Abstract
Following the abolishment of dual nomenclature, Stilbospora is recognised as having priority over Prosthecium. The type species of Stilbospora, S. macrosperma, is the correct name for P. ellipsosporum, the type species of Prosthecium. The closely related genus Stegonsporium is maintained as distinct from Stilbospora based on molecular phylogeny, morphology and host range. Stilbospora longicornuta and S. orientalis are described as new species from Carpinus betulus and C. orientalis, respectively. They differ from the closely related Stilbospora macrosperma, which also occurs on Carpinus, by longer, tapering gelatinous ascospore appendages and by distinct LSU, ITS rDNA, rpb2 and tef1 sequences. The asexual morphs of Stilbospora macrosperma, S.longicornuta and S. orientalis are morphologically indistinguishable; the connection to their sexual morphs is demonstrated by morphology and DNA sequences of single spore cultures derived from both ascospores and conidia. Both morphs of the three Stilbospora species on Carpinus are described and illustrated. Other species previously recognised in Prosthecium, specifically P.acerophilum, P. galeatum and P. opalus, are determined to belong to and are formally transferred to Stegonsporium. Isolates previously recognised as Stegonsporium pyriforme (syn. Prosthecium pyriforme) are determined to consist of three phylogenetically distinct lineages by rpb2 and tef1 sequence data, two of which are described as new species (S. protopyriforme, S. pseudopyriforme). Stegonsporium pyriforme is lectotypified and this species and Stilbospora macrosperma are epitypified. Based on DNA sequence data, the North American Stegonsporium acerophilum is recorded from Europe for the first time, and new hosts from Acer sect. Acer are reported for S. opalus and S. pyriforme. Stilbospora and Stegonsporium are classified within the revived family Stilbosporaceae. Prosthecium appendiculatum, P. auctum and P. innesii are shown to be unrelated to the Stilbosporaceae and are recognised in three distinct genera, Phaeodiaporthe appendiculata, Alnecium auctum n. gen. and Calosporella innesii within Diaporthaceae, Gnomoniaceae and Sydowiellaceae, respectively. The generic types of these three monotypic genera are briefly described, illustrated and lecto- and epitypfied.
Keywords: Alnecium, Calospora, Calosporella, ITS, LSU, molecular phylogeny, Phaeodiaporthe, rpb2, systematics, tef1
INTRODUCTION
Wehmeyer (1941) gave an account of the diaporthalean genus Prosthecium Fresen. 1862, basically characterised by inconspicuous or light-coloured ectostromatic discs, scant prosenchymatous entostroma and large, several-celled, appendaged ascospores and several-celled conidia, with two subgenera. Later Barr (1978) separated species of the subgenus Pseudoprosthecium, distinguished by elongate ascospore appendages, as Hapalocystis Auersw. ex Fuckel (see also Jaklitsch & Voglmayr 2004). While the asexual genus Stilbospora had been widely regarded as being linked to Prosthecium (Winter 1887, Petrak 1923, Barr 1978), the genus Stegonsporium was long thought to be the asexual morph of the pleosporalean genus Splanchnonema (Kirk et al. 2001). However, Voglmayr & Jaklitsch (2008) confirmed Stilbospora macrosperma Pers. as the asexual morph of Prosthecium ellipsosporum, and they clearly showed that also Stegonsporium belongs to Prosthecium.
In their account of Prosthecium, Voglmayr & Jaklitsch (2008) re-defined the genus Prosthecium, confining it to parasites of Carpinus with Stilbospora asexual morphs and Acer with Stegonsporium asexual morphs, respectively. They documented Prosthecium ellipsosporum from Carpinus betulus, and described five species of Prosthecium with Stegonsporium asexual morphs from Acer, concluding that they were highly host specific, being mostly confined to a single host species. In addition, two distinct species of Prosthecium having Stegonsporium asexual morphs were each found to co-occur on the European Acer pseudoplatanus and on the North American Acer saccharum. All hosts of the maple-inhabiting species were revealed to belong to Acer section Acer.
Recent changes of the International Code of Nomenclature (ICN) for unified nomenclature raised the question of appropriate generic classification of the species currently classified within Prosthecium. Stilbospora macrosperma Pers., the type species of Stilbospora Pers. 1801, was confirmed as the asexual morph of Prosthecium ellipsosporum, the generic type of Prosthecium, by Voglmayr & Jaklitsch (2008), thus these genera are synonyms. Because the genus Stilbospora Pers. (Persoon 1801) is older than Prosthecium, the use of Prosthecium would require conservation (Crous et al. 2012). As the asexual morph placed in Stilbospora is more common and conspicuous than the sexual morph, it seems practical to follow the principle of priority and recognise Stilbospora over Prosthecium.
Stilbospora and Stegonsporium are closely related genera both of which have sexual morphs that have been placed in Prosthecium (Voglmayr & Jaklitsch 2008). Both share similar acervular conidiomata with simple, hyaline paraphyses and hyaline, cylindrical, septate conidiophores, annellidic conidiogenous cells and brown, septate conidia with a hyaline sheath. Stilbospora is characterised by ellipsoid to oblong euseptate ascospores and conidia with usually three transverse eusepta, whereas Stegonsporium has mostly pyriform conidia with 2–7 transverse and 1–3 longitudinal distosepta, and also the ascospores are distoseptate (Voglmayr & Jaklitsch 2008). Since 2008, numerous additional Stegonsporium collections from various Acer species were studied to provide additional data on distribution and host specificity. Of special interest were trees of the North American Acer saccharum and A. grandidentatum grown in European parks to evaluate the high host specificity revealed in Voglmayr & Jaklitsch (2008). In addition, southern European Acer species not yet investigated for their Stegonsporium parasites were sampled to re-assess host ranges. All accessions were cultured and characterised by means of DNA sequence data.
Stilbospora macrosperma (syn. Prosthecium ellipsosporum) was found to be a common fungus on Carpinus betulus in Europe, and numerous fresh collections of S. macrosperma were made and examined (Voglmayr & Jaklitsch 2008). In one of these collections, long, tapering ascospore appendages were observed, which strongly deviated from the short, ellipsoid ascospore appendages of S. macrosperma, and such specimens were subsequently recollected several times from the same site. Collections from the south-eastern European Carpinus orientalis proved to be significantly different from S. macrosperma in having ascospore appendages that are blunt and tapering similarly to those in Stegonsporium galeatum. Of these collections, cultures were obtained from both ascospores and conidia for pure culture and DNA studies, which revealed two distinct species closely related to S. macrosperma.
Relegation of Prosthecium into synonymy with Stilbospora also raises the problem of proper generic classification of other species currently classified in Prosthecium. In the most recent taxonomic revision of Prosthecium, Barr (1978) accepted P.appendiculatum, P. auctum, P. innesii (as P. platanoides), P. acrocystis and P. stylosporum in addition to the generic type P. ellipsosporum. We collected fresh material of the first three species and included them in our phylogenetic analyses to reveal their phylogenetic affinities. They proved to be unrelated to Prosthecium ellipsosporum. Accordingly, we dispose them in three distinct genera belonging to three different families below.
MATERIALS AND METHODS
Sample sources
Collection data, hosts, herbarium, culture and GenBank accession numbers of the specimens used for phylogenetic analyses are provided in Table 1. Single spore isolates were prepared and grown on 2 % malt extract agar (MEA; 2 % w/v malt extract, 2 % w/v agar-agar; Merck, Darmstadt, Germany). Details of the specimens used for morphological investigations are listed in the Taxonomy section after the respective descriptions.
Table 1.
Hosts, origin, herbarium, culture and GenBank accession numbers of the specimens used for phylogenetic analyses. For details on collection data, see Voglmayr & Jaklitsch (2008) and lists of specimens examined. Taxa, hosts and origins in bold denote new species, hosts and origins, respectively, for the species. Type species marked by an asterisk (*); E = epitype, H = holotype, N = neotype.
| Taxon | Host | Origin | Voucher, culture number | Type | GenBank accession no. | |||
|---|---|---|---|---|---|---|---|---|
| LSU | ITS | tef1 | rpb2 | |||||
| Alnecium auctum* | Alnus glutinosa | Austria | WU 30206, PAT = CBS 124263 (ex teleomorph) | E | KF570154 | KF570154 | KF570200 | KF570170 |
| Phaeodiaporthe appendiculata* | Acer campestre | Austria | WU32448, D76 = CBS 123809 (ex teleomorph) | KF570155 | KF570155 | |||
| Acer campestre | Austria | WU32449, D77 = CBS 123821 (ex teleomorph) | E (Diaporthe appendiculata, Phaeodiaporthe keissleri) | KF570156 | KF570156 | |||
| Stegonsporium acerinum | Acer saccharum | Canada | WU 28047, D43 = CBS 120525 (ex teleomorph) | E | EU039996 | EU039968 | EU040024 | |
| Acer saccharum | Canada | WU 28047, D42 = CBS 120524 (ex anamorph) | E | EU039995 | EU039969 | EU040023 | KF570171 | |
| S. acerophilum | Acer grandidentatum | UK, England | WU 32468, D65 = CBS 125042 (ex anamorph) | KF570202 | KF570174 | |||
| Acer saccharum | Canada | WU 28048, D44 = CBS 120602 (ex teleomorph) | EU039981 | EU040029 | ||||
| Acer saccharum | Canada | WU 28049, D45 = CBS 120601 (ex anamorph) | EU040030 | KF570172 | ||||
| Acer saccharum | Czech Republic | WU 32465, D81 = CBS 125028 (ex anamorph) | KF570157 | KF570157 | KF570203 | |||
| Acer saccharum | UK, England | WU 32467, D64 = CBS 125033 (ex anamorph) | KF570201 | |||||
| Acer saccharum | USA | WU 28050, D5 = CBS 117025 (ex teleomorph) | E | EU039993 | EU039982 | EU040027 | KF570173 | |
| Acer saccharum | USA | WU 28050, D6 = CBS 117026 (ex anamorph) | E | EU039994 | EU039985 | EU040028 | ||
| Acer saccharum | USA | WU 28051, D23 = CBS 117035 (ex anamorph) | EU039984 | EU040026 | ||||
| Acer saccharum | USA | WU 28052, D24 = CBS 117036 (ex anamorph) | EU039983 | EU040025 | ||||
| S. galeatum | Acer heldreichii | UK, Scotland | WU 32469, D70 = CBS 125035 (ex anamorph) | KF570204 | KF570176 | |||
| Acer pseudoplatanus | Austria | WU 28055, D38 = CBS 119744 (ex anamorph) | EU040012 | |||||
| Acer pseudoplatanus | Austria | WU 28056, D41 = CBS 120523 (ex teleomorph) | E | EU040013 | KF570175 | |||
| Acer pseudoplatanus | Austria | WU 28057, D1 (culture lost) (ex teleomorph) | EU039988 | EU039966 | EU040014 | |||
| Acer pseudoplatanus | Austria | WU 28058, D3 = CBS 117024 (ex teleomorph) | EU039989 | EU039967 | EU040011 | |||
| S. opalus | Acer hyrcanum | Austria | WU 32783, D59 = CBS 124485 (ex anamorph) | KF570205 | ||||
| Acer monspessulanum | France | WU 32470, PR15 (ex anamorph) | KF935260 | KF570210 | KF935261 | |||
| Acer obtusatum | Austria | WU 28059, D40 = CBS 120603 (ex anamorph) | EU039990 | EU040018 | KF570177 | |||
| Acer obtusatum | Croatia | WU 28060, D52 = CBS 121690 (ex teleomorph) | EU040022 | KF570179 | ||||
| Acer obtusatum | Italy | WU 28061, D51 = CBS 121691 (ex teleomorph) | EU040021 | |||||
| Acer obtusatum | Slovenia | WU 28062, D48 = CBS 120598 (ex teleomorph) | H | EU039997 | EU039980 | EU040020 | ||
| Acer obtusatum | Slovenia | WU 28062, D47 = CBS 120599 (ex anamorph) | H | EU039979 | EU040019 | KF570178 | ||
| Acer obtusatum | UK, England | WU 32761, D63 = CBS 125032 (ex anamorph) | KF570207 | |||||
| Acer opalus | France | WU 28241, D60 = CBS 124474 (ex anamorph) | KF570206 | KF570180 | ||||
| Acer opalus | UK, England | WU 32762, D69 = CBS 125034 (ex anamorph) | KF570208 | KF570181 | ||||
| Acer sempervirens | Greece | WU 32763, D89 (ex anamorph) | KF570158 | KF570158 | KF570209 | KF570182 | ||
| S. protopyriforme | Acer pseudoplatanus | Austria | WU 28064, D29 = CBS 117040 (ex teleomorph) | H | EU039991 | EU039977 | EU040016 | |
| Acer pseudoplatanus | Austria | WU 28064, D30 = CBS 117041 (ex anamorph) | H | EU039992 | EU039976 | EU040017 | ||
| Acer pseudoplatanus | Austria | WU 28067, D10 = CBS 117030 (ex anamorph) | EU039978 | EU040015 | KF570183 | |||
