS 193.62 | Air, Pakistan | HF934913 | HF934896 | HG779143 | HF934815 |
| C. eragrostidis | CBS 189.48 | Sorghum seed, Java | HG778986 | HG779033 | HG779154 | HG779164 |
| C. gladioli | CBS 210.79 | Gladiolus sp. leaf, Romania | HG778987 | HG779034 | HG779123 | HG779165 |
| C. graminicola | BRIP 23186 | Aristida ingrata, Australia | JN192376 | JN600986 | JN600964 | – |
| C. hawaiiensis | CBS 173.57 T | Oryza sativa, Hawaii | HG778988 | HG779035 | HG779140 | HG779166 |
| CBS 448.72 | Salt-marsh soil, Kuwait | HG778989 | HG779036 | HG779142 | HG779167 | |
| CBS 727.96 | Substrate unknown, USA | HG778990 | HG779037 | HG779141 | HG779168 | |
| C. heteropogonis | CBS 284.91T | Heteropogon contortus leaf, Australia | HF934919 | HF934893 | HF934919 | HF934821 |
| CBS 511.91 | Heteropogon contortus leaf, Australia | HF934918 | HF934894 | HF934918 | HF934820 | |
| C. intermedia | CBS 334.64 | Avena versicolor, USA | HG778991 | HG779038 | HG779155 | HG779169 |
| C. ischaemi | CBS 630.82 T | Ischaemum indicum leaf, Solomon Islands | HG778992 | HG779039 | HG779131 | HG779170 |
| C. lunata | CBS 730.96 NT | Lung biopsy, USA | HF934911 | HF934900 | JX256429 | HF934813 |
| C. oryzae | CBS 169.53T | Oryza sativa seed, Vietnam | HF934906 | HF934867 | HF934808 | HF934808 |
| C. ovariicola | CBS 285.91 | Eragrostis parviflora, Australia | HG778993 | HG779040 | HG779144 | HG779171 |
| CBS 286.91 | Eragrostis parviflora, Australia | HG778994 | HG779041 | HG779145 | HG779172 | |
| C. perotidis | CBS 350.90 T | Perotis rara, Australia | HG778995 | HG779042 | HG779138 | HG779173 |
| C. prasadii | CBS 143.64 T | Jasminum sambac, India | HG778996 | HG779043 | HG779147 | HG779174 |
| CBS 144.64 | Substrate unknown, England | HG778997 | HG779044 | HG779149 | HG779175 | |
| C. protuberata | CBS 376.65 T | Deschampsia flexuosa leaf, Scotland | HG778998 | HG779045 | HG779135 | HG779176 |
| C. ravenelii | CBS 127709 | Unknown | HG778999 | HG779046 | HG779109 | HG779177 |
| C. robusta | CBS 624.68 T | Dichanthium annulatum leaf, USA | HG779000 | HG779047 | HG779125 | HG779178 |
| C. senegalensis | CBS 149.71 | Substrate unknown, Nigeria | HG779001 | HG779048 | HG779128 | HG779179 |
| C. spicifera | CBS 198.31 | Capsicum anuum, Cyprus | HF934916 | HF934905 | HG779136 | HF934818 |
| CBS 199.31 | Cucurbita maxima, Cyprus | HF934915 | HF934903 | HG779137 | HF934817 | |
| C. trifolii | CBS 173.55 | Trifolium repens, USA | HG779023 | HG779077 | HG779124 | HG779208 |
| C. tripogonis | BRIP 12375 T | Tripogon jacquemonti, India | JN192388 | JN601002 | JN600980 | – |
| C. tuberculata | CBS 146.63T | Zea mays leaf, India | HF934907 | HF934866 | HG779157 | HF934809 |
| C. uncinata | CBS 221.52 T | Oryza sativa leaf, Vietnam | HG779024 | HG779078 | HG779134 | HG779209 |
| CBS 531.70 | Oryza sativa seeds, Denmark | HG779025 | HG779079 | HG779132 | HG779210 | |
| C. verruciformis | CBS 537.75 | Lobibyx sp. feather, New Zealand | HG779026 | HG779080 | HG779133 | HG779211 |
| C. verruculosa | CBS 149.63 | Elaeis guineensis, Nigeria | HF934909 | HF934891 | HG779110 | HF934811 |
| CBS 150.63 | Punica granatum leaf, India | HF934908 | HF934892 | HG779111 | HF934810 | |
| Curvularia sp. I Lineage A | CBS 144.63 T | Muehlenbeckia sp. leaf, India | HG779002 | HG779049 | HG779108 | HG779180 |
| (= C. muehlenbeckiae sp. nov.) | UTHSC 08-2905 | Chest, USA | HE861836 | HG779050 | HF565484 | HG779189 |
| Curvularia sp. I Lineage B | UTHSC 07-2791 | Cornea, USA | HG779003 | HG779057 | HG779105 | HG779181 |
| (= C. hominis sp. nov.) | UTHSC 07–3105 | Nasal sinus, USA | HG779004 | HG779058 | HG779104 | HG779182 |
| UTHSC 07-3184 | Nasal sinus, USA | HG779005 | HG779059 | HG779099 | HG779183 | |
| UTHSC 07-3581 | Nail, USA | HG779006 | HG779060 | HG779102 | HG779184 | |
| UTHSC 08-849 | Eye, USA | HE861837 | HG779051 | HF565483 | HG779185 | |
| UTHSC 08-1296 | Nail, USA | HG779007 | HG779061 | HG779103 | HG779186 | |
| UTHSC 08-2418 | Bronchial wash, USA | HG779008 | HG779062 | HG779096 | HG779187 | |
| UTHSC 09-464 T | Cornea, USA | HG779011 | HG779065 | HG779106 | HG779191 | |
| UTHSC 08-2517 | Foot, USA | HG779009 | HG779063 | HG779107 | HG779188 | |
| UTHSC 08-3737 | Bronchial wash, USA | HG779010 | HG779064 | HG779101 | HG779190 | |
| UTHSC 09-1692 | Nasal sinus, USA | HG779012 | HG779066 | HG779097 | HG779192 | |
| UTHSC 09-2197 | Nasal sinus, USA | HE861835 | HG779052 | HF565485 | HG779193 | |
| UTHSC 09-2532 | Nasopharynx, USA | HG779013 | HG779067 | HG779100 | HG779194 | |
| UTHSC 09-3403 | Unknown tissue, USA | HG779014 | HG779068 | HG779098 | HG779195 | |
| Curvularia sp. II | UTHSC 08-3414T | Ankle, USA | HE861833 | HG779056 | HF565488 | HG779200 |
| (= C. americana sp. nov.) | UTHSC 07-2649 | Toe tissue, USA | HE861834 | HG779054 | HF565486 | HG779196 |
| UTHSC 08-84 | Nasal sinus, USA | HG779015 | HG779069 | HG779115 | HG779197 | |
| UTHSC 08-278 | Peritoneal dialysis fluid, USA | HE861832 | HG779055 | HF565487 | HG779198 | |
| UTHSC 08-2697 | Leg, USA | HG779016 | HG779070 | HG779117 | HG779199 | |
| UTHSC 09-2907 | Toe nail, USA | HG779017 | HG779071 | HG779114 | HG779201 | |
| UTHSC 09-2806 | Bone marrow, USA | HG779018 | HG779072 | HG779112 | HG779202 | |
| UTHSC 09-2863 | Bronchial wash, USA | HG779019 | HG779073 | HG779113 | HG779203 | |
| UTHSC 10-1276 | Maxillary sinus, USA | HG779020 | HG779074 | HG779116 | HG779204 | |
| Curvularia sp. III Lineage A | UTHSC 07-2764T | Toe nail, USA | HG779021 | HG779075 | HG779151 | HG779205 |
| (= C. chlamydospora sp. nov.) | UTHSC 08-1283 | Nasal sinus, USA | HG779022 | HG779076 | HG779152 | HG779206 |
| Curvularia sp. III Lineage B | UTHSC 09-2092T | Nasal sinus, USA | HE861842 | HG779053 | HF565459 | HG779207 |
| (= C. pseudolunata sp. nov.) | ||||||
| Exserohilum turcicum | CBS 330.64 | Zea mays, USA | HF934950 | HF934887 | HG779153 | HF934852 |
1 BRIP: Queensland Plant Pathology Herbarium, Queensland, Australia; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; UTHSC: Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, Texas; T ex-type strain; NT ex-neotype strain (Manamgoda et al. 2012).
2 Sequences generated during this study appear in bold; other sequences originate from Manamgoda et al (2012), da Cunha et al. (2013) and Amaradasa et al. (2014).
Phenotypic study
Colony morphology and growth rates were studied on potato carrot agar (PCA; 20 g of potatoes, 20 g of carrots, 20 g of agar, 1 L of distilled water) and oatmeal agar (OA; 30 g of filtered oat flakes, 20 g of agar, 1 L of distilled water) after 7 d of incubation at 25 °C in the dark. Microscopic features were studied in lactic acid from colonies on the same media after 10–21 d of incubation. Size ranges in the species descriptions are derived from at least 30 measurements.
Cryo-Scanning Electron microscopy
Relevant areas of fungal cultures were carefully selected by means of a stereo microscope (Nikon SMZ1500, Nikon, Amsterdam, The Netherlands). Small (c. 3 × 5 mm) agar blocks were carefully cut out with a surgical blade (no. 11, Swan-Morton, Sheffield, UK), while disturbing of fungal structures was kept to a minimum during cutting and transferring of the samples to a copper cup (diam 10 mm, height 8 mm). Agar blocks were glued to the copper cup with frozen tissue medium (KP-Cryoblock, Klinipath, Duiven, The Netherlands). The copper cup was placed on an agar surface inside a closed Petri dish to prevent drying of the sample. The sample was quickly frozen in nitrogen slush and immediately transferred to a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan) equipped with an Oxford CT1500 cryostation. The sample was viewed at 2.5 kV and ice was removed by sublimation after heating of the SEM-stage to −85 °C. Then the sample was sputter-coated in the cryostation by means of a gold target for three times 90 s holding the sample at different angles for an optimal coating. Electron micrographs were acquired with the F3 or F4 scan at 5 kV and contrast levels digitally enhanced in Adobe® Photoshop® Creative Suite v. 6.
Molecular study
DNA extraction of Curvularia spp. I–III was performed with the PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA, USA) as described by da Cunha et al. (2013). DNA extraction of isolates of the other species studied was carried out from colonies growing on malt extract agar (Oxoid, Basingstoke, England) with the UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). Amplification and sequencing of the ITS and RNA polymerase II second largest subunit (RPB2) were performed with primers ITS5 + ITS4 (White et al. 1990) and 5F2 + 7cR (O’Donnell et al. 2007) following the protocols of Amaradasa et al. (2014). Amplification of the gpd and LSU genes were performed with primers gpd1 + gpd2 (Berbee et al. 1999) and LR0R + LR5 (Vilgalys & Hester 1990) as described in Manamgoda et al. (2012). The ITS PCR products were purified and sequenced at Macrogen Europe (Amsterdam) using a 3739 XL DNA analyser (Applied Biosystems). The gpd, LSU and RPB2 loci were sequenced at the CBS-KNAW Fungal Biodiversity Centre (Utrecht, The Netherlands), using the BigDye terminator sequencing kit v. 3.1 (Applied Biosystems) and an ABI PrismTM 3100 DNA sequencer (Applied Biosystems). The program SeqMan Pro (Lasergene, Madison, WI, USA) was used to obtain consensus sequences from the complementary sequences of each isolate. Sequences of the clinical isolates were aligned with those of a set of 60 isolates representing 33 species of Curvularia, and two phylogenetically related genera of Pleosporaceae, i.e. Bipolaris (nine spp.) and Exserohilum (one sp., used as outgroup) using ClustalX v. 1.81 (Thompson et al. 1997), followed by manual adjustments with a text editor. Individual alignments of ITS, LSU, gpd and RPB2 and a concatenated 4-locus dataset were analysed with maximum likelihood (ML) using MEGA5 (Tamura et al. 2011) with partial deletion of gaps, substitution models proposed by this program and 1 000 bootstrap replicates. Bootstrap support values (bs) ≥ 70 % were considered significant. Incongruence among data sets was tested by a visual inspection of all groups with ≥ 70 bs in the partial trees to search for potentially conflicting groups. A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities using MrBayes v. 3.1.1 (Ronquist & Huelsenbeck 2003). The best models of nucleotide substitution for each locus for the Bayesian analysis were determined using MrModeltest v. 2.3 (Nylander 2004). Two analyses of four MCMC chains were run from random trees for 4 598 100 generations and sampled every 100 generations, resulting in 45 981 trees, of which 25 % were discarded as the burn-in phase. Posterior probabilities (pp) were determined from the remaining trees. The sequences generated during this study and the alignments used in the phylogenetic analyses were deposited in GenBank (Table 1) and TreeBASE (submission ID http://purl.org/phylo/treebase/phylows/study/TB2:S14881), respectively.
RESULTS
Phylogenetic study
After removing ambiguously aligned regions, we obtained ITS, LSU, gpd and RPB2 alignments of 533, 830, 434, and 793 positions of which 64 (12 %), 39 (4.69 %), 111 (25.57 %) and 259 (32.66 %) were variable, respectively. MEGA5 proposed a K2 + G + I model for the ITS and RPB2 loci, K2 + I for LSU, T92 + G for gpd and GTR + G + I for the concatenated 4-locus dataset. These models were used in the ML analyses. Partial trees (not shown) were congruent except for the following clades: Curvularia gladioli CBS 210.79 grouped with C. ischaemi CBS 630.82 (93 % bs) in the ITS tree, but in the RPB2 tree the former isolate grouped with Curvularia trifolii CBS 173.55 (77 % bs), while the CBS 630.82 grouped with Curvularia coicis CBS 192.29 (100 % bs). These incongruencies affected species that are not closely related to Curvularia sp. I–III of da Cunha et al. (2013) and therefore the four loci were combined. Partial trees revealed that RPB2 was the most informative locus with 35 clades with significant bs, followed by gpd with 23. ITS and LSU both showed only 10 clades with significant bs. The ITS and LSU ML trees provided good support for a clade representing the genus Bipolaris, but Curvularia species appeared in several clades, some of which had low bootstrap support. The gpd ML tree separated Bipolaris and Curvularia as two clades with 93 % and 70 % bs, respectively, whereas these clades showed 99 % and 95 % bs in the RPB2 tree. In the concatenated 4-locus ML tree (not shown) the Bipolaris and Curvularia clades had 100 % and 97 % bs, respectively. For Bayesian analysis, MrModeltest proposed a SYM + I + G model for the ITS locus and GTR + I + G for LSU, gpd and RPB2. These models were incorporated in the analysis. The consensus tree obtained from the Bayesian analysis (Fig. 1) agreed with the topology of the ML tree (not shown) for the 4-locus dataset.
Fig. 1.
Bayesian consensus tree obtained from the combined ITS, LSU, gpd and RPB2 alignment of Curvularia and related genera. The scale bar represents the average number of substitutions per site. Bootstrap values ≥ 70 % and posterior probabilities ≥ 0.95 (in italics) are given near the internodes. The new species proposed in this study are shown in the coloured boxes. Ex-type and ex-neotype isolates for each species are indicated with a ‘T’ or ‘NT’, respectively, after the isolate number.