| Acer pseudoplatanus | Austria | WU 32765, PR5 (ex anamorph) | KF570214 | |||||
| Acer pseudoplatanus | Austria | WU 32764, PR9 (ex anamorph) | KF570215 | |||||
| Acer pseudoplatanus | Austria | WU 32766, PR10 (ex anamorph) | KF570213 | |||||
| Acer pseudoplatanus | Czech Republic | WU 32767, D80 = CBS 124480 (ex anamorph) | KF570212 | KF570184 | ||||
| Acer pseudoplatanus | UK, Scotland | WU 32768, D61 = CBS 125030 (ex anamorph) | KF570211 | |||||
| S. pseudopyriforme | Acer heldreichii | UK, England | WU 32772, D67 = CBS 125044 (ex anamorph) | KF570216 | KF570186 | |||
| Acer pseudoplatanus | Austria | WU 28072, D7 = CBS 117027 (ex anamorph) | EU039974 | EU040007 | ||||
| Acer pseudoplatanus | Austria | WU 28063, D8 = CBS 117028 (ex anamorph) | EU039973 | EU040008 | KF570187 | |||
| Acer pseudoplatanus | Austria | WU 28066, D9 = CBS 117029 (ex anamorph) | EU039975 | EU040009 | ||||
| Acer pseudoplatanus | Austria | WU 28065, D50 = CBS 120597 (ex anamorph) | EU040010 | |||||
| Acer pseudoplatanus | Austria | WU 32769, D72 = CBS 120597 (ex anamorph) | H | KF570159 | KF570159 | KF570218 | ||
| Acer pseudoplatanus | Austria | WU 32770, PR4 (ex anamorph) | KF570219 | |||||
| Acer pseudoplatanus | Austria | WU 32771, PR7 (ex anamorph) | KF570220 | |||||
| Acer pseudoplatanus | Slovenia | WU 28074, D49 = CBS 120526 (ex anamorph) | EU040006 | KF570185 | ||||
| Acer velutinum | UK, England | WU 32773, D68 = CBS 125045 (ex anamorph) | KF570217 | |||||
| S. pyriforme* | Acer heldreichii | UK, England | WU 32781, D85 = CBS 124487 (ex anamorph) | KF570160 | KF570160 | KF570223 | KF570190 | |
| Acer monspessulanum | Croatia | WU 32774, D87 (ex anamorph) | KF570161 | KF570161 | KF570224 | KF570191 | ||
| Acer pseudoplatanus | Austria | WU 28069, D2 = CBS 117023 (ex teleomorph) | EU039987 | EU039971 | EU040001 | |||
| Acer pseudoplatanus | Austria | WU 28070, D11 = CBS 117031 (ex anamorph) | EU039972 | EU040004 | KF570188 | |||
| Acer pseudoplatanus | Austria | WU 28071, D22 = CBS 117034 (ex anamorph) | EU039970 | EU040005 | ||||
| Acer pseudoplatanus | Austria | WU 28068, D39 = CBS 120522 (ex teleomorph) | E (Stegonsporium pyriforme, Prosthecium pyriforme) | EU040003 | ||||
| Acer pseudoplatanus | Denmark | WU 28073, D46 = CBS 120600 (ex teleomorph) | EU040002 | KF570189 | ||||
| Acer pseudoplatanus | France | WU 32778, PR2 (ex anamorph) | KF570228 | |||||
| Acer pseudoplatanus | France | WU 32777, PR11 (ex anamorph) | KF570226 | |||||
| Acer pseudoplatanus | Italy | WU 32775, PR6 (ex anamorph) | KF570229 | |||||
| Acer pseudoplatanus | Italy | WU 32776, PR12 (ex anamorph) | KF570227 | |||||
| Acer pseudoplatanus | UK, England | WU 32779, D62 = CBS 125031 (ex anamorph) | KF570221 | |||||
| Acer pseudoplatanus | UK, England | WU 32780, D66 = CBS 125043 (ex anamorph) | KF570222 | |||||
| Acer pseudoplatanus | UK, England | WU 32782, PR1 (ex anamorph) | KF570225 | |||||
| Stilbospora longicornuta | Carpinus betulus | Austria | WU 32453, D32 (ex anamorph) | KF570162 | ||||
| Carpinus betulus | Austria | WU 32452, D33 = CBS 118180 (ex anamorph) | KF570163 | KF570230 | KF570192 | |||
| Carpinus betulus | Austria | WU 32452, D34 = CBS 118396 (ex teleomorph) | KF570231 | KF570193 | ||||
| Carpinus betulus | Austria | WU 32450, D71 = CBS 122529 (ex teleomorph) | H | KF570164 | KF570164 | KF570232 | KF570194 | |
| S. macrosperma* | Carpinus betulus | Austria | WU 24708, D25 = CBS 115073 (ex teleomorph) | E | EU039965 | EU039999 | KF570195 | |
| Carpinus betulus | Austria | WU 28053, D53 = CBS 121692 (ex anamorph) | EU039986 | EU039998 | ||||
| Carpinus betulus | Austria | WU 28054, D54 = CBS 121693 (ex anamorph) | EU040000 | |||||
| Carpinus betulus | Austria | WU 32456, D55 = CBS 121882 (ex anamorph) | KF570233 | |||||
| Carpinus betulus | Austria | WU 32457, D56 = CBS 121694 (ex anamorph) | KF570234 | |||||
| Carpinus betulus | Austria | WU 32458, D57 = CBS 121883 (ex anamorph) | KF570235 | KF570196 | ||||
| Carpinus betulus | Netherlands | WU 27695, D58 = CBS 121695 (ex anamorph) | KF570236 | |||||
| Carpinus betulus | UK, England | WU 32460, D86 (ex anamorph) | KF570165 | KF570165 | ||||
| S. orientalis | Carpinus orientalis | Croatia | WU 31858, D92 (ex anamorph) | KF570166 | KF570166 | |||
| Carpinus orientalis | Greece | WU 32462, D90 = CBS 135075 (ex teleomorph) | H | KF570167 | KF570167 | KF570237 | KF570197 | |
| Carpinus orientalis | Greece | WU 32463, D91 (ex anamorph) | KF570168 | KF570168 | KF570238 | KF570198 | ||
| Carpinus orientalis | Montenegro | WU 32464, D93 (ex anamorph) | KF570169 | KF570169 | KF570239 | KF570199 | ||
Morphology
Morphological observations and measurements were carried out on a stereo-microscope and after mounting in tap water or 3 % KOH on a compound microscope using Nomarski differential interference contrast (DIC). Images were recorded with a Zeiss AxioCam ICc3 digital camera. Measurements are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses.
DNA extraction, PCR and sequencing
DNA extraction follows the procedure described in Voglmayr & Jaklitsch (2008). For the collections marked with PR in Table 1, DNA was directly extracted from conidiomata using the protocol described in Voglmayr & Jaklitsch (2011). The D1, D2 region of the LSU rDNA region was amplified with primers LR0R (Moncalvo et al. 1995) and TW14 (White et al. 1990), and the complete ITS rDNA region with primers ITS4 and ITS5 (White et al. 1990). Alternatively, a c. 1.6 kb fragment of partial nuSSU-complete ITS-partial LSU was amplified with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990). A c. 1.3 kb fragment of the tef1 (translation elongation factor 1 alpha) gene was amplified with primers EF1728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2006). A c. 1 kb fragment of RNA polymerase II subunit B (rpb2) was amplified using the primer pair fRPB2-5f and fRPB2-7cr (Liu et al. 1999). PCR products were purified using the enzymatic PCR cleanup described in Werle et al. (1994) according to Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington) with the same primers as in PCR and an automated DNA sequencer (ABI 3130xl or 3730xl Genetic Analyzer, Applied Biosystems); in the partial SSU-ITS-partial LSU fragment the additional primers ITS4 and LR3 (Vilgalys & Hester 1990) were used.
Data analysis
To reveal the phylogenetic position of the new species of Stilbospora and the diverse species formerly classified within Prosthecium, a phylogenetic analysis was performed with LSU rDNA sequences. Sequences of representative species of Diaporthales were selected from Castlebury et al. (2002) and supplemented with sequences from GenBank; two accessions of Gaeumannomyces (Magnaporthaceae) were included as outgroup. GenBank accession numbers are given in the phylogenetic tree (Fig. 1). For a more detailed analysis of the phylogenetic relationships of Stilbospora, Stegonsporium and Prosthecium appendiculatum and to test the ability of the ITS for species delimitation, an ITS rDNA matrix was analysed, including a representative sample of Diaporthe species selected from Gomes et al. (2013) and Chrysoporthe as outgroup. For detailed investigations of species relationships and delimitation within Stilbospora and Stegonsporium, rpb2 and tef1 sequences of a representative sample were separately analysed, with Melanconiella sequences from Voglmayr et al. (2012) as outgroup. Melanconiella was selected as outgroup for the rpb2 and tef1 matrices, because it was the phylogenetically closest group for which verified sequences were available covering the complete sequence range used in the current phylogenetic analyses. The GenBank accession numbers of sequences used in the phylogenetic analyses of ITS, tef1 and rpb2 are given in Table 1. To determine the phylogenetic position of Prosthecium auctum within Gnomoniaceae, a slightly reduced multigene matrix (ITS, LSU, rpb2, tef1) from Sogonov et al. (2008) was used, with Melanconis selected as outgroup; for the GenBank accession numbers see table 1 of Sogonov et al. (2008).
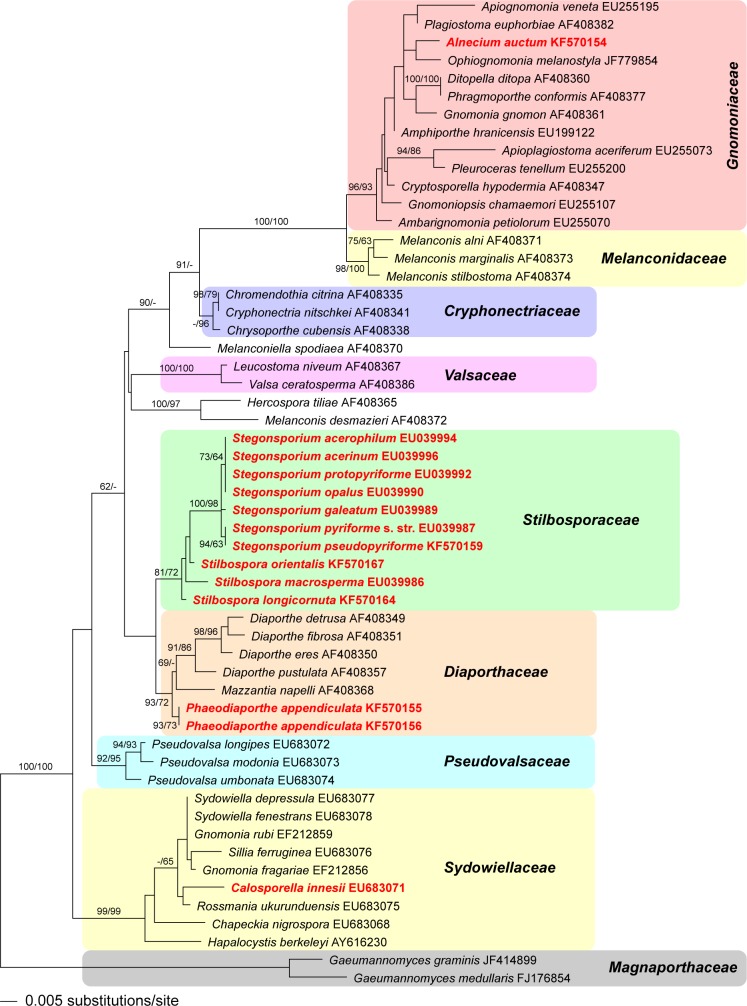
Fig. 1.
Phylogram of the best ML tree (lnL = −5619.238224) revealed by RAxML from an analysis of the LSU matrix of selected Diaporthales, showing the phylogenetic position of taxa formerly classified within Prosthecium (marked in red). ML and MP bootstrap support values above 60 % are given at the first and second position, respectively, above or below the branches. GenBank accession numbers are given following the taxon names. Note the polyphyly of the genus Prosthecium, with its generic type, P. ellipsosporum, corresponding to Stilbospora macrosperma within Stilbosporaceae. For the other former Prosthecium species the new generic names are given in the tree (Alnecium, Phaeodiaporthe and Calosporella).
All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit v. 7.0.4.1 (Hall 1999). After the exclusion of excessive leading and trailing gap regions, the LSU matrix contained 1 687 characters. The ITS, tef1, rpb2 and combined data matrices contained 561, 1 450, 1 177 and 3 361 characters, respectively.
Maximum parsimony (MP) analyses were performed with PAUP v. 4.0 b10 (Swofford 2002), using 1 000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, COLLAPSE = MAXBRLEN, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. Bootstrap analysis with 1 000 replicates was performed in the same way, but using 10 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate; in addition, each replicate was limited to 1 million rearrangements in the LSU, ITS, tef1 and the combined analyses. To identify how many additional steps are required to reveal Stilbospora as a monophyletic clade in the LSU analyses, the MP heuristic search was repeated with the same settings, but applying the constraint that Stilbospora and Stegonsporium are monophyletic sister groups.
For maximum likelihood (ML) analyses, 500 rounds of random addition of sequences as well as 1 000 fast bootstrap replicates were computed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro & Michalak 2012), using the GTRGAMMA and GTRCAT models of sequence substitution, respectively. For the multigene analyses, partitioned substitution models were implemented for each gene. The final matrices used for phylogenetic analyses were deposited in TreeBASE (http://www.treebase.org) and are available under http://purl.org/phylo/treebase/phylows/study/TB2:S14652.
RESULTS
Molecular phylogenetic analyses
Of the 1 687 characters included in the LSU analyses, 187 were parsimony informative. MP analyses revealed 290 MP trees of 583 steps (not shown). The ML analyses revealed a tree of lnL = −5619.2382, which is shown as phylogram in Fig. 1, with ML and MP bootstrap support above 60 % given at first and second position above/below the branches. Tree topologies between the strict consensus tree of the MP and the ML tree are largely compatible; minor differences concern a few non-supported nodes in the medium part of the tree backbone: MP analyses (not shown) reveal the sequential arrangement Pseudovalsaceae-Hercospora tiliae/Melanconis desmazieri clade - Diaporthaceae-Stilbosporaceae-Valsaceae-Melanconiella, while the topology of the remaining clades is compatible with the ML tree. In addition, there are a few minor topological differences of non-supported nodes within Sydowiellaceae and Gnomoniaceae. In both MP and ML analyses, monophyly of the Stegonsporium-Stilbospora clade is moderately supported. While Stegonsporium is revealed as a monophyletic lineage with high to maximum support (100 % / 98 % ML / MP bootstrap support), Stilbospora is revealed as a paraphyletic lineage basal to the Stegonsporium clade in both MP and ML analyses, however without bootstrap support, and only a single additional step (584 steps altogether) is required to reveal Stilbospora as a monophyletic sister group to Stegonsporium (data not shown).
The three additional species included in the study currently classified within Prosthecium but belonging elsewhere were revealed to be unrelated to the Stegonsporium-Stilbospora clade (Fig. 1). Prosthecium auctum was found to belong to the Gnomoniaceae, where its closest relatives remain unclear. Prosthecium appendiculatum is placed within Diaporthaceae close to Diaporthe, whereas Prosthecium innesii is placed within Sydowiellaceae (Fig. 1).