The 4-locus tree (Fig. 1) revealed that C. carica-papayae, listed as a synonym of C. aeria by Sivanesan (1987), is a phylogenetically distinct species. The concatenated tree also corroborated that isolates in Curvularia spp. I–III of da Cunha et al. (2013) are different from accepted species of this genus represented in the CBS collection (Fig. 1). However, Curvularia spp. I and III were each split into two lineages that are sufficiently distant from each other to represent different species. These lineages were named here Ia, Ib and IIIa, IIIb, accordingly. One of them, Ib, shows considerable genetic variation, but is treated here as a single taxon because its complex topology does not seem to suggest a clear separation of species within it. The ITS and LSU ML trees did not provide enough resolution to separate lineages within Curvularia spp. I and III, but showed 87 % and 99 % bs for Curvularia sp. II. The gpd ML tree gave 80 % bs to Curvularia sp. II and separated lineages Ia (98 % bs) and Ib (52 % bs) of Curvularia sp. I, but did not separate the two lineages of Curvularia sp. III. The RPB2 ML tree gave 100 % bs to Curvularia sp. II and provided enough resolution to separate lineages Ia, Ib, IIIa and IIIb with bs ≥ 75 %. Curvularia sp. Ia, Ib, II, IIIa and IIIb are morphologically and phylogenetically different from other members of the genus and therefore are proposed here as new taxa. These species were respectively named C. muehlenbeckiae, C. hominis, C. americana, C. chlamydospora and C. pseudolunata and described in alphabetical order in the Taxonomy section.
Within the Curvularia clade, six well-supported lineages were associated with certain combinations of morphological features. These lineages were named the americana-, eragrostidis-, hominis-, lunata-, spicifera- and trifolii-clades (Fig. 1). The eragrostidis-clade (82 % bs, 1 pp) was formed by C. eragrostidis, C. graminicola and C. intermedia, and is characterised by producing inconspicuously distoseptate (i.e. the two cell wall layers within the conidium are difficult to distinguish in mature conidia), straight to somewhat unequal-sided, 4-celled conidia. In mature conidia of C. graminicola, all septa are accentuated by dark transverse bands, whereas in C. eragrostidis and C. intermedia, only the median septum is accentuated (Sivanesan 1987, Alcorn 1998). The americana- (99 % bs, 1 pp), hominis- (99 % bs, 1 pp) and lunata- (93 % bs, 0.99 pp) clades included species with mostly 4-celled, inconspicuously distoseptate conidia with the central cells usually darker than the end cells. In these clades the conidia are often curved at the third cell from base, and this cell is usually larger than the others (the only exceptional case is C. brachyspora, in which both central cells are more or less the same size (Sivanesan 1987)). This morphology, however, is also observed in C. ischaemi, which falls outside these three clades. The americana-clade included C. americana and C. verruculosa. These species appeared in Fig. 1 as two distinct species separated by relatively long branches. The hominis-clade included two new species, C. hominis and C. muehlenbeckiae. One isolate of the latter taxon, CBS 144.63, had been labelled ‘C. lunata’ in the CBS collection, but in this study it proved to be phylogenetically quite distant from the ex-neotype strain of that species, CBS 730.96. The lunata-clade was formed by C. aeria, C. brachyspora, C. caricapapayae, C. chlamydospora, C. lunata, C. prasadii and C. pseudolunata. Accentuated septa can be observed in all members of this clade and elongate blackish stromata have been reported in C. carica-papayae and C. aeria (Mathur & Mathur 1959, Ellis 1966, 1971, Sivanesan 1987). This kind of stromata is also produced by old cultures of the ex-neotype strain of C. lunata, CBS 730.96 (unpubl. data). Isolates of C. chlamydospora and C. pseudolunata can produce aggregates of brown chlamydospores in culture (Fig. 3k and 6i). The spicifera-clade (98 % bs, 1 pp) was formed by C. australiensis, C. ellisii, C. hawaiiensis, C. perotidis and C. spicifera. Members of this clade produce conspicuously distoseptate conidia that are straight in all species, except in C. ellisii which produces both straight and curved conidia (Sivanesan 1987). Three taxa of this clade are agents of opportunistic infections in humans, i.e. C. australiensis, C. hawaiiensis and C. spicifera (McGinnis et al. 1986, de Hoog et al. 2000). The trifolii-clade (95 % bs, 1 pp) included C. akaii, C. heteropogonis, C. gladioli and C. trifolii. These species produce 4-celled, usually curved, inconspicuously distoseptate conidia which, in contrast to those seen in the other clades discussed here, show a strongly protruding hilum (Sivanesan 1987, Boerema & Hamers 1989, Alcorn 1990). Two other species in our study produce conidia with a protruding hilum, i.e. C. cymbopogonis and C. protuberata. Their conidia, however, are 5-celled (Sivanesan 1987).
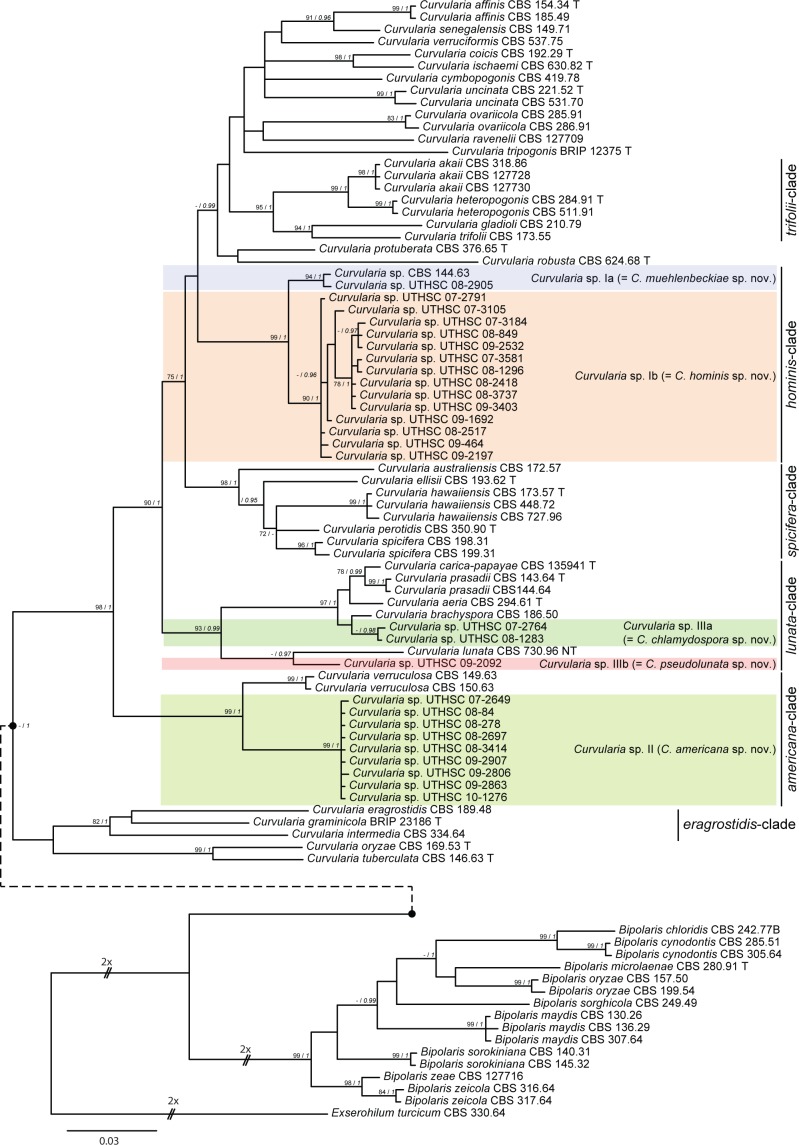
Fig. 3.
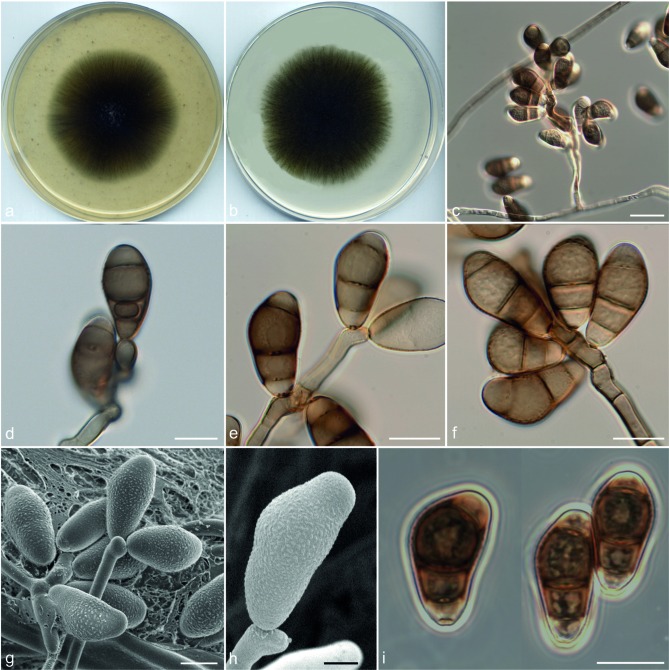
Curvularia chlamydospora (a–d, h–k: CBS 136984; e–g: FMR 11040). a, b. Colonies on OA and PCA, respectively, at 25 °C after 7 d; c–g, i, j. conidiophores and conidia; h. microconidiation; k. chlamydospore. — Scale bars: c, i, k = 20 μm; d–h = 10 μm; j = 5 μm.