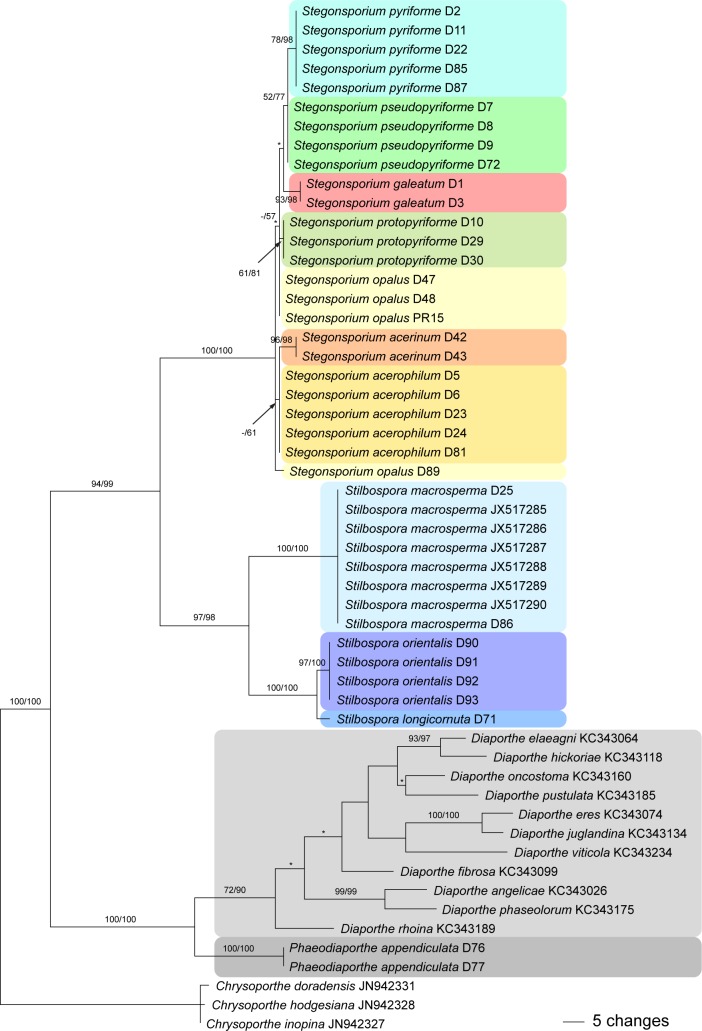
The ITS matrix contained 561 characters, of which 187 were parsimony informative. MP analyses revealed 24 MP trees of 484 steps, one of which is shown as phylogram in Fig. 2, with MP and ML bootstrap support above 50 % given at first and second position, respectively, above/below the branches. Tree topologies of the backbone of all MP trees were identical except for minor differences of topologies without bootstrap support within Diaporthe and Stegonsporium (nodes marked by an asterisk in Fig. 2). Stilbospora and Stegonsporium were revealed as highly supported monophyletic lineages (97–100 % bootstrap support), and sister group relationship of the two genera received high support as well. Within Stilbospora, sister group relationship of S. macrosperma to the highly supported S. longicornuta / S. macrosperma clade received maximum support. Contrarily, resolution of the ITS trees was comparatively low within Stegonsporium, where only S. acerinum, S. galeatum, S. pyriforme and S. protopyriforme were revealed as monophyletic lineages.
Fig. 2.
One of 24 phylograms of length 484 revealed by an MP analysis of 561 characters of the ITS alignment of Phaeodiaporthe appendiculata, Diaporthe spp., Stegonsporium and Stilbospora, with Chrysoporthe as outgroup. MP and ML bootstrap support values above 50 % are given at the first and second position, respectively, above or below the branches. Asterisks (*) denote nodes collapsed in the strict consensus tree of all MP trees.
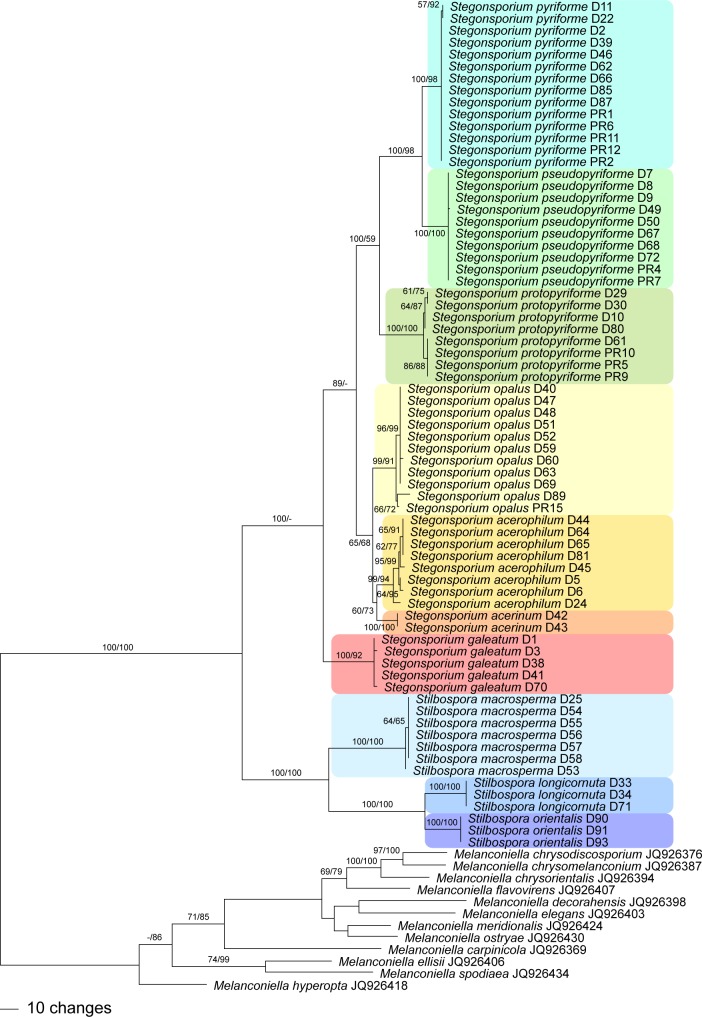
The tef1 matrix contained 1 450 characters, of which 474 were parsimony informative. MP analyses revealed 112 MP trees of 1 318 steps, one of which is shown as phylogram in Fig. 3, with MP and ML bootstrap support above 55 % given at first and second position, respectively, above/below the branches. Tree topologies of the backbone of all MP trees were identical except for minor differences of topologies without bootstrap support within the species. The ML analyses revealed a tree of –ln = 8378.8858, the topology of which was fully compatible with the MP strict consensus tree except for minor differences lacking bootstrap support within the outgroup (Melanconiella spp.; data not shown).
Fig. 3.
One of 112 phylograms of length 1 318 revealed by an MP analysis of 1 450 characters of the tef1 alignment of Stegonsporium and Stilbospora, with Melanconiella as outgroup. The backbone of all MP trees was identical, and minor topological differences were observed only within the highly supported terminal clades. MP and ML bootstrap support values above 55 % are given at the first and second position, respectively, above or below the branches.
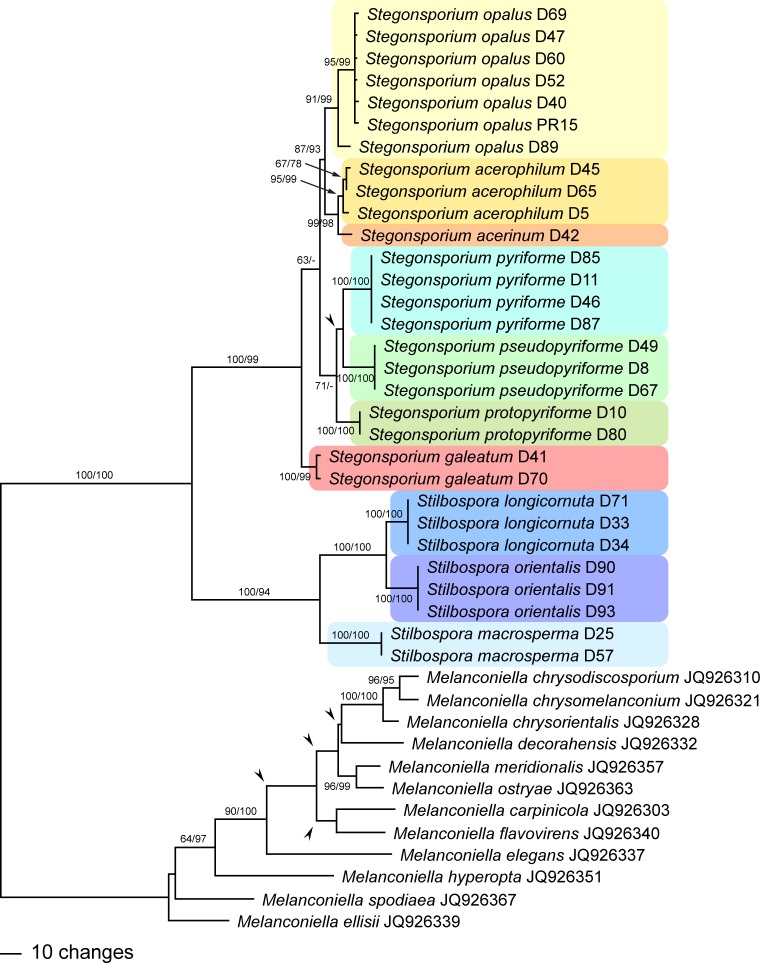
The rpb2 matrix contained 1 177 characters, of which 380 were parsimony informative. MP analyses revealed ten MP trees of 865 steps, one of which is shown as phylogram in Fig. 4, with MP and ML bootstrap support above 60 % given at first and second position, respectively, above/below the branches. The ML analyses revealed a tree of –ln = 5871.9762, the topology of which, apart from minor topological differences without bootstrap support within the outgroup (Melanconiella), differed from the MP strict consensus tree in the placement of the three species of the S. pyriforme s.l. clade. They did not form a monophylum, but were paraphyletically placed at the base of the Stegonsporium clade (i.e., S. pyriforme, then S. pseudopyriforme, then S. protopyriforme; data not shown). However, this placement did not receive any ML bootstrap support.
Fig. 4.
One of 10 phylograms of length 865 revealed by an MP analysis of 1 177 characters of the rpb2 alignment of Stegonsporium and Stilbospora, with Melanconiella as outgroup. Arrowheads denote nodes collapsing in the strict consensus tree of all MP trees. MP and ML bootstrap support values above 60 % are given at the first and second position, respectively, above or below the branches.
Both tef1 and rpb2 analyses revealed fully compatible phylogenetic relationships within the Stegonsporium-Stilbospora lineage. Sister group relationship of the genera Stegonsporium and Stilbospora received maximum bootstrap support (Fig. 3, 4). Within Stilbospora, S. longicornuta is sister species to S. orientalis, and both species are sister clade of S. macrosperma, all with maximum bootstrap support. Stegonsporium acerophilum is sister species of S. acerinum, and both species are sister clade of S. opalus, and all three form a sister group relationship with the three cryptic species of the S. pyriforme s.l. clade. Stegonsporium galeatum is sister species to all other Stegonsporium species, which receives significant bootstrap support only in the MP analyses (89 % in tef1, 63 % in rpb2). Monophyly of the S. pyriforme s.l. clade reveals maximum (MP) or low (59 %, ML) bootstrap support in the tef1 analyses (Fig. 3), while support is low in the rpb2 analyses (71 % MP bootstrap support, Fig. 4). In addition, the rpb2 MP strict consensus tree reveals a polytomy of the three cryptic species (S. protopyriforme, S. pseudopyriforme and S. pyriforme).
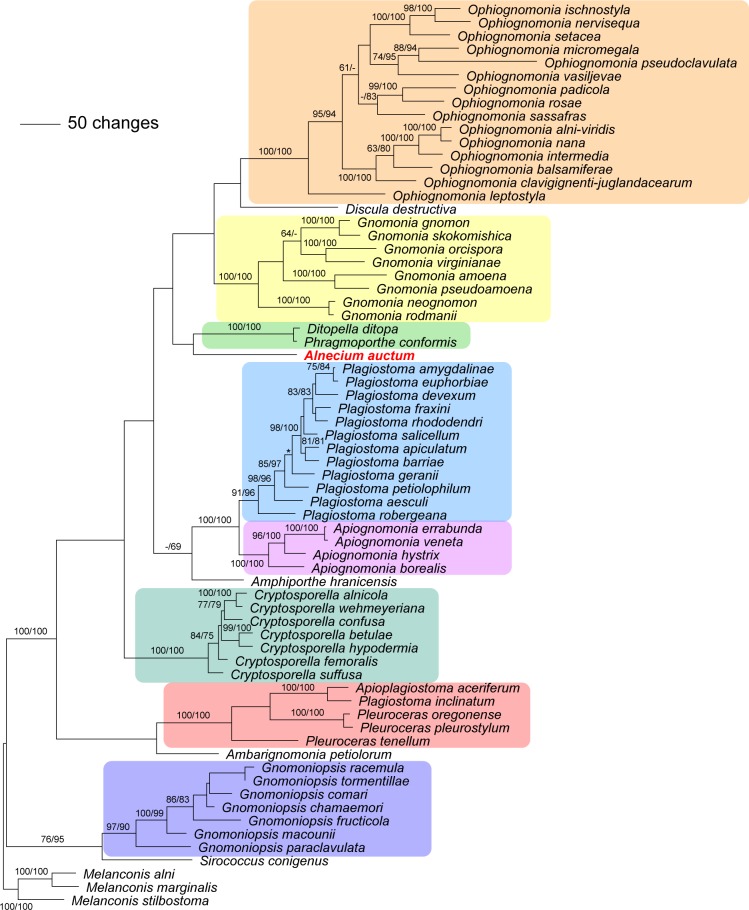
The combined matrix used for phylogenetic analyses of Gnomoniaceae contained 3 361 characters (581 from ITS, 1 220 from LSU, 1 089 from rpb2 and 471 from tef1), of which 789 were parsimony informative. MP analyses revealed three MP trees of 4 870 steps, one of which is shown as phylogram in Fig. 5, with MP and ML bootstrap support above 60 % given at first and second position above/below the branches. The MP trees differed slightly in the position of Plagiostoma petiolophilum and P. geranii. The ML analyses revealed a tree of –ln = 27717.1585, the topology of which differed in the unsupported deeper nodes but was compatible with the MP tree in the nodes receiving significant bootstrap support (data not shown). In the MP analyses, Alnecium auctum was sister to the Ditopella ditopa/Phragmoporthe conformis clade (Fig. 5), whereas in the ML analyses it was basal to the Amphiporthe /Apiognomonia/Plagiostoma clade (not shown); however, none of these placements received bootstrap support.
Fig. 5.
One of three phylograms of length 4 870 revealed by an MP analysis of 3 361 characters of the combined ITS-LSU-rpb2-tef1 alignment of Gnomoniaceae, with Melanconis as outgroup, showing the phylogenetic position of Alnecium auctum. MP and ML bootstrap support values above 60 % are given at the first and second position, respectively, above or below the branches. The asterisk (*) denotes the node collapsed in the strict consensus tree of all MP trees.
Taxonomy
Stilbosporaceae Link [as ‘Stilbosporei’], Abh. Königl. Akad. Wiss. Berlin 1824: 180. 1826, emend.
Accepted genera: Stilbospora Pers. (type genus), Stegonsporium Corda.