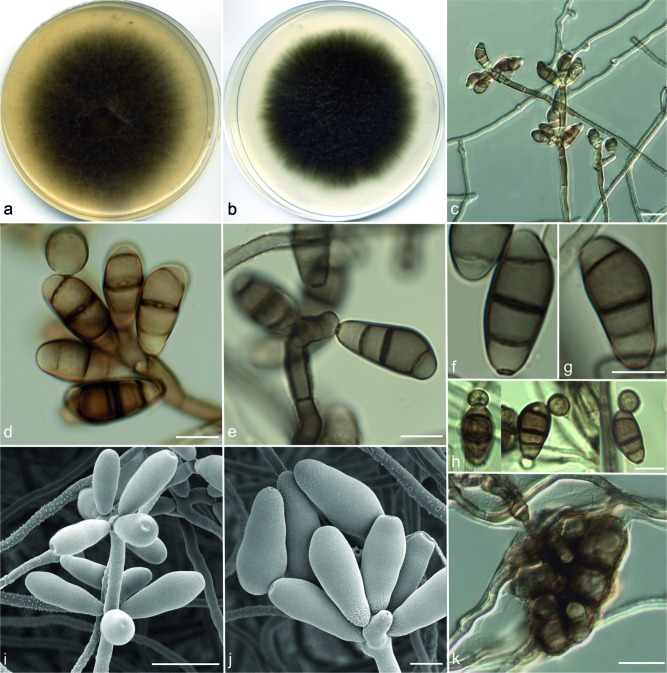
Fig. 6.
Curvularia pseudolunata (CBS 136987). a, b. Colonies on OA and PCA, respectively, at 25 °C after 7 d; c–h. conidiophores and conidia; i. chlamydospores. — Scale bars: c = 20 μm; d–i = 10 μm.
Not all Curvularia species were included in the six clades previously mentioned, and other well-supported lineages were observed. Curvularia oryzae and C. tuberculata, for example, appeared as sister taxa with 99 % bs and 1 pp. These species are morphologically very different, i.e. the conidia of C. oryzae are 3-distoseptate and smooth while those of C. tuberculata are 3–8-distoseptate and tuberculate at maturity (Sivanesan 1987). We preferred not to name morphologically heterogeneous lineages because future studies including more taxa might reveal more homogeneous groupings within such lineages.
Taxonomy
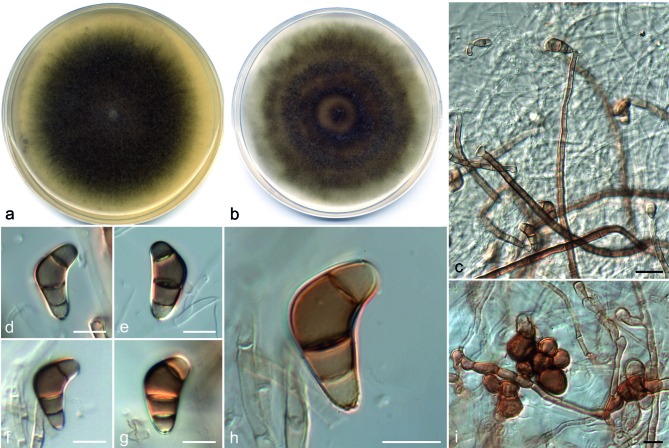
Curvularia americana Da Cunha, Madrid, Gené & Cano, sp. nov. — MycoBank MB806052; Fig. 2
Fig. 2.
Curvularia americana (a, b, d–j: CBS 136983; c, k: FMR 11674 ). a, b. Colonies on OA and PCA, respectively, at 25 °C after 7 d; c–i. conidiophores and conidia; j, k. microconidiation. — Scale bars: c–i = 10 μm.
Etymology. The name refers to the continent where this species was found.
Vegetative hyphae septate, branched, subhyaline to brown, smooth to asperulate, 1.5–4 μm wide, anastomosing. Conidiophores semi- to macronematous, mononematous, septate, usually simple, slightly geniculate, subhyaline to dark brown, smooth to asperulate, with cell walls often thicker than those of the vegetative hyphae, 60–299 × 2–5 μm. Conidiogenous cells terminal or intercalary, polytretic, proliferating sympodially, subcylindrical to slightly swollen, 8–22 × 4–8 μm. Conidia 4(–5)-celled, straight to slightly curved, 13–28 × 7–15 μm, usually with the third cell unequally sided and larger than the others, second and third cells pale brown to brown, apical and basal cell subhyaline, apical cell smooth-walled, intermediate smooth (slightly verruculose under SEM), basal cell often verruculose; hilum non-protruding, flat, darkened and thickened, 1.5–3 μm wide. Microconidiation sometimes present, forming 1-celled, pale brown, globose conidia 5–6 μm wide. Chlamydospores not observed. Sexual morph not observed.
Culture characteristics — Colonies on OA and PCA attaining 62 and 69 mm diam, respectively, in 7 d at 25 °C, funiculose and greenish grey to dark green at the centre, effuse and greyish white towards the periphery, with a fimbriate margin; reverse olive to dark green.
Specimens examined. USA, Minnesota, culture from ankle (human), 2008, D.A. Sutton (holotype CBS H-21465, culture ex-type FMR 11551 = UTHSC 08-3414 = CBS 136983); California, culture from maxillary sinus (human), 2010, D.A. Sutton (FMR 11500 = UTHSC 10-1276); Ohio, culture from peritoneal dialysis fluid (human), 2008, D.A. Sutton (FMR 11691 = UTHSC 08-278); Oklahoma, culture from toe nail (human), 2009, D.A. Sutton (FMR 11005 = UTHSC 09-2907); Tennessee, culture from leg (human), 2008, D.A. Sutton (FMR 11674 = UTHSC 08-2697); Texas, culture from toe tissue (human), 2007, D.A. Sutton (FMR 11687 = UTHSC 07-2649); Texas, culture from bronchial wash (human), 2009, D.A. Sutton (FMR 11514 = UTHSC 09-2863); Utah, culture from nasal sinus (human), 2008, D.A. Sutton (FMR 11693 = UTHSC 08-84); Virginia, culture from bone marrow (human), 2009, D.A. Sutton (FMR 11515 = UTHSC 09-2806).
Notes — Curvularia americana is similar to C. lunata and C. prasadii in conidial morphology. However, the conidia of C. lunata are slightly narrower, up to 13 μm wide (Manamgoda et al. 2012) and, in contrast to C. americana, all septa in conidia of C. prasadii are accentuated and up to 2.4 μm wide (Mathur & Mathur 1959, Ellis 1966, 1971). The phylogenetic study placed C. lunata and C. prasadii in the lunata-clade, a lineage relatively distant from C. americana. The 4-locus tree indicated that C.americana is the sister taxon of C. verruculosa, but these species were separated by a considerable genetic distance (Fig. 1). The conidia of C. verruculosa are slightly larger (20–40 × 12–17 μm) than those of C. americana and show distinctly verruculose intermediate cells (Tandon & Bilgrami 1962, Ellis 1966, 1971, Sivanesan 1987).
Curvularia chlamydospora Madrid, Da Cunha, Gené & Guarro, sp. nov. — MycoBank MB806053; Fig. 3
Etymology. The name refers to the presence of chlamydospores.