Family of Diaporthales. Pseudostromata inconspicuous, immersed in bark of trees and shrubs. Ostioles inconspicuous, convergent in groups, not projecting. Ectostromatic disc absent or inconspicuous and light-coloured, rarely brown. Entostroma prosenchymatous, pale-coloured, scarcely differentiated from the surrounding bark tissue. Perithecia loosely disposed or crowded in valsoid groups in a single layer, black. Centrum of broad multiguttulate, collapsing bands. Asci first sessile, becoming free, containing 8 ascospores, with or without a more or less cylindrical, slightly refractive canal in the apex; walls thick, appearing bitunicate. Ascospores ellipsoid to oblong, brown, with several eu- or distosepta, sometimes with one oblique or longitudinal septum in one to several cells; with a gelatinous appendage at each end. Conidiomata acervular, with paraphyses. Conidiophores cylindrical, hyaline. Conidiogenous cells annellidic. Conidia brown, cylindrical, clavate to pyriform, with several eu- or distosepta, with or without oblique or longitudinal septa, surrounded by a narrow hyaline sheath.
Notes — The molecular phylogenetic analysis of LSU data (Fig. 1) confirm monophyly of the Stegonsporium-Stilbospora clade, previously classified as genus Prosthecium (Voglmayr & Jaklitsch 2008) and included within Melanconidaceae (Barr 1978). However, Stilbospora and Stegonsporium form a distinct phylogenetic lineage and cannot be retained within that family, the type of which, Melanconis stilbospora, is phylogenetically distant (Fig. 1). We therefore classify both genera in the separate family Stilbosporaceae, which was already established by Link (1826, as Stilbosporei). Above we emend the family and restrict it to the genera Stilbospora and Stegonsporium based on currently available data.
Key to accepted genera of Stilbosporaceae
1. Ascospores and conidia with three transverse eusepta, ellipsoid to oblong; asci without a refractive canal in the apex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Stilbospora
1. Ascospores and conidia with more than three transverse distosepta, ascospores sometimes and conidia always with additional longitudinal distosepta, ascospores ellipsoid to oblong, conidia mostly pyriform; asci with a cylindrical, slightly refractive canal in the apex . . . . . . . . . . . . Stegonsporium
Stilbospora Pers., Neues Mag. Bot. 1: 93. 1794, emend.
Type species. Stilbospora macrosperma Pers., Syn. Meth. Fung. (Göttingen) 1: 96. 1801, lectotype selected by Clements & Shear (1931).
Pseudostromata inconspicuous, immersed in bark, lifting it and causing fissures. Ectostroma inconspicuous, rarely widely erumpent, limited to a light grey, amber to brown disc of a gel matrix containing numerous, tightly packed periphyses extending from ostioles. Ostioles inconspicuous, cylindrical, with pale brownish walls, convergent in groups, not projecting, invisible or appearing as subhyaline to brownish circles in the disc. Entostroma confined to an inconspicuous loose network of hyaline to brownish, (1.5–)2–4(–6) μm wide hyphae, enclosing more or less circular groups of usually tightly packed perithecia filling the area of the entostroma, or disposed in a valsoid ring; sometimes more compact above perithecia around convergent ostioles. Perithecia depressed globose to lenticular, dark brown to nearly black when mature, disposed in one layer. Peridium of a dark brown textura angularis in face view. Asci first sessile, becoming free; ellipsoid to fusoid, containing 8 uni- or biseriate ascospores, without a refractive canal in the apex. Ascospores ellipsoid to oblong, brown, 3-euseptate; with a gelatinous appendage at each end. Conidiomata immersed in bark, acervular, with circular outline, appearing as dark brown to black spots of 0.5 to several mm, containing simple, septate, hyaline paraphyses and hyaline, unbranched cylindrical conidiophores. Conidiogenous cells annellidic. Conidia brown, ellipsoid or oblong, often slightly curved, truncate at the base, 3-euseptate; with a hyaline sheath.
Notes — Sutton (1975) provided an account about synonymy and lectotypification of the genus. The genus is characterised by acervular conidiomata that occur in bark of trees and shrubs, presence of septate paraphyses, cylindrical hyaline annellidic conidiophores, and brown, thick-walled, cylindrical conidia that have several (usually three) transverse eusepta and a narrow hyaline sheath. Ascospores are similar to conidia, but bear a hyaline appendage at each end. The genus contains numerous species that require critical revision, a task far beyond the scope of the current manuscript; it is likely that most of these species are not congeneric with the generic type, S. macrosperma. The three confirmed Stilbospora species treated here occur on Carpinus, have indistinguishable conidia and can morphologically only be identified by their ascospore appendages.
Key to accepted species of Stilbospora
1. Ascospores with rounded ascospore appendages shorter than wide (3.5–8 μm long and 9–14 μm wide), widespread on Carpinus betulus. . . . . . . . . . . . . . . . . S. macrosperma
1. Ascospores with straight or curved ascospore appendages gradually tapering towards their acute distal ends . . . . . . . . . . . . . . . . . 2
2. Ascospore appendages elongate, horn-like, 20–44 μm long and 5–8 μm wide, on Carpinus betulus . . . . . . . . . . . . . . . . . S. longicornuta
2. Ascospore appendages bell-shaped, 9.5–19 μm long and 8.5–15 μm wide at the base, on Carpinus orientalis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. orientalis
Stilbospora longicornuta Voglmayr & Jaklitsch, sp. nov. — MycoBank MB805344; Fig. 6
Fig. 6.
Stilbospora longicornuta. a, b. Pseudostroma in transverse section showing perithecia immersed in the scant entostroma; c. bark fissure with scarcely erumpent ectostroma; d. ostioles; e. mature vital ascus; f–l. vital ascospores with long, gradually tapering, horn-like gelatinous appendages with l. showing a longitudinal septum; m. conidiophores (annelides), young conidia and filamentous paraphyses; n. conidiophore (annelide) with young conidium; o–r. vital conidia surrounded by gelatinous sheath (a–d. WU 32452; e–r. WU 32450 (holotype)). –– Scale bars: a, b = 1 mm; c = 0.5 mm; d = 0.2 mm; e–m = 20 μm; n–r = 10 μm.
Etymology. Referring to the long, often curved, horn-like ascospore appendages.
Holotype. AUSTRIA, Oberösterreich, Schärding, Raab, between Gautzham and Wetzlbach, grid square 7648/1, E13°14’18" N48°21’32", on dead, corticated branches of Carpinus betulus attached to the tree, holomorph, 2 Nov. 2007, H. Voglmayr D71 (WU 32450, holotype; ex-holotype culture CBS 122529 (from ascospores)); ex-type sequences KF570164 (ITS-LSU), KF570194 (rpb2), KF570232 (tef1).
Pseudostromata c. 1–5 mm diam, whitish, pale yellowish, brown to light olive green in section, containing up to 40 perithecia. Ostioles inconspicuous and often invisible at the surface. Perithecia (205–)230–335(–390) μm diam (n = 30). Asci clavate to ellipsoid, (125–)150–190(–210) × (20–)21–24(–25.5) μm (n = 36), thick-walled, containing 8 uni- or biseriate ascospores; apex without a refractive canal. Ascospores dark brown, ellipsoid to oblong, (26–)29–35.5(–40) × (9.5–)10–12.5(–13.5) μm, l/w = (2.3–)2.5–3.3(–4.1) (n = 30), with 3 eusepta, multiguttulate; appendages at both ends projecting for (20–)24–34(–44) μm and (5–)5.5–7(–8) μm wide at the base (n = 60), straight or curved, gradually tapering towards their distal ends. Conidiomata acervular, circular. Conidiogenous cells annellidic. Conidia dark brown, oblong, (27–)32.5–41.5(–49) × (9–)11–12.5(–14) μm, l/w = (2.5–)2.8–3.5(–4.2) (n = 96), usually with 3 eusepta, multiguttulate; surrounded by a 1–1.5 μm wide hyaline sheath.
Distribution — Known only from the type locality where it has been repeatedly collected for several years.
Habitat & Host range — Corticated, dead branches of Carpinus betulus attached to the trees, apparently very rare.
Additional selected specimens examined (all from the type locality). AUSTRIA, Oberösterreich, Schärding, Raab, between Gautzham and Wetzlbach, grid square 7648/1, E13°14’18" N48°21’32", 6 Feb. 2004, H. Voglmayr D35 (from conidia) (WU 32451); 8 May 2004, H. Voglmayr D33 (from conidia), D34 (from ascospores) (WU 32452); 21 May 2004, H. Voglmayr D32 (from conidia) (WU 32453);21 July 2007, H. Voglmayr (WU 32454), 14 Aug. 2007, H. Voglmayr (WU 32461).
Notes — Stilbospora longicornuta is distinct from S. macrosperma, which occurs on the same host, Carpinus betulus, by its long, tapering, horn-like ascospore appendages. Ascospore appendages of S. orientalis are also tapering but distinctly shorter and blunter; in addition, the latter occurs on a distinct host, Carpinus orientalis, in Southern Europe. Stilbospora longicornuta appears to be very rare, as it is only known from the type locality despite intense searches at various localities for many years.
Stilbospora macrosperma Pers., Syn. Meth. Fung. (Göttingen) 1: 96 (1801); Fig. 7
Fig. 7.
Stilbospora macrosperma. a. Pseudostroma in transverse section showing perithecia immersed in the entostroma, flanked by a conidioma; b. pseudostroma in transverse section showing ostiolar canals; c. bark fissures with erumpent red-brown ectostromatic discs; d. mature vital ascus; e–j. vital ascospores with cap-like, ellipsoid, gelatinous appendages; k. conidiophores (annelides), young conidia and filamentous paraphyses; l. vital conidia surrounded by gelatinous sheath (a–j. WU 32455; k, l. WU 32459). –– Scale bars: a, c = 1 mm; b = 0.5 mm; d–l = 20 μm.
= Stilbospora macrospora Pers., Neues Mag. Bot. 1: 94. 1794.
= Prosthecium ellipsosporum Fresen., Beitr. Mykol. 2: 62. 1852.
Typification. Without place and date, L 0118581 (Herb. Lugd. Bat. 90. O.H. No. 910.264-837, holotype). AUSTRIA, Niederösterreich, Rekawinkel, grid square 7862/1, on a trunk of Carpinus betulus, 20 Oct. 2001, W. Jaklitsch W.J. 1840, D25 (holomorph) (WU 24708, epitype here designated; ex-epitype culture CBS 115073; MBT176014); ex-epitype sequences EU039965 (ITS), AY616229 (LSU), KF570195 (rpb2), EU039999 (tef1).
Pseudostromata c. 1–10 mm diam, pale yellowish, ochre, brown to olive brown in section, containing up to 80 perithecia. Ostioles inconspicuous and often invisible at the surface, embedded in a brownish ectostromatic disc. Perithecia (410–)490–620(–700) μm diam (n = 70). Asci clavate to ellipsoid, (185–)190–240(–275) × (23–)26–30(–33) μm (n = 33), thick-walled, containing 8 uni- or biseriate ascospores; apex without a refractive canal. Ascospores dark brown, ellipsoid to oblong, (31.5–)35–42(–49) × (11–)12–14(–17) μm, l/w = (2.1–)2.7–3.3(–3.8) (n = 187), with usually 3 eusepta, multiguttulate; with subglobose to ellipsoid appendages at both ends projecting for (3.5–)4.5–7(–8) μm and (9–)10.5–13(–14) μm wide (n = 56). Conidiomata acervular, circular. Conidiogenous cells annellidic. Conidia dark brown, oblong, (34–)38–46(–55) × (10.5–)11.7–14(–15) μm, l/w = (2.3–)2.8–3.9(–4.8) (n = 100), usually with 3 eusepta, multiguttulate; surrounded by a 1–1.5 μm wide hyaline sheath.
Distribution — Widespread in Europe throughout the natural range of its host.
Habitat & Host range — Corticated, dead branches, logs or stumps of Carpinus betulus.
Additional selected specimens examined (all on corticated twigs, logs or stumps of Carpinus betulus). AUSTRIA, Burgenland, Hornstein, Lebzelterberg, holomorph, grid square 8064/4, 16 Sept. 2007, H. Voglmayr (WU 32455); Niederösterreich, Mödling, Gießhübl, Wassergspreng, grid square 7963/1, 2 Apr. 2006, H. Voglmayr D55 (WU 32456); Oberösterreich, Kopfing, Au, grid square 7548/3, 15 Apr. 2006, H. Voglmayr D56 (WU 32457); Natternbach, Leitenbachtal, Leithen E Teucht, grid square 7648/2, 17 Apr. 2006, H. Voglmayr D57 (WU 32458); same area, 3 Nov. 2007, H. Voglmayr (WU 32459). – NETHERLANDS, Utrecht, Rijnsweerd, near the Centraalbureau voor Schimmelcultures, 15 Nov. 2006, H. Voglmayr D58 (WU 27695). – UK, England, Surrey, Richmond, Richmond Park, 16 Nov. 2008, H. Voglmayr D85 (WU 32460).
Notes — This is a well-known and distinct species, which is rather common on Carpinus betulus throughout its range. For details about synonymy and typification see e.g. Sutton (1975). Stilbospora macrosperma differs from S. longicornuta and S. orientalis by its cap-like, rounded ascospore appendages, which are shorter than wide (Fig. 7e–j). The species was first described as Stilbospora macrospora Pers., but the later Stilbospora macrosperma Pers. was sanctioned by Fries (1832) and thus has to be used. The name S. macrosperma has priority over Prosthecium ellipsosporum. To ensure nomenclatural stability of the generic type, a recent collection for which a culture and ITS, LSU, rpb2 and tef1 sequences are available, is here designated as epitype.
Stilbospora orientalis Voglmayr & Jaklitsch, sp. nov. — MycoBank MB805345; Fig. 8
Fig. 8.
Stilbospora orientalis. a, b. Pseudostroma in transverse section showing perithecia immersed in the entostroma, flanked by conidiomata in a; c. bark fissure with scarcely erumpent ectostroma; d. mature vital ascus; e–j. vital ascospores with bell-shaped, tapering gelatinous appendages; k, l. conidiophores (annelides) with conidia; m–r. vital conidia surrounded by gelatinous sheath (all from WU 32462 (holotype)). — Scale bars: a–c = 0.5 mm; d = 20 μm; e–r = 10 μm.
Etymology. Referring to its host, Carpinus orientalis.
Holotype. GREECE, Kerkyra (Corfu), E Ano Korakiana, c. 1 km W of Analipsis, small shady ravine, on dead, corticated branches of Carpinus orientalis attached to the tree, holomorph, 23 Apr. 2012, H. Voglmayr & W. Jaklitsch D90 (WU 32462), holotype; ex-holotype culture CBS 135075 (from ascospores); ex-type sequences KF570166 (ITS-LSU), KF570197 (rpb2), KF570237 (tef1).