Vegetative hyphae septate, branched, subhyaline to brown, smooth-walled, 1.5–4 μm wide, anastomosing. Conidiophores semi- to macronematous, mononematous, septate, usually simple, geniculate or bent at the apex, brown to dark brown, smooth to asperulate, 22–323 × 2–5 μm. Conidiogenous cells terminal or intercalary, polytretic, proliferating sympodially, subcylindrical to irregularly shaped, 7–18 × 5–10 μm. Conidia 4-celled, mostly slightly curved, 16–25 × 7–12 μm wide in the broadest part, smooth-walled (basal cell verruculose under SEM), usually with the central septum appearing slightly accentuated, the third cell from the base slightly larger and unequal sided, second and third cells darker than the others, brown to dark brown, end cells paler; hilum non-protruding, flat, darkened and thickened, 1.5–3 μm wide. Chlamydospores present, initially as intercalary chains but later forming clusters of swollen cells, 13–80 μm, smooth to verruculose and thick-walled. Microconidiation present, forming conidia 1–2-celled, pale brown, globose to subglobose, 4–6 μm diam. Sexual morph not observed.
Culture characteristics — Colonies on OA attaining 76 mm diam in 7 d at 25 °C, funiculose, greenish grey or dark green, margin fimbriate; reverse olive grey to dark green. Colonies on PCA attaining 68 mm diam at the same temperature and time of incubation, funiculose at the centre, effuse towards the periphery, dark green, with a fimbriate margin; reverse dark green.
Specimens examined. USA, Montana, culture from toe nail (human), 2007, D.A. Sutton (holotype CBS H-21466, culture ex-type FMR 11709 = UTHSC 07-2764 = CBS 136984); Nevada, culture from nasal sinus (human), 2008, D.A. Sutton (FMR 11040 = UTHSC 08-1283).
Notes — Curvularia chlamydospora is superficially similar to three species producing 4-celled conidia with an accentuated median septum, namely C. brachyspora, C. eragrostidis and C. intermedia. However, the third cell from base is usually larger and more pigmented than the second one in C. chlamydospora, while in the three similar taxa both intermediate cells are rather equal in size and pigmentation. These species have not been reported to produce chlamydospores in culture and have wider conidia, i.e. 10–14 μm in C. brachyspora, 11–20 μm in C. eragrostidis and 13–20 in C. intermedia (Sivanesan 1987). Curvularia eragrostidis and C. intermedia reside in the eragrostidis-clade, while C. chlamydospora belongs to the lunata-clade. Curvularia brachyspora appeared as the sister taxon of C. chlamydospora but this relationship received poor statistical support (Fig. 1).
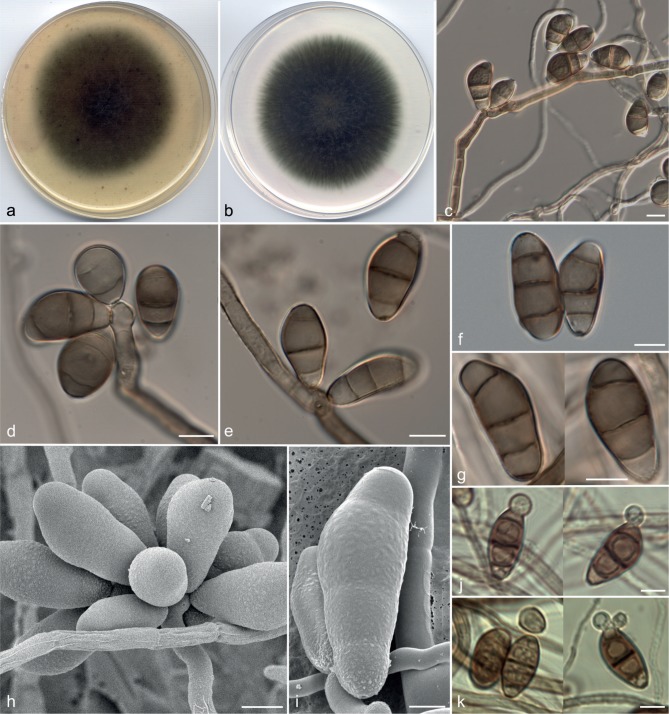
Curvularia hominis Da Cunha, Madrid, Gené & Cano, sp. nov. — MycoBank MB806054; Fig. 4
Fig. 4.
Curvularia hominis (CBS 136985). a, b. Colonies on OA and PCA, respectively, at 25 °C after 7 d; c, e–i. conidiophores and conidia; d. conidium showing two wall layers. — Scale bars: c = 20 μm; d–g, i = 10 μm; h = 5 μm.
Etymology. The name refers to the origin of the isolates, all of which were isolated from clinical human specimens.
Vegetative hyphae septate, branched, subhyaline to brown, smooth to slightly asperulate 1.5–5 μm wide, anastomosing. Conidiophores semi- to macronematous, mononematous, septate, simple or branched, geniculate towards the apex, subhyaline to dark brown, smooth to asperulate, with cell walls often thicker than those of the vegetative hyphae, 55–325 × 2–5 μm wide. Conidiogenous cells terminal or intercalary, polytretic, proliferating sympodially, subcylindrical to irregularly shaped, 6–26 ×4–9 μm; conidiogenous loci usually somewhat thickened and darkened. Conidia 4–5-celled, slightly curved, 18–30 × 7–14 μm wide in the broadest part, with the third cell from the base often larger and unequal sided, intermediate cells usually verruculose and darker than the others, brown, end cells subhyaline to pale brown and smooth-walled; hilum non-protruding, flat, darkened and thickened, 1.5–3 μm wide. Microconidiation and chlamydospores were not observed. Sexual morph not observed.
Culture characteristics — Colonies on OA and PCA attaining 70–72 mm diam in 7 d at 25 °C, funiculose and dark green at the centre, floccose and olive to white towards the periphery, with a fimbriate margin; reverse olive to dark green.
Specimens examined. USA, Florida, culture from cornea (human), 2009, D.A. Sutton (holotype CBS H-21467, culture ex-type FMR 11539 = UTHSC 09-464 = CBS 136985); Arkansas, culture from nasal sinus (human), 2007, D.A. Sutton (FMR 11172 = UTHSC 07-3184); Louisiana, culture from eye (human), 2008, D.A. Sutton (FMR 11688 = UTHSC 08-849); Minnesota, culture from nail (human), 2007, D.A. Sutton (FMR 11698 = UTHSC 07-3581); Minnesota, culture from nasal sinus (human), 2009, D.A. Sutton (FMR 11527 = UTHSC 09-2197); Ohio, culture from nasal sinus (human), 2009, D.A. Sutton (FMR 11535 = UTHSC 09-1692); Texas, culture from nasal sinus (human), 2007, D.A. Sutton (FMR 11704 = UTHSC 07-3105); Texas, culture from nail (human), 2008, D.A. Sutton (FMR 11683 = UTHSC 08-1296); Texas, culture from bronchial wash (human), 2008, D.A. Sutton (FMR 11680 = UTHSC 08-2418); Texas, culture from bronchial wash (human), 2008, D.A. Sutton (FMR 11542 = UTHSC 08-3737); Texas, culture from foot (human), 2008, D.A. Sutton (FMR 11678 = UTHSC 08-2517); Texas, culture from nasopharynx (human), 2009, D.A. Sutton (FMR 11521 = UTHSC 09-2532); Texas, culture from tissue (human), 2009, D.A. Sutton (FMR 11509 = UTHSC 09-3403); Utah, culture from cornea (human), 2007, D.A. Sutton (FMR 11708 = UTHSC 07-2791).
Notes — Although all isolates of this fungus were obtained from humans, the species might also be common in the environment. Curvularia hominis resembles other species of the genus with 4-celled conidia and an asymmetrically swollen, dark third cell, such as C. aeria, C. carica-papayae, C. lunata and C. prasadii, but differs from them in producing conidia with verruculose intermediate cells (Fig. 4e–i). The latter four species are members of the lunata-clade, whereas C. hominis and C. muehlenbeckiae form a distinct lineage, the hominis-clade (Fig. 1).