Pseudostromata c. 1–2 mm diam, indistinct in face view, white to pale yellowish in section, containing up to 25 perithecia. Ostioles inconspicuous and often invisible at the surface. Perithecia (290–)310–390(–440) μm diam (n = 35). Asci clavate to ellipsoid, (160–)185–235(–240) × 23–27 μm (n = 11), thick-walled, containing 8 uni- or biseriate ascospores; apex without a refractive canal. Ascospores dark brown, broadly ellipsoid to oblong, rarely fusoid and curved, (17.5–)25–32(–38) × (9.5–)10.5–12(–14) μm, l/w = (1.3–)2.1–3(–4.1) (n = 97), with (1–)3 eusepta, multiguttulate; appendages at both ends projecting for (9.5–)11–16(–19) μm and (8.5–)10–13.5(–15) μm wide at the base (n = 48), straight or curved, gradually tapering towards their distal ends. Conidiomata acervular, circular in outline. Conidiogenous cells annellidic. Conidia dark brown, oblong, (27–)31–37(–46) × (8.5–)9.5–11.5(–13) μm, l/w = (2.3–)2.8–3.7(–4.8) (n = 120), usually with 3 eusepta, multiguttulate; surrounded by a hyaline, 1–1.5 μm wide sheath.
Distribution —South-eastern Europe (Croatia, Greece, Montenegro).
Habitat & Host range —Corticated dead branches of Carpinus orientalis.
Additional selected specimens examined (all on corticated dead branches of Carpinus orientalis). CROATIA, Istria, Vrsar, soc. Melanconiella chrysorientalis and Melanconiella spodiaea, asexual morph, 14 May 2010, H. Voglmayr & W. Jaklitsch (WU 31858). – GREECE, Kerkyra (Corfu), c. 3 km S Ano Korakiana, small shady ravine, asexual morph, 23 April 2012, H. Voglmayr & W. Jaklitsch D91 (WU 32463). – MONTENEGRO, NE Ulcinj, dry mixed forest, asexual morph, 27 Aug. 2012, H. Voglmayr & I. Greilhuber D93 (WU 32464).
Notes — Stilbospora orientalis is well characterised by its host Carpinus orientalis and the bell-shaped, tapering ascospore appendages, which are reminiscent of Stegonsporium galeatum, a species growing on Acer pseudoplatanus. Stilbospora orientalis is closest relative of S. longicornuta, with which it shares tapering ascospore appendages. These are, however, distinctly shorter and wider (10–19 × 8.5–15 vs 20–44 × 5–8 μm).
Stegonsporium Corda, in Opiz, Naturalientausch 11: 458. 1827, emend.
Type species. Stegonsporium pyriforme (Hoffm.) Corda, Icon. Fungorum (Prague) 3: 23. 1839.
Pseudostromata inconspicuous, immersed in bark and lifting it slightly, causing fissures to c. 1 mm. Ectostroma largely hidden by surrounding lobes of the bark, limited to an amber to light brownish disc of a gel matrix containing numerous tightly packed periphyses 1.5–3(–5) μm wide. Ostioles inconspicuous, cylindrical, with pale brownish walls, convergent in groups, not projecting, invisible or appearing as subhyaline to pale yellowish brown circles in the disc. Entostroma confined to an inconspicuous loose network of hyaline to brownish, (1.5–) 2–4(–6) μm wide hyphae, enclosing more or less circular groups of usually tightly packed perithecia filling the area of the entostroma, or disposed in a valsoid ring; sometimes more compact above perithecia in the centre around convergent ostioles. Perithecia depressed globose to lenticular, dark brown to nearly black when mature, disposed in one layer. Peridium of a dark brown textura angularis in face view. Asci first sessile, becoming free; ellipsoid or clavate, containing 8 uni- or biseriate ascospores, a more or less cylindrical, slightly refractive canal in the apex; walls thick, appearing bitunicate at least when young. Ascospores ellipsoid to oblong, brown, mostly 5-distoseptate, sometimes with one oblique or longitudinal distoseptum in one to several cells; with a gelatinous appendage at each end. Conidiomata immersed in bark, acervular, with circular outline, appearing as dark brown to black spots of 0.5 to several mm, containing simple hyaline paraphyses and hyaline cylindrical septate conidiophores. Conidiogenous cells annellidic. Conidia brown, pyriform to oval, ellipsoid or oblong, truncate and hyaline at the base, with several distosepta and one, rarely two longitudinal distosepta in one to several cells, and a hyaline sheath; basal cell morphologically distinct from others.
Notes — Van Warmelo & Sutton (1981) provided a detailed account about synonymy, orthography and typification of Stegonsporium, which is followed here. Based on thorough morphological studies of conidiomata, conidiophores and conidia, they only accepted S. pyriforme and S. acerinum and excluded numerous species from the genus. As currently circumscribed, the genus Stegonsporium is morphologically, ecologically and phylogenetically coherent and distinct. The genus is characterised by acervular conidiomata that occur in bark of trees and shrubs, presence of paraphyses, cylindrical hyaline annellidic conidiophores, and brown, thick-walled, obovate, pyriform to clavate conidia that are subdivided by both transverse and longitudinal distosepta and have a narrow hyaline sheath (Sutton 1980, van Warmelo & Sutton 1981, Voglmayr & Jaklitsch 2008). Also sexual morphs are characterised by ascospores that are brown and distoseptate, but they often lack longitudinal septa and bear a hyaline appendage at each end (Voglmayr & Jaklitsch 2008). For detailed species descriptions, see Voglmayr & Jaklitsch (2008).
Key to accepted species of Stegonsporium (modified from Voglmayr & Jaklitsch 2008)
1. Ascospores with a sheath readily breaking in mounts, oblong; appendages bell-shaped to pyriform, 40–60 × 13–20(–22) μm; conidia (36–)40–49(–53) × (16–)18–21.5(–23) μm; on Acer pseudoplatanus and A. heldreichii in Europe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. galeatum
1. Ascospores without a sheath, ellipsoid; appendages (sub)globose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. Ascospores rarely (less than 20 %) with a longitudinal distoseptum, (30–)35–45(–50) × 13–17(–21) μm; asci (28–)30–43(–51) μm wide; conidia 30–40(–50) × 14–18(–20) μm; mostly on Acer pseudoplatanus, rarely on A. heldreichii, A. monspessulanum and A. velutinum in Europe; three cryptic species only distinguishable by sequence data . . . . . . . . . . . . . . . . . S. protopyriforme, S. pseudopyriforme, S. pyriforme
2. Ascospores commonly (in more than 40 % of the spores) with one longitudinal distoseptum in 1–3 cells . . . . . . . . . . . . . . . . . 3
3. Ascospores 30–40(–44) × 14–18 μm; asci 26–32(–35) μm wide; conidia 30–40 ×14–18 μm, with narrow lenticular cell lumina; on Acer saccharum, A. grandidentatum. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. acerophilum
3. Ascospores 40–50(–55) × 17–22(–25) μm. . . . . . . . . . . . . . . . . 4
4. Conidia (46–)50–58(–61) × 24–31 μm; on Acer saccharum in North America . . . . . . . . . . . . . . . . . . . . . . . . . . S. acerinum
4.Conidia (32–)37–44(–51) × 18–22(–24) μm; on Acer hyrcanum, A. monspessulanum, A. obtusatum, A. opalus and A. sempervirens in Europe . . . . . . . . . . . . . . . . . S. opalus
Stegonsporium acerinumPeck, Bull. Torrey Bot. Club 25: 326.1898
= Prosthecium acerinum Voglmayr & Jaklitsch, Mycol. Res. 112, 8: 892. 2008.
Typification. CANADA, Ontario, Ottawa, on bark of Acer saccharum, 14 Sept. 1897, J.M. Macoun (NYS f52, holotype); Québec, Ville de Québec, Plains d’Abraham, Parc des Champs-de-Bataille, on dead corticated branches of Acer saccharum, 26 July 2006, H. Voglmayr D42, D43 (WU 28047, holotype of Prosthecium acerinum, epitype of Stegonsporium acerinum here designated; ex-epitype culture CBS 120525; MBT176015); ex-epitype sequences EU039996 (LSU), EU039968 (ITS), KF570171 (rpb2), EU040024 (tef1).
Notes — Stegonsporium acerinum is well distinguished from the other accepted Stegonsporium species by distinctly larger conidia. The holotype of Prosthecium acerinum, which is a well-developed specimen and for which cultures and ITS, LSU, rpb2 and tef1 sequence data are available, is here designated as epitype for S. acerinum to stabilise the nomenclatural connection of both names.
Stegonsporium acerophilum(M.E. Barr) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB805346
Basionym. Dictyoporthe acerophila M.E. Barr, Mycol. Mem. 7: 188. 1978.
≡ Prosthecium acerophilum (M.E. Barr) Jaklitsch & Voglmayr, Mycol. Res. 112, 8: 892. 2008.
Typification. USA, New Hampshire, White Mountains National Forest, near Pinkham Notch, on dead corticated branches of Acer saccharum, 29 July 1963, M.E. Barr 4007 (NY 00921994, holotype of Dictyoporthe acerophila); Tennessee, Knoxville, wood lot of the Agricultural Sciences of the University of Tennessee, on dead corticated branches of Acer saccharum, 23 May 2003, W. Jaklitsch & H. Voglmayr W.J. 2204, D5, D6 (WU 28050, epitype designated by Voglmayr & Jaklitsch (2008); ex-epitype cultures CBS 117025, CBS 117026; MBT176010); ex-epitype sequences EU039993 (LSU), EU039982 (ITS), KF570173 (rpb2), EU040027 (tef1).
New records. CZECH REPUBLIC, Morava, Lednice, park of the castle, grid square 7166/4, on dead corticated branches of Acer saccharum, 6 Sept. 2008, H. Voglmayr & I. Greilhuber D81 (WU 32465, living culture CBS 125028), D82 (WU 32466, living culture CBS 124482). – UK, England, Surrey, Kew, Royal Botanic Gardens Kew, on dead corticated branches of Acer saccharum, 13 Sept. 2007, H. Voglmayr D64 (WU 32467, living culture CBS 125033); same place, same date, on dead corticated branches of Acer grandidentatum, H. Voglmayr D65 (WU 32468, living culture CBS 125042).
Notes — The basionym of S. acerophilum, Dictyoporthe acerophila, was epitypified by Voglmayr & Jaklitsch (2008). The species is common on Acer saccharum and close relatives in North America. We report it here for the first time for Europe.
Stegonsporium galeatum (Höhn.) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB805347
Basionym. Massaria galeata Höhn., Ann. Mycol. 3: 403. 1905.
≡ Prosthecium galeatum (Höhn.) Jaklitsch & Voglmayr, Mycol. Res. 112, 8: 895. 2008.
Typification. AUSTRIA, Niederösterreich, Puchberg am Schneeberg, Aug. 1905, F. Höhnel, Herb. Höhnel A3831a (FH - lectotype); Puchberg am Schneeberg, at the Schneebergbahn between Hauslitzsattel and Hengsthütte, grid square 8261/1, 880 m s.m., on dead corticated branches of Acer pseudoplatanus, 11 June 2006, H. Voglmayr D41 (WU 28056, epitype designated in Voglmayr & Jaklitsch (2008); ex-epitype culture CBS 120523); ex-epitype sequences KF570175 (rpb2), EU040013 (tef1).
New records. UK, Scotland, Scottish borders, SW Stobo, Royal Botanic Garden Edinburgh, Dawyck Botanic Garden, on dead corticated branches of Acer heldreichii, 4 Sept. 2007, H. Voglmayr & W. Jaklitsch D70 (WU 32469, living culture CBS 125035).
Notes — The basionym of S. galeatum, Massaria galeata, was lecto- and epitypified by Voglmayr & Jaklitsch (2008). Acer heldreichii is a new host for this species.
Stegonsporium opalus(Voglmayr & Jaklitsch) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB805348
Basionym. Prosthecium opalus Voglmayr & Jaklitsch, Mycol. Res. 112, 8: 897. 2008.
Typification. SLOVENIA, Vipava, Mt Nanos massif, Rebrnice NE Lozice, at the road to Podraška Tura, SW-exposed steep slope, mixed deciduous thermophilous forest, 500–560 m s.m., on dead corticated branches of Acer obtusatum, holomorph, 23 Sept. 2006, H. Voglmayr & W. Jaklitsch D47, D48 (WU 28062, holotype; ex-type cultures CBS 120598 (from ascospores), CBS 120599 (from conidia)); ex-type sequences EU039997 (LSU), EU039980 (ITS), KF570178 (rpb2), EU040020 (tef1).
New records. AUSTRIA, Wien, Landstraße, Botanical Garden of the University (HBV), grid square 7864/1, on dead corticated branches of Acer hyrcanum, 16 June 2007, H. Voglmayr D59 (WU 32783; living culture CBS 124485). – FRANCE, Dept. Alpes de Haute Provence, Gorges du Verdon, Rougon, Point Sublime, on dead corticated branches of Acer opalus, 8 Aug. 2007, I. Greilhuber D60 (WU 28241, living culture CBS 124474); Dept. Var, Pont de l’Artuby, on dead corticated branches of Acer monspessulanum, 28 July 2011, H. Voglmayr & I. Greilhuber PR15 (WU 32470). – GREECE, Crete, Askifou Sfakion, on dead corticated branches of Acer sempervirens, 20 Nov. 2011, W. Jaklitsch D89 (WU 32763). – UK, England, Surrey, Kew, Royal Botanic Gardens Kew, on dead corticated branches of Acer obtusatum, 13 Sept. 2007, H. Voglmayr D63 (WU 32761, living culture CBS 125032); same place, same date, on dead corticated branches of Acer opalus, H. Voglmayr D69 (WU 32762, living culture CBS 125034).
Notes — The collections from Acer monspessulanum and A. sempervirens differ slightly in tef1 and rpb2 sequences from the other collections of S. opalus, indicating some host specialization.
Stegonsporium protopyriforme Voglmayr & Jaklitsch, sp. nov. — MycoBank MB805349
Etymology. Referring to its similarity to S. pyriforme.
Holotype. AUSTRIA, Niederösterreich, Mödling, Gießhübl, Wassergspreng, grid square 7963/1, on dead corticated branches of Acer pseudoplatanus, holomorph, 27 Nov. 2004, H. Voglmayr D29, D30 (WU 28064, holotype; ex-type cultures CBS 117040, CBS 117041); ex-type sequences EU039991, EU039992 (LSU), EU039976, EU039977 (ITS), EU040016, EU040017 (tef1).
Stegonsporium protopyriforme differs from its closest phylogenetic neighbours, S. pyriforme and S. pseudopyriforme, by unique fixed alleles in two tree loci (rpb2, tef1) based on alignments of the separate loci deposited in TreeBASE as study S14652: rpb2 positions 7, 40: A; 205, 277, 481, 1087, 1120, 1133: C; 76, 85, 289, 496, 503, 664, 847, 1099: T; tef1 positions 10, 51, 54, 218, 289, 622, 1277: A; 8, 18, 47, 100, 101, 112, 153, 307, 506, 515, 630, 961, 985, 1036, 1075, 1120, 1147, 1278, 1307, 1382: C; 14, 80, 81, 201, 509, 1226, 1336: G; 44, 78, 79, 214, 221, 322, 607, 718, 874, 940, 1342, 1393, 1396, 1444: T.
Additional selected specimens examined (all from dead corticated branches of Acer pseudoplatanus). AUSTRIA, Oberösterreich, Schärding, Raab, Großrothmayr, grid square 7647/2, 20 June 2003, H. Voglmayr D10 (WU 28067; living culture CBS 117030); Niederösterreich, Neunkirchen, Prigglitz, Kleewiese, grid square 8261/4, 20 Sept. 2008, H. Voglmayr PR9 (WU 32764); Perchtoldsdorf, Bierhäuslberg, grid square 7863/3, H. Voglmayr PR10 (WU 32766); Steiermark, Graz-Umgebung, Peggau, Ruine Peggau, grid square 8758/3, 26 Oct. 2007, H. Voglmayr PR5 (WU 32765). – CZECH REPUBLIC, Morava, Lednice, park of the castle, grid square 7166/4, 6 Sept. 2008, H. Voglmayr D80 (WU 32767, living culture CBS 124480). – UK, Scotland, Scottish borders, SW Stobo, Royal Botanic Garden Edinburgh, Dawyck Botanic Garden, 4 Sept. 2007, H. Voglmayr & W. Jaklitsch D61 (WU 32768, living culture CBS 125030).
Notes — As there are no clear-cut morphological features that distinguish S. protopyriforme from S. pyriforme and S. pseudopyriforme, the rpb2 and tef1 sequences are here used for formal description of the species. Reliable species identification is hence only possible with sequence data.
Stegonsporium pseudopyriforme Voglmayr & Jaklitsch, sp. nov. — MycoBank MB805350
Etymology. Referring to its similarity to S. pyriforme.
Holotype. AUSTRIA, Oberösterreich, Natternbach, Leitenbachtal, Leithen E Teucht, grid square 7648/2, on dead branches of Acer pseudoplatanus, holomorph, 15 Aug. 2007, H. Voglmayr D72 (WU 32769, holotype; ex-type culture CBS 125046); ex-type sequences KF570159 (ITS-LSU), KF570218 (tef1).
Stegonsporium pseudopyriforme differs from its closest phylogenetic neighbours, S. pyriforme and S. protopyriforme, by unique fixed alleles in two tree loci (rpb2, tef1) based on alignments of the separate loci deposited in TreeBASE as study S14652: rpb2 positions 439: A; 1, 34, 850, 901, 1084, 1112: C; 830, 1133, 1144: G; 391, 973, 1156: T; tef1 positions 274, 286: A; 21, 99, 251, 601: C; 100, 719: G; 185, 1030, 1327, 1402, 1438: T.
Additional selected specimens examined (all from dead corticated branches of Acer pseudoplatanus except where noted). AUSTRIA, Kärnten, Klagenfurt-Land, St. Margareten im Rosental, Zabrde, grid square 9452/4, 21 June 2003, W. Jaklitsch W.J. 2260, D8 (WU 28063; living culture CBS 117028); Niederösterreich, St. Aegyd/Neuwald, Lahnsattel, Donaudörfl, grid square 8259/1, 27 Sept. 2006, H. Voglmayr D50 (WU 28065; living culture CBS 120597); Pernitz, Muggendorf, Steinwandklamm, grid square 8061/4, 9 June 2007, H. Voglmayr PR4 (WU 32770); Oberösterreich, Natternbach, Leitenbachtal, Leithen E Teucht, grid square 7648/2, 21 June 2003, H. Voglmayr D9 (WU 28066; living culture CBS 117029); Steiermark, Graz-Umgebung, Peggau, Lurgrotte, grid square 8758/3, 26 Oct. 2007, H. Voglmayr PR7 (WU 32771); Wien, Döbling, Himmelstraße, grid square 7763/2, 18 June 2003, W. Jaklitsch D7 (WU 28072; living culture CBS 117027). – SLOVENIA, Vipava, Nanos, 23 Sept. 2006, H. Voglmayr & W. Jaklitsch D49 (WU 28074; living culture CBS 120526). – UK, England, Surrey, Kew, Royal Botanic Gardens Kew, on Acer heldreichii, 13 Sept. 2007, H. Voglmayr D67 (WU 32772, living culture CBS 125044); same place, same date, on Acer velutinum, H. Voglmayr D68 (WU 32773, living culture CBS 125045).
Notes — As there are no clear-cut morphological features that distinguish S. pseudopyriforme from S. pyriforme and S. protopyriforme, the rpb2 and tef1 sequences are here used for formal description of the species. Reliable species identification is hence only possible with sequence data.
Stegonsporium pyriforme (Hoffm.) Corda, Icon. Fungorum 3: 23. 1839; Fig. 9
Fig. 9.

Lectotype of Stilbospora pyriformis (Hoffmann, Deutschlands Flora, Zweiter Theil (Erlangen): t. 13, f. 2 (1795), housed in the library of the Department of Botany and Biodiversity Research, University of Vienna).
Basionym. Stilbospora pyriformis Hoffm. (as‘piriformis’), Deutschl. Fl., Zweiter Theil (Erlangen): t. 13, f. 2. 1795.
= Prosthecium pyriforme Jaklitsch & Voglmayr, Mycol. Res. 112, 8: 898. 2008.
Typification. Hoffmann, Deutschlands Flora, Zweiter Theil (Erlangen): t. 13, f. 2 (1795), housed in the library of the Department of Botany and Biodiversity Research, University of Wien, Vienna (iconotype, lectotype of Stilbospora pyriformis here designated, MBT176673, MBT176674). – AUSTRIA, Wien, Landstraße, Botanical Garden of the University of Vienna (HBV), map grid 7864/1, on dead corticated branches of Acer pseudoplatanus, holomorph, 20 Nov. 2003, H. Voglmayr (WU 28075, holotype of Prosthecium pyriforme); Wien, Landstraße, Botanical Garden of the University of Vienna (HBV), grid square 7864/1, on dead corticated branches of Acer pseudoplatanus, holomorph, 4 Feb. 2006, H. Voglmayr D39 (WU 28068, epitype of Stilbospora pyriformis and Prosthecium pyriforme here designated; ex-epitype culture CBS 120522; MBT176016); ex-epitype sequence EU040003 (tef1).
Additional selected specimens examined (all from dead corticated branches of Acer pseudoplatanus except where noted). AUSTRIA, Wien, Donaustadt, Lobau, close to Panozzalacke, grid square 7865/1, 29 Oct. 2002, W. Jaklitsch W.J. 2017, D2 (WU 28069; living culture CBS 117023); same area, 17 June 2003, H. Voglmayr D11 (WU 28070; living culture CBS 117031); 7 July 2003, W. Jaklitsch W.J. 2273, D22 (WU 28071; living culture CBS 117034). – CROATIA, Istrija, between Golaš and Bale, on Acer monspessulanum, 16 Sept. 2010, H. Voglmayr & W. Jaklitsch D87 (WU 32774). – DENMARK, Nordjylland, Tranum, Fosdalenvej, 25 Aug. 2006, H. Voglmayr & W. Jaklitsch D46 (WU 28073; living culture CBS 120600). – FRANCE, Dept. Alpes de Haute Provence, Castellane, 1 Aug. 2008, H. Voglmayr & I. Greilhuber PR11 (WU 32777); Camping des Gorges du Verdon, 1 Aug. 2008, H. Voglmayr & I. Greilhuber PR2 (WU 32778). – ITALY, Latium, Viterbo, Lago di Vico, 28 July 2009, H. Voglmayr & W. Jaklitsch PR6 (WU 32775); Soriano nel Cimino, Monte Cimino, 26 Nov. 2009, H. Voglmayr & W. Jaklitsch PR12 (WU 32776). – UK, England, Northumberland, West Woodburne, 4 Sept. 2007, H. Voglmayr & W. Jaklitsch D62 (WU 32779, living culture CBS 125031); Surrey, Royal Botanic Gardens Kew, 13 Sept. 2007, H.Voglmayr D66 (WU 32780, living culture CBS 125043); Surrey, Richmond, Richmond Park, on Acer heldreichii, 16 Nov. 2008, H.Voglmayr D85 (WU 32781, living culture CBS 124487); Surrey, Staines, Laleham Park, 15 Nov. 2008, H. Voglmayr PR1 (WU 32782).
Notes — Hoffmann (1795) does not give a locality or host of the collection upon which his description and illustration were based, but they agree well with the current fungus. No type collection of Stilbospora pyriformis by Hoffman appears to be extant, therefore the illustration by Hoffmann (1795) is here selected as lectotype, which is epitypified by a recent, well documented collection. Unfortunately, for the type of Prosthecium pyriforme, which could serve as epitype for S. pyriformis, no cultures or sequences are available. Therefore, a collection from the same tree as the type of P. pyriforme, for which a culture and the tef1 sequence are available, is here selected as epitype for both S. pyriformis and Prosthecium pyriforme to stabilise the nomenclatural connection of both names.
Reclassification of Prosthecium taxa not contained within the Stilbosporaceae
Alnecium Voglmayr & Jaklitsch, gen. nov. — MycoBank MB805342
Etymology. Referring to its host, Alnus, and to the genus Prosthecium in which it has been previously classified.
Type species. Alnecium auctum (Berk. & Broome) Voglmayr & Jaklitsch.
Genus of Gnomoniaceae, Diaporthales. Perithecia immersed in groups, black, with erumpent necks. Ascospores ellipsoid, 1-septate, thick-walled, hyaline, in age eventually becoming 3-septate and pale brown, with a gelatinous appendage at each end.
Alnecium auctum (Berk. & Broome) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB805343; Fig. 10
Fig. 10.
Alnecium auctum. a. Bark fissure with scarcely erumpent ectostroma and four ostioles in surface view; b. pseudostroma in vertical section; c. pseudostroma in transverse section, showing perithecia and brown entostromata; d, e. scarcely erumpent ectostroma and compressed ostioles in surface view; f. erumpent ostioles in surface view; g. mature dead ascus; h–u. dead ascospores with blunt gelatinous appendages; v–aa. multiguttulate vital ascospores with blunt gelatinous appendages with g–aa in water (a–c, g–o. WU 30206 (epitype); d, e, p–u. K(M) 188100 (lectotype); f, v–aa WU 32163). –– Scale bars: a, d–f = 200 μm; b, c = 0.5 mm; g = 20 μm; h–aa = 10 μm.
Basionym. Sphaeria aucta Berk. & Broome, Ann. Mag. Nat. Hist., ser. II, 9: 323. 1852.
≡ Aglaospora aucta (Berk. & Broome) Kuntze, Revis. Gen. Pl. (Leipzig) 3, 2: 441. 1898.
≡ Calospora aucta (Berk. & Broome) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 191. 1870. (‘1869–70’).
≡ Cryptospora aucta (Berk. & Broome) Tul. & C. Tul., Select. Fung. Carpol. (Paris) 2: 152. 1863.
≡ Melanconis aucta (Berk. & Broome) Wehm., Revision of Melanconis, Pseudovalsa, Prosthecium & Titania, Univ. Michigan Stud., Scientific Ser. 14: 58. 1941.
≡ Prosthecium auctum (Berk. & Broome) Petr., Ann. Mycol. 21, 3/4: 325. 1923.
≡ Pseudovalsa aucta (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 2: 138. 1883.
Typification. UK, England, Wiltshire, Spye Park, on branches of Alnus glutinosa, spring (without date), C.E. Broome, in: Rabenhorst, Fungi Europaei Exsiccati 143 (K(M) 188100, lectotype here designated). – AUSTRIA, Kärnten, St. Margareten im Rosental, village area, at the brook Tumpfi, grid square 9452/4, on branches of Alnus glutinosa, 2 Nov. 2008, W. Jaklitsch W.J. 3231 (WU 30206, epitype of Sphaeria aucta here designated; ex-epitype culture CBS 124263 = PAT; MBT176655).
Pseudostromata c. 1.5–2 mm diam, indistinctly pustulate in face view, containing 3–8 perithecia. Ectostromatic disc inconspicuous, brown to grey, scarcely erumpent through a circular to elongate cortical crack. Entostroma poorly developed, small, central, olive-grey. Ostioles erumpent, 1–8, cylindrical to conic, black. Perithecia black, 400–800 μm diam. Asci broadly fusoid to saccate, (90–)105–120(–130) × (29–)31–42(–47) μm (n = 26), containing 8 uni- to triseriate ascospores; apex without a distinct ring. Ascospores hyaline to subhyaline, ellipsoid to oblong, (28–)32–37(–45) × (9–)11–14(–16) μm, l/w = (2.2–)2.4–3.0(–3.9) (n = 140), with 1 euseptum, with age eventually becoming light brown and 3-septate, not to slightly constricted at septum, multiguttulate when fresh, thick-walled, with rounded ends and hyaline cylindrical appendages at both ends projecting for 2.5–5 μm and 3–5 μm wide at the base. Asexual morph unknown.
Distribution — Europe.
Habitat & Host range —Corticated dead branches of Alnus glutinosa.
Selected specimens examined (all from Alnus glutinosa). Without place, substrate, date and collector, Herb. Berkeley (K(M) 188099, possibly an isotype). – AUSTRIA, Kärnten, St. Margareten im Rosental, village area, at the brook Tumpfi, grid square 9452/4, 29 May 1992, W. Jaklitsch (WU 16100); same area, 7 Jan. 1994, W. Jaklitsch (WU 15536); same area, 1 May 2002, W. Jaklitsch W.J. 1887 (WU 32114); St. Margareten im Rosental, Wograda, grid square 9452/3, 27 May 1997, W. Jaklitsch W.J. 1082 (WU 32112); Oberösterreich, Raab, between Gautzham and Wetzlbach, alluvial forest at Wiesbach, grid square 7648/1, 25 Mar. 2000, H. Voglmayr W.J. 1433 (WU 28176); St. Willibald, Großer Salletwald, grid square 7648/1, 27 Dec. 2013, H. Voglmayr (WU 32163); Unterach am Attersee, at Stockwinkl/Egelsee, grid square 8147/3, 25 May 1996, W. Jaklitsch W.J. 885 (WU 32111). – SPAIN, Bizkaia, Uarka Auzoa, 30 Oct. 2010, W. Jaklitsch (WU 31388).