Curvularia muehlenbeckiae Madrid, Da Cunha, Gené, Guarro & Crous, sp. nov. — MycoBank MB806055; Fig. 5
Fig. 5.
Curvularia muehlenbeckiae (CBS 144.63). a, b. Colonies on OA after 7 d and on PCA after 5 d, respectively, at 25 °C; c–h. conidiophores and conidia. — Scale bars: c–g = 10 μm; h = 5 μm.
Etymology. The name refers to the substrate from which the ex-type strain was obtained, Muehlenbeckia sp.
Vegetative hyphae septate, branched, subhyaline to brown, smooth-walled, 1.5–5 μm wide, anastomosing. Conidiophores semi- to macronematous, mononematous, septate, simple to branched, straight or flexuous, geniculate towards the apex, subhyaline to dark brown, smooth to asperulate with cell walls often thicker than those of the vegetative hyphae, 21.5–398 × 2–5 μm with subnodulose and nodulose intercalary swellings up to 9.5 μm wide, swellings coinciding with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, subcylindrical to irregularly shaped, mono- to polytretic, proliferating sympodially; intercalary conidiogenous cells 5–18 μm long, terminal conidiogenous cells 5–25 μm long. Conidia 4-celled, asymmetrical to more or less curved at the third cell from base, 17–26 × 8.5–12 μm, intermediate cells dark brown and usually verruculose, end cells paler and smooth-walled or less ornamented than central cells.Chlamydospores and microconidiation not observed. Sexual morph not observed.
Culture characteristics — Colonies on OA attaining 76 mm diam in 7 d at 24 °C, cottony to funiculose, pale grey at the centre, dark olive towards the periphery, with a fimbriate margin; reverse olivaceous-black. Colonies on PCA attaining 40 mm diam at the same temperature and period of incubation, radiate, funiculose, dark olive with a slightly fimbriate margin; reverse concolorous with surface.
Specimens examined. INDIA, from Muehlenbeckia sp. leaf, 1962, K.S. Bilgrami (holotype CBS H-10451, culture ex-type CBS 144.63). – USA, Utah, culture from chest (human), 2008, D.A. Sutton (UTHSC 08-2905 = FMR 11671 = CBS 136986).
Notes — This species is the sister taxon of C. hominis, which has slightly larger conidia (18–30 × 7–14 μm) with a similar ornamentation consisting of small but conspicuous warts. Some Curvularia species outside the hominis-clade produce conidia ornamented with warts, e.g. C. tuberculata, C. verruculosa and C. verruciformis. The first two species produce larger conidia, i.e. 23–52 × 13–20 μm and 20–40 × 12–17 μm, respectively, and the third one differs from members of the hominis-clade by having mostly 5-celled, more strongly ornamented conidia (Jain 1962, Agarwal & Sahni 1963, Ellis 1966, Sivanesan 1987). Curvularia species with warted conidia appear in different clades, suggesting that this kind of ornamentation evolved several times in Curvularia.
Curvularia pseudolunata Da Cunha, Madrid & Gené, sp. nov. — MycoBank MB806056; Fig. 6
Etymology. The name refers to the morphological resemblance and phylogenetic closeness of this species to Curvularia lunata.
Vegetative hyphae septate, branched, subhyaline to brown, smooth-walled, 1.5–5 μm wide. Conidiophores macronematous, mononematous, septate, unbranched, geniculate near the apex, brown, smooth-walled, 100–350 × 2–4.5 μm. Conidiogenous cells mostly terminal, polytretic, proliferating sympodially, subcylindrical, subglobose to irregularly shaped, 4.5–30 × 6–10 μm. Conidia 4-celled, mostly curved, 20–27 × 8–12 μm, with the third cell from base usually unequally sided, larger and darker than the others, brown, second and end cells subhyaline to pale brown, smooth-walled, basal cell often verruculose; hilum non-protruding, flat, darkened and thickened, 1.5–2.5 μm wide. Chlamydospores abundant, initially as intercalary chains, later forming clusters of swollen cells, up to 60 μm diam, smooth- and thick-walled. Microconidiation not observed. Sexual morph not observed.
Culture characteristics — Colonies on OA attaining 71 mm diam in 7 d at 25 °C cottony to lanose, greenish grey, with a fimbriate margin; reverse dark green. Colonies on PCA attaining 78 mm diam at the same temperature and time of incubation, lanose at the centre, floccose towards the periphery, greyish green, with a fimbriate margin; reverse olive green.
Specimen examined. USA, California, culture from nasal sinus (human), 2009, D.A. Sutton (holotype CBS H-21468, cultures ex-type FMR 11529 = UTHSC 09-2092 = CBS 136987).
Notes — Curvularia pseudolunata is morphologically similar to C. lunata and these taxa grouped together in the 4-locus phylogeny (Fig. 1). However, the conidia of C. lunata are slightly larger (21–31 × 9–13 μm) and this species is separated from C.pseudolunata by a considerable genetic distance.
DISCUSSION
Traditionally, Curvularia and Bipolaris have been distinguished by conidial features, i.e. euseptate and typically curved at a swollen intermediate cell in Curvularia, but straight to slightly curved and distinctly distoseptate in Bipolaris (Kwon-Chung & Bennett 1992, de Hoog et al. 2000, Revankar & Sutton 2010). We agree with the view of authors like Alcorn (1983b), Sivanesan (1987) and Seifert et al. (2011) that both genera have distoseptate conidia. Phylogenetic studies (Berbee et al. 1999, Manamgoda et al. 2012) have demonstrated that species with conspicuously distoseptate conidia previously placed in Bipolaris actually belong in Curvularia, e.g. members of the spicifera-clade (Fig. 1). Furthermore, in the new species described herein, two wall layers were often evident in young conidia (Fig. 2f, 4d, 5f) and septa are already visible at this stage; however, in mature conidia the layers may appear so close to one another that the conidia may look euseptate under the light microscope (Fig. 2g, 4i, 5e). A recently described pleosporalean genus, Porocercospora, the causal agent of buffalograss false-smut disease, also shows two-layered conidial cell walls, but mature conidia often seem to have both eu- and distosepta, depending on how closely together the two cell wall layers cohere near the septa. This genus is phylogenetically closely related to Bipolaris and Curvularia and is similar to them in having tretic conidiogenesis and darkly pigmented mycelium. However, Porocercospora has conidiophores without a geniculate rachis, conidiogenous cells with inconspicuous, non-darkened conidiogenous loci and long, obclavate to cylindro-obclavate conidia (Amaradasa et al. 2014).
Although Bipolaris and Curvularia cannot be distinguished based on the morphology of their conidial septa, other morphological features seem to be of diagnostic value. None of the species of Bipolaris s.str. included in this study (Fig. 1) and in previous works (Berbee et al. 1999, Manamgoda et al. 2012) has conidia curved at an intermediate swollen cell. As described by Berbee et al. (1999) for ‘Cochliobolus group 1’, the conidia of Bipolaris s.str. can show a gentle curve that continues along the whole length of the conidium. Conidia ornamented with small to coarse warts are produced by some Curvularia species, e.g., C. tuberculata, C. verruciformis and C. verruculosa, but this kind of ornamentation has not been reported in Bipolaris s.str. (Jain 1962, Tandon & Bilgrami 1962, Agarwal & Sahni 1963, Ellis 1966, Sivanesan 1987). Another helpful character is the morphology of the hilum. None of the species in Bipolaris s.str. has conidia with a strongly protruding hilum, but it is observed in several members of Curvularia s.str., such as C. cymbopogonis and C. protuberata, as well as all species in the trifolii-clade (Sivanesan 1987). A protruding hilum is also observed in a closely related genus, Exserohilum, which also includes clinically relevant and plant-pathogenic species (McGinnis et al. 1986, de Hoog et al. 2000). Members of this genus sometimes form curved conidia, but the hilum is different from those seen in Curvularia spp. In Exserohilum the hilum appears as a protrusion of the cell wall that is not delimited by a septum and that often appears double-walled, with the outer wall forming an enveloping collar or ‘hilar bubble’ around it (Alcorn 1983b, 1988). In Curvularia, by contrast, when the hilum protrudes, it appears single-walled in light microscopy and is delimited by a septum (Nelson & Hodges 1965, Sivanesan 1987, Zhang et al. 2004). Conidial size might also be helpful to distinguish Bipolaris s.str. from Curvularia s.str. Among the species falling in the Bipolaris clade in Berbee et al. (1999) and Manamgoda et al. (2012), the longest conidia are those of B. zeae, up to 225 μm long (Sivanesan 1987). Conidia of Curvularia s.str. tend to be shorter. Among species in the Curvularia clade (Berbee et al. 1999, Manamgoda et al. 2012), the longest conidia are produced by C. tripogonis and are up to 130 μm long (Sivanesan 1987).
Boedijn (1933) divided Curvularia into three groups of species, i.e. groups Maculans, Lunata and Geniculata. The Maculans group was characterised by producing 4-celled, straight or somewhat asymmetrical conidia with the central cells darker and larger than the end cells. This group included C. maculans (currently considered a synonym of C.eragrostidis), C. cesatii (this species was transferred to the genus Endophragmiella as E. cesatii by Hughes in 1979), C. intermedia and C. spicifera, all of which were unable to produce stromata in culture. The Lunata group included species with 4-celled, more or less curved conidia in which one of the intermediate cells is enlarged and darker than the others. Some of its members were C.lunata, C. ramosa and C. trifolii. This group was reported to produce subcylindrical stromata in culture. The Geniculata group was proposed for species with 5-celled conidia which often produced stromata, such as C. geniculata, C. affinis, C. fallax and C. falcata (this species was synonymized with C.senegalensis by Sivanesan in 1987). In our phylogenetic study, three species of Boedijn’s Maculans group were included, i.e. C.eragrostidis, C. intermedia and C. spicifera. The group is polyphyletic since only the former two species grouped together in the eragrostidis-clade, a lineage characterised by rather straight, inconspicuously distoseptate, 4-celled conidia. This lineage also included C. graminicola (Fig. 1). Curvularia spicifera clustered in a different clade with other species whose conidia show evident distosepta. No DNA sequences have as yet been analysed for Endophragmiella cesatii, which, as indicated previously, originally was considered a Curvularia species and a member of the Maculans group (Boedijn 1933). Its morphology clearly suggests a phylogenetically distant fungus (Hughes 1979, 1980). The genus Endophragmiella is considered a member of the Lasiosphaeriaceae, Sordariales by Seifert et al. (2011). Boedijn’s Lunata group also proved to be polyphyletic since two of its members, C. lunata and C. trifolii, clustered in separate, relatively distant clades in Fig. 1. Two members of Boedijn’s Geniculata group included in this study, C. affinis and C. senegalensis, formed a well-supported clade. CBS isolates of C. geniculata could not be clearly distinguished molecularly from isolates labelled C.senegalensis in a study by da Cunha et al. (2013); other authors also suggested that these taxa might be conspecific (Hosokawa et al. 2003, Sun et al. 2003). Unfortunately, no ex-type strains of these species are available and epitypification is necessary to clarify their taxonomy. Isolate CBS 155.34, labelled ‘Syntype’ of C. fallax has 4-celled conidia and clustered with morphologically similar isolates of Curvularia spp. in a preliminary phylogeny (not shown). CBS 155.34 is possibly mislabelled and therefore was excluded from the analysis.
The well-documented Bipolaris s.l. opportunists, i.e. B. australiensis, B. hawaiiensis and B. spicifera (McGinnis et al. 1986), were transferred to Curvularia (Manamgoda et al. 2012), suggesting that pathogenicity to vertebrates in this group of fungi might be restricted to the latter genus (Manamgoda et al. 2012). Interestingly, however, a recent study by da Cunha et al. (2012) reported that two species of Bipolaris s.str., B. cynodontis and B. setariae, represented 8.6 % and 1 %, respectively, of a total of 104 clinical Bipolaris s.l. isolates from the USA. These species were isolated from various anatomical sites, including the eyes, legs, nasal sinuses, nails and lower respiratory tract. Their pathogenicity still needs to be demonstrated and no cases of human disease by these fungi have been published yet.
The ITS locus has been widely used in the identification of plant pathogenic and clinically relevant fungi and recently has been proposed as a universal barcode marker for these organisms (Iwen et al. 2002, Schoch et al. 2012). It has been used by some authors to identify isolates of Curvularia from clinical samples and plants (Fryen et al. 1999, Bagyalakshmi et al. 2008, Dyer et al. 2008, Chowdhary et al. 2011, Funnell-Harris et al. 2013). This marker, however, is not optimal for species identification since it provided little resolution for closely related Curvularia species in our study. Similar results were published by da Cunha et al. (2012), in which an ITS tree gave < 70 % bs for clades representing C.spicifera and C. hawaiiensis, two of the main clinically relevant members of the genus. Other authors have found limited species resolution in ITS phylogenies of other members of the Pleosporales (Pryor & Gilbertson 2000, de Hoog & Horré 2002, Pryor & Bigelow 2003, Park et al. 2008, Brun et al. 2013), indicating that additional genes need to be used for reliable species identification in this group of fungi. Protein-coding loci have been reported to be phylogenetically more informative than rDNA in Ascomycota (Schoch et al. 2009) and this is confirmed here in Curvularia. In our work, species discrimination improved with the gpd and RPB2 loci, which revealed more than double the percentage of variable sites seen in ITS. These protein-coding loci are promising markers for future phylogenetic studies in Curvularia and related genera.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures) and Janneke Bloem (DNA isolation, amplification and sequencing of some of the isolates studied) for their invaluable assistance. The study has been economically supported in part by the Spanish Ministry of ‘Economía y Competitividad’, grant CGL 2011-27185.
REFERENCES
- Agarwal GP, Sahni VP. 1963. Curvularia verruciformis Agarwal & Sahni, a new fungus from Jabalpur (M.P.). Current Science 32: 276–277. [Google Scholar]
- Alcorn JL. 1983a. On the genera Cochliobolus and Pseudocochliobolus. Mycotaxon 16: 353–379. [Google Scholar]
- Alcorn JL. 1983b. Generic concepts in Drechslera, Bipolaris and Exserohilum. Mycotaxon 17: 1–86. [Google Scholar]
- Alcorn JL. 1988. The taxonomy of ‘Helminthosporium’ species. Annual Review of Phytopathology 26: 37–56. [Google Scholar]
- Alcorn JL. 1990. Additions to Bipolaris, Cochliobolus and Curvularia. Mycotaxon 39: 361–392. [Google Scholar]
- Alcorn JL. 1998. A new Cochliobolus species and its Curvularia anamorph. Proceedings of the Royal Society of Queensland 107: 1–4. [Google Scholar]
- Amaradasa BS, Madrid H, Groenewald JZ, Crous PW, Amundsen K. 2014. Porocercospora seminalis gen. et comb. nov. the causal organism of buffalograss false smut. Mycologia 106: 77–85. [DOI] [PubMed] [Google Scholar]
- Bagyalakshmi R, Therese KL, Prasanna S, Madhavan HN. 2008. Newer emerging pathogens of ocular nonsporulating molds (NSM) identified by polymerase chain reaction (PCR)-based DNA sequence technique targeting internal transcribed spacer (ITS) region. Current Eye Research 33: 139–147. [DOI] [PubMed] [Google Scholar]
- Barr ME. 1979. A classification of Loculoascomycetes. Mycologia 71: 935–957. [Google Scholar]
- Berbee ML, Pirseyedi M, Hubbard S. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91: 964–977. [Google Scholar]
- Boedijn KB. 1933. Über einige phragmosporen Dematiazen. Bulletin du Jardin Botanique de Buitenzorg 13: 120–134. [Google Scholar]
- Boerema GH, Hamers MEC. 1989. Check-list for scientific names of common parasitic fungi. Series 3b: Fungi on bulbs: Amaryllidaceae and Iridaceae. Netherlands Journal of Plant Pathology 95: 1–32 (Supplement 3). [Google Scholar]
- Brun S, Madrid H, Gerrits van den Ende B, Andersen B, Marinach-Patrice C, et al. 2013. Multilocus phylogeny and MALDI-TOF analysis of the plant pathogenic species Alternaria dauci and relatives. Fungal Biology 117: 32–40. [DOI] [PubMed] [Google Scholar]
- Chowdhary A, Randhawa HS, Singh V, Khan ZU, Ahmad S, et al. 2011. Bipolaris hawaiiensis as etiologic agent of allergic bronchopulmonary mycosis: first case in a pediatric patient. Medical Mycology 49: 760–765. [DOI] [PubMed] [Google Scholar]