Notes — Alnecium auctum has been classified within various different genera (see synonymy above), which suggests substantial uncertainties about its generic affiliation. Petrak (1923) and Barr (1978) classified A. auctum in Prosthecium, while Wehmeyer (1941) placed it in Melanconis. Its phylogenetic placement in the Gnomoniaceae is unexpected, as no earlier mycologist ever combined it in a genus that then was thought to be affiliated to this family. The Gnomoniaceae contains predominantly members that colonize non-woody material such as leaves, culms and stalks of herbaceous plants or leaves of trees and shrubs. The family has been characterised as having mostly small, non- or rudimentarily stromatic ascomata and small, hyaline to yellowish, thin-walled ascospores (Barr 1978, Monod 1983). Only few genera of the family inhabit bark of trees. The phylogenetic position of Ditopella or Phragmoporthe was already determined by Castlebury et al. (2002), and Amphiporthe, Cryptosporella and Plagiostoma were added later (Mejia et al. 2008, Sogonov et al. 2008). Except for being thin-walled, ascospores of Plagiostoma micromegala (Barr 1978) or P. petrakii (Monod 1983) have some similarity with those of A. auctum, but these species are non-stromatic and occur in herbaceous material. However, Plagiostoma now also contains the genus Cryptodiaporthe Petr. (Mejia et al. 2008, 2011, Sogonov et al. 2008), whose species generally inhabit bark of trees and shrubs. Alnecium shares the configuration of ascomata in indistinct or reduced prosenchymatous pseudostromatic tissues with the enlarged concept of Plagiostoma, but has thick-walled ascospores that turn brown in age, whereas ascospores of bark-inhabiting species of Plagiostoma basically resemble those of Diaporthe in being thin-walled and remaining hyaline. Also ascospores of Amphiporthe are Diaporthe-like. Ditopella and Phragmoporthe occur also on Alnus. They differ from Alnecium by the absence of a stroma except for a rudimentary clypeus around ostioles, thin-walled ascospores and polysporous asci (Ditopella) or hyaline phragmospores (Phragmoporthe). Finally, ascospores of Cryptosporella are hyaline, thin-walled, elongate and non-appendaged. As none of the bark-inhabiting genera of the Gnomoniaceae in the current circumscription is congruent with A. auctum, the establishment of a new genus is necessary, even more as a clear phylogenetic affiliation to another genus could not be shown (Fig. 1, 5).
In the species description, Berkeley & Broome (1852: pl. X, f. 11) provided a good illustration, which clearly shows the features of the species. We studied two authentic collections from K, one without date but giving the same collection site as the original description, which was distributed as Rabenhorst, Fungi Europaei Exsiccati 143, and a second from the Herbarium Berkeley without any collection data. The first collection was selected as the lectotype, because the data agree with the description, it is in better condition and duplicates of this exsiccatum should also be present in other herbaria. In the lectotype only ascospores but no asci could be seen. To ensure nomenclatural stability, a recent well-developed specimen for which a culture and ITS-LSU, tef1 and rpb2 sequences are available is here selected as epitype. The ascospores are initially hyaline and 1-septate, with age eventually becoming light brown and 3-septate, with a small gelatinous appendage at each end (Fig. 10). The asexual morph of this fungus is unknown. The slow-growing, dark grey to black colonies of CBS 124263 produced only sterile black ostiolate pycnidia on PDA.
Calosporella J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2, 4: 442. 1897 (‘1908’)
Type species. Calosporella innesii (Curr.) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2, 4: 442. 1897 (‘1908’).
Notes — As currently circumscribed, Calosporella is monotypic, but the taxonomic history of the genus as well as species name to be applied is complicated. Schröter (1897) erected the genus Calosporella as a replacement name for Calospora Sacc., which he considered to be a homonym of Calospora Fuckel 1970, the latter being typified with C. hapalocystis (Berk. & Broome) Fuckel (= Hapalocystis berkeleyi Auersw. ex Fuckel; see Jaklitsch & Voglmayr 2004). However, as Calospora Fuckel 1870 and Calospora Nitschke ex Niessl 1875 are nomina nuda (Holm 1975), Calospora Sacc. 1883 is a valid name. In describing the genus Calospora, Saccardo (1883) listed 13 species without designating a generic type, and he remarked that the first two species, C. platanoidis (Pers.) Sacc. and C. innesii (Curr.) Sacc., are scarcely distinct. Subsequently, both taxa were commonly considered to be conspecific, and the epithet platanoidis was mostly used for the species. Clements & Shear (1931) lectotypified Calospora Sacc. with C. platanoidis. However, as Wehmeyer (1941) pointed out, the type specimen of its basionym Sphaeria platanoidis Pers. is not congeneric with the current fungus, because it has widely erumpent greyish stromata and fusoid, 2-celled, 4-guttulate, hyaline spores which are constricted at the septum. As material of Persoon is not sent out on loan by L, the true identity of S. platanoidis cannot be clarified. The widely used concept of Sphaeria platanoidis (e.g. Saccardo 1883, Höhnel 1918, Clements & Shear 1931, Barr 1978) was based on a misconception by Fries who distributed the current fungus as S. platanoidis in his Scler. suec. 186 (Wehmeyer 1941). Because S. platanoidis is not a sanctioned name, the name cannot be lectotypified by material of Fries, and the concept of S. platanoidis is bound to the type specimen of Persoon. Therefore the name Calospora, lectotypified with C. platanoidis, cannot be applied for the current fungus and the next available generic name is Calosporella, and also the epithet platanoidis cannot be retained.
In his description of Calosporella, Schröter (1897) listed only C. innesii (as ‘junnesii’) and gave Sphaeria platanoidis Pers. as a doubtful synonym. Höhnel (1918: 116) lectotypified Calosporella with C. platanoidis, which was subsequently also followed by Clements & Shear (1931). However, this lectotypification is superfluous and formally incorrect as the only species listed in Schröter (1897) is C. innesii, which therefore has to be the nomenclatural type. In addition, S. platanoidis is not congeneric with C. innesii (see above).
Calosporella innesii (Curr.) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2, 4: 442. 1897 (‘1908’); Fig. 11
Fig. 11.
Calosporella innesii. a, e. Ectostromatic discs and ostioles in surface view; b. pseudostroma in vertical section; c, g. pseudostromata in transverse section, showing perithecia and pale brown entostromata; d. transverse section of ectostromatic disc and ostioles; f. ectostromatic disc and ostioles in side view; h, i. mature dead asci; j–m. vital ascospores with tapering gelatinous appendages; n–y. dead ascospores with tapering gelatinous appendages with h–u in water and v–y in 3 % KOH (a, b, h, n–q. WU 32447; c, d, j–m. WU 32161 (epitype); e–g, i, r–y. K(M) 188103 (lectotype)). –– Scale bars: a–g = 0.5 mm; h, i = 20 μm; j–y = 10 μm.
Basionym. Sphaeria innesii Curr., Trans. Linn. Soc. London 22: 281. 1858 (‘1859’).
≡ Calospora innesii (Curr.) Sacc., Syll. Fung. (Abellini) 2: 231. 1883.
≡ Diaporthe innesii (Curr.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 204. 1870 (‘1869–70’).
≡ Prosthecium innesii (Curr.) Wehm., Revision of Melanconis, Pseudovalsa, Prosthecium & Titania, Univ. Michigan Stud., Scientific Ser. 14: 98. 1941.
≡ Valsa innesii (Curr.) Sacc., Syll. Fung. (Abellini) 2: 231. 1883.
= Valsa aglaeostoma Berk. & Broome, Ann. Mag. Nat. Hist., ser. III, 3: 368. 1859
≡ Aglaospora aglaeostoma (Berk. & Broome) Kuntze, Revis. Gen. Pl. (Leipzig) 3, 2: 441. 1898.
≡ Pseudovalsa aglaeostoma (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 2: 137. 1883.
Typification. AUSTRIA, Wien, Donaustadt, Lobau, Panozzalacke, grid square 7865/1, on dead corticated branches of Acer pseudoplatanus, 8 Mar. 2008, H. Voglmayr & W. Jaklitsch D78 (WU 32161, epitype of Sphaeria innesii here designated; ex-epitype culture CBS 123810; MBT176656). – UK, England, Surrey, Weybridge, on dead corticated branches of Acer pseudoplatanus, Apr. 1855, without collector, Herb. F. Currey (K(M) 188103, lectotype of Sphaeria innesii here designated, MBT176658; K(M) 188104, K(M) 188105, isotypes).
Pseudostromata c. 1–3 mm diam, pustulate in face view, circular, containing up to 20 perithecia. Ectostromatic disc erumpent, conspicuous, white to light grey, darkening with age, fusoid to circular, containing 1–15(–20) distinctly protruding ostioles. Entostroma crumbly, brownish. Ostioles erumpent, cylindrical, black. Perithecia black, 400–600 μm diam. Asci broadly fusoid, 60–100 × 16–22 μm, containing 8 bi- to triseriate ascospores; apex without a distinct ring. Ascospores hyaline, fusoid to oblong, (20.5–)24–30(–34) × (6.5–)8–9.5(–10.5) μm, l/w = (2.3–)2.8–3.5(–4.1) (n = 111), with 3–4 eusepta, not to slightly constricted at septum, multiguttulate, with rounded ends and hyaline tapering appendages at both ends projecting for 5–7 μm, 3–4.5 μm wide at the base. Asexual morph unknown.
Distribution — Europe.
Habitat & Host range —Corticated dead branches of Acer pseudoplatanus.
Selected specimen examined (all on Acer pseudoplatanus). Without collection data, Herb. F. Currey (K(M) 188101, K(M) 188102, K(M) 188110, K(M) 188111); Herb. M.C. Cooke (K(M) 188109). – AUSTRIA, Kärnten, St. Margareten im Rosental, shrubs in village area, grid square 9452/4, 4 Apr. 2008, W. Jaklitsch W.J.3187 (WU 32447); same area, 22 Jan. 1995, W. Jaklitsch W.J. 465 (WU 32118); Trieblach, above Cihuc, grid square 9452/2, 14 Apr. 2001, W. Jaklitsch W.J. 1739 (WU 32121); Niederösterreich, Schwarzensee, grid square 7962/3, 25 Feb. 1996, W. Jaklitsch W.J. 822 (WU 32119); Wien, Grinzing, Himmelstraße, grid square 7763/2, 18 June 2003, W. Jaklitsch W.J. 2255 (WU 32122); Unterer Reisenbergweg, grid square 7763/2, 17 Apr.1999,W. Jaklitsch W.J. 1303 (WU 32120). – UK, England, East Bergholt, Sept. 1855, without collector, Herb. F. Currey (K(M) 188106, K(M) 188108); same area, Oct. 1855, without collector, Herb. F. Currey (K(M) 188107).
Notes — Calosporella innesii is characterised by hyaline, mostly 3-septate ascospores with small apical appendages and has been transferred to Prosthecium by Wehmeyer (1941) based on its phragmospores with apical appendages. This generic classification has subsequently been commonly followed. Barr (1978) argued that the correct epithet for the taxon should be Prosthecium platanoidis due to priority, but the type collection of this species is apparently not congeneric with the current fungus (see notes above). The name to be applied for the species is therefore C. innesii.
In the original description, Currey (1858) did not provide any details about collections. There are numerous authentic collections of the Currey herbarium in K, one of which is here selected as lectotype. To ensure nomenclatural stability, a recent well-developed specimen for which a culture is available is here selected as epitype. The appendages can be seen well in water mounts even from old herbarium specimens, but they quickly disappear in KOH mounts.
Phaeodiaporthe Petr., Ann. Mycol. 17, 2/6: 99. 1920 (‘1919’)
Type species. Phaeodiaporthe keissleri Petr., Ann. Mycol. 17, 2/6: 99. 1920 (‘1919’).
Notes — Petrak (1919) erected the genus Phaeodiaporthe as a ‘Diaporthe with dark spores’, but later he (Petrak 1921) considered it as a synonym of Melanconiella. However, phylogenetic analyses show that Phaeodiaporthe is neither closely related to Melanconiella nor to Prosthecium, but it is placed within Diaporthaceae where it forms a distinct clade (Fig. 1). Therefore we reinstate the genus Phaeodiaporthe here.
Phaeodiaporthe appendiculata (G.H. Otth) Lar.N. Vassiljeva, Pyrenomycetes of the Russian Far East, 2. Valsaceae (Vladivostok): 29. 1994; Fig. 12
Fig. 12.
Phaeodiaporthe appendiculata. a, d, e, g. Ectostromatic discs and ostioles in surface view; b. pseudostroma in vertical section; c, f, h. pseudostromata in transverse section, showing perithecia, whitish to brownish entostromata and faint blackish marginal zones; i, j. mature dead asci with apical ascal ring in i; k–r. living ascospores with blunt gelatinous appendages; s–ac. dead ascospores with blunt gelatinous appendages with i, k–r, y–aa in water and j, s–x, ab, ac in 3 % KOH (a–c, i. WU 32449 (epitype); d, e, f, j, s–x. B 700021801 (holotype of Diaporthe appendiculata); g, h, y–ac (lectotype of Phaeodiaporthe keissleri); k–r. WU 32448). –– Scale bars: a–c, e, f, h = 0.5 mm; d, g = 1 mm; i, j = 20 μm; k–ac = 10 μm.
Basionym. Diaporthe appendiculata G.H. Otth, Mitth. Naturf. Ges. Bern: 100. 1871 (‘1870’).
≡ Melanconiella appendiculata (G.H. Otth) Sacc., Syll. Fung. (Abellini) 11: XXIX. 1895.
≡ Melanconis appendiculata (G.H. Otth) Wehm., Revision of Melanconis, Pseudovalsa, Prosthecium & Titania, Univ. Michigan Stud., Scientific Ser. 14: 60. 1941.
≡ Prosthecium appendiculatum (G.H. Otth) M.E. Barr, Mycol. Mem. 7: 187. 1978.
= Phaeodiaporthe keissleri Petr., Ann. Mycol. 17, 2/6: 99. 1920 (‘1919’).
Typification. AUSTRIA, Wien, Donaustadt, Lobau, Panozzalacke, grid square 7865/1, 8 Mar. 2008, H. Voglmayr D77 (WU 32449, epitype of Diaporthe appendiculata and of Phaeodiaporthe keissleri here designated; ex-epitype culture CBS 123821; MBT176659). – CZECH REPUBLIC, Mährisch-Weißkirchen (Hranice na Moravě), park of the military school, dead twigs of Acer sp., 6 Feb. 1919, F. Petrak 3662, Flora Bohemiae et Moraviae exsiccata. Vol. 33, Ser. II - Abt. 1: Pilze 1621 (distributed as Melanconiella appendiculata; W, lectotype of Phaeodiaporthe keissleri here designated, MBT176657). – SWITZERLAND, Bern, on dead twigs of Acer platanoides from late autumn to spring, without date, G. Otth 29 (as Valsa appendiculata; B 700021801, holotype of Diaporthe appendiculata).