- Cunha KC da, Sutton DA, Fothergill AW, Cano J, Gené J, et al. 2012. Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. Journal of Clinical Microbiology 50: 4061–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha KC da, Sutton DA, Fothergill AW, Gené J, Cano J, et al. 2013. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagnostic Microbiology and Infectious Disease 76: 168–174. [DOI] [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson TH. 2007. Compendium of soil fungi. 2nd ed IHW-Verlag, Germany. [Google Scholar]
- Dyer ZA, Wright RS, Rong IH, Jacobs A. 2008. Back pain associated with endobronchial mucus impaction due to Bipolaris australiensis colonization representing atypical allergic bronchopulmonary mycosis. Medical Mycology 46: 589–594. [DOI] [PubMed] [Google Scholar]
- Ebright JR, Chandrasekar PH, Marks S, Fairfax MR, Aneziokoro A, McGinnis MR. 1999. Invasive sinusitis and cerebritis due to Curvularia clavata in an immunocompetent adult. Clinical Infectious Diseases 28: 687–689. [DOI] [PubMed] [Google Scholar]
- Ellis MB. 1966. Dematiaceous Hyphomycetes VII: Curvularia, Brachysporium, etc. Mycological Papers 106: 1–57. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, United Kingdom. [Google Scholar]
- Ellis MB. 1976. More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, United Kingdom. [Google Scholar]
- Eriksson O. 1981. The families of bitunicate ascomycetes. Opera Botanica 60: 1–220. [Google Scholar]
- Faurel L, Schotter G. 1965. Champignons coprophiles du Tibesti. Revue Mycologique 30: 330–351. [Google Scholar]
- Fryen A, Mayser P, Glanz H, Füssle R, Breithaupt H, Hoog GS de. 1999. Allergic fungal sinusitis caused by Bipolaris (Drechslera) hawaiiensis. European Archives of Otorhinolaryngology 256: 330–334. [DOI] [PubMed] [Google Scholar]
- Funnell-Harris DL, Prom LK, Pedersen JF. 2013. Isolation and characterization of the grain mold fungi Cochliobolus and Alternaria spp. from sorghum using semiselective media and DNA sequence analysis. Canadian Journal of Microbiology 59: 87–96. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ. 2000. Atlas of clinical fungi. 2nd ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Hoog GS de, Horré R. 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45: 259–276. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Tanaka C, Tsuda M. 2003. Conidium morphology of Curvularia geniculata and allied species. Mycoscience 44: 227–237. [Google Scholar]
- Hughes SJ. 1979. Relocation of species of Endophragmia auct. with notes on relevant generic names. New Zealand Journal of Botany 17: 139–188. [Google Scholar]
- Hughes SJ. 1980. Endophragmiella cesatii. Fungi Canadenses 162: 1–2. [Google Scholar]
- Ismail Y, Johnson RH, Wells MV, Pusavat J, Douglas K, Arsura EL. 1993. Invasive sinusitis with intracranial extension caused by Curvularia lunata. Archives of Internal Medicine 153: 1604–1606. [PubMed] [Google Scholar]
- Iwen PC, Hinrichs SH, Rupp ME. 2002. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human pathogens. Medical Mycology 40: 87–109. [DOI] [PubMed] [Google Scholar]
- Jain BL. 1962. Two new species of Curvularia. Transactions of the British Mycological Society 45: 539–544. [Google Scholar]
- Jiang YL, Zhang TY. 2007. Notes on soil dematiaceous hyphomycetes. Mycosystema 26: 17–21. [Google Scholar]
- Kamalam A, Ajithadass K, Sentamilselvi G, Thambiah AS. 1992. Paronychia and black discoloration of a thumb caused by Curvularia lunata. Mycopathologia 118: 83–84. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Bennett JW. 1992. Medical Mycology. Lea & Febiger, USA. [Google Scholar]
- Lopes JO, Jobim NM. 1998. Dermatomycosis of the toe web caused by Curvularia lunata. Revista do Instituto de Medicina Tropical de São Paulo 40: 327–328. [DOI] [PubMed] [Google Scholar]
- Luttrell ES. 1963. Taxonomic criteria in Helminthosporium. Mycologia 55: 643–674. [Google Scholar]
- Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, et al. 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Diversity 56: 131–144. [Google Scholar]
- Mathur RL, Mathur BL. 1959. A new species of Curvularia from the leaves of Jasminum sambac. Current Science 28: 448–449. [Google Scholar]
- McGinnis MR, Rinaldi MG, Winn RE. 1986. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. Journal of Clinical Microbiology 24: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RR, Hodges CS. 1965. A new species of Curvularia with a protuberant conidial hilum. Mycologia 57: 822–825. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v. 2. Software distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. [Google Scholar]
- O’Donnell K, Sarver BAJ, Brandt M, Chang DC, Noble-Wang J, et al. 2007. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria including isolates from the 2005–06 multistate contact lens-associated U.S. keratitis outbreaks. Journal of Clinical Microbiology 45: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C, Berbee ML, Shoemaker RA, Loria R. 2000. Molecular phylogenetic support from ribosomal DNA sequences for origin of Helminthosporium from Leptosphaeria-like loculoascomycete ancestors. Mycologia 92: 736–746. [Google Scholar]
- Park MS, Romanosky CE, Pryor BM. 2008. A reexamination of the phylogenetic relationship between the causal agents of carrot black rot, Alternaria radicina and A. carotiincultae. Mycologia 100: 511–527. [DOI] [PubMed] [Google Scholar]
- Pryor BM, Bigelow DM. 2003. Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium and Stemphylium. Mycologia 95: 1141–1154. [DOI] [PubMed] [Google Scholar]
- Pryor BM, Gilbertson RL. 2000. Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycological Research 104: 1312–1321. [Google Scholar]
- Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clinical Microbiology Reviews 23: 884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist S, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences USA 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, et al. 2009. The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, Kendrick B. 2011. The genera of hyphomycetes. CBS Biodiversity Series 9. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Sivanesan A. 1984. The bitunicate ascomycetes and their anamorphs. Strauss & Kramer, Liechtenstein. [Google Scholar]
- Sivanesan A. 1987. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers 158: 154–185. [Google Scholar]
- Sun G, Oide S, Tanaka E, Shimizu K, Tanaka C, Tsuda M. 2003. Species separation in Curvularia ‘geniculata’ group inferred from Brn1 gene sequences. Mycoscience 44: 239–244. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon RN, Bilgrami KS. 1962. A new pathogenic species of genus Curvularia. Current Science 31: 254. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX Windows interface: flexible strategies for multiple alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Ueyama A. 1981. Pseudocochliobolus australiensis, the ascigerous state of Bipolaris australiensis. Mycologia 73: 88–96. [Google Scholar]
- Tsuda M, Ueyama A, Nishihara N, 1977. Pseudocochliobolus nisikadoi, the perfect stage of Helminthosporium coicis. Mycologia 69: 1109–1120. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococcus. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In: Gelfand M, Sninsky JI, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, USA. [Google Scholar]
- Yanagihara M, Kawasaki M, Ishizaki H, Anzawa K, Udagawa S, et al. 2010. Tiny keratotic brown lesions of the interdigital web between the toes of a healthy man caused by Curvularia species infection and a review of cutaneous Curvularia infections. Mycoscience 51: 224–233. [Google Scholar]
- Zhang M, Zhang TY, Wu YM. 2004. A new name and a new variety in Curvularia. Mycosystema 23: 177–178. [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. 2012. Pleosporales. Fungal Diversity 53: 1–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Crous PW, Gruyter J de, Woudenberg JHC, et al. 2009. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]