Pseudostromata c. 1–3 mm diam, pustulate in face view, containing up to 10 perithecia, often confined by a faint blackish marginal zone. Ectostromatic disc brown to blackish, circular, erumpent through a cortical rupture, containing 2–12 ostioles. Entostroma whitish to brownish. Ostioles erumpent, convergent, cylindrical to conic, black. Perithecia 450–800 μm diam, black. Asci clavate to broadly fusoid, (145–)150–178(–190) × (27–)29–38(–44) μm (n = 22), containing 8 biseriate ascospores; apex with a distinct ring when fresh. Ascospores dark to blackish brown, ellipsoid to oblong, (26–)31–38(–43) × (12.5–)14–17(–19.5) μm, l/w = (1.8–)2.0–2.4(–2.9) (n = 212), with 1 euseptum, constricted at septum, distinctly multiguttulate, with rounded ends and blunt, hyaline, cap-like appendages at both ends projecting for 2.5–8 μm and 5–7 μm wide at the base. Asexual morph unknown.
Distribution — Europe.
Habitat & Host range —Corticated dead branches of Acer campestre and A. platanoides.
Additional specimens examined (all on corticated twigs of Acer campestre). AUSTRIA, Wien, Ottakring, Wilhelminenberg, grid square 7763/4, 9 Mar. 2008, H. Voglmayr D76 (WU 32448, culture CBS 123809); Mauer, Maurer Wald, grid square 7863/1, 4 Nov. 2000, W. Jaklitsch; Niederösterreich, Hagenbrunn, Bisamberg-east side, grid square 7664/3, 1 Nov. 2000, W. Jaklitsch W.J. 1690 (WU 32110).
Notes — Phaeodiaporthe appendiculata has been classified within various genera, which shows the uncertainties about its phylogenetic affiliation. Petrak (1919) described this species as Phaeodiaporthe keissleri, giving Aesculus or Acer as possible hosts. Later, Petrak (1921) recognized P. keissleri to be synonymous with Diaporthe appendiculata, which he classified as Melanconiella appendiculata based on some morphological similarities to M. spodiaea. He subsequently distributed the type collection of P. keissleri as Melanconiella appendiculata in his Flora Bohemiae et Moraviae exsiccata, from which the copy in W is here designated as lectotype. Subsequently, Wehmeyer (1941) who did not accept Melanconiella as a distinct genus, transferred it to Melanconis, while Barr (1978) argued for inclusion in Prosthecium, despite the 2-celled ascospores and the conspicuous refractive apical ring in the ascus in P. appendiculata. Vassilyeva (1994) formally transferred Diaporthe appendiculata to Phaeodiaporthe. A recent well-developed specimen, for which a culture and an ITS-LSU sequence are available, is here selected as epitype of both Diaporthe appendiculata and Phaeodiaporthe keissleri to stabilize nomenclatural connection of both names. The appendages are highly refractive in water mounts even from old herbarium specimens, but they are less distinct in KOH mounts.
DISCUSSION
Phylogeny and nomenclature of Stegonsporium and Stilbospora
While Stegonsporium forms a highly supported monophylum in the LSU analyses, Stilbospora is not resolved as monophyletic but as paraphyletic basal to Stegonsporium. However, this topology receives no bootstrap support, and only a single additional step is required in the MP analyses to resolve Stilbospora as a monopyhletic sister clade to Stegonsporium. This indicates that the LSU alone does not always contain enough phylogenetic resolution to reveal reliably well-supported phylogenetic relationships on the generic level. Morphologically, Stilbospora is a homogeneous genus; both ascospores as well as conidia are euseptate and highly similar in all species (Fig. 6, 7, 8), and the main diagnostic features of Stilbospora species are their differently shaped ascospore appendages. In addition, all Stilbospora species studied so far grow on hosts belonging to the genus Carpinus, whereas the species of Stegonsporium are found on Acer section Acer. Furthermore, all species of Stegonsporium have distoseptate ascospores and conidia. Contrary to the LSU analysis, the status of Stilbospora and Stegonsporium as distinct sister clades is highly supported by ITS, tef1 and rpb2 sequence data (Fig. 2, 3, 4). These morphological, ecological and molecular phylogenetic arguments strongly support the recognition of Stilbospora and Stegonsporium as distinct genera.
Many species of Stilbospora and Stegonsporium have recently been classified within Prosthecium based on their teleomorphs (Voglmayr & Jaklitsch 2008). However, the recent changes of the international code of nomenclature (ICN; McNeill et al. 2012) for unified nomenclature, the nomenclatural status of Prosthecium, Stilbospora and Stegonsporium as competing genera has to be re-evaluated. Based on priority, Stilbospora takes precedence over Prosthecium. As this genus is well-known and well-defined, there is little reason to argue for retaining Prosthecium via conservation, and the latter is therefore relegated into synonymy of Stilbospora.
The current study revealed two new Stilbospora species, which are distinct morphologically as well as phylogenetically. Sister group relationship of S. longicornuta to S. orientalis is highly supported in ITS, rpb2 and tef1 analyses, which is also in line with morphology, as both share long, tapering ascospore appendages, compared to the short cap-like appendages of S. macrosperma.
Including significantly more accessions as well as an additional sequence marker (rpb2), the current study confirms the findings of Voglmayr & Jaklitsch (2008) that Stegonsporium pyriforme consists of three phylogenetically separate entities, which biologically clearly represent distinct species. No significant morphological or ecological differences between these three entities could be found, rendering them cryptic species. We previously refrained from establishing formal names for them (Voglmayr & Jaklitsch 2008); however, considering the genetic homogeneity within and the high genetic distances between these three ‘S. pyriforme’ clades, which are even higher than between the morphologically clearly distinct S. acerinum, S. acerophilum and S. opalus (Fig. 3, 4), we suggest that these three lineages should not be retained within a single species. Thus, we recognize them here as three distinct species, S. pyriforme s.str., S. protopyriforme and S. pseudopyriforme, based on their differences in DNA sequences.
Generic reclassification of Prosthecium appendiculatum, P. auctum and P. innesii
Within Prosthecium Barr (1978) recognised P. appendiculatum, P. auctum and P. innesii (as P. platanoidis) based on ascospore septation and appendages. According to our phylogenetic analyses, P. appendiculatum, P. auctum and P. innesii are neither closely related to the generic type, P. ellipsosporum (= Stilbospora macrosperma) nor to each other, as they fall within Diaporthaceae, Gnomoniaceae and Sydowiellaceae, respectively. Within these families their closest relatives could not be revealed by molecular phylogenetic analyses due to lack of backbone support. In addition, their distinctive morphological characters do not match other genera. Therefore we classify these three species in three separate genera, viz. Phaeodiaporthe, Alnecium and Calosporella.
New host and species records
The North American Stegonsporium acerophilum is here first recorded for Europe from Acer saccharum and its close relative A. grandidentatum grown in parks (arboreta) in the UK and the Czech Republic. This is remarkable, as both species are rarely planted outside arboreta, which means that individual trees are spatially highly separated from each other. Limited dispersal abilities of the large conidia and ascospores in combination with highly localized occurrence of its hosts indicate that the parasite may have been dispersed as an endophyte by transporting living trees. Distinct host specificity of Stegonsporium species was corroborated by the fact that indigenous neighbouring Acer pseudoplatanus trees were only infected by Stegonsporium pyriforme s.l., which has never been observed on A. saccharum.
Also Stegonsporium opalus co-occurs with its hosts, Acer opalus and its close relative, A. obtusatum, as it is recorded from France as well as the UK, the latter being far outside the natural range of its hosts. In the present study we recorded S. opalus for the first time also from Acer hyrcanum in Austria. Stegonsporium opalus from Acer hyrcanum, A. obtusatum and A. opalus formed a homogeneous lineage in the phylogenetic analyses of rpb2 and tef1 sequences (Fig. 3, 4). In addition, S. opalus is recorded here for the first time on Acer monspessulanum from France and on the eastern mediterranean A. sempervirens from Crete (Greece). The isolate from A. sempervirens was genetically distinct in its ITS, tef1 and rpb2 sequences from S. opalus originating from Acer hyrcanum, A. obtusatum and A. opalus (Fig. 2, 3, 4). The French isolate from A. monspessulanum had a slightly deviating tef1 sequence, while its ITS and rpb2 sequences were identical to those of typical S. opalus (Fig. 2, 3, 4).These differences are considered to be within the intraspecific variability of S. opalus, but may indicate some evolutionary differentiation on these hosts.
New hosts were also recorded for Stegonsporium galeatum (A. heldreichii; Scotland) as well as for the various Stegonsporium pyriforme lineages: Acer monspessulanum (Croatia) as well as Acer heldreichii (UK) are new hosts for S. pyriforme s.str., and Acer heldreichii and A. velutinum (both from UK) are new hosts for S. pseudopyriforme. However, it remains unclear whether these Acer species are regular hosts for these Stegonsporium species, as they have not yet been sampled within their natural distribution range but only in arboreta in the UK.
Acknowledgments
Irmgard Greilhuber is gratefully acknowledged for organising collecting trips, Christian Scheuer (GZU) and Anton Igersheim (W) for information on the type collection of Phaeodiaporthe keissleri, József Geml (L) for providing information on the type collection of Stilbospora macrosperma, the fungarium curators of K and B for sending type specimens, Walter Till (WU) for handling herbarium loans and Chris Yeates for providing literature.
REFERENCES
- Barr ME. 1978. The Diaporthales of North America. Mycological Memoirs 7: 1–232. [Google Scholar]
- Berkeley MJ, Broome CE. 1852. Notices of British fungi. Annals and Magazine of Natural History, Series 2, 9: 317–329 (nos 615–639). [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WM, Vasilyeva LN. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Clements FE, Shear CL. 1931. Genera of Fungi. 2nd ed Wilson, New York, USA. [Google Scholar]
- Crous PW, Groenewald JZ, Lombard L, Wingfield MJ. 2012. Homortomyces gen. nov., a new dothidealean pycnidial fungus from the Cradle of Humankind. IMA Fungus 3: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey F. 1858. (‘1859’). Synopsis of the fructification of the compound Sphaeriae of the Hookerian herbarium. The Transactions of the Linnean Society of London 22: 257–287, pl. 45–49. [Google Scholar]
- Fries E. 1832. Systema Mycologicum, vol. 3. Ernestus Mauritius, Greifswald, Germany. [Google Scholar]
- Gomes RR, Glienke C, Videira CIR, Lombard l, Groenewald JZ, Crous PW. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hoffmann GF. 1795 Deutschlands Flora oder Botanisches Taschenbuch. 2. Theil für das Jahr 1795. Cryptogamie. Johann Jakob Palm, Erlangen, Germany. [Google Scholar]
- Höhnel F. 1918. Mycologische Fragmente. Annales Mycologici 16: 35–174. [Google Scholar]
- Holm L. 1975. Nomenclatural notes on pyrenomycetes. Taxon 24: 475–488. [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. 2006. (‘2005’). Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2004. Hapalocystis occidentalis - a new species of Diaporthales from North America and a key to the species of Hapalocystis. Studies in Mycology 50: 229–234. [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. 2001. Ainsworth & Bisby’s Dictionary of the Fungi, 9th edn CAB International, Wallingford, UK. [Google Scholar]
- Link HF. 1826. Entwurf eines phytologischen Pflanzensystems nebst einer Anordnung der Kryptogamen. Abhandlungen der königlichen Akademie der Wissenschaften zu Berlin aus dem Jahre 1824: 145–194. [Google Scholar]
- Liu YL, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, et al. 2012 International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International BotanicalCongress Melbourne, Australia, July 2011. Regnum Vegetabile 154. Koeltz, Koenigstein, Germany. [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF., Jr 2008. Phylogenetic placement and taxonomic review of the genus Cryptosporella and its synonyms Ophiovalsa and Winterella (Gnomoniaceae, Diaporthales). Mycological Research 112: 23–35. [DOI] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF., Jr 2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68: 211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalvo JM, Wang HH, Hseu RS. 1995. Phylogenetic relationships in Ganoderma inferrred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87: 223–238. [Google Scholar]
- Monod M. 1983. Monographie taxonomique des Gnomoniaceae I. Beihefte zur Sydowia 9: 1–315. [Google Scholar]
- Persoon CH. 1801. Synopsis Methodica Fungorum, vol.1 Henricus Dieterich, Göttingen, Germany. [Google Scholar]
- Petrak F. 1919. Mykologische Notizen. I. Annales Mycologici 17: 59–100. [Google Scholar]
- Petrak F. 1921. Mykologische Notizen. II. Annales Mycologici 19: 17–128. [Google Scholar]
- Petrak F. 1923. Mykologische Notizen VI. Annales Mycologici 21: 182–335. [Google Scholar]
- Saccardo PA. 1883. Sylloge Fungorum, vol. 2 Saccardo, Pavia, Italy. [Google Scholar]
- Schröter J. 1897. (‘1908’). Pilze. In: Cohn F. (ed), Kryptogamen-Flora von Schlesien 3.2. Kern, Breslau, Germany. [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF., Jr 2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sutton BC. 1975. Coelomycetes. V. Coryneum. Mycological Papers 138: 1–223. [Google Scholar]
- Sutton BC. 1980 The coelomycetes. Fungi imperfecti with Pycnidia acervuli and stromata. CABI Publishing, Kew, Surrey, England. [Google Scholar]
- Swofford DL. 2002 PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]
- Vassiljeva LN. 1994 Pyrenomycetes of the Russian Far East. 2. Valsaceae. Institute of Biology and Pedology, Far East Branch, Russian Academy of Sciences, Vladivostok, Russia. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch W. 2008. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch W. 2011. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae). Fungal Diversity 46: 133–170. [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, Jaklitsch W. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Warmelo KT van, Sutton BC. 1981. Coelomycetes VII. Stegonsporium. Mycological Papers 145: 1–45. [Google Scholar]
- Wehmeyer LE. 1941. A revision of Melanconis, Pseudovalsa, Prosthecium and Titania. University of Michigan Studies, Scientific Series 14: 1–161 (repr. Bibl. Mycol. 41 (1973), Cramer, Lehre). [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990 Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA. [Google Scholar]
- Winter G. 1887. Ascomyceten: Gymnoasceen und Pyrenomyceten. Rabenhorst Kryptogamenflora 2: 1–928. [Google Scholar]